Abstract
Conjugate addition of organometallics to carbonyl based Michael acceptors is a widely used method that allows the building of new carbon-carbon (C-C) bonds and the introduction of chirality in a single step. However, conjugate additions to the simplest Michael acceptors, namely unprotected, unsaturated carboxylic acids, are considered to be prohibited by the fact that acid-base reactions overpower any other type of reactivity, including nucleophilic addition. Here we describe a transient protecting group strategy that allows efficient catalytic asymmetric additions of organomagnesium reagents to unprotected α,β-unsaturated carboxylic acids. This unorthodox pathway is achieved by preventing the formation of unreactive carboxylate salts by means of a reactive intermediate, allowing modifications of the carbon chain to proceed unhindered, while the stereochemistry is controlled with a chiral copper catalyst. A wide variety of β-chiral carboxylic acids, obtained with excellent enantioselectivities and yields, can be further transformed into valuable molecules through for instance catalytic decarboxylative cross-coupling reactions.
Subject terms: Asymmetric catalysis, Synthetic chemistry methodology, Homogeneous catalysis
Unprotected carboxylic acids are classically deemed to be unsusceptible to conjugated additions due to acid-base neutralization. Here, the authors show that the use of an appropriate Lewis acid allows the catalytic addition of organometallics to unprotected carboxylic acids, also in an asymmetric fashion.
Introduction
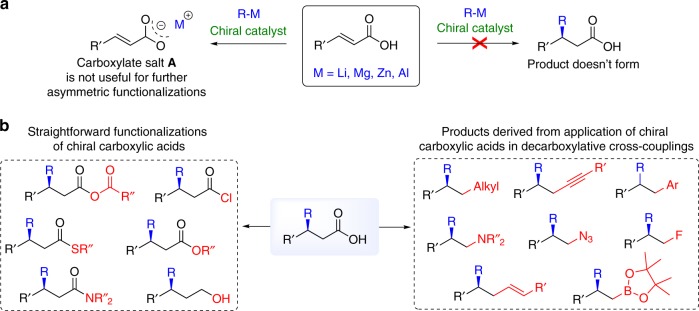
Unprotected carboxylic acids are essential constituents of biologically active compounds and essential precursors in the synthesis of numerous useful derivatives1–3. As such, they are often produced industrially and used in the production of polymers, pharmaceuticals, solvents, and food additives. One of the simplest ways to generate target carboxylic acids would exist of taking simple, readily available variants, and modifying the carbon chain by, for example, introducing functional groups, forming additional carbon–carbon bonds, and introducing chirality. This implies (asymmetric) conjugate addition of organometallics, a highly important and widely used method, that allows the introduction of carbon–carbon (C–C) bonds and chirality in a single step4–7. However, applying this method to unprotected α,β-unsaturated carboxylic acids is inhibited by a fundamental problem, namely that upon mixing with common organometallics the acidity of the carboxylic acids and the basicity of the organometallics invariably leads to deprotonation and the formation of carboxylate salts, as the organometallic functions primarily as a base instead of as a nucleophile (Fig. 1a). Once the salt is formed, further organic reactions become unfeasible because of the inherent low reactivity and insolubility of salts in organic solvents. Consequently, even though the first effort to do this, in a non-enantioselective manner, dates back to 19538, and despite many further attempts9–13, to our knowledge no examples of direct applications of unsaturated carboxylic acids in either catalytic or stoichiometric enantioselective reactions with hard organometallics, nor with organoboron or organosilicon reagents, are known.
Fig. 1.
State of the art in conjugate additions to unsaturated carboxylic acids and potential value of addition products. a Fundamental problem that prevents the development of conjugate additions to unprotected unsaturated carboxylic acids: mixing of organometallics with carboxylic acids leads to an acid–base reaction, resulting in a carboxylate salt A nearly unreactive toward further reactions. b Overview of transformations of the carboxylic acid functional group leading to its chiral derivatives and new structural motives: carboxylic acids can undergo straightforward functionalization and decarboxylative cross-coupling reactions
This is unfortunate, given that carboxylic acids are not just the main precursors of various carbonyl compounds and common components of biologically active compounds but, perhaps even more importantly, because of their potential for application in decarboxylative coupling reactions that have witnessed tremendous progress in recent years and would allow access to a variety of β-chiral functionalized molecules in a simple manner (Fig. 1b)14–19. Therefore, developing a successful strategy for asymmetric conjugate additions to carboxylic acids would also allow direct access to β-chiral carboxylic acids and other chiral functional molecules, starting from simple substrates and without any derivatizations or protecting and deprotecting steps.
So far, chiral β-substituted carboxylic acids are mainly obtained by kinetic resolution or asymmetric hydrogenation reactions20–23. However, these methods are often limited to aryl substituents in the β-position of the substrate, require precious transition metal catalysts and make use of molecular frameworks, in which all the carbons have already been preinstalled. Thus, all C–C bonds must be formed in preceding reactions. Another common, indirect way is through asymmetric additions to premade α,β-unsaturated esters, thioesters, or amides using chiral catalysts or chiral auxiliaries, followed by a hydrolysis step4–7,24–28.
Our goal was to develop a general platform for direct catalytic synthesis of enantiopure β-substituted carboxylic acids from simple carboxylic acid-building blocks via C–C bond-forming reactions. Making this possible via additions of organometallics directly to unprotected α,β-unsaturated carboxylic acids would present a unique and important step forward in organic synthesis, but requires circumventing the fundamental issue of the acid–base reactions hindering the desired carbon–carbon bond-forming process.
Here, we describe a strategy that allows highly efficient direct catalytic asymmetric additions of organomagnesium reagents to unprotected α,β-unsaturated carboxylic acids by preventing the formation of unreactive carboxylate salts. This allows modifications of the carbon chain to proceed unhindered, while the stereochemistry is controlled with a chiral copper catalyst. The catalytic system is scalable, does not require cryogenic temperatures, does not rely on precious metals, and allows the catalyst to be reused.
