Abstract
Kidney function/dysfunction may affect liver function/dysfunction and vice versa. Liver function is indicated by the observed concentrations of several liver enzymes. Kidney function is indicated by the glomerular filtration rate. Consequently, it is logical to study associations between liver enzymes and glomerular filtration rate indicted by the stages of glomerular function (GF). Thus, this study was undertaken to evaluate the associations between selected liver enzymes and the stages of GF for US adults aged >= 20 years. Data (N = 9523) for US adults for the years 2003–2014 from National Health and Nutrition Examination Survey were analyzed to estimate variabilities in concentrations associated with liver enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphate (APH), and γ-glutamyl transferase (GGT) across the stages of GF and to assess variabilities in associations that perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) may have with these enzymes across the stages of GF. Those with eGFR >90 mL/min/1.73 m2 were defined as being in GF-1, those with eGFR between 60 and 89 mL/min/1.73 m2 were defined as being in GF-2, those with eGFR between 45 and 59 mL/min/1.73 m2 were defined as being in GF-3A, those with eGFR between 15 and 44 mL/min/1.73 m2 were defined as being GF-3B/4. Regression models stratified by GF stages with ALT, AST, APH, and GGT as dependent variables were fitted to evaluate the associations of interest. Adjusted levels of ALT decreased with deteriorating kidney function from 25.3 IU/L at GF-1 to 20.9 IU/L at GF-3B/4 for obese adults and from 21.4 IU/L at GF-1 to 16.4 IU/L at GF-3B/4 for nonobese adults. Adjusted levels of AST followed inverted U-shaped distributions with increases from GF-1 to GF-2 followed by decreases from GF-2 to GF-3B/4. Adjusted levels of APH followed inverted U-shaped distributions with increases from GF-1 to GF-3A followed by decreases from GF-3A to GF-3B/4. Adjusted levels of GGT followed inverted U-shaped distribution among obese participants with point of inflection located at GF-3A. For the total population, obese had higher adjusted levels than nonobese at GF-1, GF-2, and GF-3A for ALT, APH, and GGT. Male-female differences in adjusted levels of ALT and GGT continued narrowing as kidney function deteriorated from GF-1 to GF-3B/4. The differences in ALT widened among nonobese smokers and nonsmokers as kidney function deteriorated. The concentrations of liver enzymes across GF stages varied by gender, race/ethnicity, smoking status, and obesity and more often than not, were indicated by inverted U-shaped curves with points of inflections located at G-2 or GF-3A. The associations between PFOA/PFOS with liver enzymes varied in magnitude and/or direction by stages of GF as kidney function deteriorated.
Keywords: Chemistry, Environmental health, Environmental pollution, Environmental risk assessment, Environmental science, Environmental toxicology, Liver, Kidney, Liver enzymes, PFAA, NHANES
1. Introduction
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphate (APH), and γ-glutamyl transferase (GGT) are among the liver enzymes whose observed levels in serum are used to indicate the health of the human liver. Hepatocellular damage not attributable to alcohol are indicated by elevated levels of ALT and GGT. AST/ALT ratio is used to differentiate alcoholic from nonalcoholic fatty liver disease (Toshikuni et al., 2014). Elevated levels of ALT may be indicative of the existence of other medical problems such as viral hepatitis, diabetes, congestive heart failure, liver damage and others. Abnormal levels of APH may indicate issues related to not only liver but also bone, gall bladder, kidney tumors, and infections. GGT is a marker of alcohol related liver disease and has been linked to increased risk of cardiovascular disease, diabetes, metabolic syndrome and all-cause mortality (Koenig and Seneff, 2015). GGT along with APH are also used as biomarkers of biliary disease.
The National Health and Nutrition Examination Survey (NHANES, www.cdc.gov/nchs/nhanes/index.htm) conducted by the US Centers for Disease Control and Prevention releases nationally representative data in public domain for over 200 chemicals every two years. Chemicals for which data are released include liver enzymes. Quite a few authors have used these and other data to evaluate association of perfluoroalkyl acids (PFAA) with liver enzymes (Gallo et al., 2012, Darrow et al., 2016, Lin et al., 2010, Olsen and Zobel, 2007, Olsen et al., 2012), association of liver enzymes with insulin resistance (Sheng et al., 2018), gestational diabetes (Zhu et al., 2018; Kong et al., 2018), type 2 diabetes (Zhang et al., 2018; Chen et al., 2016), type 1 diabetes (Stadler et al., 2017), and others. We could not, however, locate a study that has studied the variabilities in the levels of liver enzymes associated with diseases and disorders of the kidney.
Among others as mentioned above, Lin et al. (2010) and Jain and Ducatman (2019a) have investigated associations between selected PFAA and liver enzymes. Lin et al. (2010) used NHANES 1999–2000 and 2003–2004 data for US adults aged >= 18 years and investigated how levels of ALT and GGT were associated with the levels of perfluorooctanoic acid (PFOA) and perfluorononanoic acid (PFNA). A positive association between PFOA and ALT and GGT was reported and association between PFOA and liver enzymes was found to be more evident among obese participants (Lin et al., 2010). With this as a motivating factor, Jain and Ducatman (2019a) used more recent NHANES data for 2011–2014 and evaluated associations of PFOA, perfluorooctane sulfonic acid (PFOS), perfluorodecanoic acid (PFDA), perfluorohexane sulfonic acid (PFHxS), and PFNA with ALT, AST, APH, and GGT by fitting models stratified by obesity status. These authors reported positive associations of ALT with PFOA, PFHxS, and PFNA among obese participants only; and positive associations of PFOA and PFNA with GGT among obese participants only. Among nonobese participants, none of the PFAA was found to be associated with any of the four liver enzymes investigated by Jain and Ducatman (2019a). Jain and Ducatman (2019b) reported the levels of PFOA, PFOS, PFDA, PFHxS, and PFNA to vary across the stages of glomerular function (GF) with the levels of PFAA to be lower at GF-3A (eGFR between 45 and 59 mL/min/1.73 m2) or GF-3B/4 (eGFR between 15 and 44 mL/min/1.73 m2) as compared to the levels at GF-1 (eGFR >90 mL/min/1.73 m2) and GF-2 (eGFR between 60 and 89 mL/min/1.73 m2) or during the advanced stages of kidney function. This article raised the possibility if the associations between PFAA and liver enzymes may vary across different stages of GF.
With motivations as listed above, this study was undertaken to evaluate (i) how the unadjusted and adjusted levels of ALT, AST, APH, and GGT vary as kidney function deteriorates with aging, and (ii) how associations between liver enzymes and PFAA vary across the stages of GF. Date from NHANES for the period 2003–2014 were selected for this study and this study was limited to US adults aged >= 20 years.
2. Materials and methods
2.1. Data source and description
Data on demographics, liver function biomarkers, blood pressure, glycohemoglobin, serum creatinine, total dietary nutrients intake, PFOA and PFOS, and serum cotinine for US adults aged >= 20 years from NHANES for the period 2003–2014 were downloaded and match merged. Liver function biomarkers selected for analysis were: ALT, AST, APH, and GGT.
For the purpose of this study, those who reported being current users of insulin and/or diabetic pills and/or had glycohemoglobin levels ≥6.5% were considered to be diabetic. Those with average systolic blood pressure >130 mm Hg and/or diastolic blood pressure >80 mm Hg and/or self-reported being current users of blood pressure medications were considered to be hypertensive. CKD-EPI equation (Levey et al., 2009) was used to compute eGFR based on the observed values of serum creatinine. Estimated glomerular filtration (eGFR) is conventionally defined to correspond to stages of kidney disease (Inker et al., 2014). Those with eGFR >90 mL/min/1.73 m2 were defined as being in glomerular function-1 (GF-1, most individuals in this category do not have kidney disease), those with eGFR between 60 and 89 mL/min/1.73 m2 were defined as being in GF-2, those with eGFR between 45 and 59 mL/min/1.73 m2 were defined as being in GF-3A, those with eGFR between 30 and 44 mL/min/1.73 m2 were defined as being in GF-3B, those with eGFR between 15 and 29 mL/min/1.73 m2 were defined as being in GF-4, and those with eGFR <15 mL/min/1.73 m2 were defined as being in GF-5 (Inker et al., 2014). Those who had serum cotinine levels >= 10 ng/mL were defined as smokers. Obesity was defined as having body mass index ≥30 kg/m2.
After removing pregnant females and participants with missing values for body mass index, diabetes status, hypertension status, fasting, alcohol consumption, and serum cotinine, and those who self-reported being on dialysis during the last 12 months or were in GF-5, 9523 (6121 nonobese and 3402 obese) participants remained available for analysis. Sample size details are given in Table 1.
Table 1.
Unweighted sample sizes by the stages of glomerular function by gender, race/ethnicity, and smoking status for US adults aged >= 20 years. Data from National Health and Nutrition Examination Survey 2003–2014.
| Obesity status | Demographic group* | Glomerular function stage |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GF-1 |
GF-2 |
GF-3A |
GF-3B |
|||||||
| N | % | N | % | N | % | N | % | N | ||
| Nonobese | Total | 3562 | 58.2 | 2026 | 33.1 | 335 | 5.8 | 178 | 2.9 | 6121 |
| Males | 1722 | 54.9 | 1148 | 36.6 | 179 | 5.7 | 87 | 2.8 | 3136 | |
| Females | 1840 | 61.6 | 878 | 29.4 | 176 | 5.9 | 91 | 3.1 | 2985 | |
| Nonsmokers | 2508 | 55.2 | 1582 | 34.8 | 306 | 6.7 | 147 | 3.2 | 4543 | |
| Smokers | 1054 | 66.8 | 444 | 28.1 | 49 | 3.1 | 31 | 2.0 | 2745 | |
| NHW | 1388 | 46.7 | 1228 | 41.3 | 238 | 8.0 | 118 | 4.0 | 2972 | |
| NHB | 702 | 65.9 | 284 | 26.6 | 50 | 4.7 | 30 | 2.8 | 1066 | |
| HISP | 1032 | 71.2 | 348 | 24.0 | 50 | 3.5 | 19 | 1.3 | 1449 | |
| OTHR | 440 | 69.4 | 166 | 26.2 | 17 | 2.7 | 11 | 1.7 | 634 | |
| Obese | Total | 1946 | 57.2 | 1148 | 33.4 | 200 | 5.9 | 108 | 3.2 | 3402 |
| Males | 822 | 55.5 | 540 | 36.5 | 80 | 5.4 | 39 | 2.6 | 1481 | |
| Females | 1124 | 56.5 | 608 | 31.7 | 120 | 6.3 | 69 | 3.6 | 1921 | |
| Nonsmokers | 1435 | 55.1 | 890 | 34.2 | 182 | 7.0 | 97 | 3.7 | 2604 | |
| Smokers | 511 | 64.0 | 258 | 32.3 | 18 | 2.3 | 11 | 1.4 | 798 | |
| NHW | 701 | 46.6 | 619 | 41.2 | 120 | 8.0 | 63 | 4.2 | 1503 | |
| NHB | 538 | 61.8 | 259 | 29.7 | 52 | 6.0 | 22 | 2.5 | 871 | |
| HISP | 617 | 69.2 | 233 | 26.1 | 24 | 2.7 | 18 | 2.0 | 892 | |
| OTHR | 90 | 66.2 | 37 | 27.2 | 4 | 3.0 | 5 | 3.7 | 136 | |
*NHW: non-Hispanic white, NHB: non-Hispanic black, HISP: Hispanics, OTHR: other unclassified race/ethnicities.
2.2. Software and statistical analysis
University Edition SAS (www.sa.com) was used to analyze all data for this study. All analyses used sampling weight and sampling design characteristics that included information on number of strata and clusters. Specifically SAS Procs FREQ and SURVEYREG were used to conduct all analyses. For univariate analyses, in an analysis of variance set up, SAS Proc SURVEYREG was used to compute unadjusted geometric means (UGM) for all four liver enzymes for each GF stage for the total population, by gender and smoking status. Since, obesity has been found to be associated with higher levels of liver enzymes (Jain and Ducatman, 2019a), UGMs were separately computed for obese and nonobese participants. Data for UGMs are given in Table 2. Adjusted geometric means (AGM) stratified by obesity status were computed with log10 transformed values of each of the four liver enzymes used as dependent variables for each GF stage. However, in order to have adequate sample size for each model, data for GF-3B and GF-4 were combined. Thus, since there were four liver enzymes and four GF stages to analyze, a total of 16 models for nonobese participants and 16 models for obese participants were fitted. In addition, each model was fitted twice, once with PFOA as an independent variable and once with PFOS as an independent variable. Independent variables included in each model were: gender (male, female), race/ethnicity (non-Hispanic white or NHW, non-Hispanic black or NHB, all Hispanics or HISP, and all other unclassified race/ethnicities or OTHR), smoking status (nonsmoker, smoker) as categorical variables; age, log10 transformed values of body mass index, log10 transformed values of PFOA or PFOS, diabetes status (no coded as 0, yes coded as 1), hypertension status (no coded as 0, yes coded as 1), fasting time in hours, poverty income ratio, survey year to account for any changes in liver enzyme values over time, and daily alcohol consumption in gm as continuous/binary variables. Data on AGMs for nonobese participants are given in Table 3 and data for obese participants are given in Table 4. Table 5 provides adjusted slopes for associations between diabetes and hypertension status with log10 transformed values of each of the four liver enzymes. Table 6 provides adjusted slopes for associations between log10 transformed values of PFOA and PFOS with log10 transformed values of each of the four liver enzymes.
