Abstract
From pneumonia to pericarditis, from sepsis to splenic abscess, Streptococcus pneumoniae is the causative agent of a diverse array of pyogenic disease. With the introduction of vaccines and effective antibiotic treatments, the incidence of complicated streptococcal infection has declined. We report a case of S. pneumoniae bacteremia, in the setting of occult sinusitis, complicated by hemophagocytic lymphohistiocytosis (HLH), disseminated intravascular coagulation (DIC), and recurrent pneumococcal infection. Although severe streptococcal infection has been associated with immunodeficiency or splenectomy, no such predisposition was identified in our patient. We discuss the association of streptococcal infection with HLH and DIC and review occult sinusitis as a source of pneumococcal bacteremia, with the goal of enhancing the “illness scripts” of general medical practitioners to include such entities.
KEY WORDS: pneumococcus, Streptococcus pneumoniae, bacteremia, hemophagocytic lymphohistiocytosis, sinusitis
INTRODUCTION
Streptococcus pneumoniae was first described independently by Louis Pasteur and George Sternberg in the year 1881.1 The lancet-shaped diplococcus was identified as a lethal organism and causative agent in pneumonia, meningitis, and otitis media. However, the incidence of invasive pneumococcal infection has declined dramatically in the post-antibiotic, post-vaccine era,2 and the suppurative and inflammatory complications of pneumococcal disease are infrequently encountered in the current clinical care of immunocompetent adults.3 While streptococcal infection has been associated with hemophagocytic lymphohistiocytosis (HLH) in patients with hyposplenism or other immunocompromised states, no cases have been described in healthy adults. We report a case of streptococcal bacteremia from occult sinusitis, complicated by HLH, DIC, and meningitis.
CASE DESCRIPTION
A 46-year-old Filipino man with a history of gout and hypertension presented to the emergency department with 1 day of left shoulder pain, subjective fevers, and mottled skin. He had recently completed an empiric 6-day course of prednisone for a gout flare in his left ankle. Chronic medications included amlodipine, metoprolol, rosuvastatin, and methocarbamol. He worked as a certified nurse anesthetist and denied needlestick injuries or recreational drug use. Upon initial evaluation, his vital signs were notable for a fever of 103.7 °F (39.8 °C), heart rate of 107 beats per minute, blood pressure of 141/101 mmHg, respiratory rate of 31 breaths per minute, and oxygen saturation of 99% on room air. Physical examination revealed a well-appearing, alert gentleman with painful active range of motion of his left shoulder, and blanching, net-like, violaceous patches on the forearms, shins, and feet. The remainder of his examination was unremarkable, with no auscultated heart murmurs and no cutaneous stigmata of infectious endocarditis. Bloodwork was notable for a white blood cell count of 12.7 × 103/μL (normal 4.0–10.0 × 103/μL) with 17% band forms, and platelets of 30 × 103/μL (normal 140–440 × 103/μL). Serum lactate was initially 5.1 mmol/L (normal 0.5–2.22 mmol/L) and increased to a peak of 8.7 mmol/L following 3 L of normal saline administration, despite normotension. Ferritin was greater than 100,000 ng/mL (normal 18–370 ng/mL). Plain radiography of the shoulder and chest were unremarkable, and a right upper quadrant ultrasound revealed increased hepatic echogenicity without biliary dilation. Attempted aspiration of the left shoulder was unsuccessful.
Blood cultures obtained upon initial presentation revealed gram-positive cocci in pairs and chains, subsequently speciating as pan-sensitive Streptococcus pneumoniae. Concurrent with the peak of his lactic acidosis, the patient began to complain of bilateral leg pain which was accompanied by a rise in creatine kinase from an admission value of 137 U/L to a peak of 22,000 U/L (normal 24–195 U/L). CT of the bilateral lower extremities was unremarkable, but MRI revealed diffuse muscular edema throughout both calves, as well as a small amount of fluid in the superficial and deep fascia bilaterally. These findings collectively suggested myositis and fasciitis. Both transthoracic and transesophageal echocardiography revealed no evidence of cardiac valvular vegetation. However, a CT of the patient’s sinuses revealed right maxillary sinus disease with obstruction of the right infundibulum (Fig. 1). Given the absence of a competing source, this occult sinusitis was presumed to be the cause of the patient’s bacteremia.
Fig. 1.
