Abstract
Background
The US National Viral Hepatitis Action Plan depends on additional providers to expand hepatitis C virus (HCV) treatment capacity in order to achieve elimination goals. Clinical pharmacists manage treatment and medication within interdisciplinary teams. The study’s objective was to determine sustained virologic response (SVR) rates for clinical pharmacist–delivered HCV therapy in an open medical system.
Methods
Investigators conducted a multicenter retrospective cohort study of patients initiating direct-acting antivirals from January 1, 2014, through March 12, 2018. Data included demographics, comorbidities, treatment, and clinical outcomes. The primary outcome of SVR was determined for patients initiating (intent-to-treat) and those who completed (per-protocol) treatment. Chi-square tests were conducted to identify associations between SVR and adverse reactions, drug–drug interactions, and adherence.
Results
A total of 1253 patients initiated treatment; 95 were lost to follow-up, and 24 discontinued therapy. SVR rates were 95.1% (1079/1134) per protocol and 86.1% (1079/1253) intent to treat. The mean age (SD) was 57.4 (10.1) years, the mean body mass index (SD) was 28.7 (6.2) kg/m2, 63.9% were male, 53.7% were black, 40.3% were cirrhotic, 88.4% were genotype 1, and 81.6% were treatment-naïve. Patients missing ≥1 dose had an SVR of 74.9%; full adherence yielded 90% (P < .0001).
Conclusions
HCV treatment by clinical pharmacists in an open medical system resulted in high SVR rates comparable to real-world studies with specialists and nonspecialists. These findings demonstrate the success of a clinical pharmacist–delivered method for HCV treatment expansion and elimination.
Keywords: hepatitis C virus, clinical pharmacists, HCV elimination, direct-acting antiviral, interdisciplinary
A clinical pharmacist-driven model for hepatitis C virus treatment at four open health-systems yielded sustained virologic response rates comparable to published rates of specialists and non-specialists. This model can be implemented to assist with national hepatitis C virus elimination goals.
The number of acute hepatitis C virus (HCV) cases in the United States has risen steadily since 2010, and it increased from 2015 to 2016 by 21.8%, from 2436 to 2967 patients infected [1]. Approximately 3.5 million people in the United States are living with chronic HCV. HCV mortality surpassed that of HIV in 2006 and has surpassed >60 other nationally notifiable infectious diseases combined since 2013 [2]. Rising mortality rates are of increasing concern, as only 50% of infected patients are diagnosed and aware of their HCV infection and only 17% have been prescribed HCV treatment [3]. Highly effective direct-acting antiviral (DAA) agents are available, yet accompanied by challenges of drug–drug interactions (DDIs) and medication access [1, 4, 5].
In 2017, the United States National Academy of Sciences released a domestic hepatitis elimination strategy that incorporated pharmacies as additional venues to expand access to prevention, care, and cure [6]. Additional HCV providers are needed not only to diagnose HCV infection, but also to close the gap between HCV diagnosis and treatment. Successful models that expand the HCV treatment workforce to include midlevel providers and nonspecialist primary care providers have been published [7, 8]. The multifaceted roles of the clinical pharmacist in HCV management have previously been outlined as well [9–12]. Clinical pharmacists as HCV treatment providers have been demonstrated to achieve high SVR rates within self-contained systems such as the US Veterans Affairs Health System and the Indian Health Services. The Veterans Affairs Health System is the largest treater of HCV in the nation and is on track for HCV elimination. These self-contained settings may not be reflective of the majority of open practices in the United States, which encounter other barriers to care including but not limited to insurance restrictions, out-of-pocket costs, and medication procurement through a specialty pharmacy [9, 13–16]. The effectiveness of a clinical pharmacist–driven HCV delivery model in an open system has not yet been reported.
Several US practice sites have clinical pharmacist–driven treatment models, in which the clinical pharmacist selects the appropriate HCV medication and duration for patients as part of an interdisciplinary team and under a collaborative practice agreement with a physician. This multicentered cohort study evaluates sustained virologic response (SVR) rates from 4 clinical pharmacist–driven HCV treatment models within hepatology, infectious diseases, and primary care clinics to assess this approach for HCV care delivery.
METHODS
Investigators conducted a multicenter observational retrospective cohort study using electronic medical record data from patients who initiated HCV treatment at 4 US institutions with a clinical pharmacist–driven treatment model within an interdisciplinary health care team. The study was reviewed and approved by the institutional review board at each of the participating institutions, and waivers of informed consent were granted.