Results
Racemic reaction development
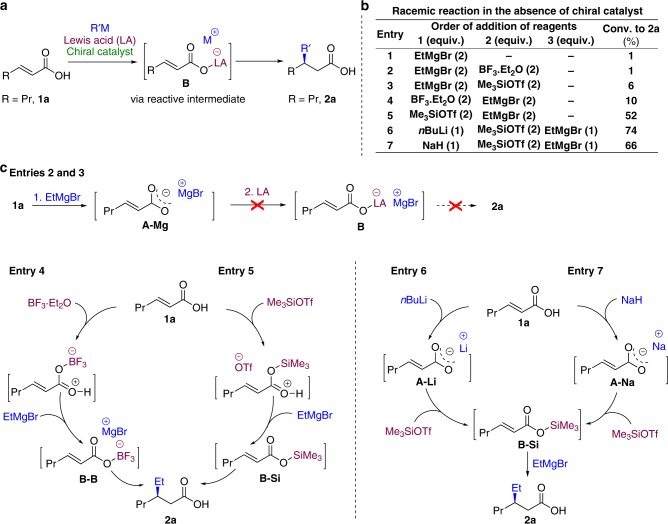
We realized that the fundamental issue of the acid–base reactions leading to the formation of unreactive carboxylate salts could be circumvented by in situ formation of a reactive intermediate B from or instead of the carboxylate salt A (Fig. 2a). This would require an intermediate that is reactive toward enantioselective conjugate additions, is easily formed under organometallic addition reaction conditions and would lead to the final carboxylic acid product without additional chemical reactions, simply upon reaction quenching or product isolation29,30. We set out to identify a compound that fulfils all of these stringent requirements, drawing on our past experience with combining Lewis acids (LA) and highly reactive organometallics in conjugate additions28,31,32. We speculated that using common LA such as trimethylsilyl triflate (Me3SiOTf, OTf = OSO2CF3) or boron trifluoride (BF3·Et2O) as electrophiles might lead to the formation of soluble and unstable silyl intermediates or boron intermediates, with anticipated high reactivity toward conjugate addition of Grignard reagents and can lead directly to the final unprotected carboxylic acid product. This particular choice of LA was based on our previous studies, where these LA were found to be compatible with Grignard reagents and successfully used to enhance reaction rates with various electrophiles28,31,32.
Fig. 2.
Reaction development. a Our approach, based on the use of Lewis acid to promote in situ formation of a transient intermediate B that can undergo conjugate addition of organometallic reagent. b Conjugate addition of EtMgBr to the substrate 1a in the absence of chiral catalyst with varying conditions. c Rationalization of the experimental data in entries 1–7 obtained for conjugate addition of EtMgBr in various conditions
To address the feasibility of this strategy, we first investigated the noncatalyzed addition of EtMgBr to trans-2-hexenoic acid 1a (Fig. 2b). At 0 °C, a complex mixture of products (including 20% of 2a) was observed in the absence of any catalyst or additives, indicating that the system is dominated by various side reactions at such a relatively high temperature. As expected, no conversion of the substrate 1a was seen at −55 °C (Fig. 2b, entry 1), because only the Mg-carboxylate A–Mg was formed and precipitated out of the reaction mixture (and hydrolyzed back to the substrate during quenching of the reaction). We proceeded by adding the reactive LA Me3SiOTf or BF3·Et2O to the mixture, hoping they would react with the Mg-carboxylate A–Mg to form a more reactive boron or silyl intermediate (B–Si or B–B depending on the Lewis acid used), but unfortunately this only had a minor effect (Fig. 2b, c, entries 2 and 3). Anticipating poor reactivity of the Mg-carboxylate A–Mg toward the silyl and boron electrophiles, we decided to add the latter first and EtMgBr second, and to our delight this yielded the addition product 2a with 10% conversion with BF3·Et2O and 52% conversion with Me3SiOTf (Fig. 2b, entries 4 and 5, respectively). The much higher conversion toward the addition product 2a obtained with Me3SiOTf can be rationalized by the higher electrophilicity of the latter in comparison with BF3·Et2O.
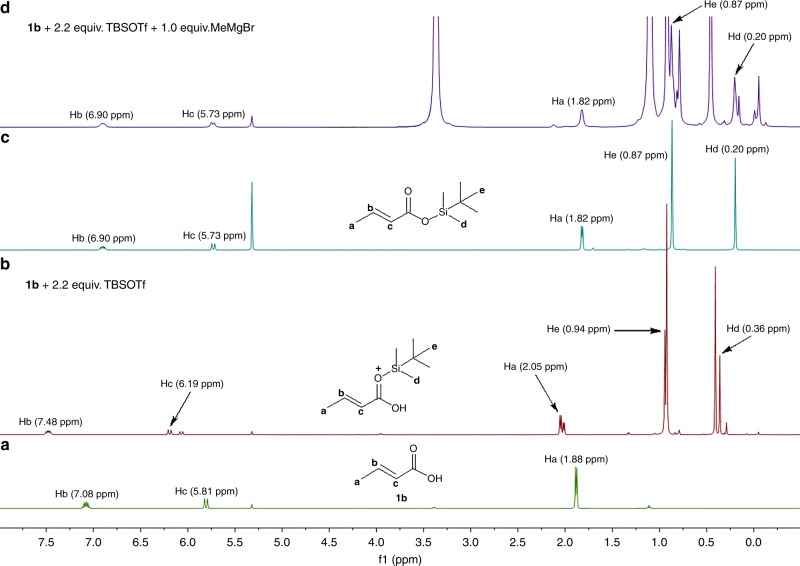
We believe that the sequence of addition of the reagents is important, because the nucleophilicity of the Mg-carboxylate A–Mg is insufficient to react with the boron or silyl electrophile (presumably due to aggregates formation), and thus nearly no reactive intermediates are generated (Fig. 2b, c, entries 2 and 3). Conversely, when either Lewis acid is added first, it combines with the carboxylic acid 1a to form an initial complex, after which addition of EtMgBr leads to abstraction of the proton and concomitant formation of intermediates B–B or B–Si (depending on the Lewis acid used), that are reactive toward conjugate addition (Fig. 2b, c, entries 4 and 5, respectively). Hence, the formation of the Mg-carboxylate A–Mg is avoided, and conjugate addition to form the product 2a can proceed. To get further support for this rationalization, we attempted to observe both the silyl and the boron intermediates derived from Me3SiOTf and BF3·Et2O, respectively, using 1H NMR spectroscopy (Fig. 3). However, we failed to do so due to the apparent low stability of the Me3Si- and BF3 intermediates. To overcome this problem, we decided to switch to a more bulky tBuMe2Si intermediate, which is expected to be more stable and could derive from tBuMe2SiOTf. First, we carried out the conjugate addition reaction in the same conditions as those of entry 5 in Fig. 2b, using tBuMe2SiOTf instead of Me3SiOTf. Similar results were obtained in terms of conversion to the addition product, but the product was obtained as a mixture of protected and free carboxylic acid 2a. We then carried out 1H NMR spectroscopic experiments using tBuMe2SiOTf and crotonic acid 1b instead of 1a in order to have simpler 1H NMR spectra (Fig. 3). NMR spectroscopic measurements were carried out for four samples derived from (a) crotonic acid 1b; (b) mixing 1b with 2.2 equiv. of tBuMe2SiOTf; (c) synthesized and isolated tBuMe2Si-ester of 1b; and (d) mixing 1b with 2.2 equiv. of tBuMe2SiOTf followed by addition of 1.0 equiv. of MeMgBr. Upon addition of tBuMe2SiOTf to 1b, a new species appeared, corresponding to a complex between the two reagents (Fig. 3b). Addition of 1 equiv. of MeMgBr (just enough to deprotonate but not to be added) led to the formation of the tBuMe2Si-ester of 1b with signals comparable with that of independently synthesized and isolated tBuMe2Si-ester of 1b (compare Fig. 3c, d). These results confirm indeed that tBuMe2SiOTf allows in situ protection of 1a, and that Me3SiOTf acts as a traceless protecting group for carboxylic acids and enables the conjugate addition reaction. We assume that BF3·Et2O works in a similar manner to Me3SiOTf. The importance of our strategy of in situ generation of transient intermediates is further highlighted by the difficulties to access pure isolated BF3- and TMS-protected unsaturated carboxylic acids. Our attempts to synthesize and isolate these compounds independently (even more bulky and stable silyl esters) resulted in a very low yields (below 20%) and tedious procedures for purification.
