Abstract
Babesiosis is an emerging health risk, and clinicians need to be aware of its different clinical manifestations. In our cohort of 38 patients, almost half did not recall a tick bite, and diagnosis was delayed due to the nonspecific nature of symptoms. Sixty-eight percent of patients required hospitalization, with 21% requiring intensive care unit stay. Coinfection with Lyme, anaplasma, or both Lyme and anaplasma was seen in 24%, 5%, and 8% of the patients, respectively. None of the patients in our cohort died from their disease.
Keywords: babesiosis, parasitic infection, tick-borne illness
Babesiosis is an increasingly frequent worldwide disease caused by a tick-borne malaria-like protozoa that infects host erythrocytes [1]. Although mainly transmitted by ticks, rare cases have been reported after blood transfusion or solid organ transplantation [2, 3]. Perinatal transmission has also been reported [4]. There are multiple Babesia species that cause human disease, including B. microti, B. divergens, B. duncani, and B. venatorum. The number of reported cases in the United States has been increasing, from about 1100 cases in 2011 to nearly 1800 cases in 2014 and almost 2400 in 2017 [5]. The clinical course can vary from an asymptomatic infection to a rapidly fatal fulminant infection [6]. We describe the largest series of Midwestern patients with babesiosis to date based on our institutional experience over a 14-year period.
METHODS
We performed a retrospective review of all adult patients diagnosed with babesiosis at 3 Midwestern sites (2 MN, 1 WI) of the Mayo Clinic between January 1, 2005, and December 31, 2017. The study was approved by our institutional review board. To avoid the ambiguity of serologic diagnosis, our case definition required the diagnosis to be established by blood smear or polymerase chain reaction (PCR) in addition to a clinical syndrome compatible with babesiosis [7]. Using these criteria, our chart review identified 38 patients diagnosed with and treated for babesiosis. We reviewed each patient chart for demographic information, including age at diagnosis, sex, race, presence of spleen, medical comorbidities (ie, diabetes mellitus or iatrogenic immunosuppression), and date of diagnosis. Additionally, we evaluated the clinical presentation for the presence of specific signs and symptoms, including malaise, chills, fevers, subjective fever, arthralgias, nausea, and anorexia. We also identified what the highest level of care (outpatient, inpatient, intensive care unit [ICU]) was for each episode. Finally, we recorded duration from symptoms to diagnosis, index hemoglobin, percent parasitemia on smear, method of diagnosis, treatment course, and ultimate outcome.
We collected and managed study data using Research Electronic Data Capture (REDCap) electronic data capture tools, hosted at the Mayo Clinic. REDCap is a secure, web-based application designed to support data capture for research studies [8].
We analyzed data using the Fisher exact test or chi-square analysis for nominal data, t test for continuous parametric data, and the median test for nonparametric continuous data. We considered P values <.05 significant. We performed all statistical analysis using JMP 14 (SAS institute, Cary, NC).
RESULTS
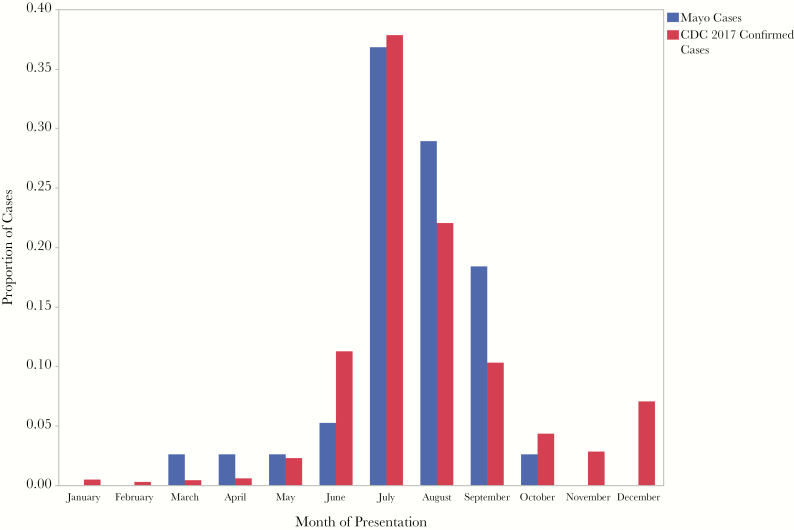
We summarize the demographics of the 38 patients whom we identified as having babesiosis in Table 1. The average age was 63 years, with 15 males and 23 females. Comorbidities included diabetes mellitus (11%), asplenia (13%), and iatrogenic immunosuppression (8%). Only 55% of the patients remembered being bitten by a tick. The most common presenting symptom was malaise (84%), with many patients complaining of fever (71%) and chills (52%). Diagnosis was made with combination PCR and peripheral blood smear in 50% of patients, whereas 50% were diagnosed with PCR alone. In 27% of patients with positive PCR, peripheral smear was negative (Figure 1). The mean length of illness before diagnosis was 15.6 days. Most of the patients were diagnosed during the summer months (Figure 2).