Study Setting and Participants
The 4 participating institutions included Creighton University (CU), Temple University Health System (TUHS), University of Illinois Hospital and Health Sciences System (UI Health), and Vanderbilt University Medical Center (VUMC). Institutions utilized an HCV treatment model in which a board-certified clinical pharmacist within an interdisciplinary team selected the appropriate DAA regimen and duration for patients according to existing standards of care and national guidance at the time of treatment. Clinics included hepatology, infectious diseases, and primary care. Although the specific workflow varied by site, all pathways were comprised of a generalized 6-step process in which the clinical pharmacist performed: (1) evaluation of treatment candidacy, (2) selection of treatment regimen, (3) facilitation of medication access, (4) provision of patient education at treatment initiation and throughout follow-up, (5) adherence counseling and assessment, and (6) assessment of laboratory values to ensure safety and efficacy.
Adult patients were identified through the pharmacists’ local electronic medical record HCV treatment lists, and data were included if a dual or triple all-oral DAA HCV treatment regimen was initiated between January 1, 2014, and March 12, 2018, under the care of the clinical pharmacist at each investigator’s respective site. Patients were excluded if they had not yet reached 12 weeks after treatment completion by September 7, 2018, or if treatment was not provided by the clinical pharmacist–driven pathway described.
Data Collection
Study data were collected from electronic medical records and managed using REDCap electronic data capture tools hosted at the University of Illinois at Chicago [17]. Baseline data were collected from the 12 months before treatment initiation as available. Treatment data were collected from the initial HCV evaluation through SVR assessment, treatment discontinuation, or determination of loss to follow-up as of September 7, 2018.
Patient Characteristics
Baseline data collection included gender, age, ethnicity, body mass index (BMI), insurance type, HCV genotype, HCV RNA levels, HCV treatment history, fibrosis stage and method of determination, Child-Turcotte-Pugh (CTP) class in patients with cirrhosis, hepatitis B virus (HBV) and/or HIV coinfection, solid organ transplant history, dialysis status, comorbid diabetes or psychiatric illness, baseline alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, serum creatinine, platelets, total bilirubin, albumin, and DDI with planned DAA regimen. Patient-reported history of and/or current alcohol use, illicit substance use, and intravenous drug use (IVDU) were determined from chart documentation. HCV treatment-related data collected included DAA regimen, intended length of therapy, and reason for treatment discontinuation if applicable. HCV RNA results were collected through 12 or more weeks after treatment completion. Investigators reviewed adverse drug reactions (ADRs) and DAA adherence as documented by any member of the health care team after treatment initiation.
Outcomes
The primary outcome was SVR, defined as an undetectable HCV RNA polymerase chain reaction test result at a minimum of 12 or more weeks after HCV treatment completion. SVR rates were defined for the overall sample and stratified by patient characteristics, including race, age, gender, genotype, fibrosis stage, treatment history, and comorbidities. Secondary outcomes included effect of baseline DDIs, on-treatment ADRs, and medication adherence on SVR.
Statistical Analysis
The primary analysis utilized an intent-to-treat (ITT) analytic approach in which patients who were lost to follow-up (LTFU) or discontinued therapy were included as treatment failures. Therefore, no patients in the study sample were excluded from the primary analysis. In a secondary analysis, a per-protocol (PP) analytic approach was performed, in which patients who discontinued therapy or were LTFU were excluded. Patients were considered LTFU if they did not have SVR data available at a minimum of 12 or more weeks after the completion of therapy for any reason, including death, at the end of the study period. Patients were considered to have discontinued treatment if they did not complete their initially prescribed length of treatment for any reason, including provider instruction, self-discontinuation, or death.
Data were analyzed using SAS 9.4 software (SAS Institute, Cary, NC, USA). Descriptive statistics were provided to define the overall study population. Categorical variables were conveyed as frequencies and proportions, whereas continuous variables, including baseline age, BMI, ALT level, and AST level, were represented as the mean and standard deviation.
SVR rates and corresponding 95% confidence intervals (using the Clopper-Pearson method) were determined across the entire multicenter sample, as well as separately by baseline demographic and clinical factors. To identify significant associations between SVR rates and DDIs, ADRs, or adherence, χ 2 tests were used, with P values <.05 regarded as statistically significant.