Fig. 3.
1H NMR experiments carried out in CD2Cl2 at −55 °C using substrate 1b, tBuMe2SiOTf and MeMgBr. a Crotonic acid 1b. b Mixture of 1b with 2.2 equiv. of tBuMe2SiOTf. c Isolated pure tBuMe2Si-ester of 1b. d Mixture of 1b with 2.2 equiv. of tBuMe2SiOTf followed by addition of 1.0 equiv. of MeMgBr
To investigate whether carboxylate salts are in general unreactive toward the formation of silyl or boron intermediate or whether this depends on the nature of the metal ion, Li and Na-carboxylates A–Li and A–Na were prepared from nBuLi and NaH, respectively, and subjected to the reaction with Me3SiOTf and EtMgBr (Fig. 2b, c, entries 6 and 7). Now the silyl intermediate B–Si was formed in both cases, and addition of 1 equiv. of EtMgBr resulted in the formation of the product 2a with 74 and 66% conversions, respectively (Fig. 2b, c, entries 6 and 7). These results indicate that once the intermediate B–Si is formed, conjugate addition works well, but that when the metal carboxylate A is formed first, the success of the reaction depends on the counter ions (Fig. 1c). Since metal-carboxylates A–Mg, A–Li, and A–Na are all poorly soluble and precipitate in the reaction solvent, the differing reactivities could also originate from solubility differences. However, we found that the solubility of Li-carboxylate A–Li and Na-carboxylate A–Na is lower than that of Mg-carboxylate A–Mg. More specifically, the solubilities of A–Mg and A–Na in tBuOMe are 0.4318 mM and 0.1225 mM, respectively, while solubility of A–Li is under 1H NMR detection limit (see Supplementary Figs. 1–3). Thus the difference in reactivity has nothing to do with a better solubility, and can be attributed to a higher nucleophilicity of the Li- and Na-carboxylate most likely. Possible explanation for such a difference in reactivity might be higher aggregation state of the Mg-carboxylate that diminishes its nucleophilicity.
Development of catalytic asymmetric reaction
Having established that the boron and silyl intermediates B–B and, especially, B–Si are indeed formed under the right conditions and lead to the racemic product 2a, we shifted our attention to the question whether this reaction system would be susceptible to asymmetric catalysis in order to both accelerate the conjugate addition toward higher yields and achieve enantiocontrol. As copper is known to be an efficient catalyst for asymmetric conjugate addition reactions5–7, we started our investigation by selecting various chiral ligands that can bind to Cu(I). As expected, initial experiments in CH2Cl2 showed no addition of the highly reactive EtMgBr to 1a when performing the reaction in the presence of 5 mol% of L1/Cu(I)-catalyst at −78 °C. Raising the temperature to 0 °C resulted in 79% conversion with only 20% toward noncatalyzed addition product 2a and many byproducts (Table 1, entries 1 and 2). At this point, we decided to investigate catalytic reactions in the presence of Me3SiOTf (via the formation of the most reactive intermediate B–Si) and copper complexes with various chiral diphosphine ligands in CH2Cl2 at −78 °C. Several chiral catalytic systems result in both acceleration of the conjugate addition and in significant enantiodiscrimination (Table 1, entries 3–7). The superior yield and enantioselectivity obtained with diphosphine Tol-BINAP ligand (R)-L4 (Table 1, entry 6) prompted us to select it as the optimal ligand for this reaction. Subsequently, a thorough optimization process was executed using the catalytic system derived from L4/CuBr·SMe2 involving the evaluation of various parameters and reaction conditions (for complete set of data see Supplementary Tables 1–5). In particular, the effect of different solvents was studied. With the exception of THF, all solvents tested were effectively tolerated, providing 2a with good yields and ee (Table 1, entries 8–11). We were especially pleased to find exceptionally high yield (95%) and enantioselectivity (92% ee) in tBuOMe (Table 1, entry 11).
Table 1.