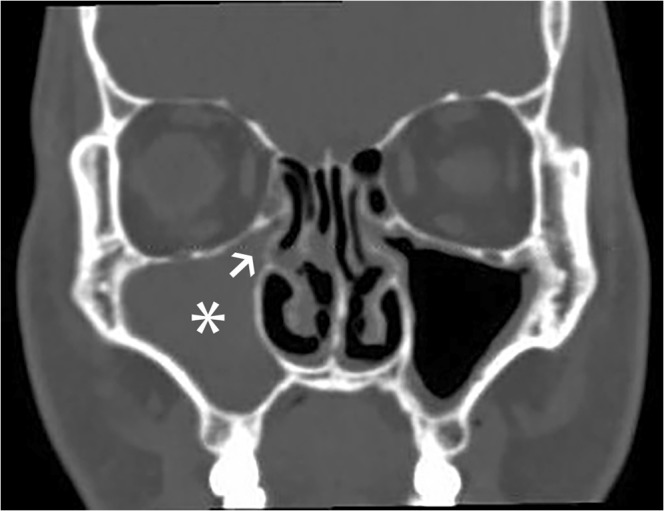
CT scan of the head (coronal image), demonstrating right maxillary sinus opacification (asterisk) and infundibular obstruction (arrowhead)
The markedly elevated ferritin, fevers, hepatitis, and coagulopathy raised suspicion for HLH. He was started on high-dose steroids (dexamethasone 20 mg daily) while awaiting further testing. A triglyceride level was 1578 mg/dL (normal < 199 mg/dL), NK cell cytotoxicity was 0.0 lytic units (normal ≥ 2.6), and an interleukin-2 level was 1409 U/mL (normal range 109–663 U/mL). Bone marrow biopsy revealed a hypercellular marrow with increased megakaryopoiesis and histologically confirmed hemophagocytosis, confirming the diagnosis of HLH (Fig. 2). Additional infectious evaluation included negative testing for respiratory viruses, HIV, HCV, HBV, HSV 1/2, Lyme, Anaplasma, and Ehrlichia. EBV and CMV serologies were consistent with prior exposure. Over several days, his skin lesions darkened and were no longer blanching, an appearance consistent with retiform purpura (Fig. 3). Serologic investigation for vasculitis revealed normal complement levels and negative ANA, ANCA, and dsDNA. Skin biopsy revealed fibrin deposition within vessels (Fig. 4), but no evidence of vasculitis. An extensive evaluation for coagulopathies was unrevealing, including workup for thrombotic thrombocytopenic purpura, antiphospholipid syndrome, paroxysmal nocturnal hemoglobinuria, cryoglobulinemia, inherited coagulopathies, and cryofibrinogenemia. Fibrinogen levels downtrended over several days from an initial value of 453 mg/dL to a nadir of 112 mg/dL (normal 144–436 mg/dL), and D-dimer was elevated to greater than 33.89 mg/L (normal 0.17–0.54 mg/L), leading to a presumptive diagnosis of disseminated intravascular coagulation (DIC) secondary to invasive pneumococcal disease.
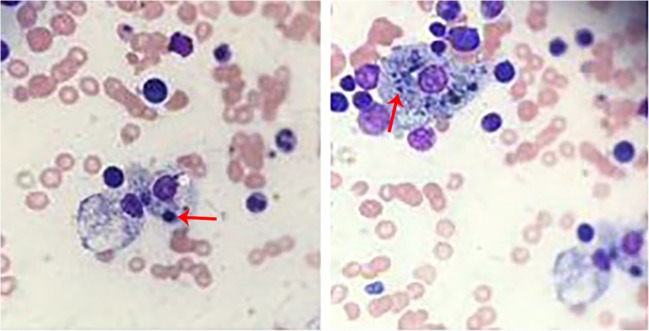
Fig. 2.
Bone marrow aspirate, demonstrating hemophagocytosis of erythrocytes (arrow, left) and platelets (arrow, right)
Fig. 3.