Table 2.
Unadjusted geometric means with 95% confidence intervals in IU/L for liver function tests (LFT) by stages of glomerular function for US adults aged >= 20 years by obesity status by gender and smoking status. Data from National Health and Nutrition Examination Survey 2003–2014.
| Glomerular function stage |
SSD* | |||||
|---|---|---|---|---|---|---|
| GF-1 | GF-2 | GF-3A | GF-3B/4 | |||
| Nonobese | LFT** | |||||
| Total | ALT | 21.4 (21.0–21.8) | 21.7 (21.3–22.2) | 20.7 (19.7–21.7) | 18.2 (16.9–19.6) | GF-1 > GF-3B/4 (p < 0.01), GF-2 > GF-3B/4 (p < 0.01), GF-3A > GF-B/4 (p < 0.01) |
| AST | 23.3 (23.0–23.6) | 24.5 (24.1–24.9) | 25.5 (24.6–26.5) | 23.6 (22.3–24.9) | GF-1< GF-2 (p < 0.01), GF-1< GF-3A (p < 0.01) | |
| APH | 61.4 (60.5–62.2) | 62.4 (61.3–63.4) | 67.3 (64.4–70.2) | 70.7 (66.2–75.6) | GF-1< GF-3A (p < 0.01), GF-1< GF-3B/4 (p < 0.01), GF-2< GF-3A (p < 0.01), GF-2< GF-3B/4 (p < 0.01), GF-3A < GF-3B (p < 0.01) | |
| GGT | 18.7 (18.2–19.3) | 19.4 (18.7–20.1) | 20.0 (18.3–21.8) | 20.0 (17.8–22.5) | ||
| Males | ALT | 25.8 (25.4–26.1) | 24.8 (24.3–25.2) | 22.1 (21.2–23.0) | 19.6 (18.2–21.2) | GF-1 > GF-2 (p = 0.03), GF-1 > GF-3A (p = 0.02), GF-1 > GF-3B/4 (p < 0.01), GF-2 > GF-3B/4 (p < 0.01), GF-3A > GF-3B/4 (p = 0.02) |
| AST | 25.3 (25.0–25.6) | 25.6 (25.3–26.0) | 25.1 (24.4–25.9) | 24.3 (22.9–25.8) | ||
| APH | 66.9 (66.0–67.7) | 63.2 (62.2–64.1) | 63.5 (61.2–65.9) | 66.9 (62.3–71.8) | GF-1 > GF-2 (p < 0.01), GF-2< GF-3B4 (p = 0.04) | |
| GGT | 24.7 (24.1–25.3) | 24.0 (23.4–24.7) | 21.8 (20.5–23.3) | 22.9 (20.1–26.1) | ||
| Females | ALT | 18.0 (17.8–18.3) | 19.2 (18.9–19.5) | 19.0 (18.3–19.8) | 17.9 (16.9–18.8) | GF-1< GF-2 (p = 0.01) |
| AST | 21.3 (21.1–21.5) | 23.2 (22.9–23.5) | 23.9 (23.3–24.6) | 24.2 (23.3–25.1) | GF-1< GF-2 (p < 0.01), GF-1< GF-3A (p < 0.01), GF-1 < GF-3B/4 (p = 0.02), GF-2 < GF-3A (p < 0.01) | |
| APH | 58.6 (58.0–59.3) | 61.4 (60.5–62.4) | 64.8 (62.8–66.9) | 69.5 (65.9–73.3) | GF-1< GF-2 (p < 0.01), GF-1< GF-3A (p < 0.01), GF-1< GF-3B/4 (p < 0.01) | |
| GGT | 15.7 (15.4–16.1) | 16.8 (16.4–17.3) | 18.3 (17.2–19.4) | 19.4 (17.3–21.6) | GF-1< GF-3A (p < 0.01) | |
| Nonsmokers | ALT | 21.3 (21.0–21.5) | 22.0 (21.7–22.4) | 20.5 (19.9–21.1) | 18.5 (17.6–19.5) | GF-1 > GF-3B/4 (p = 0.04), GF-2 > GF-3B/4 (p < 0.01) |
| AST | 23.0 (22.8–23.2) | 24.6 (24.3–24.9) | 24.6 (24.1–25.1) | 24.3 (23.4–25.1) | GF-1< GF-2 (p < 0.01), GF-1< GF-3A (p < 0.01) | |
| APH | 60.7 (60.1–61.3) | 60.9 (60.2–61.7) | 63.4 (61.9–65.0) | 68.0 (64.9–71.3) | GF-1< GF-3A (p < 0.01), GF-1< GF-3B/4 (p < 0.01), GF-2< GF-3A (p < 0.01), GF-2< GF-3B/4 (p < 0.01) | |
| GGT | 18.1 (17.7–18.4) | 19.6 (19.2–20.1) | 19.4 (18.5–20.3) | 20.0 (18.2–21.8) | GF-1< GF-2 (p < 0.01), GF-1< GF-3A (p = 0.02) | |
| Smokers | ALT | 22.2 (21.7–22.7) | 21.4 (20.7–22.2) | 18.5 (17.1–19.9) | 17.6 (15.4–20.0) | GF-1 > GF-3A (p < 0.01), GF-1 > GF-3B/4 (p < 0.01), GF-2 > GF-3A (p < 0.01), GF-2 > GF-3B/4 (p < 0.01) |
| AST | 23.7 (23.3–24.2) | 23.8 (23.2–24.5) | 23.3 (21.5–25.1) | 24.0 (22.0–26.2) | GF-2 < GF-3B/4 (p = 0.03) | |
| APH | 66.8 (65.8–67.9) | 67.8 (66.6–69.1) | 72.3 (67.9–77.0) | 73.9 (67.3–81.1) | GF-1< GF-3A (p=<0.01), GF-2 < GF-3A (p = 0.02) | |
| GGT | 23.7 (22.9–24.5) | 22.7 (21.6–23.9) | 23.4 (20.3–26.9) | 24.3 (19.6–30.2) | ||
| Obese | ||||||
| Total | ALT | 25.9 (25.2–26.6) | 24.7 (23.9–25.5) | 21.7 (20.1–23.4) | 20.3 (17.7–23.3) | GF-1 > GF-3A (p < 0.01), GF-1 > GF-3B/4 (p < 0.01), GF-2 > GF-3A (p < 0.01), GF-2 > GF-3B/4 (p = 0.03) |
| AST | 24.2 (23.8–24.7) | 24.6 (23.9–25.3) | 25.5 (23.7–27.4) | 24.9 (22.2–28.0) | ||
| APH | 67.9 (66.8–69.1) | 68.1 (66.7–69.6) | 69.8 (66.5–73.3) | 78.9 (73.1–85.2) | GF-1 < GF-3B/4 (p < 0.01), GF-2 < GF-3B/4 (p < 0.01), GF-3A < GF-3B/4 (p = 0.03) | |
| GGT | 24.7 (23.9–25.5) | 24.9 (23.7–26.2) | 22.0 (20.3–23.8) | 26.9 (22.0–32.8) | GF-1 > GF-3A (p = 0.03) | |
| Males | ALT | 33.8 (33.1–34.5) | 29.1 (28.4–29.9) | 23.3 (22.1–24.6) | 22.9 (19.2–27.4) | GF-1 > GF-2 (p < 0.01), GF-1 > GF-3A (p < 0.01) |
| AST | 27.4 (26.9–27.8) | 26.1 (25.5–26.6) | 25.1 (24.2–26.1) | 24.1 (21.2–27.4) | ||
| APH | 67.7 (66.8–68.6) | 65.1 (64.1–66.2) | 64.0 (59.9–68.3) | 71.7 (64.1–80.2) | ||
| GGT | 32.3 (31.4–33.1) | 28.9 (27.8–30.0) | 25.4 (23.0–28.1) | 29.3 (23.5–36.6) | GF-1 > GF-2 (p < 0.01), GF-1 > GF-3A (p < 0.01), GF-2 < GF-3A (p = 0.04) | |
| Females | ALT | 20.9 (20.5–21.3) | 22.0 (21.4–22.6) | 19.1 (18.3–20.0) | 18.2 (16.6–20.0) | |
| AST | 21.4 (21.1–21.7) | 23.1 (22.6–23.7) | 22.7 (21.9–23.5) | 22.7 (20.7–24.8) | GF-1< GF-3A (p = 0.03) | |
| APH | 69.0 (68.0–70.0) | 69.2 (67.7–70.8) | 72.5 (69.6–75.6) | 74.6 (69.4–80.1) | GF-1 < GF-3B/4 (p < 0.01) | |
| GGT | 20.5 (20.0–21.1) | 22.4 (21.4–23.4) | 21.1 (19.3–23.0) | 20.7 (18.2–23.5) | GF-1< GF-2 (p = 0.03), | |
| Nonsmokers | ALT | 26.0 (25.5–26.6) | 25.1 (24.5–25.7) | 20.8 (20.0–21.6) | 19.6 (17.9–21.4) | GF-1 > GF-3A (p < 0.01), GF-1 > GF-3B/4 (p < 0.01),GF-2 > GF-3A (p < 0.01), GF-2 > GF-3B/4 (p = 0.02) |
| AST | 24.1 (23.8–24.4) | 24.6 (24.1–25.1) | 23.7 (23.1–24.3) | 23.2 (21.5–25.1) | ||
| APH | 68.3 (67.6–69.0) | 66.5 (65.4–67.5) | 68.6 (65.6–71.6) | 73.5 (68.8–78.5) | GF-1 > GF-3B/4 (p < 0.01), GF-2< GF-3B/4 (p < 0.01), GF-3A < GF-3B/4 (p = 0.01 | |
| GGT | 24.3 (23.7–25.0) | 24.6 (23.7–25.5) | 22.4 (20.8–24.1) | 22.9 (20.3–25.9) | ||
| Smokers | ALT | 26.6 (25.8–27.5) | 26.0 (24.9–27.2) | 19.5 (16.6–22.9) | 18.2 (14.5–22.9) | |
| AST | 23.8 (23.3–24.4) | 24.2 (23.3–25.0) | 22.3 (19.8–25.1) | 21.1 (18.8–23.6) | GF-1 > GF-3B (p = 0.03), GF-2 > GF-3B (p = 0.04) | |
| APH | 68.7 (67.5–69.9) | 70.2 (68.1–72.4) | 74.5 (67.0–82.9) | 77.8 (69.1–87.6) | ||
| GGT | 28.4 (27.3–29.6) | 29.2 (27.4–31.2) | 25.4 (20.1–32.2) | 22.7 (15.6–33.1) | ||
*Statistically significant differences with p-values adjusted for multiple comparisons using Tukey-Kremer method.
**ALT = alanine aminotransferase, AST = aspartate aminotransferase, APH = alkaline phosphate, GGT = γ-glutamyl transferase
Table 3.
Adjusted geometric means with 95% confidence intervals in IU/L for selected liver function tests (LFT) by stage of glomerular function by gender, race/ethnicity, and smoking status for nonobese US adults aged >= 20 years. Data from National Health and Nutrition Examination Survey 2003–2014.
