Abstract
Careya arborea Roxb. (Family: Lecythidaceae) is commonly called as Slow match tree and is an important medicinal plant. Its different parts viz. bark, leaves and seeds have been reported to show many pharmacological activities. High-performance thin-layer chromatography (HPTLC) is a simple, fast and precise technique for the detection of phytochemicals present in the plant. Therefore, the objective of the present study was to characterize the phytochemical profile for various secondary metabolites using HPTLC for C. arborea bark, leaves and seeds extracts, which revealed the confirmation of these phytochemicals. The present study suggested that the bark contains all the classes of compounds tested namely alkaloids, anthracene derivatives, arbutin derivatives, bitter compounds, cardiac glycosides, coumarin derivatives, essential oils, flavonoids, lignans, pungent-tasting principles, saponins, triterpenes and valepotriates. Whereas, alkaloids are not detected in leaves, and alkaloids and arbutin derivatives are not detected in seeds.
Keywords: Careya arborea, HPTLC, Secondary metabolites, Phytochemical profile, Bark, Leaves, Seeds
Introduction
People have been using plants as traditional medicines for the treatment of various diseases and improving health from many years ago until the present time (SM Rates 2001). Medicinal plants serve as raw materials for herbal drugs, which are safe, efficient and cost-effective health care for people (Trivedi 2006). In recent years, the pharmacological effects of medicinal plants have been considered as a promising future for the management of health care. The detailed information about the phytochemical profile of the medicinal plant is useful for describing its therapeutic value as well as helps in the discovery of new therapeutic agents. The discovery of new chemical structures with pharmaceutical activities largely depends on chance, and it will, therefore, be made more likely by screening large numbers of compounds (Mukherjee 2008). Plant-based drugs, mainly their secondary metabolites, have provided an outstanding contribution to modern therapeutics (Rungsung et al. 2015).
The sum of all biochemical activities in a plant cell is called metabolism (Rungsung et al. 2015) and the products are known as metabolites. Plant-metabolites are organic compounds, which can be classified into primary metabolites and secondary metabolites. Primary metabolites include carbohydrates, proteins, lipids and nucleic acids. These are found in all plants and are beneficial for their growth and development. Plants synthesize secondary metabolites, which include alkaloids, flavonoids, saponins, terpenoids, steroids, glycosides, tannins, volatile oils etc. (Shakya 2016). These are not essential for growth, energy conservation or for primary metabolic pathways. They are required for the plant to interact with its environment and other organisms (Oksman-caldentey and Barz 2002). Most species of plants are capable of producing such secondary metabolites.
Fruits, flowers and seeds are usually rich in secondary metabolites, especially in annual plants. In perennial plant species, high amounts of secondary metabolites are found in bulbs, roots, rhizomes and the bark of roots and stems (Pagare 2015). Sarath and Sudha Bai (2019) compared the phytochemicals present in bark, leaves and seeds of Putranjiva roxburghii Wall. (Putranjivaceae) and they found that the quantity of secondary metabolites namely alkaloids, flavonoids, phenolic compounds, tannins and terpenoids was maximum in leaves, followed by bark and the seeds. Seeds contain comparatively lesser amount of all major classes of therapeutically promising secondary phytocompounds. Lerato et al. (2017) showed that most of the phytocompounds such as tannins, terpenoids, flavonoids, saponins, steroids, cardiac glycosides, phenols and coumarins were present in the leaves of T. violacea as compared to the stem and roots.
These phytoconstituents are responsible for the pharmacological properties of the plants. They include alkaloids having antispasmodic, antimalarial, analgesic, diuretic activities (Bribi 2018); terpenoids are known for their antiviral, antihelminthic, antibacterial, anticancer, antimalarial, anti-inflammatory properties (Guangyi 2005). Glycosides are reported for antifungal and antibacterial properties (Compean 2014). Phenols, flavonoids have antioxidant, anti-allergic, antibacterial properties (Karak 2019), and Saponins are reported to have anti-inflammatory, antiviral, plant defence activities (Barbosa 2014; Ram et al. 2015). Therefore, the analysis of these constituents in plants will definitely help in determining various biological activities of plants (Sharma and Paliwal 2013).