RESULTS
Baseline Characteristics of the Study Patients
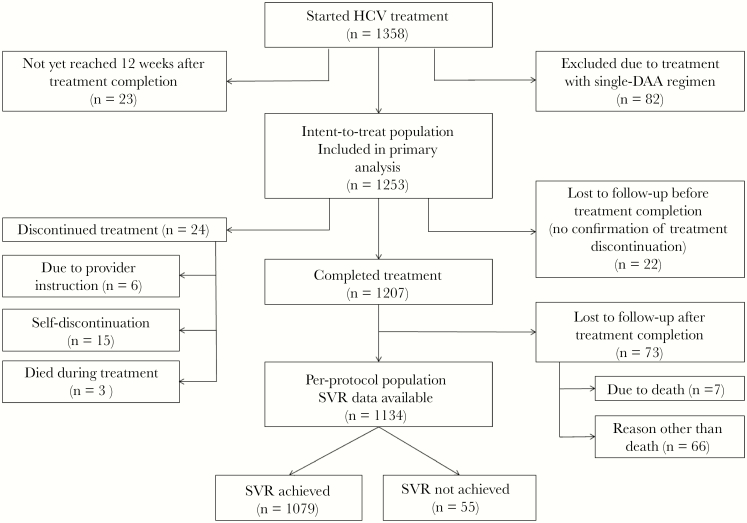
In total, 1253 patients from 4 institutions received dual or triple DAA treatment under a clinical pharmacist–driven interdisciplinary HCV treatment model (Figure 1). The few patients at the 4 institutions who did not receive treatment via the pharmacist-driven model had extenuating circumstances (ie, provider unavailability, or a patient presented with HCV medication from an outside facility). Exact numbers were not available due to the rarity of this occurrence, and these patients were not included in pharmacists’ HCV treatment lists. Ninety-five (7.6%) patients were LTFU, of whom 22 (23.2%) were lost during HCV treatment and 73 (76.9%) were lost after treatment completion. An additional 24 patients discontinued therapy. For the overall population, baseline demographic and treatment data are summarized in Table 1. The majority of the patients were born between 1945 and 1965 (74.1%), male (63.9%), black (53.7%), treatment-naïve (81.6%), and genotype 1 (88.4%). The overall baseline cirrhosis rate was 40.3%; 82.8% of cirrhotic patients were CTP class A, 13.7% CTP class B, and 3.6% CTP class C. Other common comorbidities included psychiatric illness (33.5%), diabetes mellitus (type 1 or 2, 24.2%), and HIV coinfection (18%). Over half of the study cohort received ledipasvir/sofosbuvir ± ribavirin (60.4%), and 83.8% of the population received 12 weeks of therapy. Baseline DDIs were identified in 47.6% of patients. Rates of alcohol use, other illicit substance use, or IVDU within 6 to 12 months before the initiation of treatment were 31.1%, 15.9%, and 5.6%, respectively.
Figure 1.
Patient attrition. Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus; SVR, sustained virologic response.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | n = 1253 |
| Mean age (SD), y | 57.4 (10.1) |
| Mean BMI (SD), kg/m2 | 28.7 (6.2) |
| Mean ALT (SD), U/L | 65.6 (62) |
| Mean AST (SD), U/L | 63.2 (48.9) |
| Male, No. (%) | 801 (63.9) |
| Ethnicity, No. (%) | |
| African American/black | 673 (53.7) |
| Caucasian | 387 (30.9) |
| Hispanic | 157 (12.5) |
| Other | 36 (2.9) |
| Insurance, No. (%) | |
| Medicaid/Medicaid Managed Care | 455 (36.3) |
| Medicare Part D Plan | 374 (29.8) |
| Private (commercial) insurance | 330 (26.3) |
| No insurance | 57 (4.5) |
| Other/unknown | 37 (3) |
| Born between 1945–1965 (baby boomer), No. (%) | 928 (74.1) |
| HIV coinfection, No. (%) | 225 (18) |
| HBV coinfection, No. (%) | 16 (1.3) |
| Diabetes mellitus, No. (%) | 303 (24.2) |
| History of solid organ transplantation, No. (%) | 90 (7.2) |
| Documentation of psychiatric illness, No. (%) | 420 (33.5) |
| Hepatocellular carcinoma, No. (%) | 49 (3.9) |
| Treatment-naïve, No. (%) | 1022 (81.6) |
| Drug–drug interactions present at baseline, No. (%) | 596 (47.6) |
| On dialysis, No. (%) | 38 (3) |
| Genotype, No. (%) | |
| 1 | 1108 (88.4) |
| 1A | 779 (70.3)a |
| 1B | 299 (27)a |
| Not otherwise specified | 30 (2.7)a |
| 2 | 57 (4.5) |
| 3 | 66 (5.3) |
| 4 | 12 (1) |
| 6 | 6 (0.5) |
| Other | 4 (0.3) |
| METAVIR score, No. (%) | |