| Non-obese adults |
Glomerular filtration stage |
||||
|---|---|---|---|---|---|
| LFT** | GF-1 | GF-2 | GF-3A | GF-3B/4 | |
| ALT | Total | 21.4 (21.0–21.9) | 21.3 (20.5–22.0) | 18.7 (17.3–20.3) | 16.4 (16.2–16.5) |
| Males (M) | 24.6 (23.8–25.3) | 23.0 (22.1–23.8) | 19.6 (17.9–21.5) | 16.6 (16.4–16.8) | |
| Females (F) | 18.6 (18.1–19.2) | 19.7 (18.8–20.6) | 17.9 (16.3–19.7) | 16.2 (16.0–16.3) | |
| Non-Hispanic White (NHW) | 21.2 (20.6–21.9) | 20.9 (20.2–21.6) | 18.8 (17.7–20.0) | 17.0 (16.5–17.4) | |
| Non-Hispanic Black (NHB) | 19.6 (19.0–20.3) | 19.5 (18.6–20.5) | 16.8 (14.7–19.2) | 13.8 (13.6–14.0) | |
| Hispanics (HISP) | 23.5 (22.6–24.6) | 23.3 (21.6–25.2) | 20.1 (17.2–23.5) | 14.2 (13.9–14.6) | |
| Others (OTHR) | 21.4 (20.5–22.4) | 21.5 (19.6–23.7) | 19.4 (15.1–25.0) | 21.5 (21.2–21.9) | |
| Nonsmokers (NSM) | 21.8 (21.3–22.2) | 21.9 (21.1–22.7) | 20.7 (19.1–22.4) | 19.1 (18.8–19.3) | |
| Smokers (SM) | 21.0 (20.3–21.8) | 20.7 (19.6–21.8) | 17.0 (15.1–19.2) | 14.1 (13.9–14.2) | |
| SSD* | M > F (p < 0.01), NHW > NHB (p < 0.01), NHW < HISP (p < 0.01), NHB < HISP (p < 0.01), NHB < OTHR (p < 0.01), HISP > OTHR (p = 0.02) | M > F (p < 0.01), NHB < HISP (p < 0.01) | NSM > SM (p < 0.01) | M > F (p < 0.01), NHW > HISP (p < 0.1), NHW > NHB (p < 0.01), NHW < OTHR (p < 0.01), NHB < OTHR (p = 0.01), HISP < OTHR (p < 0.01), NSM > SM (p < 0.01) | |
| AST | Total | 23.4 (23.0–23.7) | 24.6 (24.1–25.2) | 24.2 (22.6–25.9) | 23.1 (22.2–23.9) |
| Males (M) | 24.9 (24.4–25.4) | 25.4 (24.8–26.1) | 24.4 (22.7–26.4) | 22.4 (21.3–23.7) | |
| Females (F) | 21.9 (21.4–22.4) | 23.9 (23.2–24.6) | 24.0 (22.1–26.2) | 23.7 (23.2–24.1) | |
| Non-Hispanic White (NHW) | 23.1 (22.6–23.6) | 24.0 (23.3–24.7) | 24.2 (23.0–25.5) | 22.3 (21.0–23.6) | |
| Non-Hispanic Black (NHB) | 23.5 (22.8–24.2) | 24.7 (23.7–25.7) | 22.9 (20.2–25.9) | 21.4 (20.8–22.0) | |
| Hispanics (HISP) | 24.4 (23.6–25.1) | 25.2 (24.0–26.4) | 23.6 (21.1–26.5) | 20.5 (20.2–20.8) | |
| Others (OTHR) | 22.6 (21.9–23.3) | 24.7 (23.5–26.0) | 26.4 (21.8–31.8) | 28.9 (26.9–31.0) | |
| Nonsmokers (NSM) | 23.6 (23.3–24.0) | 25.0 (24.5–25.6) | 25.8 (24.3–27.4) | 25.7 (25.4–26.0) | |
| Smokers (SM) | 23.1 (22.5–23.8) | 24.2 (23.2–25.3) | 22.8 (20.6–25.2) | 20.7 (19.0–22.5) | |
| SSD* | M > F (p < 0.01), NHW < HISP (p = 0.01), HISP < OTHR (p < 0.01) | M > F (p < 0.01) | NSM > SM (p = 0.01) | M < F (p < 0.01), NHW > NHB (p < 0.01), NHW < OTHR (p < 0.01), NHB < OTHR (p < 0.01), HISP < OTHR (p < 0.01), NSM > SM (p < 0.01) | |
| APH | Total | 63.8 (62.5–65.1) | 68.8 (67.2–70.4) | 73.8 (69.6–78.3) | 72.1 (69.6–74.7) |
| Males (M) | 67.0 (65.4–68.6) | 67.4 (65.4–69.3) | 74.3 (69.0–80.0) | 71.6 (67.9–75.6) | |
| Females (F) | 60.7 (59.3–62.2) | 70.3 (68.3–72.3) | 73.3 (67.9–79.2) | 72.6 (71.2–74.1) | |
| Non-Hispanic White (NHW) | 61.7 (60.0–63.4) | 65.6 (64.0–67.2) | 70.2 (66.2–74.5) | 71.0 (68.8–73.3) | |
| Non-Hispanic Black (NHB) | 61.5 (59.8–63.2) | 66.4 (63.9–69.0) | 70.5 (63.1–78.8) | 64.0 (59.2–69.1) | |
| Hispanics (HISP) | 69.2 (67.0–71.4) | 76.3 (72.9–79.8) | 81.8 (70.6–94.7) | 69.5 (67.6–71.5) | |
| Others (OTHR) | 63.1 (60.6–65.7) | 67.4 (63.0–72.1) | 73.3 (64.6–83.3) | 85.7 (81.7–89.8) | |
| Nonsmokers (NSM) | 61.6 (60.5–62.8) | 64.5 (62.9–66.1) | 68.9 (65.2–72.8) | 72.5 (71.5–73.4) | |
| Smokers (SM) | 66.0 (64.0–68.0) | 73.4 (70.8–76.0) | 79.1 (72.7–86.2) | 71.8 (67.8–75.9) | |
| SSD* | M > F (p < 0.01), NHW < HISP (p < 0.01), NHB < HISP (p < 0.01), HISP > OTHR (p < 0.01), NSM < SM (p < 0.01) | M < F (p = 0.01), NHW < HISP (p < 0.01), NHB < HISP (p < 0.01), HISP > OTHR (p < 0.01), NSM < SM (p < 0.01) | NSM < SM (p < 0.01) | NHW < OTHR (p < 0.01), NHB < HISP (p = 0.01), NHB < OTHR (p < 0.01), HISP < OTHR (p < 0.01) | |
| GGT | Total | 20.9 (20.2–21.6) | 22.3 (21.2–23.5) | 21.7 (19.2–24.6) | 25.3 (23.1–27.8) |
| Males (M) | 24.1 (23.0–25.4) | 24.7 (23.5–26.0) | 22.8 (19.6–26.5) | 26.4 (22.4–31.1) | |
| Females (F) | 18.1 (17.2–18.9) | 20.1 (18.8–21.5) | 20.7 (17.6–24.4) | 24.3 (23.5–25.1) | |
| Non-Hispanic White (NHW) | 18.5 (17.6–19.4) | 19.9 (19.0–20.9) | 20.1 (17.4–23.2) | 17.9 (17.0–19.0) | |
| Non-Hispanic Black (NHB) | 23.0 (21.7–24.3) | 23.2 (21.4–25.2) | 23.4 (18.6–29.3) | 22.3 (18.8–26.5) | |
| Hispanics (HISP) | 22.6 (21.2–24.1) | 25.2 (22.5–28.2) | 22.0 (16.7–28.9) | 25.3 (24.1–26.5) | |
| Others (OTHR) | 19.8 (18.3–21.4) | 21.3 (18.8–24.1) | 21.6 (17.5–26.7) | 40.7 (35.2–47.0) | |
| Nonsmokers (NSM) | 19.0 (18.4–19.6) | 20.9 (19.9–22.0) | 21.6 (19.6–23.8) | 27.8 (26.9–28.8) | |
| Smokers (SM) | 23.0 (21.7–24.3) | 23.8 (22.0–25.8) | 21.9 (17.1–27.9) | 23.1 (19.6–27.2) | |
| SSD* | M > F (p < 0.01), NHW < NHB (p < 0.01), NHW < HISP (p < 0.01), NHB > OTHR (p = 0.01), HISP > OTHR (p = 0.04), NSM < SM (p < 0.01) | M > F (p < 0.01), NHW < NHB (p < 0.01), NHW < HISP (p < 0.01), NSM < SM (p < 0.01) | NHW < HISP (p < 0.01), NHW < OTHR (p < 0.01), NHB < OTHR (p < 0.01), HISP < OTHR (p = 0.01), NSM > SM (p = 0.01) | ||
*Statistically significant differences with p-values adjusted for multiple comparisons using Tukey-Kramer method.
**ALT = alanine aminotransferase, AST = aspartate aminotransferase, APH = alkaline phosphate, GGT = γ-glutamyl transferase
Table 4.
Adjusted geometric means with 95% confidence intervals in IU/L for selected liver function tests (LFT) by stages of glomerular function by gender, race/ethnicity, and smoking status for obese US adults aged >= 20 years. Data from National Health and Nutrition Examination Survey 2003–2014.
| Obese adults |
Glomerular filtration stage |
||||
|---|---|---|---|---|---|
| LFT** | GF-1 | GF-2 | GF-3A | GF-3B/4 | |
| ALT | Total | 25.3 (24.3–26.3) | 23.4 (22.1–24.7) | 23.8 (21.9–25.9) | 20.9 (19.6–22.4) |
| Males (M) | 30.4 (28.8–32.0) | 26.1 (24.2–28.2) | 26.4 (24.4–28.6) | 24.1 (21.1–27.5) | |
| Females (F) | 21.0 (20.0–22.0) | 20.9 (19.8–22.2) | 21.5 (19.5–23.7) | 18.2 (17.9–18.4) | |
| Non-Hispanic White (NHW) | 26.0 (25.0–27.0) | 23.7 (22.8–24.7) | 24.2 (22.1–26.5) | 24.6 (23.5–25.7) | |
| Non-Hispanic Black (NHB) | 21.9 (21.0–22.8) | 20.1 (18.9–21.3) | 20.3 (19.5–21.1) | 16.8 (14.5–19.5) | |
| Hispanics (HISP) | 29.6 (28.1–31.1) | 26.2 (23.9–28.6) | 23.3 (21.8–24.9) | 23.1 (22.0–24.4) | |
| Others (OTHR) | 24.2 (21.4–27.4) | 24.0 (20.5–28.2) | 28.3 (22.1–36.3) | 20.0 (18.8–21.3) | |
| Nonsmokers (NSM) | 25.8 (24.8–26.9) | 24.6 (23.5–25.8) | 21.8 (20.4–23.3) | 19.9 (19.5–20.3) | |
| Smokers (SM) | 24.7 (23.3–26.2) | 22.2 (20.4–24.2) | 26.1 (22.3–30.4) | 22.0 (19.5–24.8) | |
| SSD* | M > F (p < 0.01), NHW > NHB (p < 0.01), NHW < HISP (p < 0.01), NHB < HISP (p < 0.01), HISP > OTHR (p = 0.01) | M > F (p < 0.01), NHW > NHB (p < 0.01), NHB < HISP (p < 0.01), NSM > SM (p = 0.01) | M > F (p < 0.01), NHW > NHB (<0.01), NHB < HISP (p < 0.01), NHB < OTHR (p = 0.04) | M > F (p < 0.01), NHW > NHB (p < 0.01), NHB < HISP (p < 0.01), NHB < OTHR (p < 0.01), NSM > SM (p < 0.01), NHW > OTHR (p < 0.01) | |
| AST | Total | 24.2 (23.4–25.0) | 24.4 (23.5–25.3) | 26.1 (24.9–27.4) | 25.1 (24.5–25.7) |
| Males (M) | 26.5 (25.4–27.5) | 25.3 (23.8–26.9) | 27.3 (26.2–28.4) | 26.1 (24.5–27.8) | |
| Females (F) | 22.1 (21.4–22.9) | 23.5 (22.6–24.4) | 24.9 (23.4–26.6) | 24.2 (23.8–24.6) | |
| Non-Hispanic White (NHW) | 23.8 (23.2–24.5) | 24.0 (23.2–24.8) | 26.9 (25.2–28.6) | 27.1 (26.4–27.7) | |
| Non-Hispanic Black (NHB) | 22.9 (22.2–23.7) | 23.0 (22.1–24.0) | 23.1 (22.1–24.3) | 20.9 (19.6–22.3) | |
| Hispanics (HISP) | 26.3 (25.3–27.4) | 25.4 (23.7–27.2) | 27.2 (25.9–28.6) | 24.4 (24.1–24.7) | |
| Others (OTHR) | 23.8 (21.7–26.1) | 25.2 (23.1–27.5) | 27.3 (24.9–30.1) | 28.9 (27.5–30.4) | |
| Nonsmokers (NSM) | 24.6 (23.9–25.3) | 25.3 (24.6–26.0) | 24.8 (24.3–25.4) | 25.3 (24.8–25.7) | |
| Smokers (SM) | 23.8 (22.8–24.8) | 23.5 (22.1–25.0) | 27.4 (24.7–30.4) | 25.0 (24.2–25.8) | |
| SSD* | M > F (p < 0.01), NHW < HISP (p < 0.01), NHB < HISP (p < 0.01) | M > F (p = 0.04), NSM > SM (p = 0.03) | M > F (p < 0.01), NHW > NHB (p < 0.01), NHB < HISP (p < 0.01), NHB < OTHR (p < 0.01) | NHW > NHB (p < 0.01), NHW < HISP (pp < 0.01), NHB < HISP (p < 0.01), NHB < OTHR (p < 0.01), HISP < OTHR (p < 0.01) | |
| APH | Total | 68.7 (66.8–70.5) | 72.2 (69.5–75.1) | 77.4 (72.4–82.7) | 72.1 (67.4–77.2) |
| Males (M) | 68.2 (66.0–70.5) | 70.0 (66.9–73.3) | 72.1 (67.9–76.5) | 71.2 (64.7–78.3) | |
| Females (F) | 69.1 (67.1–71.2) | 74.5 (71.5–77.7) | 83.1 (76.6–90.1) | 73.1 (69.7–76.6) | |
| Non-Hispanic White (NHW) | 68.3 (66.3–70.3) | 68.6 (66.7–70.5) | 72.5 (68.6–76.6) | 78.4 (73.8–83.4) | |
| Non-Hispanic Black (NHB) | 66.7 (64.6–68.9) | 70.3 (67.0–73.7) | 78.6 (76.7–80.5) | 77.5 (69.1–86.8) | |
| Hispanics (HISP) | 74.3 (72.2–76.6) | 75.6 (72.0–79.4) | 83.4 (78.4–88.8) | 58.9 (52.4–66.2) | |
| Others (OTHR) | 65.6 (60.4–71.3) | 74.7 (65.8–84.9) | 75.5 (60.7–93.9) | 75.6 (71.8–79.5) | |
| Nonsmokers (NSM) | 67.8 (66.1–69.5) | 69.3 (66.5–72.1) | 70.7 (66.6–75.1) | 71.9 (68.7–75.2) | |
| Smokers (SM) | 69.5 (66.9–72.3) | 75.3 (71.3–79.6) | 84.7 (76.3–93.9) | 72.3 (64.1–81.5) | |
| SSD* | NHW < HISP (p < 0.01), NHB < HISP (p < 0.01), HISP > OTHR (p = 0.02) | M < F (p < 0.1), NHW < HISP (p < 0.01), NHB < HISP (p = 0.04), NSM < SM (p < 0.01) | M < F (p < 0.01), NHW < NHB (p < 0.01), NHW < HISP (p < 0.01), NSM < SM (p < 0.01) | NHW > HISP (p < 0.01), NHB > HISP (p < 0.01), HISP < OTHR (p < 0.01) | |
| GGT | Total | 26.1 (24.9–27.3) | 26.8 (24.7–29.1) | 27.8 (25.5–30.3) | 24.4 (21.6–27.6) |
| Males (M) | 30.9 (29.3–32.7) | 29.4 (26.8–32.3) | 28.8 (26.3–31.7) | 25.2 (21.0–30.4) | |
| Females (F) | 22.0 (20.7–23.3) | 24.4 (22.2–26.9) | 26.8 (24.4–29.5) | 23.7 (21.7–25.8) | |
| Non-Hispanic White (NHW) | 25.3 (24.2–26.6) | 24.8 (23.3–26.3) | 25.8 (23.2–28.6) | 28.9 (28.0–29.9) | |
| Non-Hispanic Black (NHB) | 24.9 (23.2–26.7) | 24.7 (22.6–26.9) | 29.0 (25.9–32.3) | 20.1 (17.4–23.2) | |
| Hispanics (HISP) | 28.5 (26.9–30.1) | 27.5 (24.9–30.4) | 24.9 (23.2–26.8) | 16.1 (14.4–17.9) | |
| Others (OTHR) | 25.7 (21.6–30.7) | 30.7 (23.9–39.5) | 32.2 (25.0–41.5) | 38.1 (28.3–51.2) | |
| Nonsmokers (NSM) | 24.9 (23.6–26.2) | 26.2 (24.6–27.9) | 23.6 (22.1–25.3) | 25.9 (23.0–29.2) | |
| Smokers (SM) | 27.3 (25.5–29.2) | 27.4 (24.1–31.2) | 32.7 (27.8–38.5) | 23.0 (20.2–26.3) | |
| SSD* | M > F (p < 0.01), NHW < HISP (p = 0.02), NHB < HISP (p = 0.02), NSM < SM (p = 0.01) | M > F (p < 0.01) | NSM < SM (p < 0.01) | NHW > NHB (p < 0.01), NHW < HISP (p < 0.01), NHB < HISP (p < 0.01), NHB < OTHR (p < 0.01), HISP < OTHR (p < 0.01), NSM > SM (p = 0.01) | |
*Statistically significant differences with p-values adjusted for multiple comparisons using Tukey-Kremer method.