Careya arborea Roxb. (Family: Lecythidaceae) is a highly valued medicinal plant known as Sthala kumbhi in Hindi and Kumbi in English (Anonymous). It is small- to medium-sized deciduous tree (Warrier 1993), distributed in different tropical regions of the world like India, Sri Lanka and Malay Peninsula (Kirtikar and Basu 1980). C. arborea Roxb. as a whole plant and its different parts (Fig. 1) has a long history of utilization for a variety of medicinal uses (Ambardar and Aeri 2013). It is traditionally used in treatment of sores, ear pain, snake bite, inflammation, piles, tumors, cough and cold, toothache, wounds, bronchitis, colic, intestinal worms, hemorrhoids, dyspepsia, dysentery, spermatorrhoea, leukoderma, epileptic fits, abscesses, ulcers and eruptive fevers particularly smallpox (Satish et al. 2010; Khaliq 2016; Kirtikar and Basu 1980; Warrier 1993).
Fig. 1.
Careya arborea Roxb
Thin-layer chromatography has the widest application in phytochemistry, since it can be applied to almost every class of compounds, except to highly volatile compounds. It can be applied to crude plant extracts in a preliminary detection for the presence of most compounds (Harborne 1998). High-performance thin-layer chromatography (HPTLC) is an efficient, sophisticated and automated form of the thin-layer chromatography (TLC) with better and advanced separation efficiency and detection limits (Saibaba and Shanmuga 2016). It is faster, easier and more flexible as compared to any other chromatographic technique (Eike and Anne 2006). The modern HPTLC technique, combined with automated sample application and densitometric scanning, is sensitive, completely reliable and suitable for use in qualitative and quantitative analysis (Srivastava 2011). Therefore, in the present study, HPTLC technique was used for the confirmation of major classes of secondary metabolites following extraction procedure of the samples in the bark, leaves and seeds.
The available literature review indicated no reports are available on the HPTLC chemical profiling of major phytochemicals present in the bark, leaves and seeds extracts of C. arborea Roxb. Hence, the objective of this research was to develop qualitative HPTLC phytochemical profile of C. arborea Roxb. bark, leaves and seeds so as to document various phytocomponents present in these plant parts and to assess the medicinal potential of the plant.
Materials and methods
Plant materials Bark, leaves and seeds were collected from the forest area of Badlapur, Mumbai (Maharashtra) and authenticated from Agharkar Research Institute, Pune, India. Plant materials were collected from ten different mature trees randomly. Plant materials were air-dried, ground into fine powder (100 g) and stored in airtight container at room temperature for further studies.
Reagents All the solvents and reagents (toluene, ethyl acetate, diethyl amine, methanol, ether, glacial acetic acid, formic acid, chloroform, n-hexane, potassium hydroxide, Gibb’s reagent, anisaldehyde, sulphuric acid, ethanol, vanillin) used were of analytical grade, purchased from Sigma-Aldrich, Darmstadt, Germany. The precoated TLC silica gel 60 F254 plates were obtained from E. Merck (Mumbai, India).
Sample preparation Sample preparation depends upon the class of compound isolated; so for each class of compound, samples are prepared differently. Samples of bark, leaves and seeds were prepared according to standard methods described by Wagner and Bladt (1984) for different phytochemicals. All the samples were filtered by Whatmann filter paper no. 1 (pore size 11 µm) before HPTLC analysis.
High-performance thin-layer chromatography (HPTLC) analysis: instrumentation and operating conditions
The chromatography was performed on TLC aluminium precoated silica gel 60 F254 Plate, with 200-µm layer thickness (E. Merck, Mumbai, India). 10 µl of samples was applied to the plate as 8-mm band length using the CAMAG Linomat 5 TLC sample applicator equipped with syringe (Hamilton, Bonaduz, Switzerland 100 μL). After the application, plates were developed vertically ascending in a glass twin-trough chamber (CAMAG, Switzerland) pre-saturated for 20 min at room temperature, with respective mobile phase. The chromatographic run length was 80 mm from the bottom edge of the plate. After development, the plate was air-dried for complete removal of mobile phase and derivatized by dipping the developed plate in respective derivatizing agent for 2 s. Mobile phases and derivatizing agents for respective secondary metabolites are mentioned in Table 1. The plate was then air-dried and heated at 110 °C on TLC plate heater for 10 min. The plate was kept in photo-documentation chamber (CAMAG REPROSTAR 3) and images were captured. Densitometric scanning was then performed at 254 nm, 366 nm and visible light using CAMAG TLC scanner 4 with winCATS software version 1.4.6.