| Noncirrhotic, not otherwise staged | 73 (5.8) |
| F0 | 103 (8.2) |
| F1 | 131 (10.5) |
| F2 | 217 (17.3) |
| F3 | 224 (17.9) |
| F4 | 505 (40.3) |
| CTP class A | 418 (82.8)b |
| CTP class B | 69 (13.7)b |
| CTP class C | 18 (3.6)b |
| Regimen, No. (%) | |
| SOF + SMV | 110 (8.8) |
| SOF + SMV + RBV | 1 (0.1) |
| LDV/SOF | 697 (55.6) |
| LDV/SOF + RBV | 60 (4.8) |
| PrOD | 4 (0.3) |
| PrOD + RBV | 12 (1) |
| DCV + SOF | 26 (2.1) |
| DCV + SOF + RBV | 6 (0.5) |
| EBR/GZR | 107 (8.5) |
| EBR/GZR + RBV | 7 (0.6) |
| SOF/VEL | 114 (9.1) |
| SOF/VEL + RBV | 17 (1.4) |
| SOF/VEL/VOX | 13 (1) |
| G/P | 79 (6.3) |
| Length of therapy, No. (%) | |
| 8 wk | 113 (9) |
| 12 wk | 1050 (83.8) |
| 16 wk | 6 (0.5) |
| 24 wk | 84 (6.7) |
| History of alcohol use in last 6–12 mo, No. (%)c | |
| No | 846 (67.5) |
| Yes | 390 (31.1) |
| History of IVDU in last 6–12 mo, No. (%)c | |
| No | 1120 (89.4) |
| Yes | 70 (5.6) |
| History of IVDU ever, No. (%)c | |
| No | 541 (43.2) |
| Yes | 539 (43) |
| History of other illicit substance use in last 6–12 mo, No. (%)c | |
| No | 1022 (81.6) |
| Yes | 199 (15.9) |
| History of other illicit substance use ever, No. (%)c | |
| No | 429 (34.2) |
| Yes | 648 (51.7) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CTP, Child-Turcotte-Pugh; DCV, daclatasvir; DDI, drug–drug interaction; EBR, elbasvir; G/P, glecaprevir/pibrentasvir; GZR, grazoprevir; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IVDU, intravenous drug use; LDV, ledipasvir; PrOD, paritaprevir/ritonavir/ombitasvir/dasabuvir; RBV, ribavirin; SMV, simeprevir; SOF, sofosbuvir; SVR, sustained virologic response; VEL, velpatasvir; VOX, voxilaprevir.
aPercentages based on number of genotype 1 patients.
bPercentages based on number of F4 patients.
cPercentages do not sum to 100% due to missing data.
Sustained Virologic Response
The overall ITT SVR rate was 86.1% (1079/1253; 95% confidence interval [CI], 84.1%–88.0%), and the PP SVR rate was 95.1% (1079/1134; 95% CI, 93.7%–96.3%). Among the 174 patients who did not achieve SVR, 95 (54.6%) were LTFU, 24 (13.8%) discontinued therapy before treatment completion, and 55 (31.6%) had a detectable HCV RNA level at least 12 weeks after treatment completion. The ITT and PP SVR rates did not differ among the 4 treatment sites (P > .05).
ITT SVR rates for specific subgroups are summarized in Figure 2 and did not differ significantly by treatment history, regimen, or comorbidities. SVR rates were higher for noncirrhotic patients than cirrhotic patients (88.8%; 95% CI, 86.3%–90.9%; vs 82.2%; 95% CI, 78.6%–85.4%). CTP class A patients had higher SVR rates than CTP class B patients (84.9%; 95% CI, 81.1%–88.2%; vs 69.4%; 95% CI, 57.3%–80.1%). ITT SVR rates were higher in patients with documented adherence vs those who self-reported any missed doses (90% vs 75%; P < .0001) (Table 2). SVR rates were 85.6%, 85.4%, and 77.1% among patients who had documented usage of alcohol, other illicit substances, and intravenous drugs within 6 to 12 months before initiating HCV treatment, respectively. These rates did not differ significantly from patients without recent use (Figure 2).
Figure 2.