**ALT = alanine aminotransferase, AST = aspartate aminotransferase, APH = alkaline phosphate, GGT = γ-glutamyl transferase.
Table 5.
Adjusted** slopes for associations between log10 transformed values of perfluorooctane acid (PFOA) and perfluorooctane sulfonic acid (PFOS) on the adjusted levels of liver function tests (LFT) along with percent changes** in the untransformed lebvels of LFT for 10% changes in the levels of PFOA and PFOS by stages of glomerular function for nonobese and obese US adults aged >= 20 years. Data from National Health and Nutrition Examination Survey 2003–2014.
| Glomerular function stage | Percent change in LFT for 10% change in PFOA and PFOS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-obese Participants | GF-1 | GF-2 | GF-3A | GF-3B/4 | GF-1 | GF-2 | GF-3A | GF-3B/4 | |
| LFT* | |||||||||
| ALT | Log10(PFOA) | 0.00882 (0.55) | 0.04761 (0.02) | 0.00083 (0.98) | −0.00099 (0.94) | 0.08 | 0.45 | 0.01 | −0.01 |
| Log10(PFOS) | −0.00806 (0.50) | 0.01128 (0.51) | −0.01335 (0.73) | −0.08768 (<0.01) | −0.08 | 0.11 | −0.13 | −0.84 | |
| AST | Log10(PFOA) | 0.01352 (0.31) | 0.02783 (0.05) | 0.00227 (0.95) | 0.05548 (0.03) | 0.13 | 0.27 | 0.02 | 0.53 |
| Log10(PFOS) | −0.01342 (0.12) | 0.00687 (0.58) | −0.01534 (0.63) | −0.00372 (0.84) | −0.13 | 0.07 | −0.15 | −0.04 | |
| APH | Log10(PFOA) | −0.00594 (0.60) | −0.00157 (0.91) | −0.01299 (0.69) | 0.01255 (0.13) | −0.06 | −0.01 | −0.12 | 0.12 |
| Log10(PFOS) | −0.00137 (0.92) | −0.01603 (0.26) | 0.00191 (0.95) | −0.05218 (<0.01) | −0.01 | −0.15 | 0.02 | −0.50 | |
| GGT | Log10(PFOA) | 0.05862 (0.01) | 0.07736 (<0.01) | −0.01617 (0.83) | 0.13030 (<0.01) | 0.56 | 0.74 | −0.15 | 1.25 |
| Log10(PFOS) | 0.00819 (0.63) | 0.02213 (0.25) | −0.03163 (0.61) | 0.01293 (0.40) | 0.08 | 0.21 | −0.30 | 0.12 | |
| Obese Participants | |||||||||
| LFT* | |||||||||
| ALT | Log10(PFOA) | 0.07691 (<0.01) | 0.03498 (0.18) | 0.05678 (0.11) | 0.16354 (<0.01) | 0.74 | 0.33 | 0.54 | 1.57 |
| Log10(PFOS) | 0.04840 (<0.01) | 0.00521 (0.79) | 0.03828 (0.09) | 0.06956 (<0.01) | 0.46 | 0.05 | 0.37 | 0.67 | |
| AST | Log10(PFOA) | 0.03872 (<0.01) | 0.02908 (0.12) | 0.03616 (0.03) | 0.04963 (<0.01) | 0.37 | 0.28 | 0.35 | 0.47 |
| Log10(PFOS) | 0.01085 (0.41) | −0.01302 (0.46) | 0.04050 (0.01) | 0.02278 (0.16) | 0.10 | −0.12 | 0.39 | 0.22 | |
| APH | Log10(PFOA) | −0.01720 (0.29) | 0.02195 (0.18) | 0.01718 (0.40) | 0.03871 (0.17) | −0.16 | 0.21 | 0.16 | 0.37 |
| Log10(PFOS) | −0.01230 (0.34) | 0.01548 (0.37) | −0.01131 (0.56) | −0.02362 (0.01) | −0.12 | 0.15 | −0.11 | −0.23 | |
| GGT | Log10(PFOA) | 0.09099 (<0.01) | 0.00947 (0.74) | 0.06276 (0.20) | 0.12278 (0.01) | 0.87 | 0.09 | 0.60 | 1.18 |
| Log10(PFOS) | 0.00340 (0.90) | −0.05827 (0.05) | 0.06186 (0.01) | 0.05180 (0.06) | 0.03 | −0.56 | 0.59 | 0.49 | |
*ALT = alanine aminotransferase, AST = aspartate aminotransferase, APH = alkaline phosphate, GGT = γ-glutamyl transferase.
**Statistically significant slopes and changes are shown in bold letters.
Table 6.
Adjusted** slopes for associations between presence of diabetes and hypertension on the adjusted levels of liver function tests (LFT) along with percent changes** in the untransformed levels of LFT by stages of glomerular function for nonobese and obese US adults aged >= 20 years. Data from National Health and Nutrition Examination Survey 2003–2014.
| Glomerular function stage | Percent change in LFT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-obese Participants | GF-1 | GF-2 | GF-3A | GF-3B/4 | GF-1 | GF-2 | GF-3A | GF-3B/4 | |
| LFT* | |||||||||
| ALT | Diabetes | 0.00638 (0.74) | 0.01165 (0.43) | 0.02855 (0.31) | 0.09177 (<0.01) | 1.5 | 2.7 | 6.8 | 23.5 |
| Hypertension | 0.02829 (<0.01) | 0.04783 (<0.01) | −0.00170 (0.94) | 0.00026 (0.97) | 6.7 | 11.6 | −0.4 | 0.1 | |
| AST | Diabetes | −0.01462 (0.36) | −0.01617 (0.15) | 0.01247 (0.6) | 0.06801 (<0.01) | −3.4 | −3.8 | 2.9 | 17.0 |
| Hypertension | 0.02623 (0.02) | 0.02744 (<0.01) | −0.00354 (0.86) | 0.02390 (<0.01) | 6.2 | 6.5 | −0.8 | 5.7 | |
| APH | Diabetes | 0.02726 (0.04) | −0.01059 (0.47) | 0.00815 (0.79) | −0.00398 (0.08) | 6.5 | −2.5 | 1.9 | −0.9 |
| Hypertension | 0.02619 (<0.01) | 0.01776 (0.01) | 0.02050 (0.27) | 0.04841 (<0.01) | 6.2 | 4.2 | 4.8 | 11.8 | |
| GGT | Diabetes | 0.03186 (0.34) | 0.00664 (0.76) | 0.06194 (0.3) | 0.10672 (<0.01) | 7.6 | 1.5 | 15.3 | 27.9 |
| Hypertension | 0.08877 (<0.01) | 0.04425 (<0.01) | −0.04226 (0.36) | 0.12720 (<0.01) | 22.7 | 10.7 | −10.2 | 34.0 | |
| Obese Participants | |||||||||
| LFT* | |||||||||
| ALT | Diabetes | 0.05512 (<0.01) | 0.02938 (0.16) | 0.00587 (0.73) | 0.09730 (<0.01) | 13.5 | 7.0 | 1.4 | 25.1 |
| Hypertension | 0.03950 (<0.01) | 0.04050 (<0.01) | 0.01430 (0.39) | 0.02272 (0.01) | 9.5 | 9.8 | 3.3 | 5.4 | |
| AST | Diabetes | 0.01958 (0.12) | 0.02855 (0.12) | 0.03227 (<0.01) | 0.04150 (0.01) | 4.6 | 6.8 | 7.7 | 10.0 |
| Hypertension | 0.01721 (0.02) | 0.02011 (0.09) | −0.00566 (0.61) | 0.01132 (0.18) | 4.0 | 4.7 | −1.3 | 2.6 | |
| APH | Diabetes | 0.01829 (0.07) | −0.01227 (0.29) | −0.04446 (<0.01) | −0.01165 (0.36) | 4.3 | −2.9 | −10.8 | −2.7 |
| Hypertension | 0.01291 (0.08) | 0.02600 (0.01) | −0.00697 (0.5) | 0.03617 (0.03) | 3.0 | 6.2 | −1.6 | 8.7 | |
| GGT | Diabetes | 0.08945 (<0.01) | 0.09871 (<0.01) | 0.05154 (0.02) | 0.02082 (0.49) | 22.9 | 25.5 | 12.6 | 4.9 |
| Hypertension | 0.06770 (<0.01) | 0.03989 (0.07) | −0.03176 (0.06) | 0.11660 (<0.01) | 16.9 | 9.6 | −7.6 | 30.8 | |
*ALT = alanine aminotransferase, AST = aspartate aminotransferase, APH = alkaline phosphate, GGT = γ-glutamyl transferase
**Statistically significant slopes and changes are shown in bold letters.
3. Results
3.1. Unadjusted geometric means (UGM)
3.1.1. Alanine aminotransferase (ALT)
For the total population, males, smokers, and nonsmokers, UGMs for ALT were found to decrease as kidney function deteriorated from GF-1 to GF-3B/4 (Table 2). In general, decrease from GF-3A to GF-3B or from GF-3B to GF-4 was higher than the decreases from GF-1 to GF-2. The differences between UGMs for GF-1 as compared to GF-3A and/or GF-3B/4 were almost always statistically significant (Table 2). Males always had higher UGMs for ALT than females but the differences were higher among obese participants. The decreases from GF-1 to GF-3B/4 for males were higher than the decreases for females.
3.1.2. Aspartate aminotransferase (AST)
There were no consistent decreasing or increasing trends in UGMs for AST across the stages of GF (Table 2). Males had higher UGMs than females for both obese and nonobese participants.
3.1.3. Alkaline phosphate (APH)
Except for males and obese smokers and nonsmokers, UGMs for APH increased with deterioration in kidney function. For males, UGMs were found to be located on a U-shaped distribution.
3.1.4. γ-glutamyl transferase (GGT)
There were no consistent decreasing or increasing trends in UGMs for GGT across the stages of GF. Males, however, did have higher UGMs for GGT than females (Table 2).