Table 1.
Mobile phases and derivatizing agents for respective secondary metabolites (Wagner et al. 1984)
| S. no. | Class of compound | Mobile phase | Derivatization | Observation |
|---|---|---|---|---|
| 1. | Alkaloids | Toluene:ethyl acetate:diethyl amine (70:20:10) v/v/v | Dragendorff reagent | Orange bands in visible light and blue bands in UV-366 nm |
| 2. | Anthracene derivatives | Ethyl acetate:methanol:water (100:13.5:10) v/v/v | Potassium hydroxide reagent | Yellow or red–brown fluorescence under UV-366 nm |
| 3. | Arbutin derivatives | Ethyl acetate:methanol:water (100:13.5:10) v/v/v | Gibb’s reagent | Blue–violet bands in visible light |
| 4. | Bitter compounds | Ethyl acetate:methanol:water (7.7:1.5:0.8) v/v/v | Anisaldehyde sulphuric acid reagent | Red–violet, brown, blue–green, blue zones under visible light |
| 5. | Cardiac glycosides | Ethyl acetate:methanol:water (81:11:8) v/v/v | Sulphuric acid reagent | Blue, Brown, green and yellowish fluorescence in UV-366 nm |
| 6. | Coumarin derivatives | Toluene:ether (1:1 saturated with 10% acetic acid) | Potassium hydroxide reagent | Intense blue–green or blue, green fluorescence under UV-366 nm |
| 7. | Essential oils | Toluene:ethyl acetate (93:7) v/v/v | Anisaldehyde sulphuric acid reagent | Blue, green, red and brown coloration in visible light |
| 8. | Flavonoids | Ethyl acetate:water:formic acid:glacial acetic acid (100:26:11:11) v/v/v/v | Sulphuric acid reagent | Blue, green, red fluorescence in UV-366 nm |
| 9. | Lignans | Toluene:ethyl acetate (7:3) v/v | Sulphuric acid reagent | Blue fluorescence under UV-366 nm |
| 10. | Pungent-tasting principles | Toluene:ethyl acetate (7:3) v/v | Vanillin sulphuric acid reagent | Lemon yellow and blue to violet bands in visible light and blue fluorescence in UV-366 nm |
| 11. | Saponins | Chloroform:glacial acetic acid:methanol:water (6.4:3.2:1.2:0.8) v/v/v/v | Anisaldehyde sulphuric acid reagent | Blue, Blue–violet, red and yellow–brown zones under visible light |
| 12. | Triterpenes | n-Hexane:ethyl acetate (1:1) v/v | Anisaldehyde sulphuric acid reagent | Blue violet, red and red–violet zones in visible light |
| 13. | Valepotriates | Toluene:ethyl acetate (75:25) v/v | Anisaldehyde sulphuric acid reagent | Violet or blue zones in visible light |
Results and discussion
Knowledge of the chemical constituents of plants is desirable because such information may be of great value in revealing new sources of compounds and precursors for the synthesis of new chemical constituents, which can be used in drugs (Mehta et al. 2017).
Preliminary phytochemical analysis is an indication of the presence or absence of phytochemicals in a plant extract based on visual inspection of color or precipitation reaction; whereas, HPTLC chemoprofile accurately and efficiently confirms the presence of these constituents. While traditional TLC is based on visual inspection of the chromatographic plate and its documentation by either tracing or photography, HPTLC features highly sensitive scanning densitometry for rapid chromatogram evaluation and documentation (Mukherjee 2008).