Development of the catalytic system for direct asymmetric conjugate addition of EtMgBr to carboxylic acid 1aa
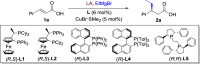
| ||||||
|---|---|---|---|---|---|---|
| Entry | L/Cu(I) | LA | Solvent | T [°C] | Conv. [%]b | ee [%]c |
| 1 | L1/Cu(I) | – | CH2Cl2 | −78 | 0 | – |
| 2 | L1/Cu(I) | – | CH2Cl2 | 0 | 79d | Rac |
| 3 | L1/Cu(I) | Me3SiOTf | CH2Cl2 | −78 | 74 | 47 |
| 4 | L2/Cu(I) | Me3SiOTf | CH2Cl2 | −78 | 70 | 9 |
| 5 | L3/Cu(I) | Me3SiOTf | CH2Cl2 | −78 | 72 | 47 |
| 6 | L4/Cu(I) | Me3SiOTf | CH2Cl2 | −78 | 87 | 56 |
| 7 | L5/Cu(I) | Me3SiOTf | CH2Cl2 | −78 | 75 | 47 |
| 8 | L4/Cu(I) | Me3SiOTf | THF | −78 | 100 | Rac |
| 9 | L4/Cu(I) | Me3SiOTf | Toluene | −78 | 62 | 80 |
| 10 | L4/Cu(I) | Me3SiOTf | Ether | −78 | 91 | 88 |
| 11 | L4/Cu(I) | Me3SiOTf | tBuOMe | −78 | 95 | 92 |
| 12e | L4/Cu(I) | tBuMe2SiOTf | tBuOMe | –78 | 95 | 95 |
| 13 | L4/Cu(I) | BF3·Et2O | tBuOMe | −78 | 19 | 92 |
| 14f | L4/Cu(I) | BF3·Et2O | tBuOMe | −78 | 77 | 97 |
| 15f | L4/Cu(I) | Me3SiOTf | tBuOMe | −78 | 99 | 97 |
| 16g | L4/Cu(I) | Me3SiOTf | tBuOMe | −78 | 100 | 95 |
| 17 | L4/Cu(I) | Me3SiOTf | tBuOMe | 0 | 95 | 88 |
| 18 | L4/Cu(I) | Me3SiOTf | tBuOMe | −20 | 97 | 97 |
aReaction conditions: 0.1 M of 1a, 5 mol% of CuBr·SMe2, 6 mol% of L, and 2–3 equiv. of LA followed by the addition of 2–3 equiv. of EtMgBr
bConversion was determined by NMR of reaction crude
cEnantiomeric excess was determined by chiral HPLC after transforming 2a to the corresponding N,N-dimethyl amide derivative
dLess than 20% of 2a formed with many other byroducts
eThe product was obtained as a mixture of silyl ester and free carboxylic acid in the ratio of 62:38, respectively
fThe reaction was performed by first forming Li-carboxylate with nBuLi followed by addition of corresponding LA and EtMgBr
gThe reaction was performed by first forming Na-carboxylate with NaH followed by the additions of Me3SiOTf and EtMgBr
In order to connect catalytic asymmetric addition with the results of our 1H NMR spectroscopic studies using tBuMe2SiOTf, we tested the addition of EtMgBr to substrate 1a using this Lewis acid. As expected, we found that the reaction proceeds with excellent conversion toward the addition product, providing the final product as a mixture of tBuMe2Si-ester and free carboxylic acid 2a in a ratio of 62:38, respectively, and high enantioselectivity (95% ee, Table 1, entry 12). This composition of the product mixture is not surprising, as under these reaction conditions tBuMe2Si-ester is expected to be relatively stable.
Catalytic reaction with L4/CuBr·SMe2 in tBuOMe was also investigated using BF3·Et2O as a Lewis acid. Although high level of enantiomeric purity (92% ee) could be obtained in this case, lower reactivity toward conjugate addition of Grignard reagent (only 19% of conversion to 2a), similar to that observed earlier for racemic reaction was also found in this case (Table 1, entry 13). In contrast, high yield and enantioselectivitiy can be obtained when using BF3·Et2O in combination with Li-carboxylate (formed by nBuLi in-situ) followed by addition of EtMgBr (Table 1, entry 14). Similar excellent results were found when using Me3SiOTf in combination with Li- and Na-carboxylates (formed by nBuLi and NaH, respectively) followed by addition of EtMgBr (Table 1, entries 15 and 16). However, Me3SiOTf was selected as the Lewis acid of choice for further studies because of the convenience of a procedure using only one organometallic reagent and highest conversion obtained (Table 1, entry 11). Since a temperature of −78 °C is not practical, particularly for large-scale synthesis, we evaluated the temperature as well (Table 1, entries 17 and 18), finding that −20 °C is the optimal temperature for the reaction with the Grignard reagent, the chiral L4/Cu(I) catalyst system, and Me3SiOTf. Under these optimized conditions, the reaction is completed in 2 h providing the final product 2a with 97% of conversion and an ee of 97% (Table 1, entry 17).
Scope of the reaction
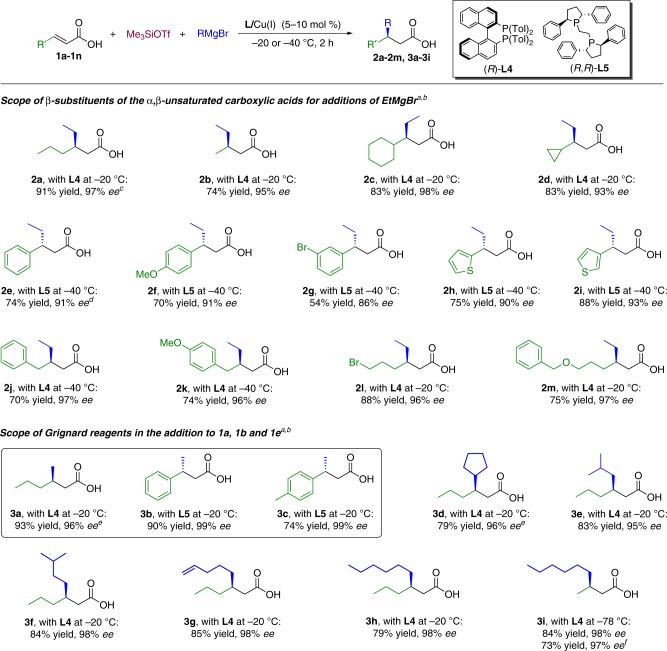
With the optimized conditions in hand, initial efforts to explore the scope of this transformation focused on investigating the effect of varying the carboxylic acid substitution at the β-position (Fig. 4). A wide variety of substrates allow efficient transformation to the corresponding chiral β-substituted carboxylic acids. The substrates with linear and branched aliphatic chains (including cyclohexyl and cyclopropyl) gave the corresponding addition products 2a–2d with high yields and excellent enantioselectivities. However, when we applied this condition to the aromatic substrate 1e, only 57% ee was obtained for product 2e.
Fig. 4.