3.2. Adjusted geometric means (AGM)
3.2.1. AGM: trends across stages of glomerular function
3.2.1.1. AGM trends: alanine aminotransferase (ALT)
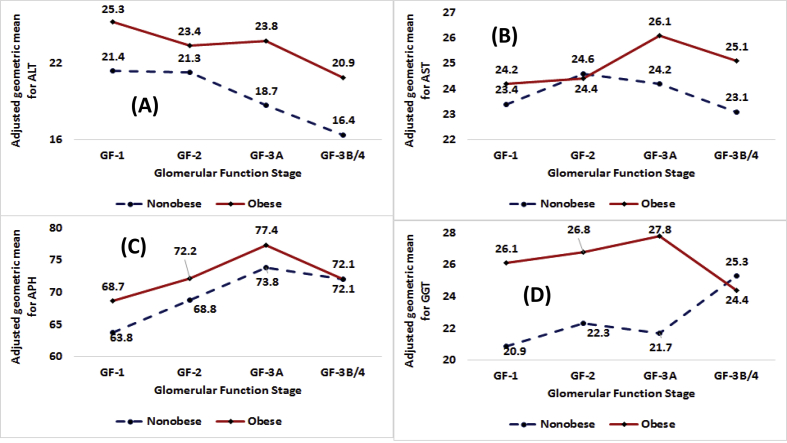
Among nonobese participants, irrespective of gender, race/ethnicity, and smoking status, AGMs decreased with deteriorating kidney function from GF1- to GF-3B/4 (Table 3, Fig. 1, Panel A, Fig. 2, Panel A, and Fig. 3, Panel A). Decrease from GF-1 to GF-3B/4 was higher for males as compared to females (8.0 IU/L vs. 2.4 IU/L), for HISP as compared to NHW and NHB (9.3 IU/L vs. 4.2 and 5.8 IU/L), and for smokers as compared to nonsmokers (6.9 vs. 2.7 IU/L).
Fig. 1.
Adjusted geometric means in IU/L by obesity status for the total population by stages of glomerular function for (A) alanine aminotransferase or ALT, (B) aspartate aminotransferase or AST, (C) alkaline phosphate or APH, and (D) γ-glutamyl transferase or GGT.
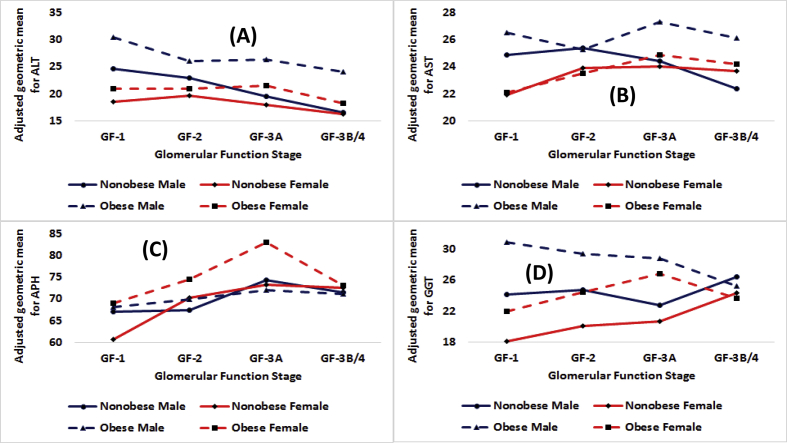
Fig. 2.
Adjusted geometric means in IU/L by obesity status for males and females by stages of glomerular function for (A) alanine aminotransferase or ALT, (B) aspartate aminotransferase or AST, (C) alkaline phosphate or APH, and (D) γ-glutamyl transferase or GGT.
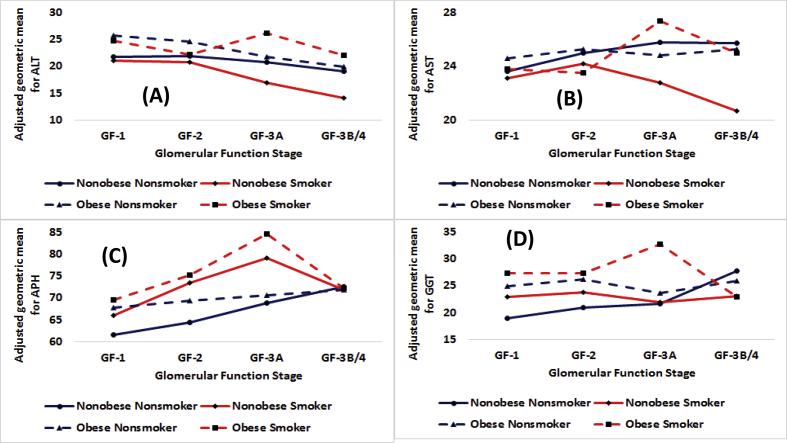
Fig. 3.
Adjusted geometric means in IU/L by obesity status for smokers and nonsmokers by stages of glomerular function for (A) alanine aminotransferase or ALT, (B) aspartate aminotransferase or AST, (C) alkaline phosphate or APH, and (D) γ-glutamyl transferase or GGT.
Among obese participants, for the total population, males, NHB, HISP, and nonsmokers, AGMs decreased in a consistent fashion from GF-1 to GF-3B/4 (Table 4, Fig. 1, Panel A, Fig. 2, Panel A, and Fig. 3, Panel A). The decreases from GF-1 to GF-3B/4 were: 4.4, 6.3, 5.1, 6.5, and 5.9 IU/L for the total population, males, NHB, HISP, and nonsmokers respectively (Table 4). AGMs for GF-3B/4 were always lower than the AGMs for GF-1 for every demographic group.
3.2.1.2. AGM trends: aspartate aminotransferase (AST)
Among nonobese participants, irrespective of gender, race/ethnicity except OTHRS, and smoking status, AGMs for AST increased from GF-1 to GF-2 or GF-3A to be followed by decreases until GF-3B/4 (Table 4, Figs. 1, 2, and 3, Panels B).
Among obese participants, for the total population, females, NHW, NHB, OTHRS, and smokers, AGMs for AST were found to follow inverted U-shaped distributions with point of inflection being at GF-3A (Table 4, Figs. 1, 2, and 3, Panels B).
3.2.1.3. AGM trends: alkaline phosphate (APH)
Among nonobese participants, irrespective of gender, race/ethnicity, and smoking status, AGMs for APH increased from GF-1 to GF-2 or GF-3A before decreasing at GF-3B/4 (Table 3, Fig. 1, Panel C, Fig. 2, Panel C, and Fig. 3, Panel C).
Among obese participants, for the total population, males, females, NHB, HISP, and smokers, AGMs for APH were found to follow inverted U-shaped distributions with point of inflection being at GF-3A (Table 4, Fig. 1, Panel C, Fig. 2, Panel C, and Fig. 3, Panel C).
3.2.1.4. AGM trends: γ-glutamyl transferase (GGT)
Among nonobese participants, for the total population, males, females, HISP, and nonsmokers, AGMs increased from GF-1 to GF-3B/4 (Table 3, Fig. 1, Panel D, and Fig. 3, Panel D). For the total population, males, females, HISP, and nonsmokers, the increases from GF-1 to GF-3B/4 were 4.4, 2.3, 6.2, .7, and 8.8 IU/L respectively. For NHW and NHB, AGMs followed inverted U-shaped distributions with point of inflection located at GF-3A (Table 3).
For obese participants, for the total population, females, NHB, and smokers, AGMs for GGT followed inverted U-shaped distributions with points of inflections located at GF-3A and AGMs at GF-3B/4 were lower than the AGMs at GF-1 or GF-2 (Table 4, Figs. 1, 2, and 3, Panels D). For males, AGMs decreased from 30.9 IU/L at GF-1 to 25.2 IU/L at GF-3B/4. For HISP, AGMs decreased from 28.5 IU/L at GF-1 to 16.1 IU/L at GF-3B/4.
3.2.2. AGM: gender, racial/ethnic, and smoking status differences
3.2.2.1. AGM: gender differences
Among nonobese participants, as compared to females, males had higher AGM for ALT for GF-1, GF-2, and GF-3B/4 (Table 3). For AST, as compared to females, males had higher AGM for GF-1 and GF-2 (p < 0.01) but at GF-3B/4, males had lower AGM than females (p < 0.01). For APH, as compared to females, males had higher AGM for GF-1 (p < 0.01) but for GF-2, males had lower GM than females (p = 0.01). For GGT, males had higher AGMs than females for GF-1 and GF-2 (p < 0.01). Male-females differences narrowed as kidney function deteriorated (Fig. 2).
Obese males had higher AGMs for ALT than obese females for GF-1, GF-2, GF-3A, and GF-3B/4 (p < 0.01, Fig. 2). Male-female differences narrowed from 9.4 IU/L for GF-1 to 5.2 IU/L for GF-2 to 4.9 IU/L for GF-3A, and finally went up a bit to 5.9 IU/L for GF-3B/4. For AST, males had higher AGMs than females by 4.1 IU/L for GF-1 (p < 0.01), by 1.8 IU/L for GF-2 (p < 0.01), and by 2.4 IU/L for GF-3A (p < 0.01). AGMs for APH differed in favor of females by 0.9 IU/L for GF-1, 4.5 IU/L for GF-2 (p < 0.01), 11.0 IU/L for GF-3A (p < 0.01), and 5.3 for GF-3B/4 (Table 4). Thus, male-female differences widened as kidney function deteriorated from GF1- to GF-3A.
3.2.2.2. AGM: racial/ethnic differences
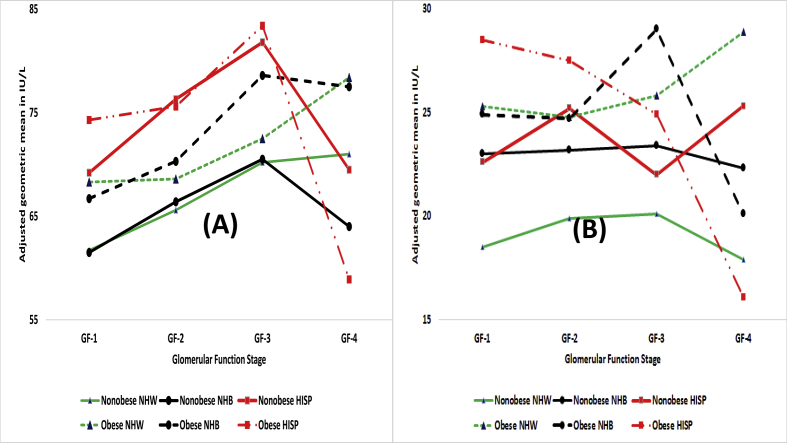
Among nonobese participants, for ALT, (i) NHW had higher AGMs than NHB for GF-1 (p < 0.01) and GF-3B/4 (p < 0.01); NHW had lower AGMs than HISP for GF-1 (p < 0.01) and GF-3B/4 (p < 0.01); and (iii) NHB had lower AGMs than HISP for GF-1 (p < 0.01) and GF-2 (p < 0.01). Thus, HISP had higher AGMs for ALT than both NHB and NHW. For AST, (i) NHW had higher AGMs than NHB for GF-3B/4 (p < 0.01) and (ii) NHW had lower AGM than HISP for GF-1 (p < 0.01). For APH, both NHW and NHB had lower AGMs than HISP for GF-1 (p < 0.01) and GF-2 (p < 0.01). For GGT, NHW had lower AGMs than both NHB and HISP for GF-1 (p < 0.01) and GF-2 (p < 0.01).
For ALT, obese NHW had higher AGMs than obese NHB for every GF stage (p < 0.01). Also, for ALT, obese NHB had lower AGMs than obese HISP for every GF stage (p<=0.02) Among obese participants, for AST, (i) NHW had higher AGMs than NHB for GF-3A and GF-3B/4 (p < 0.01), (ii) NHW had lower AGM than HISP for GF-1 and GF-3B/4 (p < 0.01)), and (iii) NHB had lower AGMs than HISP for GF-1, GF-3A, and GF-3B/4 (p < 0.01). For APH, both NHW and NHB had lower AGMs than HISP for GF-1, GF-2 and GF-3A (p<=0.01). However, for GF-3B/4, the direction of differences was reversed as both NHW and NHB had higher AGMs than HISP (p < 0.01). For GGT, both NHW and NHB had lower AGMs than HISP for GF-1 (p<=0.02). However, for GF-3B/4, the direction of differences was reversed as both NHW and NHB had higher AGMs than HISP (p < 0.01, Table 4).
3.2.2.3. AGM: smoker-nonsmoker differences
Among nonobese participants, for ALT, nonsmokers had higher AGMs than smokers for GF-3A and GF-3B/4 (p < 0.01, Table 3). For AST, nonsmokers had higher AGMs than smokers for GF-3A and GF-3B/4 (p<=0.01, Table 3). For APH, nonsmokers had lower AGMs than smokers for GF-1, GF-2, and GF-3A (p < 0.01, Table 3). Smoker-nonsmoker differences increased with as GF moved from GF-1 to GF-3A. For GGT, nonsmokers had lower AGMs than smokers for GF-1 and GF-2 (p < 0.01) but higher AGMs than smokers at GF-3B/4 (p = 0.01, Table 3).