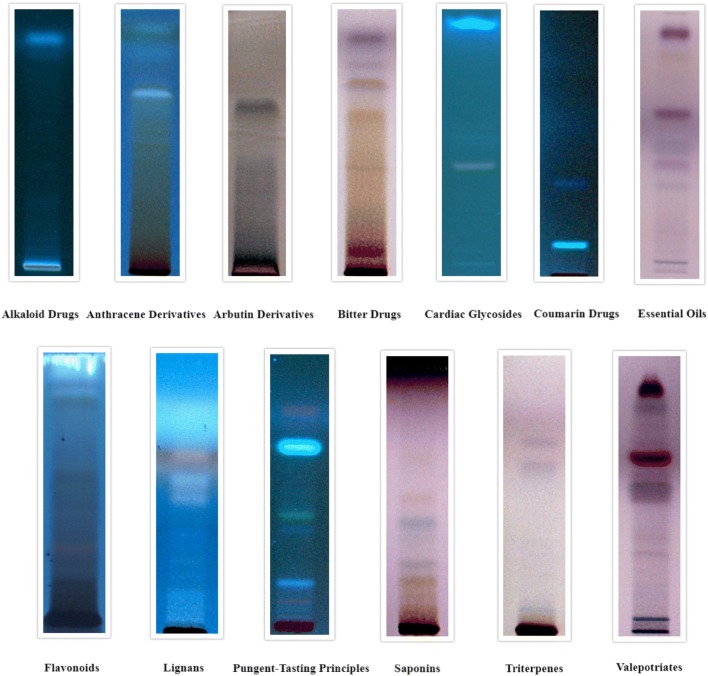
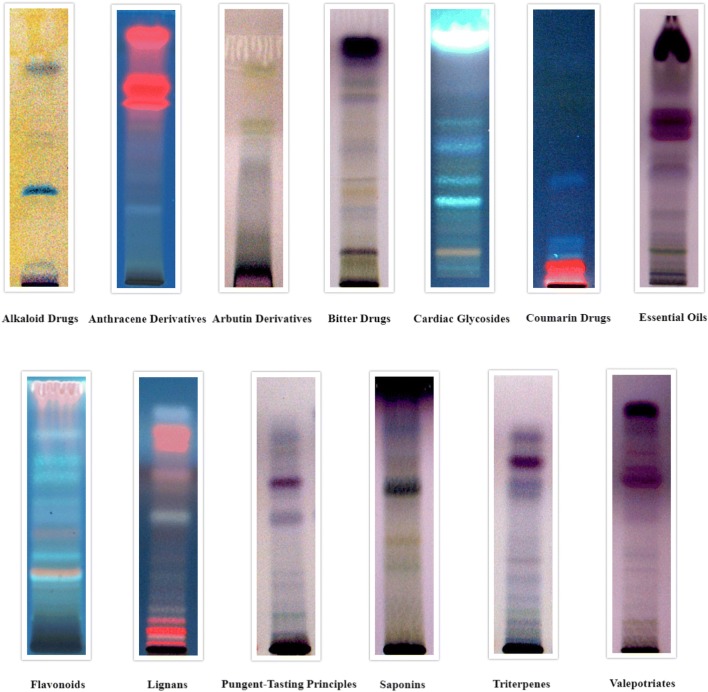
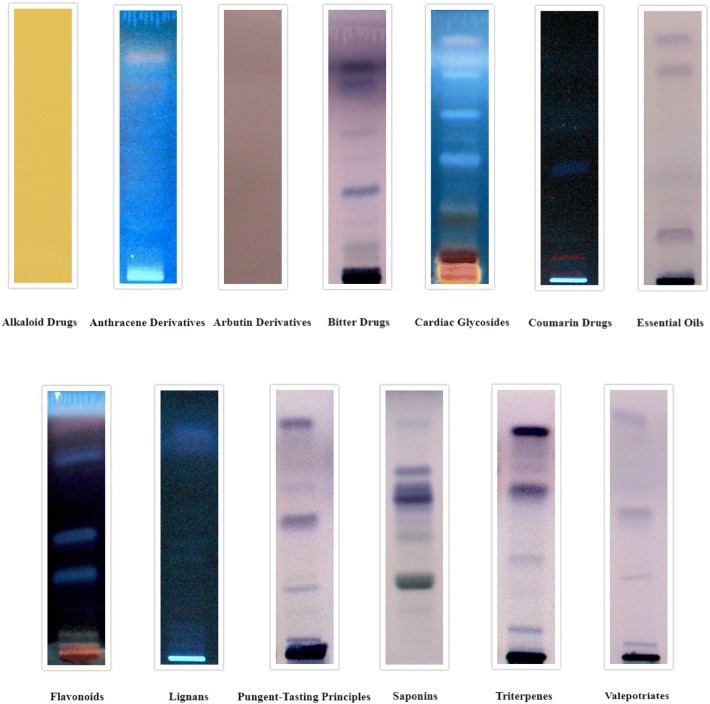
In the current study, bark, leaves and seeds extracts of C. arborea were evaluated for the detection of thirteen main classes of secondary phytocompounds namely alkaloids, anthracene derivatives, arbutin derivatives, bitter compounds, cardiac glycosides, coumarin derivatives, essential oils, flavonoids, lignans, pungent-tasting principles, saponins, triterpenes and valepotriates. This study revealed that the solvent system developed and the specific derivatizing agents used gave well-resolved bands for secondary metabolites present in C. arborea bark, leaves and seeds extracts.