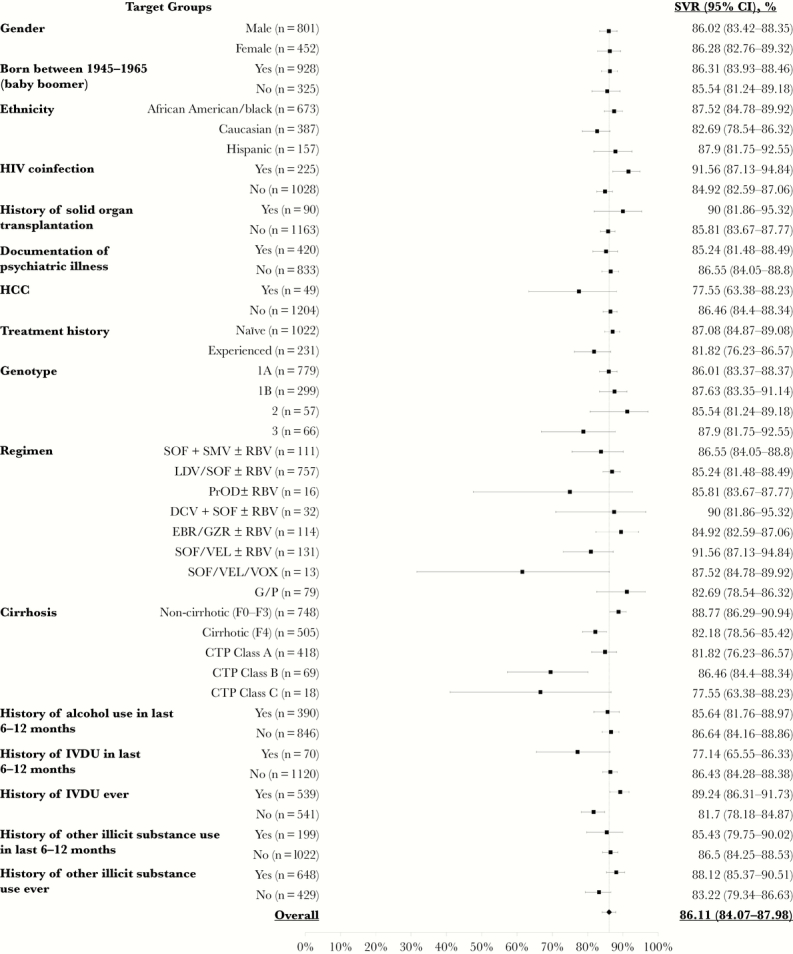
Sustained virologic response rates by patient subgroup. Abbreviations: CI, confidence interval; CTP, Child-Turcotte-Pugh; DCV, daclatasvir; EBR, elbasvir; GZR, grazoprevir; G/P, glecaprevir/pibrentasvir; HCC, hepatocellular carcinoma; IVDU, intravenous drug use; LDV, ledipasvir; PrOD, paritaprevir/ritonavir/ombitasvir/dasabuvir; RBV, ribavirin; SMV, simeprevir; SOF, sofosbuvir; SVR, sustained virologic response; VEL, velpatasvir; VOX, voxilaprevir.
Table 2.
Sustained Virologic Response Rates by Adherence, Drug–Drug Interactions, and Adverse Drug Reactions
| SVR, No. (%) | ||||
|---|---|---|---|---|
| Yes | No | P Value | ||
| Adherence | 0 missed doses | 837 (90.0) | 93 (10.0) | <.0001 |
| ≥1 missed dose | 242 (74.9) | 81 (25.1) | ||
| Presence of drug–drug interactions | Yes | 525 (88.1) | 71 (11.9) | .054 |
| No | 554 (84.3) | 103 (15.7) | ||
| Presence of adverse drug reactions | Yes | 444 (88.3) | 59 (11.7) | .071 |
| No | 635 (84.7) | 115 (15.3) | ||
Abbreviation: SVR, sustained virologic response.
Among the 73 patients lost after treatment completion, 44 (60.3%) had an end-of-treatment response with HCV RNA not detected, and 29 (39.7%) did not have an HCV RNA drawn at the end of treatment.
Safety
A total of 716 ADRs were recorded among the 1253 patients who started HCV treatment. Fatigue and headache occurred in 20.8% and 14.5%, respectively, whereas 21.9% of patients reported other ADRs of any type. SVR did not differ by presence (88.3%) or absence (84.7%) of ADRs (P = .07). Treatment discontinuations due to ADRs were uncommon and occurred in 24 patients. Of the 103 patients who received ribavirin; 17 experienced anemia, 15 required a ribavirin dosage reduction, 8 discontinued ribavirin, and 5 had an interruption in ribavirin therapy. Eleven patients died over the course of the study, 3 during treatment, 1 after discontinuing treatment early, and 7 after treatment completion but before a blood draw was performed for assessment of SVR. Medical records pertaining to death indicated that 3 deaths were related to decompensated liver disease, 3 were unrelated to liver disease, and cause of death was not available for 5 patients.
Drug–Drug Interactions
DDIs were present in 47.6% (596/1253) of patients at baseline; 798 DDIs by medication class were identified (Table 3). Changes to baseline concomitant medications were required for 184 medications to facilitate DAA safety and efficacy. SVR rates did not differ significantly by presence or absence of DDIs at baseline (88.1% vs 84.3%; P = .0543) (Table 2). The DAA regimen associated with the highest rate of DDIs was paritaprevir/ritonavir/ombitasvir/dasabuvir at 68.8%, yet it was used by only 1.3% of study patients. Ledipasvir/sofosbuvir led to the highest number of DDIs due to its frequent use, yet the DDI rate was only 50.1% with this regimen. The most common DDIs were with acid-suppressing agents, comprising 21.4% of all interactions identified.
Table 3.
