Abstract
During the 2017–2018 flu epidemic, the point-of-care Alere-i (n = 72) and reverse transcription polymerase chain reaction (n = 106) tests were compared. Patients in the point-of-care group were administered oseltamivir significantly more rapidly (9 hours vs 23 hours), they spent less time in the emergency department, and they had lower rates of antibiotic administration and hospitalization.
Keywords: influenza, nucleic-acid amplification test, oseltamivir, point of care, seasonal flu
Seasonal influenza is responsible for yearly worldwide seasonal epidemics, with millions of hospitalizations and 290 000–650 000 deaths every year [1], placing substantial stress on health care structures, especially emergency departments (EDs). Neuraminidase inhibitors (NIs) such as oseltamivir are recommended in the management of patients presenting with severe influenza, in those at risk of complications, or in cases of hospitalization [2]. The efficacy of NIs is highest when administered within 48 hours after symptom onset [3, 4] and prompt diagnosis and treatment are of utmost importance. When analyzing oseltamivir prescriptions during the flu season, the prescription rates appear low (<25%), even in high-risk in- and outpatients [5–7]. Rapid and accurate detection of the influenza virus is therefore required for optimal management of seasonal influenza. Although classic real-time reverse transcription polymerase chain reaction (RT-PCR) performed by laboratories is the standard-of-care test for flu diagnosis, it results in inherent delay. Nucleic acid amplification tests (NAATs) are rapid digital influenza diagnostic tests with good positive predictive and low negative predictive values [8]. The Alere-i Influenza A&B test is a point-of-care (POC) NAAT with good sensitivity and specificity and delivers a response within 15 minutes [9]. The test was used during the 2017–2018 epidemic season in the adult ED at the Hôpitaux Civils de Colmar (HCC). However, because of reagent shortage, standard RT-PCR tests were also used to diagnose flu during the epidemic. We examined the 2017–2018 seasonal flu epidemic, which was characterized by atypical dynamics, with successive circulation of A(H1N1)pdm09 and B/Yamagata viruses. We compared the outcomes when the Alere-i POC test vs the RT-PCR test was used in the HCC ED in terms of oseltamivir and antibiotic prescriptions, time spent in the ED, and hospitalization and isolation rates.
METHODS
We conducted a retrospective, descriptive observational study in adults, in which we compared 2 diagnostic strategies used in the ED during the 2017–2018 epidemic at HCC, a 1200-bed hospital in northeastern France.
Patients who were at least 18 years of age and were diagnosed with a molecular flu test (Alere-i Influenza A/B or classic RT-PCR) in the ED during the period between November 1, 2017, and April 30, 2018, were included. Patients who had already been administered oseltamivir, those with missing medical charts, and those who were transferred to another hospital were excluded.
The study was approved by the ethical committees of the odontology and pharmacy faculties and hospitals.
Trial Procedures
Nasopharyngeal swabs were collected in the ED. The Alere-i Influenza A & B assay (Abbott, Scarborough, ME) diagnostic test was performed in the ED according to the manufacturer’s instructions. When Alere reagents were out of stock, a standard RT-PCR was performed using duplex TaqMan hydrolysis probes for genes encoding for the matrix proteins (M) of influenza viruses A and B (Influenza A/B r-gene kit; bioMérieux, Marcy-l’Étoile, France). This technique used a cell control r-gene, which guaranteed the sample quality by preventing false-negative (pauci-cellular) samples. Samples were collected in the ED and sent to the central laboratory. The test was carried out in the hospital central laboratory, once a day but not on Sundays and days off; that is, the turnaround time ranged from an optimal turnover of 6 hours in the case of a swab arriving before 9 am (Monday through Saturday) up to 39 hours (for a swab arriving after 9 am on Saturday) or 63 hours in the case of a swab arriving on a Saturday such as December 24 or 30, 2017, after 9 am (weekend followed by a day off).
Only patients with a positive RT-PCR or Alere test were analyzed. A case report form was completed retrospectively and included anonymous demographic data (age and sex), high-risk conditions (chronic renal, hepatic, cardiac, or pulmonary diseases, diabetes, neurologic disorders, cancer, immunodepression, and pregnancy), and biological data (C-reactive protein, microbiological data) at admittance. Oseltamivir and antibiotic prescriptions and their time of initiation (ie, the time between arrival in the ED and the first administration) were noted for hospitalized patients and outpatients. Regarding hospitalizations, the duration of antibiotherapy, intensive care unit (ICU) hospitalizations, isolation measures, and mortality rates were noted.