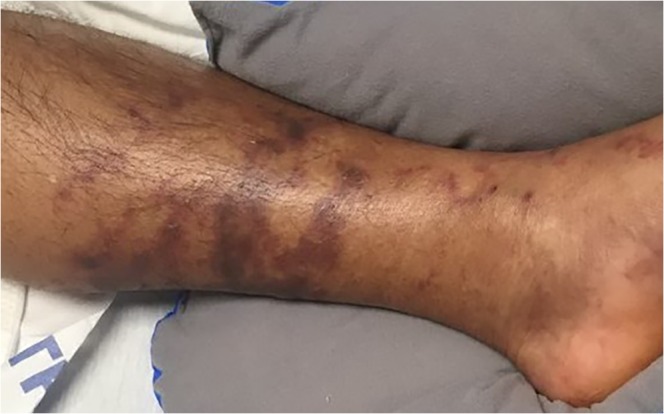
Clinical image of the distal left lower extremity, demonstrating retiform purpura
Fig. 4.
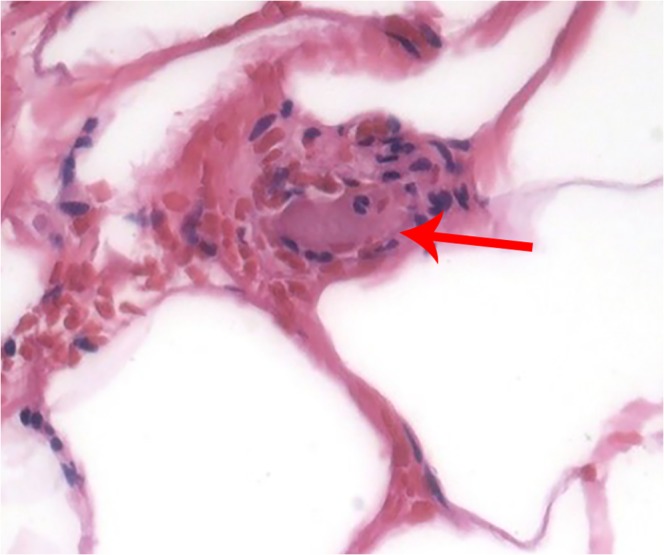
Skin biopsy, H&E stain, demonstrating fibrin deposition within an arteriole (arrow)
The patient completed a 2-week course of ceftriaxone for his bacteremia and a 5-week taper of dexamethasone for HLH. Several months later, he again presented to the emergency department and was admitted with fever, nausea, and headache. MRI of the brain revealed resolution of his sinus opacification, with only trace residual fluid in the bilateral maxillary sinuses. His cerebrospinal fluid leukocyte count was 2975 (normal <6 ); protein was 194 mg/dL (normal 15–45 mg/dL). No organisms were seen on gram stain and the culture was negative. Viral PCRs for HSV, VZV, EBV, CMV, and respiratory pathogens were negative. He responded to empiric antibiotics with resolution of CSF pleocytosis on repeat lumbar puncture, leading to a presumptive diagnosis of pneumococcal meningitis. With this concern for recurrent pneumococcal disease, the patient underwent a comprehensive evaluation for immunodeficiency, revealing normal immunoglobulin levels and negative HIV testing. Physiologic uptake in the spleen was demonstrated on SPECT. Pre- and post-vaccination pneumococcal antibody titers revealed an adequate response to vaccination and provided no evidence of a specific antibody deficiency. He also underwent genetic testing as an outpatient revealing no duplication, deletion or mutation on next-generation sequencing in common genes associated with HLH.
DISCUSSION
This was a remarkable case of S. pneumoniae bacteremia in the setting of occult sinus obstruction, complicated by DIC and HLH. Since its isolation in 1881 by Louis Pasteur, S. pneumoniae has been recognized as a virulent pathogen.4 Despite the success of pneumococcal vaccination, it remains a morbid illness, with mortality rates for bacteremia without pneumonia reaching 20%.4 Advanced age, immunosuppression, and underlying chronic disease are associated with a more fulminant course.5 Immunosuppressed states portend a particularly high risk for pneumococcal disease, with a 50-fold increase in incidence in patients with HIV.6 Other risk factors for streptococcal infection range from environmental exposures such as cigarette smoking and nursing home residence to medical comorbidities such as chronic cardiovascular, kidney, liver, or pulmonary disease.7 Special note should be made of hyposplenism and splenectomy, due to the unique role of the spleen in clearance of encapsulated bacteria, and the remarkable fatality rate of pneumococcal sepsis in asplenic patients.8 Our patient had a functional spleen and no evidence of defects in humoral immunity. His extensive evaluation demonstrated dramatic reductions in NK cell activity, which are classically thought to combat viral infection9; however, emerging data in mice with defective NK cell activity demonstrate increased susceptibility to S. pneumoniae infection as well.10 These associations require additional study, but provide one possible explanation for our patient’s recurrent infection.