Among obese participants, for ALT, nonsmokers had higher AGM than smokers for GF-1 but for GF-3B/4, the opposite was true (19.9 vs. 22.0, p<=0.01). For AST, nonsmokers had higher AGM than smokers at GF-2 (p = 0.03). For APH, nonsmokers had lower AGMs than smokers for GF-2 (p < 0.01) and GF-3A (p < 0.01). For GGT, nonsmokers had lower AGM than smokers at GF-1 and GF-3A (p < 0.01) but the reverse was found to be true for GF-3B/4 (Table 4).
3.2.2.4. AGM: obese-nonobese differences
For ALT, obese had higher AGMs than nonobese and the differences were higher at GF-3A and GF-3B/4 than at GF-1 and GF-2 (Fig. 1, Panel A). The obese nonobese differences for males and females were also in favor of obese (Fig. 2, Panel A) but obese nonobese differences were smaller for females than for males. For both smokers and nonsmokers, obese had higher AGMs than nonobese (Fig. 3, Panel A) but among nonsmokers, obese nonobese differences were almost nonexistent for GF-3A and GF-3B/4. For NHW, NHB, as well as HISP, obese did have higher AGMs (Fig. 4, Panel A) for ALT but the differences were narrowest at GF-2 and widest at GF-3B/4. Further, the differences at GF-3B/4 were smallest for NHB at 3.0 IU/L and largest for NHW at 6.4 IU/L (Fig. 4, Panel A).
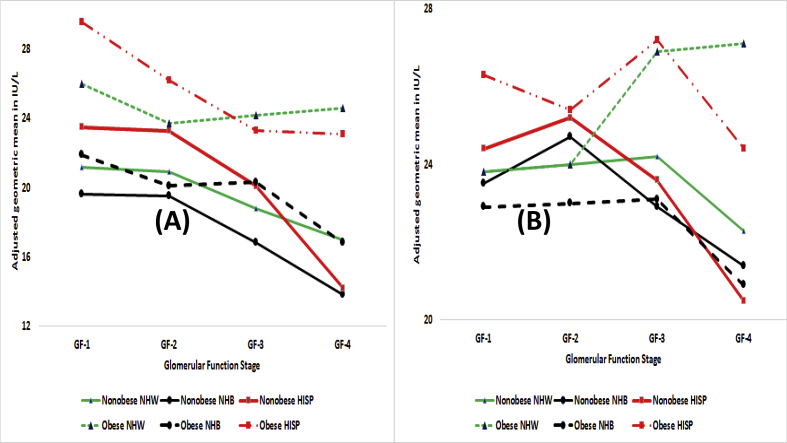
Fig. 4.
Adjusted geometric means in IU/L by obesity status by race/ethnicity (NHW: non-Hispanic white, NHB: non-Hispanic black, HISP: Hispanics) by stages of glomerular function for (A) alanine aminotransferase or ALT and (B) aspartate aminotransferase or AST.
For AST, obese had higher AGMs than nonobese at GF-3A and GF-3B/4 (Fig. 1, Panel B). The same was true for males and females (Fig. 2, Panel B) but the differences for females were almost non-existent even for GF-3A and GF-3B/4. Obese nonobese differences among both smokers and nonsmokers did not seem to exist until GF-3A (Fig. 3, Panel B). And, only among smokers, obese had higher AGMs at GF-3A and GF-3B/4. For NHB, low obese nonobese differences were observed at GF-3A and GF-3B/4 (Fig. 4, Panel B). Obese did have higher AGMs than nonobese for NHW and HISP but primarily at GF-3A and GF-3B/4).
For APH for the total population, obese did have higher AGMs but these differences narrowed as kidney function deteriorated (Fig. 1, Panel C). For females, obese did have higher AGMs than nonobese but the differences narrowed as kidney function deteriorated. For males, obese and nonobese did not seem to have any differences (Fig. 2, Panel C). Obese nonobese differences among nonsmokers narrowed as GF moved from GF-1 to GF-3B/4 and at GF-3B/4, obese nonobese differences did not exist for either smokers or nonsmokers (Fig. 3, Panel C). For NHB and NHW, obese did have higher AGMs than nonobese (Fig. 5, Panel A). For HISP, obese had higher AGM than nonobese at GF-1, there were almost no obese nonobese differences at GF-2 and GF-3A, and at GF-B/4, and obese had lower AGM than nonobese by 10.6 IU/L (Fig. 5, Panel A).
Fig. 5.
Adjusted geometric means in IU/L by obesity status by race/ethnicity (NHW: non-Hispanic white, NHB: non-Hispanic black, HISP: Hispanics) by stages of glomerular function for (A) alkaline phosphate or APH and (B) γ-glutamyl transferase or GGT.
For GGT for the total population (Fig. 1, Panel D), obese did have higher AGMs but at GF-3B/4, nonobese may have slightly higher AGM than obese. Among both males and females (Fig. 2, Panel D), obese did have higher AGMs than nonobese but there were almost no obese-nonobese differences at GF-3B/4. Among nonsmokers, obese nonobese differences narrowed as GF moved from GF-1 to GF-3B/4 resulting in almost no differences at GF-3B/4 (Fig. 3, Panel D). Obese did have higher AGMs for smokers but, at GF-3B/4, these differences ceased to exist. For GF-1, GF-2, and GF-3A, obese did have higher AGMs than nonobese for NHW, NHB, and HISP (Fig. 5, Panel B) and the same was observed for GF-3B/4 for NHW. For NHB and HISP, obese had lower AGMs than nonobese at GF-3B/4 by 2.2 IU/L for NHB and by 9.2 IU/L for HISP. To what degree, small sample sizes for HISP obese may have played a role here must be considered.
3.3. Effect of PFOA and PFOS on adjusted levels of ALT, AST, APH, and GGT
Among nonobese participants, PFOA was positively associated with ALT at GF-2 (Table 5) which translated to 0.45% increase in ALT for a 10% increase in PFOA. PFOA was positively associated with AST at GF-3B/4 (Table 5) which translated to 0.53% increase in AST for a 10% increase in PFOA. PFOA was also positively associated with GGT at GF-1, at GF-2, and at GF-3B/4 (p < 0.01, Table 5).
Among nonobese participants, PFOS was negatively associated with ALT at GF-3B/4 (Table 5) which translated to 0.84% decrease in ALT for a 10% increase in PFOS. PFOS was negatively associated with APH at GF-3B/4 (Table 5) which translated to 0.50% decrease in APH for a 10% increase in PFOS.
Among obese participants, PFOA was positively associated with ALT at GF-1 (Table 5) and at GF-3B/4 (Table 5) which translated to 0.74% increase at GF-1 and 1.57% increase at GF-3B/4 in ALT for a 10% increase in PFOA. PFOA was positively associated with AST at GF-1 and GF-3B/4 (Table 5) which translated to 0.37% increase at GF-1 and 0.47% increase at GF-3B/4 in AST for a 10% increase in PFOA. Also, PFOA was positively associated with GGT at GF-1 and GF-3B/4 which translated to 0.87% increase at GF-1 and 1.18% increase at GF-3B/4 in GGT for a 10% increase in PFOA.
Among obese participants, PFOS was positively associated with ALT at GF-1 and GF-3B/4 (p < 0.01, Table 5) which translated to 0.46% increase at GF-1 and 0.67% increase at GF-3B/4 in ALT for a 10% increase in PFOS. PFOS was positively associated with GGT at GF-3A (Table 5) which translated to 0.59% increase at GF-3B/4 in GGT for a 10% increase in PFOS.
3.4. Impact of being diabetic and hypertensive on adjusted levels of ALT, AST, APH, and GGT
For nonobese participants, having diabetes was positively associated with adjusted levels of ALT with diabetics having 23.5% higher levels than non-diabetics (Table 6). AST was also positively associated with having diabetes for GF-3B/4 with diabetics having 17% higher levels than non-diabetics. APH was positively associated with having diabetes for GF-1 meaning diabetics have 6.5% higher APH than non-diabetics. GGT was positively associated with having diabetes for GF-3B/4 (β = 0.10672, p < 0.01). This translated to diabetics having 27.9% higher GGT if in GF-3B/4 as compared to non-diabetics.
When in GF-1 and GF-2, among nonobese participants, being hypertensive was associated with higher levels of ALT (p < 0.01, Table 6), AST (p<=0.02, Table 6), APH (p<=0.01, Table 6), as well as GGT ((Table 6). Thus for ALT, hypertensives had 6.7% higher levels if in GF-1 and 11.6% higher levels if in GF-2; for AST, hypertensives had 6.2% higher levels if in GF-1 and 6.5% higher levels if in GF-2; for APH, hypertensives had 6.2% higher levels if in GF-1 and 4.2% higher levels if in GF-2; and for GGT, hypertensives had 22.7% higher levels if in GF-1 and 10.7% higher levels if in GF-2.
For obese participants, diabetics were associated with higher levels of ALT for GF-1 and GF-3B/4 (p < 0.01); with higher levels of AST for GF-3B/4 (p < 0.01); with lower levels of APH for GF-3A; and with higher levels of GGT for GF-1, GF-2, and GF-3A (p<=0.02). This translated to diabetics having (i) 13.5% higher levels of ALT for GF-1 and 25.1% higher levels for GF-3B/4; (ii) 10% higher levels of AST for GF-3B/4; (iii) 10.8% lower levels of APH for GF-3A; and (iv) 22.9% higher levels of GGT for GF-1, 25.5% higher levels for GF-2, and 12,6% higher levels for GF-3A.
Being hypertensive among obese participants was associated with higher levels of ALT for GF-1, GF-2, and GF-3B/4 (p < 0.01) meaning 9.5% higher levels for GF-1, 9.8% higher levels for GF-2, and 5.4% higher levels for GF-3B/4. Being hypertensive was also associated with higher levels of AST for GF-1 (p < 0.01) meaning 4% higher levels for GF-1. Higher APH levels were associated with hypertension for GF-2 and GF-3B/4 (p<=0.03) meaning 3% higher levels for GF-1 and 8.7% higher levels for GF-3B/4. Higher GGT levels were also associated with hypertension for GF-1and GF-3B/4 1 (p < 0.01) meaning 16.9% higher levels for GF-1 and 30.8% higher levels for GF-3B/4.
3.5. Associations of liver enzymes with other independent variables
3.5.1. Nonobese participants
For GF-1, positive associations of age with ALT, AST, APH, as well as GGT were observed (p < 0.1, Table 7). For GF-3B/4, the direction of this association was reversed for ALT, AST, and APH. Positive association between log transformed values of BMI were observed for GF-1 and GF-2 for ALT, AST, and GGT. Alcohol consumption was positively associated with liver enzymes but statistical significance was mostly observed for GF-1 and GF-2 only.
Table 7.
Adjusted regression coefficients with associated p-values for associations between various independent variables and liver function tests by stages of glomerular function for nonobese and obese US adults aged >= 20 years. Data from National Health and Nutrition Examination Survey 2003–2014.