Table 1.
Baseline Characteristics, Presenting Symptoms, and Laboratories of Patients With Babesiosis
| Outpatient (n = 12) | Hospital Ward Admission (n = 18) | Hospital ICU Admission (n = 8) | All Patients (n = 38) | |
|---|---|---|---|---|
| Age at hospitalization, No. (%) | ||||
| 18–59 y | 3 (25) | 1 (6) | 0 | 4 (11) |
| 60–69 y | 8 (67) | 10 (56) | 4 (50) | 22 (58) |
| ≥70 y | 1 (8) | 7 (39) | 4 (50) | 12 (31) |
| Sex, No. (%) | ||||
| Male | 6 (50) | 17 (94) | 8 (100) | 31 (82) |
| Female | 6 (50) | 1 (6) | 0 | 7 (18) |
| Recalled tick bite, No. (%) | 8 (67) | 10 (56) | 3 (38) | 21 (55) |
| Comorbidities, No. (%) | ||||
| Diabetes | 1 (8) | 1 (6) | 2 (25) | 4 (11) |
| Hypertension | 2 (17) | 5 (28) | 3 (38) | 10 (26) |
| Congestive heart failure | 1 (8) | 1 (6) | 1 (13) | 3 (8) |
| Chronic kidney disease | 1 (8) | 0 | 1 (13) | 2 (5) |
| Asplenia | 0 | 2 (11) | 3 (38) | 5 (13) |
| Immunosuppressive therapy | 2 (17) | 0 | 1 (13) | 3 (8) |
| Organ transplant | 0 | 0 | 0 | 0 |
| Immunodeficiency | 1 (8) | 0 | 0 | 1 (3) |
| Symptoms, No. (%) | ||||
| Arthralgia | 5 (42) | 6 (33) | 1 (13) | 12 (32) |
| Malaise | 7 (53) | 17 (94) | 8 (100) | 32 (84) |
| Chills | 5 (42) | 11 (61) | 4 (50) | 20 (52) |
| Anorexia | 5 (42) | 5 (28) | 1 (13) | 11 (29) |
| Subjective fever | 6 (50) | 14 (78) | 7 (88) | 27 (71) |
| Nausea/vomiting | 2 (17) | 4 (22) | 2 (25) | 8 (21) |
| Diarrhea | 0 | 2 (11) | 1 (13) | 3 (8) |
| Length of stay, No. (%) | ||||
| <8 d | 0 | 15 (83) | 3 (38) | 30 (79) |
| 8–14 d | 0 | 2 (11) | 4 (50) | 6 (16) |
| ≥15 d | 0 | 1 (6) | 1 (13) | 2 (5) |
| Labs (average) | ||||
| Hemoglobin, g/dL | 12.4 | 10.9 | 7.5 | 10.3 |
| WBC, ×10(9)/L | 7.5 | 6.2 | 7.8 | 7.2 |
| Platelets, ×10(9)/L | 192 | 134 | 103 | 143 |
| Alkaline phosphatase, U/L | 108 | 86 | 102 | 98.7 |
| AST, U/L | 35 | 98 | 107 | 80 |
| ALT, U/L | 30 | 66 | 71 | 55.7 |
| Bilirubin, mg/dL | 0.5 | 1.3 | 2.4 | 1.4 |
| Coinfection, No. (%) | ||||
| Lyme | 0 | 6 (33) | 3 (38) | 9 (24) |
| Anaplasma | 0 | 1 (5) | 1 (13) | 2 (5) |
| Lyme & anaplasma | 1 (8) | 2 (11) | 0 | 3 (8) |
| ID consult, No. (%) | 4 (33) | 12 (67) | 8 (100) | 24 (63) |
| Treatment | ||||
| Azithromycin + atovaquone, No. (%) | 12 (100) | 14 (78) | 0 | 26 (68) |
| Clindamycin + quinine or quinidine, No. (%) | 0 | 4 (22) | 8 (100) | 12 (32) |
| Average duration, d | 13.1 | 22.7 | 20.1 | 18.6 |
| Exchange transfusion, No. (%) | 0 | 0 | 3 (38) | 3 (8) |
| PRBC transfusion, No. (%) | 0 | 3 (17) | 8 (100) | 11 (29) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ICU, intensive care unit; ID, infectious diseases; PRBC, packed red blood cells; WBC, white blood cell count.