The diagnosis of bacteremia should always prompt an evaluation for a causative focus of infection. Immunodeficiency does not obviate a thorough workup; in fact, a localized source may be more readily identified in HIV infected patients with pneumococcal bacteremia compared to the HIV uninfected.11 Pneumonia is the predominant cause of pneumococcal blood stream infections; sinusitis and otitis media account for only 4% of cases.12 In nearly a fifth of cases of Streptococcal bacteremia, no clear source is identified.2 Although not culture-proven, the sinusitis discovered in our patient is the most likely source of his bacteremia. The absence of sinus symptoms in this case was unusual, although occult sinusitis has previously been reported as the etiology of severe sepsis and pneumococcal bacteremia in a healthy adult.13 More commonly, sinusitis presents without localizing symptoms in the intensive care unit (ICU), due to difficulty in communication with intubated patients, the placement of nasogastric feeding tubes, and lack of normal sinus clearance reflexes.14 Antimicrobial therapy is the cornerstone of treatment for bacterial sinusitis, with surgical intervention reserved for local extension of disease, refractory infection, or recurrent episodes of sinusitis.15
Since Osler first described the triad of pneumonia, endocarditis, and meningitis in 1882 (also termed “Austrian Syndrome”), the pyogenic complications of S. pneumoniae infection have been well-recognized.3 However, the illness script for pneumococcal sepsis should also include inflammatory sequelae such as DIC and HLH, as illustrated by our patient’s case. Coagulopathies in the setting of streptococcal infections range from DIC to purpura fulminans and hemorrhagic adrenalitis (e.g., Waterhouse-Friderichsen syndrome), most frequently reported in asplenic or immunocompromised hosts.16 Severe lower extremity myalgia, as seen in our patient, has been reported as a heralding sign of purpura fulminans; myositis in this setting is likely secondary to microvascular ischemia.17, 18 DIC is partially attributed to circulating tissue factor in overwhelming infections, but the inflammatory response that led to the development of HLH in our patient may have also precipitated his coagulopathy. DIC has been demonstrated in up to 50% of patients admitted to the intensive care unit (ICU) with HLH.19 It is theorized that activated macrophages in HLH secrete plasminogen activators, ultimately resulting in the accelerated degradation of fibrinogen.20 In a review of recent case reports of severe coagulopathy in pneumococcal disease, none document markers of HLH such as ferritin or triglyceride levels.21–26 Nonetheless, secondary HLH has been previously documented with invasive S. pneumoniae infection.27
Hemophagocytosis is an increasingly recognized endpoint of immune dysregulation in sepsis and can be found in the majority of septic patients with thrombocytopenia.28 The diagnosis of HLH, obscured by the absence of multi-lineage cytopenias, was not considered in our patient until we discovered a profoundly elevated ferritin. Ferritin can be a useful diagnostic adjunct in such cases: HLH was diagnosed in 61% of adults with a serum ferritin greater than 100,000 ng/mL in a retrospective study of adult patients,29 and ferritin was associated with mortality in adult ICU patients with HLH.30 However, in our experience, evaluation for HLH in septic patients with cytopenias is not routine.31 The diagnosis of HLH is established based on the criteria used in the HLH-2004 trial, though the delay associated with obtaining NK cell activity, soluble IL-2 receptor levels, and bone marrow biopsy necessitate concurrent diagnostic evaluation and empiric therapy.32 At our center, we most commonly treat HLH secondary to infection with dexamethasone 10 mg/m2 as per the HLH-2004 study when the severity of disease warrants immunosuppressive therapy. Dexamethasone is the preferred corticosteroid as it has the highest CNS penetration. The benefits of HLH-specific treatment must be carefully balanced against the risks ofimmunosuppressive therapy, especially in the setting of an active infection. Our patient’s steroid treatment may have contributed to the development of recurrent invasive pneumococcal disease, although he had completed his taper prior to his second presentation. Further research is needed regarding the routine evaluation for HLH in critically ill patients, and the impact of immunosuppressive therapy in this population.33
In conclusion, pneumococcal bacteremia can be complicated by a variety of inflammatory sequelae, including HLH and DIC. Evaluation of pneumococcal bacteremia should include an investigation of potential predisposing factors and sources, and sinus imaging should be considered in cases of pneumococcal bacteremia with an occult source. The diagnosis of HLH may be underrecognized in the setting of sepsis, and the timeframe required to obtain complete diagnostic testing can be prohibitive in critically ill patients. Treatment in patients with HLH secondary to sepsis must be weighed against the risk of further immunosuppression.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Frederick Howard, Email: Frederick.howard@yale.edu.