The results for the detection of secondary phytoconstituents in bark, leaves and seed extracts are tabulated in Table 2 which showed the presence of all the class of compound tested in bark, whereas alkaloids were not detected in leaves and alkaloids and arbutin derivatives were not detected in seeds.
Table 2.
Detection of secondary metabolites in Careya arborea bark, leaves and seeds by HPTLC
| S. no. | Class of compound | Bark | Leaves | Seeds |
|---|---|---|---|---|
| 1. | Alkaloids | D | ND | ND |
| 2. | Anthracene derivatives | D | D | D |
| 3. | Arbutin derivatives | D | D | ND |
| 4. | Bitter compounds | D | D | D |
| 5. | Cardiac glycosides | D | D | D |
| 6. | Coumarin derivatives | D | D | D |
| 7. | Essential oils | D | D | D |
| 8. | Flavonoids | D | D | D |
| 9. | Lignans | D | D | D |
| 10. | Pungent-tasting principles | D | D | D |
| 11. | Saponins | D | D | D |
| 12. | Triterpenes | D | D | D |
| 13. | Valepotriates | D | D | D |
ND not detected, D detected
The Rf value indicated the position at which a substance is located in the chromatogram. Rf values were calculated using the following formula:
The Rf values of respective compounds for all the chromatograms are depicted in Table 3 which showed number of compounds present in that particular class of phytoconstituents. Bark extracts showed the presence of 1 alkaloid, 3 anthracene derivatives, 1 arbutin derivative, 7 bitter compounds, 3 cardiac glycosides, 2 coumarin derivatives, 7 essential oils, 5 flavonoids, 5 lignans, 4 pungent-tasting principles, 4 saponins, 4 triterpenes and 6 valepotriates. Leaf extracts showed the presence of 6 anthracene derivatives, 1 arbutin derivative, 8 bitter compounds, 8 cardiac glycosides, 4 coumarin derivatives, 7 essential oils, 9 flavonoids, 7 lignans, 6 pungent-tasting principles, 5 saponins, 7 triterpenes and 6 valepotriates. Seeds showed the presence of 1 anthracene derivative, 6 bitter compounds, 6 cardiac glycosides, 3 coumarin derivatives, 4 essential oils, 3 flavonoids, 6 lignans, 5 pungent-tasting principles, 5 saponins, 5 triterpenes, and 6 valepotriates The developed chromatograms for respective secondary phytocompounds for bark, leaves and seeds are presented in Figs. 2, 3 and 4, respectively. The present study suggested that the distribution of phytochemicals is present in many parts of the plant, since many of the compounds appear to have been detected in barks, leaves and seeds.
Table 3.