Drug–Drug Interactions Identified
| DDIs With HCV Treatment Present at Baseline | No. | % of Entire Sample (n = 1253) |
|---|---|---|
| No | 657 | 52.43 |
| Yes | 596 | 47.57 |
| DDI class of medication | No. | % of Total DDIs (n = 798)a |
| Acid suppression | 268 | 21.39 |
| Antiepileptic agents | 7 | 0.56 |
| Antiretrovirals | 97 | 7.74 |
| Cardiac agents | 124 | 9.90 |
| Immunosuppressants | 70 | 5.59 |
| Psychiatric medications | 25 | 2.00 |
| Statins | 158 | 12.61 |
| Other | 49 | 3.91 |
| Management of DDI | No. | % of all Managed DDIs (n = 651) |
| ≥1 medication continued | 467 | 37.27 |
| ≥1 medication dose adjusted | 56 | 4.47 |
| ≥1 medication substituted | 56 | 4.47 |
| ≥1 medication discontinued | 72 | 5.75 |
Abbreviations: DDI, drug–drug interaction; HCV, hepatitis C virus.
aSome patients had multiple DDIs.
DISCUSSION
Results from this multisite retrospective cohort study indicate that clinical pharmacist–driven HCV care is effective. These results are important, as they are the first to report successful HCV management across multiple sites in open health care systems by a clinical pharmacist.
The overall ITT SVR rate of 86.1% from this model of care is similar to rates described in previous real-world reports of DAA therapy provided by specialists, midlevel providers, and primary care nonspecialists in the ASCEND trial [7]. The main reason for failure was LTFU, yet the majority of LTFU patients had an end-of-treatment response. The overall PP SVR rate of 95.2% is similar to the 93.8% rate of the international HCV-TARGET registry consisting of both academic and community HCV treatment sites [18].
No significant differences in SVR rates were identified by HCV regimen or treatment history. Pharmacists managed patients with a variety of comorbidities, including HBV or HIV coinfection, history of hepatocellular carcinoma, transplantation, psychiatric illness, and chronic kidney disease. SVR rates did not differ by these comorbidities, demonstrating the ability of the clinical pharmacists to manage patients with high complexity. As demonstrated in numerous other trials, presence of cirrhosis resulted in lower SVR rates than noncirrhotic patients; our population had a high rate of cirrhosis [18].
Payor coverage restrictions impacted the number of patients with advanced fibrosis, cirrhosis, and substance use who received HCV treatment. State Medicaid coverage differed among the 4 sites and changed over the course of the study period. Insurance restrictions based on staging and sobriety directly contradict current national HCV guidance and present a significant barrier to HCV elimination in the United States [19].
Consistent SVR rates regardless of recent or ongoing alcohol or illicit substance use further support that HCV treatment can be successful in these historically difficult-to-cure patient populations. These data are relevant due to national trends of increased HCV diagnoses among people who inject drugs [20]. HCV treatment in the DAA era requires effective coordination of care from an interdisciplinary medical team for patients with substance abuse [21]. Many study patients had a history of psychiatric illness; these data further support and add to existing data demonstrating successful HCV treatment among these patients [22, 23]. The literature additionally reports high rates of SVR among populations with illicit substance use and psychiatric disorders with the involvement of a pharmacist in HCV therapy management [24].
Pharmacists identified and managed 798 DDIs by medication class in 596 patients. Screening for major DDIs that reduce concentrations of DAAs is a vital responsibility for HCV treatment providers. Pharmacists are well equipped to identify and manage interactions before, during, and after a course of DAA therapy [25].
Pharmacists’ Role in Managing HCV
Clinical pharmacists have been members of HCV care teams from the interferon era to the current DAA era, and they represent a valuable resource to assist with HCV elimination via coordination of HCV care in a variety of patient settings. Interdisciplinary models that involve a pharmacist have shown higher rates of HCV treatment access than reports of traditional models [24, 26]. Previously reported data support that pharmacists can select the HCV regimen, facilitate treatment access, provide patient education throughout treatment, assess safety and efficacy, and offer mitigation strategies for ADRs to assist with treatment continuation [10–12, 16, 25, 27–31]. However, data presented here are the first to report the outcomes of clinical pharmacist–driven HCV treatment across multiple open health care system and clinical settings. The United States is on track to achieve HCV elimination after 2050, which extends 2 decades beyond the World Health Organization and US goals [32]. This study’s data further support that clinical pharmacist–driven HCV management across practice sites can assist with HCV elimination efforts nationwide.
Study Strengths and Limitations
This retrospective analysis has several strengths, the foremost being the generalizability of the findings. The clinical practice sites of the pharmacists varied from specialty (hepatology and infectious diseases) to primary care, demonstrating that the clinical pharmacist–directed HCV treatment model can be applied to a variety of settings. The lack of difference in SVR across practice sites reinforces the effectiveness of the clinical pharmacist model. The diverse patient population, representative of a wide geographic and clinical cohort, also gives strength and applicability to the findings. Our population included patients with CTP class C, which is not common in clinical trials.