Christopher Sankey, Email: christopher.sankey@yale.edu.
References
- 1.Watson DA, Musher DM, Jacobson JW, Verhoef J. A brief history of the pneumococcus in biomedical research: a panoply of scientific discovery. Clin Infect Dis. 1993;17(5):913–24. doi: 10.1093/clinids/17.5.913. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae. 2015. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/spneu15.pdf. Accessed 28 December 2018.
- 3.Taylor SN, Sanders CV. Unusual manifestations of invasive pneumococcal infection. Am J Med. 1999;107(1A):12S–27S. doi: 10.1016/S0002-9343(99)00103-5. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . In: Epidemiology and Prevention of Vaccine-Preventable Diseases. 13. Hamborsky J, Kroger A, Wolfe S, editors. Washington D.C: Public Health Foundation; 2015. [Google Scholar]
- 5.Alanee SR, Mcgee L, Jackson D, et al. Association of serotypes of Streptococcus pneumoniae with disease severity and outcome in adults: an international study. Clin Infect Dis. 2007;45(1):46–51. doi: 10.1086/518538. [DOI] [PubMed] [Google Scholar]
- 6.Yin Z, Rice BD, Waight P, et al. Invasive pneumococcal disease among HIV-positive individuals, 2000-2009. AIDS (London, England) 2012;26(1):87–94. doi: 10.1097/QAD.0b013e32834dcf27. [DOI] [PubMed] [Google Scholar]
- 7.Lipsky BA, Boyko EJ, Inui TS, Koepsell TD. Risk factors for acquiring pneumococcal infections. Arch Intern Med. 1986;146(11):2179–85. doi: 10.1001/archinte.1986.00360230105016. [DOI] [PubMed] [Google Scholar]
- 8.Rubin LG, Schaffner W. Clinical practice. Care of the asplenic patient. N Engl J Med. 2014;371(4):349–56. doi: 10.1056/NEJMcp1314291. [DOI] [PubMed] [Google Scholar]
- 9.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 10.Baranek T, Morello E, Valayer A, et al. FHL2 Regulates Natural Killer Cell Development and Activation during Streptococcus pneumoniae Infection. Front Immunol. 2017;8:123. doi: 10.3389/fimmu.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meiring S, Cohen C, Quan V, et al. HIV Infection and the Epidemiology of Invasive Pneumococcal Disease (IPD) in South African Adults and Older Children Prior to the Introduction of a Pneumococcal Conjugate Vaccine (PCV) PLoS One. 2016;11(2):e0149104. doi: 10.1371/journal.pone.0149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raz R, Elhanan G, Shimoni Z, et al. Pneumococcal bacteremia in hospitalized Israeli adults: epidemiology and resistance to penicillin. Israeli Adult Pneumococcal Bacteremia Group. Clin Infect Dis. 1997;24(6):1164–8. doi: 10.1086/513635. [DOI] [PubMed] [Google Scholar]
- 13.Hariri MA, Vice PA. Septic shock and death due to occult sinusitis. J Laryngol Otol. 1990;104(12):990. doi: 10.1017/S0022215100114574. [DOI] [PubMed] [Google Scholar]
- 14.Van zanten AR, Dixon JM, Nipshagen MD, De bree R, Girbes AR, Polderman KH. Hospital-acquired sinusitis is a common cause of fever of unknown origin in orotracheally intubated critically ill patients. Crit Care. 2005;9(5):R583–90. doi: 10.1186/cc3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ah-see KW, Evans AS. Sinusitis and its management. BMJ. 2007;334(7589):358–61. doi: 10.1136/bmj.39092.679722.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisno AL, Freeman JC. The syndrome of asplenia, pneumococcal sepsis, and disseminated intravascular coagulation. Ann Intern Med. 1970;72(3):389–93. doi: 10.7326/0003-4819-72-3-389. [DOI] [PubMed] [Google Scholar]
- 17.De souza AL, Sztajnbok J, Salgado MM, et al. Severe myalgia of the lower extremities as the first clinical feature of meningococcal purpura fulminans. Am J Trop Med Hyg. 2007;77(4):723–6. doi: 10.4269/ajtmh.2007.77.723. [DOI] [PubMed] [Google Scholar]