| Glomerular function stage | |||||
|---|---|---|---|---|---|
| Non-obese Participants | GF-1 | GF-2 | GF-3A | GF-3B/4 | |
| LFT* | |||||
| ALT | Age | 0.00117 (<0.01) | −0.00134 (<0.01) | −0.00326 (<0.01) | −0.00461 (<0.01) |
| Log10(BMI) | 0.55278 (<0.01) | 0.36422 (<0.01) | 0.41779 (0.04) | 0.01121 (0.82) | |
| Fasting time | 0.00100 (0.14) | 0.00003 (0.97) | 0.00061 (0.75) | −0.00058 (0.27) | |
| Survey Year | −0.00673 (0.04) | 0.00213 (0.45) | −0.00234 (0.7) | 0.00772 (<0.01) | |
| Alcohol Consumption | 0.00041 (<0.01) | 0.00072 (<0.01) | 0.00067 (0.01) | −0.00081 (<0.01) | |
| AST | Age | 0.00123 (<0.01) | 0.00001 (0.98) | −0.00124 (0.17) | −0.00002 (0.94) |
| Log10(BMI) | −0.04300 (0.36) | −0.09346 (0.13) | −0.07432 (0.68) | −0.38093 (<0.01) | |
| Fasting time | −0.00022 (0.69) | −0.00081 (0.25) | 0.00035 (0.85) | 0.00108 (<0.01) | |
| Survey Year | −0.00093 (0.71) | 0.00355 (0.08) | 0.00348 (0.57) | 0.01379 (<0.01) | |
| Alcohol Consumption | 0.00045 (<0.01) | 0.00081 (<0.01) | 0.00012 (0.51) | 0.00049 (0.05) | |
| APH | Age | 0.00132 (<0.01) | 0.00164 (<0.01) | 0.00082 (0.38) | −0.00457 (0.01) |
| Log10(BMI) | 0.18248 (<0.01) | 0.14377 (0.03) | 0.33088 (0.06) | −0.45189 (<0.01) | |
| Fasting time | −0.00043 (0.45) | −0.00108 (0.07) | −0.00192 (0.28) | 0.00097 (0.27) | |
| Survey Year | −0.00708 (<0.01) | −0.00591 (0.01) | −0.01199 (0.04) | −0.00281 (0.48) | |
| Alcohol Consumption | −0.00005 (0.5) | −0.00017 (0.24) | 0.00060 (<0.01) | −0.00187 (<0.01) | |
| GGT | Age | 0.00357 (<0.01) | 0.00159 (<0.01) | 0.00017 (0.93) | −0.00545 (0.16) |
| Log10(BMI) | 0.53167 (<0.01) | 0.45019 (<0.01) | 0.55649 (0.12) | −0.65744 (<0.01) | |
| Fasting time | 0.00151 (0.16) | −0.00009 (0.94) | 0.00210 (0.57) | −0.00550 (0.01) | |
| Survey Year | 0.00192 (0.68) | 0.00265 (0.51) | −0.02288 (0.04) | 0.00652 (0.17) | |
| Alcohol Consumption | 0.00104 (<0.01) | 0.00173 (<0.01) | 0.00107 (<0.01) | −0.00036 (0.61) | |
| Obese Participants | |||||
| ALT | Age | −0.00203 (<0.01) | −0.00356 (<0.01) | −0.00256 (<0.01) | −0.00652 (<0.01) |
| Log10(BMI) | 0.16916 (0.08) | 0.20850 (0.12) | 0.55588 (<0.01) | 0.40211 (<0.01) | |
| Fasting time | 0.00012 (0.89) | 0.00177 (0.34) | −0.00256 (<0.01) | −0.00109 (0.59) | |
| Survey Year | −0.00001 (1) | −0.00259 (0.56) | −0.00028 (0.95) | −0.00996 (0.21) | |
| Alcohol Consumption | 0.00064 (<0.01) | 0.00090 (0.13) | 0.00020 (0.04) | −0.00195 (<0.01) | |
| AST | Age | −0.00000 (0.99) | −0.00047 (0.23) | 0.00136 (0.05) | −0.00141 (<0.01) |
| Log10(BMI) | 0.04516 (0.45) | 0.07471 (0.44) | 0.39621 (<0.01) | −0.07178 (0.03) | |
| Fasting time | −0.00071 (0.22) | 0.00051 (0.78) | −0.00143 (0.03) | −0.00448 (<0.01) | |
| Survey Year | −0.00124 (0.66) | 0.00328 (0.39) | −0.00503 (0.01) | −0.00394 (0.57) | |
| Alcohol Consumption | 0.00058 (<0.01) | 0.00106 (0.14) | 0.00048 (<0.01) | −0.00150 (<0.01) | |
| APH | Age | 0.00100 (<0.01) | 0.00013 (0.7) | −0.00057 (0.32) | −0.00370 (<0.01) |
| Log10(BMI) | 0.18161 (<0.01) | 0.27315 (0.01) | 0.11483 (0.16) | 0.38603 (<0.01) | |
| Fasting time | 0.00049 (0.28) | −0.00183 (0.03) | −0.00055 (0.61) | 0.00144 (0.33) | |
| Survey Year | −0.00688 (0.01) | −0.00692 (0.02) | −0.01344 (<0.01) | −0.01782 (<0.01) | |
| Alcohol Consumption | 0.00020 (0.23) | −0.00009 (0.44) | 0.00050 (<0.01) | −0.00023 (0.56) | |
| GGT | Age | 0.00134 (0.02) | −0.00095 (0.2) | 0.00470 (<0.01) | −0.01172 (<0.01) |
| Log10(BMI) | 0.17025 (0.13) | 0.44200 (0.03) | 1.08678 (<0.01) | 0.77029 (<0.01) | |
| Fasting time | 0.00080 (0.45) | 0.00264 (0.34) | 0.00178 (0.21) | −0.00787 (<0.01) | |
| Survey Year | −0.00266 (0.5) | −0.01094 (0.11) | −0.00032 (0.97) | −0.01861 (0.06) | |
| Alcohol Consumption | 0.00141 (<0.01) | 0.00193 (0.05) | 0.00325 (<0.01) | 0.00113 (0.13) | |
*ALT = alanine aminotransferase, AST = aspartate aminotransferase, APH = alkaline phosphate, GGT = γ-glutamyl transferase
**Statistically significant slopes are shown in bold letters.
3.5.2. Obese participants
Age was negatively associated with ALT, AST, APH, and GGT for GF-3B/4 (p<=0.01, Table 7) but positively associated at GF-1. Alcohol consumption was positively associated with ALT, AST, APH, and GGT for GF-1. Alcohol consumption was negatively associated with ALT and AST for GF-3B/4.
4. Discussion
All data used for this study were stratified by the stages of glomerular function before analysis. Indirectly, though, age based stratification, to a degree was also achieved since age groups covered by each GF stage differed substantially from each other. Mean ages in years for those in GF-1, GF-2, GF-3A, and GF-3B/4 were: 40.0 (IQR: 28–50, SD = 14.0), 60.0 (IQR: 50–71, SD = 14.7), 72.4 (IQR: 67–80, SD = 10.0), and 74.5 (IQR: 71–80, SD = 9.5) respectively. For this reason, it may not be too incorrect to state that some of the variabilities observed in the levels of ALT, AST, APH, and GGT across stages of GF may be due to age in addition to GF stage. This must be taken into account in order to correctly interpret the results of this study. However, for nonobese participants, for ALT and only for obese participants, for ALT, AST, APH, and GGT, there was a negative association with age. A note should be made that for ALT for nonobese participants, age was negatively associated with ALT for GF-2, GF-3A, and GF-3B/4, and for obese participants, this negative associations was observed for all GF stages. Thus, direction of associations with age and liver enzymes changed over the full spectrum of glomerular function. This is in sync with how functional and structural changes in kidney are known to occur as kidneys age. Some of the functional and structural changes accompanying aging are discussed by Orr and Bridges (2017).
Narrowing of the differences between the AGMs for obese and nonobese as kidney function deteriorated to GF-3A or GF-3B was observed for the total population for APH and GGT (Fig. 1, Panels C and D); for females for APH and GGT (Fig. 2, Panel C); for males for GGT (Fig. 2, Panel D); for nonsmokers for ALT, APH, and GGT (Fig. 3, Panels A, C, and D); for smokers for APH and GGT (Fig. 3, Panels C and D); and for NHB for AST (Fig. 5, Panel B). Thus, obesity may have a role to play in how liver enzymes are processed by kidneys. These results we have are in confirmation with Kovesdy et al. (2017) according to whom, in obese, “ … compensatory hyperfiltration occurs to meet the heightened metabolic demands of the increased body weight …”. Obese having higher AGMs than nonobese are in confirmation with Lin et al. (2010) and Jain and Ducatman (2019b).
Male-female differences in adjusted levels of ALT and GGT continued narrowing as kidney function deteriorated from GF-1 to GF-2 to GF-3A to GF-3B/4 (Fig. 2, Panels A and D). For AST, for GF-3B/4, males had slightly lower levels than females (Fig. 2, Panel B). Thus, kidneys process liver enzymes for males and females differently as kidney function deteriorates.
When data were not stratified by GF stages, Jain and Ducatman (2019a) reported associations between PFOA and liver enzymes among nonobese to be statistically significantly not associated. However, Jain and Ducatman (2019a) used 2011–2014 data and we used 2003–2014 data which had relatively higher levels of PFOA than 2011–2014 data. For this reason, observing statistically significant associations between PFOA and ALT, AST, and GGT for this study should be no surprise. Increased levels of PFOA for GF-2 as compared to GF-1 as reported by Jain and Ducatman (2019b) are reflected in increasing associations between PFOA and ALT (β = 0.00882 for GF-1 and 0.04761 for GF-2, Table 5), between PFOA and AST (β = 0.01352 for GF-1 and 0.02783 for GF-2, Table 5), and between PFOA and GGT (β = 0.05862 for GF-1 and 0.07736 for GF-2, Table 5). However, even though Jain and Ducatman (2019b) reported PFOA levels at GF-3B/4 to be substantially lower than at GF-1 to GF-3A, associations of PFOA with AST (β = 0.05548) and GGT (β = 0.013030) were still found to be higher at GF-3B/4 than at GF-1 to GF-3A. We do not know the reason for this but we did show that associations between PFOA and lever enzymes do vary as kidney function deteriorates from GF-1 to GF-3B/4. Similar trends of associations between PFOA and liver enzymes being higher at GF-3B/4 than at GF-1 were seen for obese participants also (Table 5).
Jain and Ducatman (2019a) did not report any statistically significant associations for either obese or nonobese between PFOS and any of the four liver enzymes for which data were analyzed in this study. Among nonobese, in this study also, no statistically significant associations between PFOS and ALT, AST, APH, and GGT were observed for GF-1, GF-2, and GF3A. However, for GF-3B/4, statistically significant negative associations between PFOS and ALT (β = −0.09768, p < 0.01) and APH (β = −0.05218, p < 0.01, Table 5) were observed. This may be because of substantially lower levels of PFOS as compared with the levels at GF-1 to GF-3A as reported by Jain and Ducatman (2019b). However, among obese participants, PFOS was found to have positive associations at GF-1 for ALT (β = 0.04840, p < 0.01) and at GF-3A for GGT (β = 0.013030, p < 0.01). In addition, contrary to what was observed for nonobese participants, instead of a negative association, a positive association between PFOS and ALT was found at GF-3B/4 (β = 0.06956, p < 0.01) for obese participants which was higher in magnitude than association at GF-1. Thus, associations between PFAA and lever enzymes do vary across stages of GF.
Cut off values to discriminate those who are normal vs. those who are abnormal in their observed values of ALT (Schumann et al., 2002a) or 34 IU/L for females and 45 IU/L for males, AST (Schumann et al., 2002b) or 31 IU/L for females and 35 IU/L for males, APH (Schumann et al., 2011) or 98 IU/L for females and 115 IU/L for males, and GGT (Schumann et al., 2002c) or 38 IU/L for females and 55 IU/L for males, are presumably determined using samples obtained from “healthy” individuals possibly in GF-1 or maybe in GF-2. The use of these cut off values for those who are in GF-3A or GF-3B/4 may lead to underestimation of the prevalence of individuals abnormal for ALT and AST because unadjusted values of ALT and AST (Table 2) for those who were in GF-3A or GF-3B/4 were found to decrease as compared for those who were in GF-1 and GF-2. In fact, having used the cut offs recommended by Schumann et al. (2002a) for ALT we found percent with abnormal values of ALT to be 11.6%, 8.3%, 5.6%, and 4.9% for those who were in GF-1, GF-2, GF-3A, and GF-3B/4 respectively. Having used the cut offs recommended by Schumann et al. (2002b) for AST we found percent with abnormal values of AST to be 11.8%, 11.8%, 12.7%, and 12% for those who were in GF-1, GF-2, GF-3A, GF-3B/4 respectively. While we could not locate population-based or patent-based data providing information on co-occurrence of abnormal levels of ALT and AST by kidney function, it is highly unlikely that those with eGFR between 15 and 30 mL/min/1.73 m2 or GF-4 had the lowest percent with abnormal ALT and AST. This issue need more research and further consideration.
For GGT, a specific pattern of unadjusted values that could be applicable for both obese and nonobese participants could be discerned (Table 2). Percent participants with abnormal values of GGT using cut offs defined by Schumann et al. (2002c) were found to be 10.8%, 11.1%, 10.5%, and 15% for GF-1, GF-2, GF-3A, GF-3B/4 respectively. If liver and kidney functions decline in tandem, these data do make sense. For APH, an increasing pattern of unadjusted values was seen (Table 2) which means percent participants with abnormal values of APH may be overestimated for deteriorated kidney function. Percent participants with abnormal values of APH using 97.5th percentile of the distribution for normal healthy persons defined by Schumann et al. (2011) were found to be 6.5%, 6.8%, 10.2%, and 14.3% for GF-1, GF-2, GF-3A, GF-3B, and GF-4 respectively.
4.1. Concluding remarks
Exposure to PFAA as an emerging threat to kidney health has been documented by Stanifer et al. (2018) in a recent review article. Impact of exposure to selected PFAAs on concentrations of liver enzymes or liver health, as previously mentioned has also been documented (Gallo et al., 2012; Darrow et al., 2016; Lin et al., 2010; Olsen et al., 2007, 2012). Selected PFAAs have been found to be associated with the incidence of diabetes and microvascular disease (Cardenas et al., 2019). Selected PFAAs have also found to be associated with the risk of cardiovascular diseases (Huang et al., 2018), elevated blood pressure (Bao et al., 2017), elevated low density lipoprotein and total cholesterol levels (Jain and Ducatman, 2019c), and elevated levels of thyroid stimulating hormones (Jain, 2013). Long list of possible harms to human health associated with exposure to PFAAs has been of concern. Unfortunately, those already exposed to PFAAs can do very little to clear these toxins from their body in an accelerated manner; however, they can avoid or minimize new exposure by not consuming water contaminated with PFAAs, by minimizing consumption of milk products which may have been contaminated with PFAAs, by minimizing consumption of fish which has the potential to be contaminated by PFAAs, and by not cooking food in nonstick cookware.