A limitation of this study is its retrospective nature; data collection relied on previous documentation, which in some cases limited the granularity of available data (such as social history). An additional limitation is the lack of a comparator group at each institution. Each of the practice sites had implemented pharmacist-driven models for HCV care delivery up to 20 years before the study, so no patients existed for a non-pharmacist-managed comparator group. It would have been unreasonable to change the practice model for this sole purpose. The exact model of care delivery from treatment evaluation to initiation and follow-up was not standardized due to multiple study sites; however, the defined commonality was that the clinical pharmacist selected the HCV treatment and the duration of therapy. Although this limits the reproducibility, it adds diversity and generalizability to our findings. An additional limitation was collection of the DDIs by medication class instead of number of medications; the total number of DDIs may have been higher than reported.
CONCLUSIONS
The current study is the first to describe the efficacy of clinical pharmacist–driven HCV care across multiple institutions across a large and diverse patient population. HCV SVR rates were comparable to other real-world studies with specialist, nonspecialist, and nonhepatology providers. Collaborations should be established among other HCV treatment providers and clinical pharmacists in order to replicate this model of care as a method of HCV treatment expansion and a strategy geared toward HCV elimination.
Acknowledgments
We would like to acknowledge and thank all interdisciplinary members of the care teams at all sites.
Financial support. This work was supported by an investigator-sponsored research grant from Gilead Sciences Inc. (IN-US-342-4530). The contents are solely the responsibility of the authors and do not necessarily represent the official views of Gilead.
Potential conflicts of interest. The authors received investigator-sponsored research funding from Gilead Sciences, Inc. Drs Koren, Martin, and Teply have served on advisory boards for Gilead. Dr. Koren has served on an advisory panel for ViiV Healthcare. Drs Martin and Teply have served on advisory boards for AbbVie. Dr. Teply serves on the speakers’ bureau for Gilead Sciences, Inc. and AbbVie. Dr. Martin is a minor shareholder of AbbVie, Gilead, and Merck. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Drs Koren, Martin, Teply, and Zuckerman designed the study, performed data collection, and wrote and revised the manuscript. Ms. Nabulsi and Dr. Lee performed data and statistical analysis and wrote and revised the manuscript. All authors gave final approval and are accountable for the information presented.
Prior presentations. These data were presented in part as a poster presentation (Meeting Abstract 1887) at the 2018 American Association for the Study of Liver Diseases Liver Meeting on November 12, 2018.
References
- 1. Centers for Disease Control and Prevention. Surveillance for viral hepatitis – United States, 2016. Available at: https://www.cdc.gov/hepatitis/statistics/2016surveillance/index.htm. Accessed 20 June 2019. [Google Scholar]
- 2. Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis C virus in the United States, 2003–2013. Clin Infect Dis 2016; 62:1287–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014; 9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lo Re V 3rd, Gowda C, Urick PN, et al. . Disparities in absolute denial of modern hepatitis C therapy by type of insurance. Clin Gastroenterol Hepatol 2016; 14:1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ottman AA, Townsend ML, Hashem MG, et al. . Incidence of drug interactions identified by clinical pharmacists in veterans initiating treatment for chronic hepatitis C infection. Ann Pharmacother 2018; 52:763–8. [DOI] [PubMed] [Google Scholar]
- 6. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on a National Strategy for the Elimination of Hepatitis B and C. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Strom BL, Buckley GJ, eds. Washington, DC: National Academies Press;2017. [PubMed] [Google Scholar]
- 7. Kattakuzhy S, Gross C, Emmanuel B, et al. ; and the ASCEND Providers Expansion of treatment for hepatitis C virus infection by task shifting to community-based nonspecialist providers: a nonrandomized clinical trial. Ann Intern Med 2017; 167:311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arora S, Kalishman S, Thornton K, et al. . Expanding access to hepatitis C virus treatment–Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology 2010; 52:1124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mera J, Vellozzi C, Hariri S, et al. . Identification and clinical management of persons with chronic hepatitis C virus infection - Cherokee Nation, 2012–2015. MMWR Morb Mortal Wkly Rep 2016; 65:461–6. [DOI] [PubMed] [Google Scholar]