Figure 1.
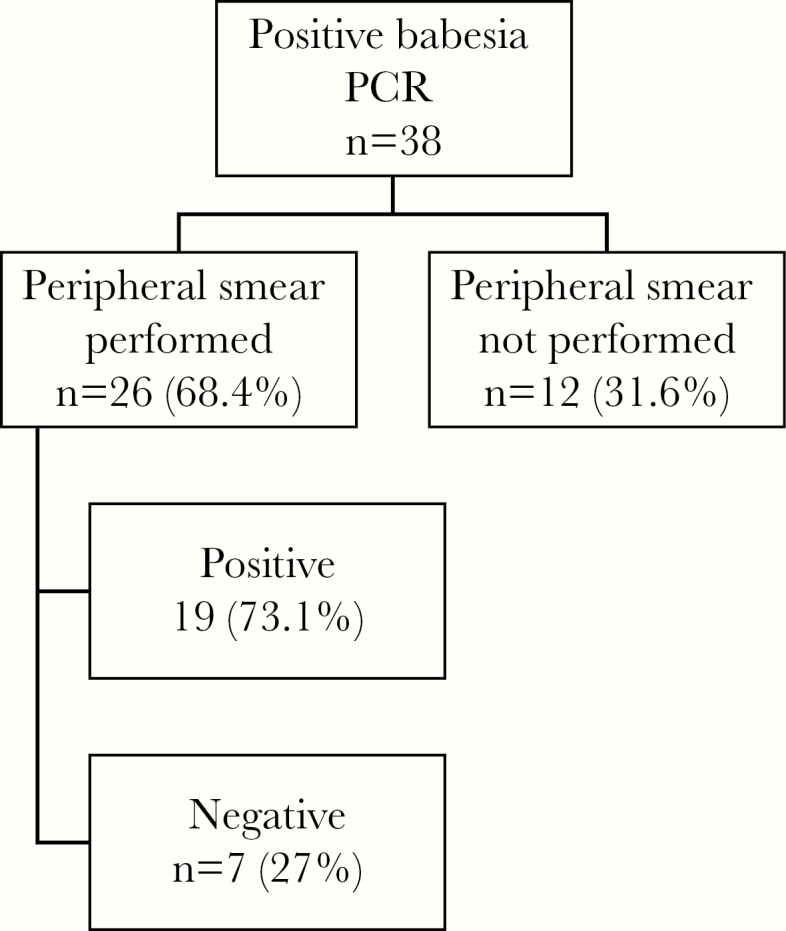
Diagnostic workflow. Abbreviation: PCR, polymerase chain reaction.
Figure 2.
Seasonality of babesiosis diagnosis—Mayo Clinic Data compared with 2017 CDC data [13]. Abbreviation: CDC, Centers for Disease Control and Prevention.
Nearly 70% of patients required hospitalization, and one-fifth of the patients were admitted to the intensive care unit. Indications for ICU admission included severe hemolytic anemia (n = 5), shock requiring vasopressor support (n = 2), and acute respiratory distress syndrome (n = 1). The mean hemoglobin in the patients managed without hospitalization was 12.4 g/dL compared with 9.8 g/dL in the hospitalized patients (P < .01) (Supplementary Table 1). Among hospitalized patients, the mean hemoglobin for ICU admissions was 7.5 g/dL vs 10.9 g/dL for those without ICU stay (P < .01). The mean parasitemia (range) was 10.1% (1.2%–28.5%) in those requiring ICU compared with 1.4% (<0.01%–9.2%) in those admitted to the medical floor (P < .01). There were also significant differences in the mean platelets and mean bilirubin in the group that was hospitalized (ICU plus regular floor patients) compared with the group managed as outpatients. In addition to Babesia infection, 28.9% of patients also had Lyme disease and 10.5% had Anaplasma coinfection. All 5 asplenic patients required hospitalization, with 3 requiring an ICU stay. Initial parasitemia of asplenic patients ranged from 2.5% to 28%, and the duration of the parasitemia varied from 10 to 142 days. Patients with smear-positive disease were admitted to the hospital 94% of the time (Supplementary Table 2).