Scope of the substrate and Grignard reagent. aFor details see Supplementary Information. Isolated yields for all the products are shown. The absolute configuration of the products obtained with (R)-L4 or (R,R)-L5 as the ligands are opposite. bReaction conditions: 0.1 M of the substrate in tBuOMe with 5 mol% (R)-L4/CuBr·SMe2 or in tBuOMe/toluene = 1/1 with 10 mol% (R,R)-L5/CuBr·SMe2, 2–3 equiv. of Me3SiOTf and RMgBr. cUsing 5 mol% (R,R)-L5/CuBr·SMe2 as a catalyst in the same condition led to 1a with 93% ee. dUsing 5 mol% (R)-L4/CuBr·SMe2 as a catalyst led to 2e with 57% ee. eThe reaction performed using 10 mol% of (R)-L4/CuBr·SMe2. fThe reaction performed at −40 °C
Further optimization (see Supplementary Table 5) revealed a different catalytic system to be optimal for aromatic substrates, based on diphosphine ligand (R,R)-L5 in combination with copper salt and lower temperatures. Using 10 mol% of L5/Cu(I) as the catalyst at −40 °C provided the product 2e with 91% enantiopurity and 74% isolated yield (Fig. 4).
An aromatic ring with an electron-donating (methoxy, 2 f) or electron-withdrawing group (Br, 2 g), as well as a heteroaromatic ring (2 h, 2i), are well tolerated, but including a m-Br-substituent in the aromatic ring led to the addition product 2g with lower yield (54%) and ee (86%). When the aromatic ring is at the γ-position, the substrates behave as aliphatic substrates, and the highest levels of ee and conversion are obtained with the catalyst L4/Cu(I) (products 2j and 2k). Finally, our catalytic system tolerates the presence of functional groups in the substrate, providing the corresponding products (2l, 2m) with high yields and ees above 96%. Next, we examined the nucleophile scope, starting our investigation with the smallest and least reactive of all Grignard reagents, MeMgBr (Fig. 4). Methylations are highly relevant for building chiral polymethylated arrays commonly found in natural products, but they pose difficulties because of the low reactivity of methylating reagents in general7,26,27. Currently, asymmetric addition of MeMgBr to α,β-unsaturated esters is only successful with aliphatic substrates26,27.
To our delight, our catalytic system solves this long-standing problem, as it works with similar efficiency for both aliphatic and aromatic substrates, leading to final methylated carboxylic acids with excellent yields and ees (3a, 3b, and 3c). All alkyl Grignard reagents afforded addition products with excellent results, independent of the chain length and branching. The sterically demanding α-, β-, and γ-branched Grignard reagents are tolerated, providing products 3d–3f with high yields and enantioselectivities (ees exceeding 95%). Grignard reagents bearing olefinic substituents also function well, affording the corresponding product 3g with excellent ee and yield. Products 3h and 3i, derived from additions of the linear Grignard reagent (nHexMgBr) to 1a and crotonic acid, respectively, were obtained with high enantiopurities and yields as well.
A few important practical aspects of this chemistry deserve to be highlighted (see Supplementary Table 6). First is the possibility to recycle the catalyst, which can be recovered from the reaction mixture with 83% isolated yield in the form of chiral Cu complex and reused in the next reaction with similar performance. Furthermore, the catalyst loading can be decreased from 5 to 1 mol% for addition reactions to aliphatic substrates, and the reaction can be carried out with similar outcome in 1 -g scale of the substrate. Finally, an additional benefit of this catalytic system is that when substrate conversion exceeds 97% most products can be obtained by simple acid–base extraction rather than time consuming column chromatography, which is important for large-scale industrial application7.
Application of the catalytic methodology
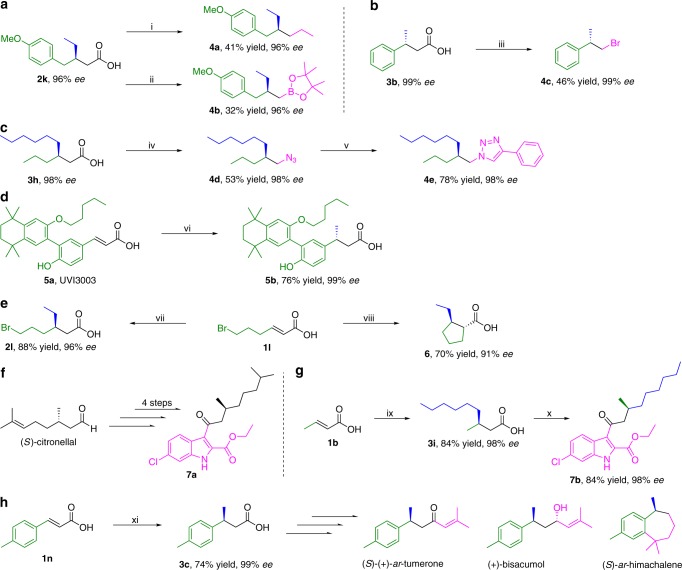
To showcase the potential of our catalytic protocol for future applications, we demonstrate that β-chiral substituted carboxylic acids can easily be transformed into a variety of valuable molecules (Fig. 5). The first applications that illustrate utility pertain to the use of our chiral products in stereoselective decarboxylative cross-coupling reactions. Decarboxylative cross-coupling reactions are developing very rapidly, and various catalytic systems utilizing aliphatic carboxylic acids (mainly achiral) leading to diverse structural motives have been established over the past decade14–19. Here, we demonstrate how useful chiral analogues of those structural motives can be obtained by combining our methodology and decarboxylative couplings (Fig. 5a–c). Nickel-catalyzed decarboxylative alkylation and borylation of product 2k afforded chiral alkane 4a and chiral β-substituted boronate ester 4b, maintaining the original enantiopurity of the starting material through the process (96% ee, Fig. 5a)16,17. Silver-catalyzed decarboxylative bromination of carboxylic acid 3b lead to the β-chiral alkyl bromide 4c with an ee of 99% (Fig. 5b)18, while Ag-catalyzed decarboxylative azidation of carboxylic acid 3h provided chiral β-substituted azide 4d, which was further transformed into chiral triazole 4e via click reaction, once again without any racemization (98% ee) (Fig. 5c)19. Although some similar molecules can be obtained via other catalytic asymmetric methodologies, these are often limited to specific structures and feature varying levels of enantioselectivities33,34. For example, chiral β-substituted boronate esters can also be obtained through transition metal catalyzed hydroboration of 1,1-disubstituted alkenes. However, these methods are only effective when an aryl group or directing carbonyl groups are present in the substrate. Applying β-substituted carboxylic acids obtained by our methodology in decarboxylative borylation offers an attractive alternative for accessing a wide range of chiral aliphatic β-substituted boronate esters.
Fig. 5.