Retention factors (Rf) of secondary metabolites present in Careya arborea bark, leaves and seeds
| S. no. | Class of Secondary metabolites | Rf values | ||
|---|---|---|---|---|
| Bark | Leaves | Seeds | ||
| 1. | Alkaloids | 0.83 | – | – |
| 2. | Anthracene derivatives | 0.60, 0.72, 0.79 | 0.39, 0.41, 0.53, 0.67, 0.71, 0.74 | 0.83 |
| 3. | Arbutin derivatives | 0.67 | 0.62 | – |
| 4. | Bitter compounds | 0.35, 0.40, 0.45, 0.59, 0.71, 0.78, 0.87 | 0.26, 0.29, 0.35, 0.40, 0.51, 0.60, 0.69, 0.76 | 0.25, 0.37, 0.51, 0.60, 0.77, 0.83 |
| 5. | Cardiac glycosides | 0.45, 0.68, 0.80 | 0.19, 0.24, 0.28, 0.37, 0.49, 0.54, 0.59, 0.68 | 0.28, 0.46, 0.54, 0.63, 0.78, 0.82 |
| 6. | Coumarin derivatives | 0.15, 0.38 | 0.16, 0.25, 0.40, 0.49 | 0.45, 0.57, 0.84 |
| 7. | Essential oils | 0.17, 0.34, 0.43, 0.50, 0.62, 0.73, 0.84 | 0.26, 0.33, 0.44, 0.47, 0.56, 0.60, 0.73 | 0.28, 0.35, 0.48, 0.60 |
| 8. | Flavonoids | 0.24, 0.57, 0.68, 0.76, 0.85 | 0.16, 0.22, 0.26, 0.39, 0.47, 0.53, 0.63, 0.67, 0.80 | 0.32, 0.47, 0.76 |
| 9. | Lignans | 0.27, 0.36, 0.50, 0.56, 0.65 | 0.16, 0.20, 0.28, 0.51, 0.65, 0.77, 0.81 | 0.18, 0.34, 0.55, 0.58, 0.68, 0.85 |
| 10. | Pungent-tasting principles | 0.42, 0.54, 0.64, 0.76 | 0.25, 0.29, 0.40, 0.49, 0.63, 0.79 | 0.17, 0.30, 0.51, 0.64, 0.81 |
| 11. | Saponins | 0.19, 0.26, 0.41, 0.50 | 0.31, 0.34, 0.41, 0.59, 0.69 | 0.23, 0.40, 0.54, 0.64, 0.82 |
| 12. | Triterpenes | 0.35, 0.63, 0.71, 0.78 | 0.16, 0.20, 0.27, 0.34, 0.62, 0.70, 0.79 | 0.25, 0.37, 0.63, 0.72, 0.85 |
| 13. | Valepotriates | 0.19, 0.31, 0.37, 0.52, 0.66, 0.84 | 0.18, 0.31, 0.36, 0.50, 0.64, 0.74 | 0.16, 0.31, 0.35, 0.55, 0.66, 0.83 |
Fig. 2.
HPTLC chromatograms of secondary metabolites for Careya arborea Roxb. bark
Fig. 3.
HPTLC chromatograms of secondary metabolites for Careya arborea Roxb. leaves
Fig. 4.
HPTLC chromatograms of secondary metabolites for Careya arborea Roxb. seeds
Conclusion
The results obtained in the present study indicates C. arborea plant parts namely bark, leaves and seeds are a rich source of secondary metabolites as most of the class of compounds were found to be present in all these three plant parts. These findings indicate the presence of various phytochemicals in bark, leaves and seeds of C. arborea may be responsible for its pharmacological activities. However, there is a need to further carry out advanced studies to isolate and identify the pure active chemical compounds, and elucidate the structure of these compounds. Furthermore, these data may be handy in probing of biochemistry of this plant in the future.