Initial treatment of intravenous (IV) clindamycin plus oral quinine (n = 11) or IV quinidine (n = 1) was used in 31% (n = 12) of patients, whereas the remaining 69% received a combination of atovaquone and azithromycin. In 75% of the patients who were initially started on a combination of quinine and clindamycin, treatment had to be modified after an average of 4 days due to side effects from quinine (hearing issues, QT prolongation, arrhythmias, hemolysis, and hypoglycemia). The median duration of treatment was 10 days. Three patients with parasitemias ranging from 12.3% to 28.5% received exchange transfusion. Patients receiving exchange transfusion required a significantly longer duration of medical treatment (Supplementary Table 3). These patients also tended to have lower presenting hemoglobin and more elevated bilirubin than the patients who did not require exchange transfusion. None of the patients died as a result of their disease. The mean duration of follow-up for our cohort was 4 years.
DISCUSSION
Babesiosis is a worldwide emerging zoonosis able to cause a wide spectrum of disease. The majority of patients who become infected are asymptomatic and never present for care. This is supported by data suggesting that 3.7% of blood donors from an endemic region are seropositive for the disease [9]. Forty percent of children and 20% of adults may experience asymptomatic infection [10]. A case series of 34 Long Island patients had a mean age of 53 years, with predominantly males (26M and 8F) [11]. Our sex distribution was closer to even, with 15 males and 23 females. Our population was also older, with a mean age of 63 years. This may simply reflect changing population demographics in the 18 years since the previous study was published.
Unsurprisingly, in our cohort, the most common month of diagnosis was July, with a clear overall peak in the summer months, consistent with previous surveillance (Figure 2) [11–13]. This corresponds to the time of the year that humans are in greatest contact with the tick vector. The vector for B. microti in the United States is the Ixodes scapularis tick. The timing of patient presentation is a diagnostic clue, as most exposure to infected nymphal ticks occurs from late spring to summer.
Rates of hospitalization for babesiosis vary widely, ranging from 16% to 87% [12, 14–16]. Our hospitalization rate of 42% is within this range but is higher than the most recent large series, which reported 16% hospital admission. The higher admissions are likely representative of referral bias in our cohort, with more severe cases being referred to our tertiary medical center being more likely to require hospital admission.
Babesiosis can be difficult to diagnose due to patients presenting with nonspecific symptoms and often not remembering a tick bite. This may explain the mean length of illness before diagnosis (15.6 days). Similar delay between symptom onset and diagnosis has been seen in previous studies [11]. All the patients in our cohort were diagnosed with babesiosis via positive PCR or positive PCR and positive blood smear. No patients had a positive blood smear without a positive PCR. There were 7 patients who had positive PCR but negative smear. This may represent less severe disease, as the PCR may continue to detect parasitic DNA even after the infection has been eradicated. Of the 7 patients in this situation, only 2 required hospitalization. Additionally, 16 of the 17 patients with positive smears were admitted to the hospital, signifying more severe disease.
Definitive babesiosis diagnosis is classically made via examination of a thin blood smear stained with Giemsa or Wright staining to identify intraerythrocyte parasites [17]. In our series, peripheral smears were obtained more frequently when infectious diseases was involved in patient care. This might reflect more comprehensive care; however, as discussed previously, this assay is less sensitive than PCR.
Although we did not report antibody titers for the patients in our cohort as we wanted a rigorous case definition, in practice titers are commonly performed with the ability to detect IgM as soon as 2 weeks after onset of illness. Serologic tests are limited by their inability to detect antibodies against B. duncani, B. divergens, or B. venatorum [6]. As previously discussed, about 3.7% of the population in endemic regions is seropositive, limiting the utility of this test.
Coinfection with Borrelia spp. or Anaplasma spp. resulted in increased severity of symptoms and a higher chance of hospitalization. More than one-quarter of the patients with babesiosis also had Lyme disease. Previous studies of patients with Lyme disease in endemic areas have found that >10% suffer from concurrent babesiosis [18]. The severity and number of symptoms in coinfected patients are worse than in those without coinfections [18]. Due to high rates of coinfection with Borrelia and Anaplasma spp., screening should be considered, especially in hospitalized patients with babesiosis.