Synthetic utility of the process. a Ni-catalyzed decarboxylative alkylation and borylation of chiral acid 2k. b Ag-catalyzed decarboxylative bromination of product 3b. c Ag-catalyzed decarboxylative azidation of chiral acid 3h followed by click reaction. d Late-stage functionalization of a RXR antagonist 5a. e Effect of the different procedures on the structure of the final asymmetric conjugate addition of EtMgBr to the carboxylic acid substrate 1l. f Reported synthetic route to a potent 15-lipoxygenase-1-inhibitor 7a. g Synthesis of the derivative 7b in two steps using current methodology. h Synthesis of chiral acid 3c, which is a key intermediate of several natural products. i) HATU, NEt3, NiCl2·glyme, 4,4'-di-t-butyl-2,2'-dipyridyl, ZnEt2, in DMF at RT; ii) N-hydroxyphthalimide, DCC, in CH2Cl2 at RT, 2h, then MgBr2·OEt2, NiCl2·6H2O, 4,4'-dimethoxy-2,2'-bipyridyl, [B2pin2Me]Li, in DMF, THF, at 0 °C 1 h ~ RT 1 h; iii) Ag(Phen)2OTf, dibromoisocyanuric acid, in 1,2-dichloroethane at 60 °C; iv) AgF, K2S2O8, MesSO2N3 in CH3CN, H2O, at 55 °C; v) phenylacetylene, CuTc, in toluene at RT; vi) CuBr·SMe2, (R,R)-L2, Me3SiOTf, MeMgBr, in tBuOMe:Toluene = 1:1, at −20 °C; vii) CuBr·SMe2, (R)-L1, Me3SiOTf, EtMgBr, in tBuOMe at −20 °C; viii) CuBr·SMe2, (R)-L1, nBuLi, Me3SiOTf, EtMgBr, in tBuOMe at −78 °C 2 h ~ RT 16 h; ix) CuBr·SMe2, (R)-L1, Me3SiOTf, nHexMgBr, in tBuOMe at −78 °C; x) SOCl2, DMF (1 drop), in CH2Cl2 at RT, 1 h, then ethyl 6-chloro-1H-indole-2-carboxylate, SnCl4, in CH2Cl2, reflux; xi) CuBr·SMe2, (S,S)-L2, Me3SiOTf, MeMgBr, in tBuOMe:Toluene = 1:1 at −20 °C
Our methodology is also sufficiently mild and robust to be applied in more complex molecules. For example, UVI3003 5a, a full antagonist of RXR (one of the retinoid receptors involved in the control of various physiological and pathological processes, including cancer and metabolic diseases) that demonstrates potent, nanomolar binding affinity35, can be functionalized successfully with our strategy without prior protection of the hydroxyl group and afford the product 5b with 76% yield and 99% ee (Fig. 5d). Another synthetically useful transformation available with our methodology is the trapping of enolate intermediates formed upon conjugate addition (Fig. 5e). For instance, while conjugate addition product 2l can be obtained with 88% yield and 96% ee, modifying the original procedure by using Li-carboxylate for asymmetric conjugate addition at −78 °C, followed by warming up to room temperature and stirring overnight, leads to intramolecular enolate trapping, affording the cyclic product 6 with contiguous stereocenters as a single diastereoisomer (70% yield, 91% ee). Recently, chiral indole derivative 7a, synthesized in four steps from the commercially available (S)-citronellal, was reported (Fig. 5f) to exhibit in vitro and ex vivo anti-inflammatory properties as a potent 15-lipoxygenase-1 inhibitor36. However, as such chiral aldehydes or carboxylic acids are rarely commercially available, synthesis of similar chiral compounds with variations of the alkyl chain is difficult, thus limiting the number of molecules available for bioactivity screening. With our methodology, a library of this type of compounds with different substituents at the β-position of the acyl group can be straightforwardly accessed in just two steps, as exemplified by the synthesis of 7b (Fig. 5g). Finally, our methodology allows us to effortlessly obtain the aromatic chiral β-substituted carboxylic acid 3c (Fig. 5h), which is a key intermediate for the synthesis of several natural products, like (S)-( + )-ar-tumerone, ( + )-bisacumol, and (S)-ar-himachalene37.
Discussion
We have shown that a wide range of β-chiral carboxylic acids are now synthetically accessible from their unsaturated analogues in one simple step and under mild conditions with high yields and enantioselectivities. Our strategy is based on activation of carboxylic acids via formation of transient silyl or boron intermediates, and it is crucial to overcoming the fundamental problem of carboxylate salt formation during the conjugate addition of organometallics to unsaturated carboxylic acid. Thus, this approach allows highly enantioselective catalytic C–C bond-forming reactions between organometallics and carboxylic acids without the use of separate protection/deprotection steps.
Methods
General procedure for the catalytic reaction
In a flame-dried Schlenk tube equipped with septum and magnetic stirring bar, the carboxylic acid substrate (0.2 mmol, 1.0 equiv.), CuBr·SMe2 (0.01 mmol, 5 mol%), and ligand L (0.012 mmol, 6 mol%) were dissolved in the solvent (2.0 mL) and stirred under nitrogen atmosphere for 20 min at RT. The mixture was cooled to −20 or −40 °C, and Me3SiOTf (0.44 mmol, 2.2 equiv.) was added. After 20 min, RMgBr (0.5 mmol, 2.5 equiv.) was added dropwise by hand in 10 min (syringe pump use is also an option), and the reaction mixture was allowed to stir for 2 h.
General work-up procedure A
Upon reaction completion, the mixture was quenched with HCl aqueous solution (2.0 mL, 1.0 M) and warmed up to RT. The resulting mixture was extracted with CH2Cl2 (10.0 mL × 3). The combined organic phase was dried over MgSO4, filtered, and the solvent was evaporated on a rotary evaporator. Pentane (1.0 mL × 3) was added to the residue, and the mixture was filtered through a cotton in a small glass pipette in order to remove the catalyst. The crude was purified by flash chromatography on the silica gel to yield the final conjugate addition product after solvent removal.
General work-up procedure B
Upon reaction completion, the mixture was quenched with saturated NaHCO3 aqueous solution (2.0 mL), warmed up to room temperature, and the organic phase was extracted. The organic phase was further extracted with saturated NaHCO3 aqueous solution (2.0 mL) for another two times. The combined aqueous phase was acidified with HCl aqueous solution (1.5 mL, 12.0 M), and extracted with CH2Cl2 (10.0 mL × 3). The combined organic phase was dried over MgSO4, filtered, and the solvent was evaporated on a rotary evaporator to yield the final conjugate addition product.