- 18.Emori K, Takeuchi N, Soneda J. A Case of Waterhouse-Friderichsen Syndrome Resulting from an Invasive Pneumococcal Infection in a Patient with a Hypoplastic Spleen. Case Rep Crit Care. 2016;2016:4708086. doi: 10.1155/2016/4708086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valade S, Azoulay E, Galicier L, et al. Coagulation Disorders and Bleedings in Critically Ill Patients With Hemophagocytic Lymphohistiocytosis. Medicine (Baltimore) 2015;94(40):e1692. doi: 10.1097/MD.0000000000001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George MR. Hemophagocytic lymphohistiocytosis: review of etiologies and management. J Blood Med. 2014;5:69–86. doi: 10.2147/JBM.S46255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cone LA, B Waterbor R, Sofonio MV. Purpura fulminans due to Streptococcus pneumoniae sepsis following gastric bypass. Obes Surg. 2004;14(5):690–4. doi: 10.1381/096089204323093507. [DOI] [PubMed] [Google Scholar]
- 22.Teo HG, Wong JY, Ting TLL. Purpura Fulminans: A rare presentation of Streptococcus Pneumoniae infection. BMJ Case Rep. 2017;2017:bcr-2017-221150. doi: 10.1136/bcr-2017-221150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komatsu M, Ishihara T, Manabe S, et al. A Case of Overwhelming Postsplenectomy Infection Caused by Streptococcus pneumoniae with Fulminant Purpura. Tokai J Exp Clin Med. 2017;42(3):130–132. [PubMed] [Google Scholar]
- 24.Asakura T, Higuchi A, Mori N. Streptococcus pneumoniae-induced purpura fulminans. QJM. 2016;109(7):499–500. doi: 10.1093/qjmed/hcw060. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez EF, Olarte KE, Ramesh MS. Purpura Fulminans Secondary to Streptococcus pneumoniae Meningitis. Case Rep Infect Dis. 2012;2012:508503. doi: 10.1155/2012/508503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bisno AL, Freeman JC. The Syndrome of Asplenia, Pneumococcal Sepsis, and Disseminated Intravascular Coagulation. Ann Intern Med 72(3):389. [DOI] [PubMed]
- 27.Ondruschka B, Habeck JO, Hädrich C, Dreßler J, Bayer R. Rare cause of natural death in forensic setting: hemophagocytic syndrome. Int J Legal Med. 2016;130(3):777–81. doi: 10.1007/s00414-015-1305-0. [DOI] [PubMed] [Google Scholar]
- 28.François B, Trimoreau F, Vignon P, Fixe P, Praloran V, Gastinne H. Thrombocytopenia in the sepsis syndrome: role of hemophagocytosis and macrophage colony-stimulating factor. Am J Med. 1997;103(2):114–20. doi: 10.1016/S0002-9343(97)00136-8. [DOI] [PubMed] [Google Scholar]
- 29.Otrock ZK, Hock KG, Riley SB, De witte T, Eby CS, Scott MG. Elevated serum ferritin is not specific for hemophagocytic lymphohistiocytosis. Ann Hematol. 2017;96(10):1667–1672. doi: 10.1007/s00277-017-3072-0. [DOI] [PubMed] [Google Scholar]
- 30.Grangé S, Buchonnet G, Besnier E, et al. The Use of Ferritin to Identify Critically Ill Patients With Secondary Hemophagocytic Lymphohistiocytosis. Crit Care Med. 2016;44(11):e1045–e1053. doi: 10.1097/CCM.0000000000001878. [DOI] [PubMed] [Google Scholar]
- 31.Greinacher A, Selleng S. How I evaluate and treat thrombocytopenia in the intensive care unit patient. Blood. 2016;128(26):3032–3042. doi: 10.1182/blood-2016-09-693655. [DOI] [PubMed] [Google Scholar]
- 32.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raschke RA, Garcia-orr R. Hemophagocytic lymphohistiocytosis: a potentially underrecognized association with systemic inflammatory response syndrome, severe sepsis, and septic shock in adults. Chest. 2011;140(4):933–938. doi: 10.1378/chest.11-0619. [DOI] [PubMed] [Google Scholar]