End Points
The primary end points were oseltamivir prescription and the length of time between arrival at the ED and first oseltamivir administration.
The secondary end points were antibiotic prescription and the length of time between arrival at the ED and first antibiotic administration, the length of time spent in the ED, the duration of hospitalization, and isolation measures.
Statistical Analysis
Qualitative variables were analyzed using the chi-square test, or the Fisher exact test if validity criteria were not met, and continuous quantitative variables were compared with the exact Mann-Whitney test, as appropriate. Differences were considered statistically significant at a level of 5% (alpha risk). All statistical analyses were performed with BiostaTGV (http://biostatgv.sentiweb.fr/).
RESULTS
Subject Characteristics
A total of 451 Alere-i POC tests were performed in the ED from November 1, 2017, to April 30, 2018, with 119 positive tests (26.4%), 50 (11.1%) invalid tests, and 282 (62.5%) negative tests. A total of 365 RT-PCR tests were performed, with 128 positive tests (35.1%), 5 (1.3%) invalid tests, and 232 (63.6%) negative tests. Forty-seven patients in the POC group and 22 in the RT-PCR group were excluded because of previous oseltamivir treatment (2 and 1 patients, respectively), transfer to another hospital (1 and 2 patients, respectively), or missing medical charts (44 and 19 patients, respectively). Finally, data from 72 and 106 patients in the POC NAAT and RT-PCR groups were analyzed, respectively.
Age, sex ratio, viral strain repartition (A/B), high-risk conditions, microbiological results, leucocytes, and CRP measures are shown in Table 1.
Table 1.
Rapid Point-of-Care vs Standard Technique Test During the 2017–2018 Flu Season: Sociodemographic Characteristics, Biologic Values, and End Point Results
| Alere-i (POC NAAT) n = 72 | Standard RT-PCR n = 106 | P Value | |
|---|---|---|---|
| Age, y | 63 (25–92) | 66 (22–95) | NS |
| M/F | 35/37 | 56/50 | NS |
| Viral strains A/B | 25/47 | 28/78 | NS |
| High-risk factors | 38 (52.8) | 69 (65.1) | NS |
| Deaths | 1 (1.4) | 4 (3.8) | NS |
| ICU | 3 (4.2) | 1 (0.9) | NS |
| Leucocytes, giga/L | 7.09 (1.98–17.40) | 7.41 (2.02–17.72) | NS |
| CRP, mg/L | 50 (2–448) | 65 (1–608) | NS |
| Microbiology | 1 sputum culture + a | 3 blood cultures + b | |
| Oseltamivir treatment | 41 (56.9) | 66 (62.3) | NS |
| Time of 1st NI delivery, h min | 9h32 (1h41–53h54) | 23h41 (1h39–101h32) | <.001 |
| Antibiotic treatment | 28 (38.9) | 59 (55.7) | .03 |
| Time of 1st ab delivery, h min | 37h57 (1h19–300h57) | 19h32 (1h22–224h58) | NS |
| Duration, d | 6 (1–15) | 6 (1–13) | NS |
| Duration of stay (ED), h min | 10h17 (1h00–52h56) | 12h52 (0h50–59h15) | .005 |
| Hospitalization | 28 (38.9) | 65 (61.3) | .003 |
| Duration, d | 8.5 (2–33) | 7.9 (1–29) | NS |
| Isolation measures | 22 (78.5) | 40 (61.5) | NS |
Data are presented as No., mean (range), or No. (%). P values* significant differences if P <0.05.
Abbreviations: ab, antibiotic; CRP, C-reactive protein; ED, emergency department; ICU, intensive care unit; NAAT, nucleic-acid amplification test; NI, neuraminidase inhibitors; NS, not significant; POC, point-of-care; RT-PCR, reverse transcription polymerase chain reaction.
a Staphylococcus aureus.
b Staphylococcus aureus (1), Haemophilus influenzae (1), Staphylococcus epidermidis (1).
Primary End Points
The proportion of patients who received oseltamivir treatment in the POC NAAT group was similar to that in the RT-PCR group. However, oseltamivir treatment was initiated earlier in the POC NAAT group, with a mean time of 9 hours 32 minutes vs 23 hours 41 minutes in the RT-PCR group (P < 0.001) (Table 1).