Compliance with ethical standards
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Ambardar N, Aeri V. A better understanding of traditional uses of Careya arborea Roxb. phytochemical and pharmacological review. TANG Assoc Human Tradit Med. 2013;3(4):1–7. [Google Scholar]
- Anonymous. The ayurvedic pharmacopoeia of India. Government of India, Ministry of Health and Family Welfare, Department of Ayush, vol V, no I, pp 110–111
- Barbosa A. An overview of biological and pharmacological activities of saponins. Int J Pharm Pharm Sci. 2014;6(8):47–50. [Google Scholar]
- Bribi N. Pharmacological activities of alkaloids: a review. Asian J Bot. 2018;1:1–6. [Google Scholar]
- Compean KL, Ynalvez RA. Antimicrobial activity of plant secondary metabolites: a review. Res J Med Plants. 2014;8(5):204–213. doi: 10.3923/rjmp.2014.204.213. [DOI] [Google Scholar]
- Eike R, Anne S. High performance thin-layer chromatography for the analysis of medicinal plants. New York: Thieme Medical Publisher; 2006. [Google Scholar]
- Guangyi W, Tang Weiping, Bidigare R. Terpenoids as therapeutic drugs and Pharmaceutical agents. Nat Products: Drug discovery and Therapeutic medicine; 2005. pp. 197–226. [Google Scholar]
- Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. 3. New York: Chapman and Hall; 1998. [Google Scholar]
- Karak P. Biological activities of flavonoids: an overview. Int J Pharm Sci Res. 2019;10(4):1567–1574. [Google Scholar]
- Khaliq HA. Pharmacognostic, physiochemical, phytochemical and pharmacological studies on Careya arborea Roxb: a review. J Phytopharmacol. 2016;5(1):27–34. [Google Scholar]
- Kirtikar KR, Basu BD (1980) Indian medicinal plants, 2nd edn. Bishen Singh Mahendra Pal Singh, Dehradun, vol 1, pp 676–683
- Lerato NM, Samkeliso T, Michael P. Preliminary phytochemical screening of crude extracts from the leaves, stems, and roots of Tulbaghia violacea. Int J Pharmacogn Phytochem Res. 2017;9(10):1300–1308. [Google Scholar]
- Mehta S, Singh PS, Saklani P. Phytochemical screening and TLC profiling of various extracts of Reinwardtia indica. Int J Pharmacogn Phytochem Res. 2017;9(4):523–527. [Google Scholar]
- Mukherjee PK. Quality control of herbal drugs: an approach to evaluation of botanicals. India: Business Horizons Pharmaceutical Publishers; 2008. [Google Scholar]
- Oksman-caldentey KM, Barz W. Plant biotechnology and transgenic plants. New York: Marcel Dekker Incorporated; 2002. [Google Scholar]
- Pagare Saurabh, Bhatia Manila, Tripathi Niraj, Pagare Sonal, Bansal YK. Secondary metabolites of plants and their role: overview. Curr Trends Biotechnol Pharm. 2015;9(3):293–304. [Google Scholar]
- Ram J, Moteriya P, Chanda S. Phytochemical screening and reported biological activities of some medicinal plants of Gujarat region. J Pharmacogn Phytochem. 2015;4(2):192–198. [Google Scholar]
- Rates SM. Plant as a source of drug. Toxicon. 2001;39(5):603–613. doi: 10.1016/S0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Rungsung W, Ratha KK, Dutta S, Dixit AK, Hazra J. Secondary metabolites of plants in drugs discovery. World J Pharm Res. 2015;4(7):604–613. [Google Scholar]
- Saibaba SV, Shanmuga P. High performance thin layer chromatography: a mini review. Res Pharm Health Sci. 2016;2(4):219–226. [Google Scholar]
- Sarath P, Sudha Bai R. A comparative evaluation of phytochemicals in bark, leaves and seeds of Putranjiva roxburghii Wall. (Putranjivaceae) J Pharmacogn Phytochem. 2019;8(1):1162–1166. [Google Scholar]
- Satish KBN, Swamy BMV, Kumar GK, Behera GM. Review on Careya arborea Roxb. Int J Res Ayurveda Pharm. 2010;1(2):306–315. [Google Scholar]
- Shakya AK. Medicinal plants: future source of new drugs. Int J Herb Med. 2016;4(4):59–64. [Google Scholar]
- Sharma V, Paliwal R. Preliminary phytochemical investigation and thin layer chromatography profiling of sequential extracts of Moringa oleifera pods. Int J Green Pharm. 2013;7:41–45. doi: 10.4103/0973-8258.111607. [DOI] [Google Scholar]
- Srivastava MM. High-performance thin- layer chromatography (HPTLC) Berlin, Heidelberg: Springer; 2011. pp. 32–60. [Google Scholar]
- Trivedi PC. Medicinal plants traditional knowledge. New Delhi: IK International Pvt. Ltd.; 2006. [Google Scholar]
- Wagner H, Bladt S, Zgainski EM. Plant drug analysis, a thin layer chromatography atlas. Berlin, Heidenberg, New York: Springer; 1984. [Google Scholar]
- Warrier PK. Indian medicinal plants-a compendium of 500 species, vol 1. Madras: Orient Longman Ltd; 1993. pp. 344–346. [Google Scholar]