- 10. Deming P, Martin MT, Chan J, et al. . Therapeutic advances in HCV genotype 1 infection: insights from the society of infectious diseases pharmacists. Pharmacotherapy 2016; 36:203–17. [DOI] [PubMed] [Google Scholar]
- 11. Sebhatu P, Martin MT. Genotype 1 hepatitis C virus and the pharmacist’s role in treatment. Am J Health Syst Pharm 2016; 73:764–74. [DOI] [PubMed] [Google Scholar]
- 12. Mohammad RA, Bulloch MN, Chan J, et al. . Provision of clinical pharmacist services for individuals with chronic hepatitis C viral infection: Joint Opinion of the GI/Liver/Nutrition and Infectious Diseases Practice and Research Networks of the American College of Clinical Pharmacy. Pharmacotherapy 2014; 34:1341–54. [DOI] [PubMed] [Google Scholar]
- 13. Mikolas LA, Jacques K, Huq M, et al. . Utilizing clinical pharmacist specialist to manage hepatitis C virus patients on direct-acting antiviral therapy. J Pharm Pract. 2018. doi:10.1177/0897190018777345 [DOI] [PubMed] [Google Scholar]
- 14. Yang S, Britt RB, Hashem MG, Brown JN. Outcomes of pharmacy-led hepatitis C direct-acting antiviral utilization management at a Veterans Affairs medical center. J Manag Care Spec Pharm 2017; 23:364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Belperio PS, Chartier M, Gonzalez RI, et al. . Hepatitis C care in the Department of Veterans Affairs: building a foundation for success. Infect Dis Clin North Am 2018; 32:281–92. [DOI] [PubMed] [Google Scholar]
- 16. Belperio PS, Chartier M, Ross DB, et al. . Curing hepatitis C virus infection: best practices from the U.S. Department of Veterans Affairs. Ann Intern Med 2017; 167:499–504. [DOI] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Thielke R, et al. . Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim JK, Liapakis AM, Shiffman ML, et al. ; HCV-TARGET Study Group Safety and effectiveness of ledipasvir and sofosbuvir, with or without ribavirin, in treatment-experienced patients with genotype 1 hepatitis C virus infection and cirrhosis. Clin Gastroenterol Hepatol 2018; 16:1811–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. AASLD/IDSA. HCV testing and linkage to care. Recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org/. Accessed 20 June 2019. [Google Scholar]
- 20. Liang TJ, Ward JW. Hepatitis C in injection-drug users - a hidden danger of the opioid epidemic. N Engl J Med 2018; 378:1169–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bajis S, Dore GJ, Hajarizadeh B, et al. . Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: a systematic review. Int J Drug Policy 2017; 47:34–46. [DOI] [PubMed] [Google Scholar]
- 22. Back D, Belperio P, Bondin M, et al. . Integrated efficacy and safety of glecaprevir/pibrentasvir in patients with psychiatric disorders. J Hepatol 2018; 68:s280–1. [Google Scholar]
- 23. Kattakuzhy S, Mathur P, Gross C, et al. . High SVR in PWID with HCV despite imperfect medication adherence: data from the Anchor Study. Hepatology. 2018; 68( S1). doi:10.1002/hep.30256. [Google Scholar]
- 24. Zuckerman A, Douglas A, Nwosu S, et al. . Increasing success and evolving barriers in the hepatitis C cascade of care during the direct acting antiviral era. PLoS One 2018; 13:e0199174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langness JA, Nguyen M, Wieland A, et al. . Optimizing hepatitis C virus treatment through pharmacist interventions: identification and management of drug-drug interactions. World J Gastroenterol 2017; 23:1618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dunn EE, Vranek K, Hynicka LM, et al. . Evaluating a collaborative approach to improve prior authorization efficiency in the treatment of hepatitis C virus. Qual Manag Health Care 2017; 26:136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wenzler E, Dickson W, Vibhakar S, et al. . Hepatitis C management and the infectious diseases pharmacist. Clin Infect Dis 2015; 61:1201–2. [DOI] [PubMed] [Google Scholar]
- 28. Smith JP. Treatment options for patients with hepatitis C: role of pharmacists in optimizing treatment response and managing adverse events. Pharmacotherapy 2008; 28:1151–61. [DOI] [PubMed] [Google Scholar]
- 29. Walters-Smith N, Marshall SM. Opportunities and considerations for pharmacist intervention in the management of the chronic hepatitis C patient. J Manag Care Pharm 2009; 15:417–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spooner LM. The expanding role of the pharmacist in the management of hepatitis C infection. J Manag Care Pharm 2011; 17:709–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olea A Jr, Grochowski J, Luetkemeyer AF, et al. . Role of a clinical pharmacist as part of a multidisciplinary care team in the treatment of HCV in patients living with HIV/HCV coinfection. Integr Pharm Res Pract 2018; 7:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Razavi H, Sanchez Y, Pangerl A, Cornberg M. Global timing of hepatitis C virus elimination: estimating the year countries will achieve the World Health Organization elimination targets. J Hepatol 2019; 70:e748. [Google Scholar]