Procedure for the preparative-scale reaction
In a flame-dried three-neck round-bottom flask equipped with the septum and mechanistic stirring bar, the substrate 1a (1.14 g, 10.0 mmol, 1.0 equiv.), CuBr·SMe2 (102.8 mg, 0.5 mmol, 5 mol%), and ligand (R)-L4 (407.3 mg, 0.6 mmol, 6 mol%) were dissolved in tBuOMe (50 mL), and the mixture was stirred under nitrogen atmosphere for 20 min at RT. The mixture was cooled to −20 °C and Me3SiOTf (3.98 mL, 22 mmol, 2.2 equiv.) was added. After 20 min, EtMgBr (8.33 mL, 25 mmol, 2.5 equiv.) was added with the syringe pump in 20 min, and the reaction mixture was allowed to stir for another 2 h at −20 °C. The reaction was quenched with water (10.0 mL) and warmed to RT. The aqueous phase was discarded, and the organic phase was extracted with saturated NaHCO3 aqueous solution (50.0 mL × 3). In this step, the chiral catalyst L4/Cu(I) remains in the organic phase, while the product 2a is in the aqueous phase. The organic phase was washed with HCl aqueous solution (10.0 mL, 1.0 M), dried over MgSO4, filtered and evaporated on a rotary evaporator. The residue was rinsed with a little amount of pentane, and dried in vacuo overnight to afford the recovered chiral catalyst L4/Cu(I) as a light yellow powder [83% yield]. The combined aqueous phase was acidified with HCl aqueous solution (50.0 mL, 12.0 M), and extracted with CH2Cl2 (100.0 mL × 3). The combined organic phase was dried over MgSO4, filtered, and evaporated on a rotary evaporator to yield the product 2a as a colorless oil [83% yield, 97% ee].
Supplementary information
Acknowledgements
Financial support from The Netherlands Organization for Scientific Research (NWO-VICI to S.R.H.), the China Scholarship Council (CSC, to X.Y.), and the Ministry of Education, Culture, and Science (Gravity program 024.001.035 to S.R.H.) is acknowledged.
Author contributions
X.Y. and S.R.H. conceived the work. X.Y. designed and performed the experiments and analyzed the data. S.R.H. guided the research.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and the Supplementary Information, as well as from the authors upon reasonable request. Supplementary Information and chemical compound information are available in the online version of the paper. For the optimization of reaction conditions, see Supplementary Tables 1–5. For practical aspects of the reaction, see Supplementary Table 6. For the experimental details and product characterization, see Supplementary Methods. For the formation, conjugate addition and NMR studies of unsaturated silyl ester, see Supplementary Methods. For the isolation, reactivity and measurement of the solubility of Mg-, Li-, and Na-carboxylates, see Supplementary Methods. For NMR analysis and HPLC traces of the compounds in this article, see Supplementary Figs. 1–75.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-11345-z.
References
- 1.Maag, H. Prodrugs of Carboxylic Acids (Springer, New York, 2007).
- 2.Gminsights.com. Carboxylic Acid Market Size, Share - Industry Outlook Report 2024. https://www.gminsights.com/industry-analysis/carboxylic-acid-market [Accessed 26 June 2019] (2019).
- 3.Gooßen LJ, Rodríguez N, Gooßen K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. 2008;47:3100–3120. doi: 10.1002/anie.200704782. [DOI] [PubMed] [Google Scholar]
- 4.Perlmutter P. Conjugate Addition Reactions in Organic Synthesis. Pergamon, Oxford: Tetrahedron Organic Chemistry, Series 9; 1992. [Google Scholar]
- 5.Alexakis, A., Krause, N. & Woodward, S. Copper-Catalyzed Asymmetric Synthesis (Wiley-VCH, Weinheim, Germany, 2014).
- 6.Harutyunyan SR, den Hartog T, Geurts K, Minnaard AJ, Feringa BL. Catalytic asymmetric conjugate addition and allylic alkylation with Grignard reagents. Chem. Rev. 2008;108:2824–2852. doi: 10.1021/cr068424k. [DOI] [PubMed] [Google Scholar]
- 7.Howell GP. Asymmetric and diastereoselective conjugate addition reactions: C–C bond formation at large scale. Org. Process Res. Dev. 2012;16:1258–1272. doi: 10.1021/op200381w. [DOI] [Google Scholar]
- 8.Wotiz JH, Matthews JS, Greenfield H. The reaction of Grignard reagents with α,β-olefinic acids. J. Am. Chem. Soc. 1953;75:6342–6343. doi: 10.1021/ja01120a539. [DOI] [Google Scholar]
- 9.Yamamoto Y, Maruyama K. RCu.BF3. 3. Conjugate addition to previously unreactive substituted enoate esters and enoic acids. J. Am. Chem. Soc. 1978;100:3240–3241. doi: 10.1021/ja00478a060. [DOI] [Google Scholar]
- 10.Cooke MP., Jr. Conjugate addition reactions of α-silylated α,β-unsaturated carboxylic acid salts. J. Org. Chem. 1987;52:5729–5733. doi: 10.1021/jo00235a016. [DOI] [Google Scholar]
- 11.Aurell MJ, Domingo LR, Mestres R, Muñoz E, Zaragozá RJ. Conjugate addition of organolithium reagents to α,β-unsaturated carboxylic acids. Tetrahedron. 1999;55:815–830. doi: 10.1016/S0040-4020(98)01073-4. [DOI] [Google Scholar]
- 12.Vautravers, N. R. & Breit, B. Rhodium (I)-catalyzed 1,4-addition of arylboronic acids to acrylic acid in water: one-step preparation of 3-arylpropionic acids. Synlett. 17, 2517–2520 (2011).