Three of the patients in the cohort were treated with exchange transfusion. Two of these patients were asplenic, leading to more severe disease. Generally, exchange transfusion is reserved for the most severely ill patients (parasitemia >10%, severe anemia, end organ dysfunction). These patients should also receive combination therapy with clindamycin and quinine. Less than one-third of the patients in the cohort were treated with this regimen, and most of these patients had to be switched to a different regimen due to side effects associated with quinine.. Less severely ill patients can be treated with atovaquone and azithromycin; however, there is also some evidence that this regimen may be effective in patients with moderate to severe disease [14]. Duration of treatment is at least 7–10 days, depending on clinical response. One patient in the cohort was treated for 210 days for babesiosis with atovaquone and azithromycin due to persistently positive blood smears. This patient was coinfected with Lyme disease and had a previous splenectomy for leukemia. His peak parasitemia was 11%. Treatment was discontinued after he proved to have negative smears for about 1 month.
None of the patients in our group died from their disease. Previous studies have found mortality rates between 5% and 10%, with rates approaching 20% for severely immunocompromised patients [19].
CONCLUSIONS
Due to the nonspecific symptoms of babesiosis, diagnosis is often delayed. Any unexplained febrile illness occurring in a patient either living in an endemic area or with a history of travel to an endemic area within last 2 months should prompt testing for babesiosis regardless of tick bite history. Once diagnosis is established, hemoglobin and parasitemia levels correlate with severity of illness and can be used for prognostication. In patients with diagnosis of Lyme disease or anaplasmosis, if there is no improvement after initiation of therapy, babesiosis should be suspected.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by Grant No. UL1 TR002377 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentation. Presented at ID Week 2018; October 3–7, 2018; San Francisco, CA.
References
- 1. Krause PJ. Human babesiosis. Int J Parasitol 2019; 49:165–74. [DOI] [PubMed] [Google Scholar]
- 2. Leiby DA. Babesiosis and blood transfusion: flying under the radar. Vox Sang 2006; 90:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwartz BS, Mawhorter SD; AST Infectious Diseases Community of Practice Parasitic infections in solid organ transplantation. Am J Transplant 2013; 13(Suppl 4):280–303. [DOI] [PubMed] [Google Scholar]
- 4. New DL, Quinn JB, Qureshi MZ, Sigler SJ. Vertically transmitted babesiosis. J Pediatr 1997; 131:163–4. [DOI] [PubMed] [Google Scholar]
- 5.Gray EB, Herwaldt BL. Babesiosis surveillance–United States, 2011–2015. MMWR Surveill Summ 2019; 68:1–11. [DOI] [PubMed] [Google Scholar]
- 6. Vannier E, Krause PJ. Human babesiosis. N Engl J Med 2012; 366:2397–407. [DOI] [PubMed] [Google Scholar]
- 7. Burgess MJ, Rosenbaum ER, Pritt BS, et al. Possible transfusion-transmitted Babesia divergens-like/MO-1 infection in an Arkansas patient. Clin Infect Dis 2017; 64:1622–5. [DOI] [PubMed] [Google Scholar]
- 8. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Popovsky MA, Lindberg LE, Syrek AL, Page PL. Prevalence of Babesia antibody in a selected blood donor population. Transfusion 1988; 28:59–61. [DOI] [PubMed] [Google Scholar]
- 10. Krause PJ, McKay K, Gadbaw J, et al. ; Tick-Borne Infection Study Group Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg 2003; 68:431–6. [PubMed] [Google Scholar]
- 11. Hatcher JC, Greenberg PD, Antique J, Jimenez-Lucho VE. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis 2001; 32:1117–25. [DOI] [PubMed] [Google Scholar]
- 12. White DJ, Talarico J, Chang HG, et al. Human babesiosis in New York State: review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med 1998; 158:2149–54. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. 2017 annual tables of infectious disease data. National Notifiable Diseases Surveillance System 2017. Available at: https://www.cdc.gov/nndss/infectious-tables.html. Accessed 6 March 2019.
- 14. Kletsova EA, Spitzer ED, Fries BC, Marcos LA. Babesiosis in Long Island: review of 62 cases focusing on treatment with azithromycin and atovaquone. Ann Clin Microbiol Antimicrob 2017; 16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hatcher JC, Greenberg PD, Antique J, Jimenez-Lucho VE. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis 2001; 32:1117–25. [DOI] [PubMed] [Google Scholar]
- 16. Joseph JT, Roy SS, Shams N, et al. Babesiosis in lower Hudson Valley, New York, USA. Emerg Infect Dis 2011; 17:843–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Homer MJ, Aguilar-Delfin I, Telford SR 3rd, et al. Babesiosis. Clin Microbiol Rev 2000; 13:451–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krause PJ, Telford SR 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 1996; 275:1657–60. [PubMed] [Google Scholar]
- 19. Vannier E, Krause PJ. Update on babesiosis. Interdiscip Perspect Infect Dis 2009; 2009:984568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.