Secondary End Points
Compared with the RT-PCR group, patients in the POC NAAT group had a lower rate of antibiotic treatment (38.9% vs 55.7%; P = .03), spent less time in the ED (10 hours 17 minutes vs 12 hours 52 minutes; P = .005), had a lower hospitalization rate (38.9% vs 61.3%; P = .003), and had a nonsignificantly higher rate of isolation in cases of hospitalization (78.5% vs 61.5%). Length of hospital stay, rate of hospitalization in the ICU, and hospital deaths are shown in Table 1.
DISCUSSION
Our study shows that implementation of a POC NAAT in the ED greatly improved the management of patients with flu during a seasonal influenza epidemic. The best results were achieved when comparing the delay in initiating oseltamivir prescription between the groups, with a median delay of 9 hours 32 minutes in the POC NAAT group vs 23 hours 41 minutes in the RT-PCR group. These results are important because NI efficacy is better when administered within 48 hours of symptom onset. Few studies have focused on this delay in the ED, and for both in- and outpatients, most NI treatments are usually initiated too late, especially when there is no laboratory confirmation to support treatment [5–7]. Current French, US Centers for Disease Control and Prevention, and Infectious Diseases Society of America guidelines suggest that patients with confirmed or suspected influenza meeting certain criteria should be treated with neuraminidase inhibitors as soon as possible. The reasons for this delay or lack in NI treatment in the RT-PCR group might be thus the difficulty in diagnosing influenza with certitude without a positive laboratory result, especially in older patients with comorbidities, and the existence of numerous differential microbes causing flu-like symptoms, particularly bacteria [10, 11].
Interestingly, time spent in the ED, antibiotic prescriptions, hospitalization rates, and, for inpatients, isolation rates were greatly improved in the POC group. We suggest that in the RT-PCR group, given the condition of highly comorbid patients, ED physicians adopted a more careful approach and favored a bacterial hypothesis in the absence of viral evidence [10]. Previous studies have analyzed the use of rapid tests in the ED and found beneficial effects on NIs and antibiotic prescriptions, length of stay in the ED, and hospitalization rates [12, 13]. These results are important in the improvement of microbial ecology through minimizing antibiotic administration, reducing stress on the ED, and increasing the availability of hospitalization beds during the flu season. However, studies have shown that chest X-rays and biological markers are important when assessing the need for antibiotic administration [12]. In our study, patients with higher CRP tended to be more frequently treated with antibiotics (data not shown).
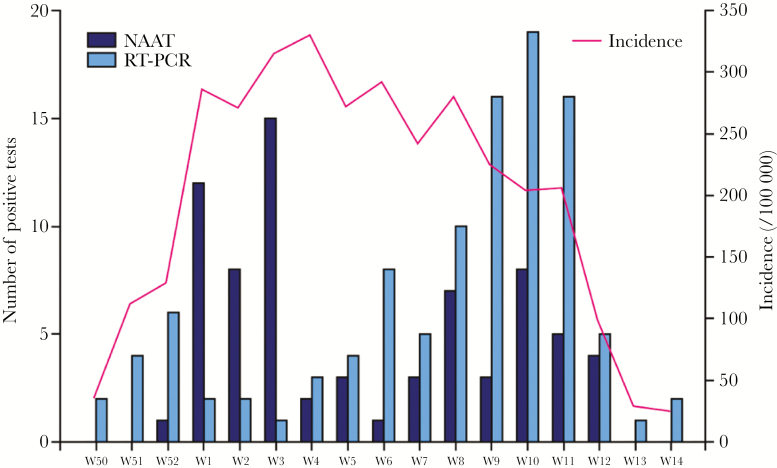
Our study has some limitations. First, it is a retrospective study, although random shortages of the Alere-i reagents allowed us to compare the patients in the 2 diagnostic groups during the same epidemic, cared for by the same medical team. We compared dates of performing NAAT or RT-PCR tests and noticed a wide overlap, underscoring that these tests were used during the same periods (Figure 1). However, in January 2018, NAATs were the most used, in contrast with RT-PCR, which was used the most in March. The shortages occurred during a period in which the B virus was more common; this may explain the slightly higher proportion of A viruses in the POC group, although this difference was not statistically significant. An unexpectedly high number of invalid tests (11%) was noted in the POC NAAT group, which could be due to the rapid introduction of the test in the ED immediately before the epidemic without supervision by the medical biology team and because the handling procedure of the Alere test is more complicated than other systems [14]. Because of the high number of health care workers performing these tests, we cannot disregard the possibility of lack of experience leading to incorrect manipulations. We did not include the invalid results in our analysis, and this exclusion may have unfairly favored POC testing. Similarly, the rapid detection flu tests in the POC NAAT group had a lack of traceability: all test results were transcribed in a global chart in which the patients’ names were not always written, resulting in a high rate of missing medical data in the Alere-i group. The involvement of laboratory-trained staff in the management of the POC test could have lowered the rate of invalid testing and improved the traceability of the results.