- 13.Liu R, et al. Pd(II)/bipyridine-catalyzed conjugate addition of arylboronic acids to α,β-unsaturated carboxylic acids. Synthesis of β-quaternary carbons substituted carboxylic acids. J. Org. Chem. 2017;82:8023–8030. doi: 10.1021/acs.joc.7b01248. [DOI] [PubMed] [Google Scholar]
- 14.Xuan J, Zhang Z-G, Xiao W-J. Visible-light-induced decarboxylative functionalization of carboxylic acids and their derivatives. Angew. Chem. Int. Ed. 2015;54:15632–15641. doi: 10.1002/anie.201505731. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz J, König B. Decarboxylative reactions with and without light – a comparison. Green. Chem. 2018;20:323–361. doi: 10.1039/C7GC02949G. [DOI] [Google Scholar]
- 16.Qin T, et al. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science. 2016;352:801–805. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, et al. Decarboxylative borylation. Science. 2017;356:1045. doi: 10.1126/science.aam7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan X, et al. Silver-catalyzed decarboxylative bromination of aliphatic carboxylic acids. Org. Lett. 2017;19:1634–1637. doi: 10.1021/acs.orglett.7b00439. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, et al. Silver-catalyzed decarboxylative azidation of aliphatic carboxylic acids. Org. Lett. 2015;17:4702–4705. doi: 10.1021/acs.orglett.5b02155. [DOI] [PubMed] [Google Scholar]
- 20.Sun X, Zhou L, Wang C-J, Zhang X. Rh-catalyzed highly enantioselective synthesis of 3-arylbutanoic acids. Angew. Chem. Int. Ed. 2007;46:2623–2626. doi: 10.1002/anie.200604810. [DOI] [PubMed] [Google Scholar]
- 21.Murai K, et al. Kinetic resolution of β-substituted olefinic carboxylic acids by asymmetric bromolactonization. Org. Lett. 2013;15:2526–2529. doi: 10.1021/ol401007u. [DOI] [PubMed] [Google Scholar]
- 22.Khumsubdee S, Burgess K. Comparison of asymmetric hydrogenations of unsaturated carboxylic acids and esters. ACS Catal. 2013;3:237–249. doi: 10.1021/cs3007389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S, Che W, Wu H-L, Zhu S-F, Zhou Q-L. Neutral iridium catalysts with chiral phosphine-carboxy ligands for asymmetric hydrogenation of unsaturated carboxylic acids. Chem. Sci. 2017;8:1977–1980. doi: 10.1039/C6SC03764J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiacchio U, et al. Syntheses of new chiral bicyclic sultams and their use as auxiliaries in asymmetric conjugate addition of Grignard reagents. Tetrahedron.: Asymmetry. 2002;13:1915–1921. doi: 10.1016/S0957-4166(02)00465-2. [DOI] [Google Scholar]
- 25.López F, Harutyunyan SR, Meetsma A, Minnaard AJ, Feringa BL. Copper-catalyzed enantioselective conjugate addition of Grignard reagents to α,β-unsaturated esters. Angew. Chem. Int. Ed. 2005;44:2752–2756. doi: 10.1002/anie.200500317. [DOI] [PubMed] [Google Scholar]
- 26.Des Mazery R, et al. An iterative catalytic route to enantiopure deoxypropionate subunits: asymmetric conjugate addition of Grignard reagents to α,β-unsaturated thioesters. J. Am. Chem. Soc. 2005;127:9966–9967. doi: 10.1021/ja053020f. [DOI] [PubMed] [Google Scholar]
- 27.Wang S-Y, Loh T-P. Highly enantioselective Cu(I)–Tol-BINAP-catalyzed asymmetric conjugate addition of Grignard reagents to α,β-unsaturated esters. Chem. Commun. 2010;46:8694–8703. doi: 10.1039/c0cc03211e. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Fernández M, Yan X, Collados JF, White PB, Harutyunyan SR. Lewis acid enabled copper-catalyzed asymmetric synthesis of chiral β-substituted amides. J. Am. Chem. Soc. 2017;139:14224–14231. doi: 10.1021/jacs.7b07344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Bandar JS, Liu RY, Buchwald SL. CuH-catalyzed asymmetric reduction of α,β-unsaturated carboxylic acids to β-chiral aldehydes. J. Am. Chem. Soc. 2018;140:606–609. doi: 10.1021/jacs.7b12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mu Y, Nguyen TT, van der Mei FW, Schrock RR, Hoveyda AH. Traceless protection for more broadly applicable olefin metathesis. Angew. Chem. Int. Ed. 2019;58:5365–5370. doi: 10.1002/anie.201901132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rong J, Oost R, Desmarchelier A, Minnaard AJ, Harutyunyan SR. Catalytic asymmetric alkylation of acylsilanes. Angew. Chem. Int. Ed. 2015;54:3038–3042. doi: 10.1002/anie.201409815. [DOI] [PubMed] [Google Scholar]
- 32.Jumde RP, Lanza F, Veenstra MJ, Harutyunyan SR. Catalytic asymmetric addition of Grignard reagents to alkenyl-substituted aromatic N-heterocycles. Science. 2016;352:433–437. doi: 10.1126/science.aaf1983. [DOI] [PubMed] [Google Scholar]
- 33.Norsikian S, Marek I, Klein S, Poisson JF, Normant JF. Enantioselective carbometalation of cinnamyl derivatives: new access to chiral disubstituted cyclopropanes–configurational stability of benzylic organozinc halides. Chem. Eur. J. 1999;5:2055–2068. doi: 10.1002/(SICI)1521-3765(19990702)5:7<2055::AID-CHEM2055>3.0.CO;2-9. [DOI] [Google Scholar]
- 34.Jang WJ, Song SM, Moon JH, Lee JY, Yun J. Copper-catalyzed enantioselective hydroboration of unactivated 1,1-disubstituted alkenes. J. Am. Chem. Soc. 2017;139:13660–13663. doi: 10.1021/jacs.7b08379. [DOI] [PubMed] [Google Scholar]
- 35.Nahoum V, et al. Modulators of the structural dynamics of the retinoid X receptor to reveal receptor function. Proc. Natl Acad. Sci. USA. 2007;104:17323–17328. doi: 10.1073/pnas.0705356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eleftheriadis N, et al. Rational development of a potent 15-lipoxygenase-1 inhibitor with in vitro and ex vivo anti-inflammatory properties. J. Med. Chem. 2015;58:7850–7862. doi: 10.1021/acs.jmedchem.5b01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Q, Kong D, Li M, Hou G, Zi G. Highly efficient Rh-catalyzed asymmetric hydrogenation of α,β-unsaturated nitriles. J. Am. Chem. Soc. 2015;137:10177–10181. doi: 10.1021/jacs.5b06418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and the Supplementary Information, as well as from the authors upon reasonable request. Supplementary Information and chemical compound information are available in the online version of the paper. For the optimization of reaction conditions, see Supplementary Tables 1–5. For practical aspects of the reaction, see Supplementary Table 6. For the experimental details and product characterization, see Supplementary Methods. For the formation, conjugate addition and NMR studies of unsaturated silyl ester, see Supplementary Methods. For the isolation, reactivity and measurement of the solubility of Mg-, Li-, and Na-carboxylates, see Supplementary Methods. For NMR analysis and HPLC traces of the compounds in this article, see Supplementary Figs. 1–75.