PFAAs are highly persistent in the environment, are bio-accumulated in food chains, biomagnified in animal species including humans, and have very long human half-lives in serum, on the order of several years for some of the longer chain PFAA species (Olsen et al., 2007; Li et al., 2018). They are stored in human liver, bone, and kidney (Maestri et al., 2006; Pérez et al., 2013). In summary, exposure to PFAAs, particularly because they stay in the body for years should be of concern to public health officials and those who are exposed to PFAAs. With long-term bio-accumulative potential being of serious concern, a voluntary 2-year phase out of PFOS was announced (https://archive.epa.gov/epapages/newsroom_archive/newsreleases/33aa946e6cb11f35852568e1005246b4.html) as a result of the negotiations between United States Environmental Protection Agency (EPA) and 3 M Company, the manufacturer of these chemicals. Under US EPA's stewardship program, “ … from a year 2000 baseline, in both facility emissions to all media of perfluorooctanoic acid (PFOA), precursor chemicals that can break down to PFOA, and related higher homologue chemicals, and product content levels of these chemicals” (https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program) a 95% reduction to be achieved no later than 2010 was announced. Jain (2018) analyzed NHANES data for US adults aged >= 20 years to monitor the progress achieved in reducing observed levels of PFOA, PFOS, perfluorodecanoic acid (PFDA), perfluorohexane sulfonic acid (PFHxS), and perfluorononanoic acid (PFNA) over 2003–2014. During this period, unadjusted concentrations of PFOA were reported (Jain, 2018) to be decreased by 50% for PFOA, by 75% for PFOS, by 32% for PFDA, by 27% for PFHxS, and by 30% for PFNA. Thus, progress in reducing the levels of these toxins has been reported. However, more work needs to be done.
In this study, we have described previously unexplored associations between variabilities in the levels of selected liver enzymes across the stages of kidney function and how PFOA and PFOS affect these associations. The conclusions arrived at in this study should be of use for public health officials who has the responsibility to manage and track the health of the populations of interest at large.
4.2. Limitations of the study
Studies like this are best done for longitudinal data. While NHANES does provide large population based representative data, these data are cross-sectional and thus, do not provide a level of confidence that a longitudinal dataset could provide. On the other hand, a longitudinal study of the size of NHANES could not be done for financial and practical reasons. A longitudinal study across the full spectrum of kidney function will probably span over lifetime. Studies with a lifetime follow up can suffer from attrition and loss to follow up and may end up providing results that are too dependent on sophisticated statistical analysis with difficult to interpret results.
Declarations
Author contribution statement
Ram B Jain: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
Data associated with this study is available at the National Health and Nutrition Examination Survey.
References
- Bao W.W., Qian Z.M., Geiger S.D., Liu E., Liu Y., Wang S.Q., Lawrence W.R., Yang B.Y., Hu L.W., Zeng X.W., Dong G.H. Gender-specific associations between serum isomers of perfluoroalkyl substances and blood pressure among Chinese: isomers of C8 Health Project in China. Sci. Total Environ. 2017 Dec 31;607–608:1304–1312. doi: 10.1016/j.scitotenv.2017.07.124. [DOI] [PubMed] [Google Scholar]
- Cardenas A., Hivert M.F., Gold D.R., Hauser R., Kleinman K.P., Lin P.D., Fleisch A.F., Calafat A.M., Ye X., Webster T.F., Horton E.S., Oken E. Associations of perfluoroalkyl and polyfluoroalkyl substances with incident diabetes and microvascular disease. Diabetes Care. 2019 doi: 10.2337/dc18-2254. pii: dc182254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Guo X., Chen Y., Dong S., Sun Y. Prevalence of abnormal serum liver enzymes in patients with type 2 diabetes mellitus: a cross-sectional study from China. Postgrad. Med. 2016;128(8):770–776. doi: 10.1080/00325481.2016.1242366. [DOI] [PubMed] [Google Scholar]
- Darrow L.A., Groth A.C., Winquist A., Shin H.M., Bartell S.M., Steenland K. Modeled perfluorooctanoic acid (PFOA) exposure and liver function in a mid-Ohio valley community. Environ. Health Perspect. 2016;124(8):1227–1233. doi: 10.1289/ehp.1510391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Leonardi G., Genser B., Lopez-Espinosa M.J., Frisbee S.J., Karlsson L. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ. Health Perspect. 2012;120(5):655–660. doi: 10.1289/ehp.1104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Jiao J., Zhuang P., Chen X., Wang J., Zhang Y. Serum polyfluoroalkyl chemicals are associated with risk of cardiovascular diseases in national US population. Environ. Int. 2018;119:37–46. doi: 10.1016/j.envint.2018.05.051. [DOI] [PubMed] [Google Scholar]
- Inker L.A., Astor B.C., Fox C.H., Isakova T., Lash J.P., Peralta C.A., Tamura M.K., Feldman H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- Jain R.B. Association between thyroid profile and perfluoroalkyl acids: data from NHANES 2007-2008. Environ. Res. 2013;126:51–59. doi: 10.1016/j.envres.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Jain R.B. Time trends over 2003-2014 in the concentrations of selected perfluoroalkyl substances among US adults aged >= 20 years: interpretational issues. Sci. Total Environ. 2018;645:946–957. doi: 10.1016/j.scitotenv.2018.07.198. [DOI] [PubMed] [Google Scholar]
- Jain R.B., Ducatman A. Selective associations of recent low concentrations of perfluoroalkyl substances with liver function biomarkers: NHANES 2011 to 2014 data on US adults aged >= 20 years. J. Occup. Environ. Med. 2019;61(4):293–302. doi: 10.1097/JOM.0000000000001532. [DOI] [PubMed] [Google Scholar]
- Jain R.B., Ducatman A. Perfluoroalkyl substances follow inverted U-shaped distributions across various stages of glomerular function: implications for future research. Environ. Res. 2019;169:476–482. doi: 10.1016/j.envres.2018.11.033. [DOI] [PubMed] [Google Scholar]
- Jain R.B., Ducatman A. Roles of gender and obesity in defining correlations between perfluoroalkyl substances and lipid/lipoproteins. Sci. Total Environ. 2019;653:74–81. doi: 10.1016/j.scitotenv.2018.10.362. [DOI] [PubMed] [Google Scholar]
- Koenig G., Seneff S. Gamma-glutamyl transferase: a predictive biomarker of cellular antioxidant inadequacy and disease risk. Dis. Markers. 2015;2015:818570. doi: 10.1155/2015/818570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M., Liu C., Guo Y., Gao Q., Zhong C., Zhou X., Chen R., Xiong G.3, Yang X., Hao L., Yang N. Higher level of GGT during mid-pregnancy is associated with increased risk of gestational diabetes mellitus. Clin. Endocrinol. 2018;88(5):700–705. doi: 10.1111/cen.13558. [DOI] [PubMed] [Google Scholar]
- Kovesdy C.P., Furth S.L., Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. Can. J. Kidney Health & Dis.. 2017;4:1–10. doi: 10.1177/2054358117698669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A.S., Stevens L.A., Schmid C.S., Zhang Y.L., Castro A.S., III, Feldman H.I., Kusak J.W., Eggers P., Van Lente F., Green T., Coresh J., MHS A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fletcher T., Mucs D., Scott K., Lindh C.H., Tallving P., Jakobsson K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 2018;75(1):46–51. doi: 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.Y., Lin L.Y., Chiang C.K., Wang W.J., Su Y.N., Hung K.Y. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. Am. J. Gastroenterol. 2010;105(6):1354–1363. doi: 10.1038/ajg.2009.707. [DOI] [PubMed] [Google Scholar]
- Maestri L., Negri S., Ferrari M., Ghittori S., Fabris F., Danesino P., Imbriani M. Determination of perfluorooctanoic acid and perfluorooctane sulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry. Rapid Commun. Mass Sp. 2006;20(18):2728–2734. doi: 10.1002/rcm.2661. [DOI] [PubMed] [Google Scholar]
- Olsen G.W., Zobel L.R. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int. Arch. Occup. Environ. Health. 2007;81(2):231–246. doi: 10.1007/s00420-007-0213-0. [DOI] [PubMed] [Google Scholar]
- Olsen G.W., Burris J.M., Ehresman D.J., Froehlich J.W., Seacat A.M., Butenhoff J.L., Zobel L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired perfluorochemical production workers. Environ. Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen G.W., Ehresman D.J., Buehrer B.D., Gibson B.A., Butenhoff J.L., Zobel L.R. Longitudinal assessment of lipid and hepatic clinical parameters in workers involved with the demolition of perfluoroalkyl manufacturing facilities. J. Occup. Environ. Med. 2012;54(8):974–983. doi: 10.1097/JOM.0b013e31825461d2. [DOI] [PubMed] [Google Scholar]
- Orr S.E., Bridges C.C. Chronic kidney disease and exposure to nephrotoxic metals. Int. J. Mol. Sci. 2017;18:1039. doi: 10.3390/ijms18051039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez F., Nadal M., Navarro-Ortega A., Fàbrega F., Domingo J.L., Barceló D., Farré M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013;59:354–362. doi: 10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Schumann G., Bonora R., Ceriotti F., Ferard G., Ferrero C.A., Franck P.F.H., Gella F.J., Hoelzel W., Jorgensen P.J., Kanno T., Kessner A., Klauke R., Kristiansen N., Lessinger J.-M., Linsinger T.P.J., Misaki, Panteghini M., Pauwels J., Schiele F., Shimmel H.G., Weidemann G., Siekmann L. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37ο C. Part 4. Reference procedure for the measurement of catalytic concentration of alanine aminotransferase. Clin. Chem. Lab. Med. 2002;40:718–724. doi: 10.1515/CCLM.2002.124. [DOI] [PubMed] [Google Scholar]
- Schumann G., Bonora R., Ceriotti F., Ferard G., Ferrero C.A., Franck P.F.H., Gella F.J., Hoelzel W., Jorgensen P.J., Kanno T., Kessner A., Klauke R., Kristiansen N., Lessinger J.-M., Linsinger T.P.J., Misaki, Panteghini M., Pauwels J., Schiele F., Shimmel H.G., Weidemann G., Siekmann L. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37ο C. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clin. Chem. Lab. Med. 2002;40:725–733. doi: 10.1515/CCLM.2002.125. [DOI] [PubMed] [Google Scholar]
- Schumann G., Bonora R., Ceriotti F., Ferard G., Ferrero C.A., Franck P.F.H., Gella F.J., Hoelzel W., Jorgensen P.J., Kanno T., Kessner A., Klauke R., Kristiansen N., Lessinger J.-M., Linsinger T.P.J., Misaki, Panteghini M., Pauwels J., Schiele F., Shimmel H.G., Weidemann G., Siekmann L. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37ο C. Part 6. Reference procedure for the measurement of catalytic concentration of γ-glutamyltransferase. Clin. Chem. Lab. Med. 2002;40:734–738. doi: 10.1515/CCLM.2002.126. [DOI] [PubMed] [Google Scholar]
- Schumann G., Klauke R., Canalias F., Bossert-Reuther S., Franck P.F.H., Gella F.J., Jorgensen P.J., Kang D., Lessinger J.-M., Panteghini M., Ceriotti F. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37ο C. Part 9. Reference procedure for the measurement of catalytic concentration of alkaline phosphate. Clin. Chem. Lab. Med. 2011;49:1439–1446. doi: 10.1515/CCLM.2011.621. [DOI] [PubMed] [Google Scholar]
- Sheng X., Che H., Ji Q., Yang F., Lv J., Wang Y., Xian H., Wang L. The relationship between liver enzymes and insulin resistance in type 2 DiabetesPatients with nonalcoholic fatty liver disease. Horm. Metab. Res. 2018;50(5):397–402. doi: 10.1055/a-0603-7899. [DOI] [PubMed] [Google Scholar]
- Stadler M., Bollow E., Fritsch M., Kerner W., Schuetz-Fuhrmann I., Krakow D., Merger S., Riedl M., Jehle P., Holl R.W. DPV Initiative and the German BMBF Competence Network Diabetes mellitus. Prevalence of elevated liver enzymes in adults with type 1 diabetes: a multicentre analysis of the German/Austrian DPV database. Diabetes Obes. Metab. 2017;19(8):1171–1178. doi: 10.1111/dom.12929. [DOI] [PubMed] [Google Scholar]
- Stanifer J.W., Stapleton H.M., Souma T., Wittmer A., Zhao X., Boulware L.E. Perfluorinated chemicals as emerging environmental threats to kidney health. Clin. J. Am. Soc. Nephrol. 2018;13(10):1479–1492. doi: 10.2215/CJN.04670418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshikuni N., Tsutsumi M., Arisawa T. Clinical differences between alcoholic liver disease and nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20(26):8393–8406. doi: 10.3748/wjg.v20.i26.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Cheng N., Ma Y., Li H., Cheng Z., Yang Y., He C., Li J., Pu H., Shen X., Ren X., Shi D., Pu R., Gan T., Ding J., Zheng T., Bai Y. Liver enzymes, fatty liver and type 2 diabetes mellitus in a Jinchang cohort: a prospective study in adults. Can. J. Diabetes. 2018 doi: 10.1016/j.jcjd.2018.02.002. pii: S1499-2671(17)30342-30348. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Hedderson M.M., Quesenberry C.P., Feng J., Ferrara A. Liver enzymes in early to mid-pregnancy, insulin resistance, and gestational DiabetesRisk: a longitudinal analysis. Front. Endocrinol. 2018;9:581. doi: 10.3389/fendo.2018.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]