Figure 1.
Number of positive RT-PCRs and Alere NAATs performed and incidence of flu in Alsace during the 2017–2018 seasonal flu epidemic week (W) 50 (2017) to week 14 (2018). Abbreviations: NAAT, nucleic-acid amplification test; RT-PCR, reverse transcription polymerase chain reaction.
Lastly, the cost of NAATs was not assessed in this study. NAATs are 2–5 times more expensive than RT-PCR or classic rapid influenza diagnostic tests. Nevertheless, this excess cost could be balanced by the reduction of costs related to decreased antibiotic prescriptions and hospitalizations.
Advancements in medical biology have allowed the use of increasingly sensitive and easy-to-use tests for lower respiratory infections such as influenza. Our findings suggest that POC NAATs in the ED improve the care of patients with seasonal flu in terms of NI and antibiotic administration, time spent in the ED, and hospitalization rates.
Acknowlegments
Financial support. None declared.
Potential conflicts of interest. The authors declare they have no potential conflict of interest or funding source. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Iuliano AD, Roguski KM, Chang HH, et al. . Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391:1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harper SA, Bradley JS, Englund JA, et al. . Expert Panel of the Infectious Diseases Society of America Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernu R, Chroboczek T, Madelaine T, et al. . on behalf the “Flu in Lyon ICUs” Study Group Early oseltamivir therapy improves the outcome in critically ill patients with influenza: a propensity analysis. Intensive Care Med 2018; 44:257–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar A. Early versus late oseltamivir treatment in severely ill patients with 2009 pandemic influenza A (H1N1): speed is life. J Antimicrob Chemother 2011; 66:959–63. [DOI] [PubMed] [Google Scholar]
- 5. Stewart RJ, Flannery B, Chung JR, et al. . Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons-United States, 2011-2016. Clin Infect Dis 2018; 66:1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinot M, Gronnwald A, Gerber V, et al. . Analysis of delays in the prescription of oseltamivir in hospitals and potential for improvement. Med Mal Infect 2019; 49:59–62. [DOI] [PubMed] [Google Scholar]
- 7. Fowlkes AL, Steffens A, Reed C, Temte JL, Campbell AP. Influenza antiviral prescribing practices and the influence of rapid testing among primary care providers in the US, 2009–2016. Open Forum Infect Dis 2019; 6(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merckx J, Wali R, Schiller I, et al. . Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: a systematic review and meta-analysis. Ann Intern Med 2017; 167:394–409. [DOI] [PubMed] [Google Scholar]
- 9. Hassan F, Crawford J, Bonner AB, et al. . Multicenter evaluation of the Alere™ i influenza A&B assay using respiratory specimens collected in viral transport media. Diagn Microbiol Infect Dis 2018; 92:294–8. [DOI] [PubMed] [Google Scholar]
- 10. Miller MR, Peters TR, Suerken CK, et al. . Predictors of influenza diagnosis among patients with laboratory-confirmed influenza. J Infect Dis 2015; 212:1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martinot M, Heller R, Martin A, et al. . Contribution of systematic RT-PCR screening for influenza during the epidemic season. Med Mal Infect 2014; 44:123–7. [DOI] [PubMed] [Google Scholar]
- 12. Semret M, Schiller I, Jardin BA, et al. . Multiplex respiratory virus testing for antimicrobial stewardship: a prospective assessment of antimicrobial use and clinical outcomes among hospitalized adults. J Infect Dis 2017; 216:936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonner AB, Monroe KW, Talley LI, et al. . Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics 2003; 112:363–7. [DOI] [PubMed] [Google Scholar]
- 14. Chen JH, Lam HY, Yip CC, et al. . Evaluation of the molecular Xpert Xpress Flu/RSV assay vs Alere i Influenza A & B assay for rapid detection of influenza viruses. Diagn Microbiol Infect Dis 2018; 90:177–80. [DOI] [PubMed] [Google Scholar]