Abstract
Pyrimethamine which is a main anti-Toxoplasma gondii drug has a serious side and toxic effects on the host. Accordingly, the development of new treatment options for toxoplasmosis with less toxic effects, low teratogenicity and parasiticidal effect against the various stage of T. gondii are dramatically crucial. Currently, natural molecules from scorpion and snake venoms are widely used as an alternative treatment against human disease, these compounds considered to be safe and to have low toxicity in comparison with synthetic drugs. Therefore, the goal of our study was to investigate the anti-Toxoplasma gondii activities of Hemiscorpius lepturus venom. We measured cytotoxicity of H. lepturus whole venom on Vero cells as well as effectiveness of this compound on viability of T. gondii applying colorimetric assay, according to mitochondrial oxidation of the MTT reagent (Methylthiazol tetrazolium 98%). The results of this study indicated that the H. lepturus whole venom has an anti-Toxoplasma effects with less toxic effect on Vero cells. Also, the T. gondii tachyzoites were treated with H. lepturus venom reached better results in comparison with Pyrimethamine-treated group. This research will serve as a base for future studies on toxoplasmosis and suggest a role for scorpion venom in promoting natural drugs.
Keywords: Toxoplasma gondii; Scorpion; Hemiscorpius lepturus venom; Anti-parasitic, in vitro
Introduction
Toxoplasma gondii is an intracellular protozoan parasite which belongs to the phylum Apicomplexa. This protozoan is considered as an unusual parasite due to be capable to infect all kinds of cells in all warm—blooded animals (Miller et al. 2009). This opportunistic parasite probably involves about one-third of the world’s population. It can be grown in virtually any warm-blooded cell line, it can be maintained indefinitely in mice and cell culture (Dubey 2010). It is a coccidian parasite with felids as the definitive host and any mammal as intermediate hosts which causes toxoplasmosis disease (Dubey 2009). This parasite has three stages of infection: tachyzoites, bradyzoites in tissue cysts and sporozoites in sporulated oocysts in its life cycle (Tenter et al. 2000). Human acquired infection through three principal routes included ingestion of tissue cysts in raw or undercooked meat, ingestion infectious oocysts from water or fruits and vegetables contaminated with cat feces and congenital infection (Dubey and Beattie 2010). The disease is one of the most prevalent parasitic infections in the human population and animals with worldwide distribution (Dubey 2008). Affliction of immunocompetent people is generally asymptomatic but may lead to acute systemic infection or ocular disease (Lass et al. 2009). In several conditions such as human immunodeficiency virus infection, treatment with chemotherapeutic agents commonly given to cancer patients, organ transplant recipients and infected pregnant women in primary during pregnancy the disease can lead to significant health problems and mortality (Di Cristina et al. 2008). Currently, treatment of toxoplasmosis is done, especially with sulfonamide drugs, notwithstanding the toxicity and side effects of the host including bone marrow suppression, elevation in serum liver enzymes, hematological abnormalities, hypersensitivity reactions, skin allergy and teratogenic effects in early in pregnancy. Treatment for this parasite has been common since 1950, and its effect in the acute phase of infection is limited (Kim et al. 2007; Schmidt et al. 2006). Moreover, other drugs such as azithromycin and spiramycin are poorly effective against tachyzoites and have no effect on the bradyzoite form (Araujo and Remington 1992). Furthermore, recent experimental studies have clearly shown drugs resistance in toxoplasmosis among acquired immunodeficiency syndrome patients (Aspinall et al. 2002). In the recent years, much progress has been made in vaccine research, but until now the researches could not access to a proper vaccine to prevent of human infection with this opportunistic parasite (Zhang et al. 2015). To prevent from penetrating of this parasite there is no vaccine available (Ahmadpour et al. 2017). Accordingly, the development of new treatment options for toxoplasmosis with less toxic effects, low teratogenicity and parasiticidal effect against the various stage of T. gondii are dramatically crucial (Montazeri et al. 2017). Nowadays alternative therapies such as natural toxins and their derivatives as well as substances derived from plants, animals and microorganisms are used to treat various parasitic diseases (Tempone et al. 2007; Bastos et al. 2008). Currently, natural molecules from scorpion and snake venoms are widely used as alternative treatment against human disease, these compounds considered to be safe and to have low toxicity in comparison with synthetic drugs, it is consequently important to use novel strategies for the development of new antibiotics from animal venoms of scorpion and snake for treating resistant pathogens (Perumal et al. 2017). Several small peptides from scorpion venom kill parasites, bacteria, fungi and viruses. Scorpion venoms contain interesting peptides, these Antimicrobial peptides manifested notable effect on some parasites such as Plasmodium, Entamoeba histolytica, Leishmania, Tryanosoma, Schistosoma and Taenia crassiceps, hence these peptides with vaste verity effect on parasites are proposed for the treatment of other parasites such as Toxoplasma gondii (Conde et al. 2000; Del Rio-Portilla et al. 2016; Borges et al. 2006).
Hemiscorpius lepturus species belongs to the Scorpionidae family and subfamily Hemiscorpiidae is the one of most medical important scorpion in Iran (Dehghani et al. 2018). Previous finding have shown that LD50 for this scorpion was 5.81 mg/kg in mice (Hassan 1984). Also Heidarpour et al. (2012) reported that LD50 of H. lepturus venom is 5 mg/kg by subcutaneous injection (SC) in white Balb/c mice. Until now 12% (11 sequences) (KX924499-KX924509 and KX874538) of H. lepturus venom component have been identified that were related to AMPs. The sequences of AMPs are unique and show similarity to other reported AMPs from venomous animals. Antimicrobial peptides are major section from scorpion venom, regarding potential activities of the AMPs they can be utilize as promising factors for therapeutic drugs (Kazemi-Lomedasht et al. 2017). So, the aim of this study was to evaluate the effects of Hemiscorpius lepturus venom on infection of Toxoplasma gondii in vero cell in vitro.
Materials and methods
Methods
Toxoplasma gondii tachyzoites
Tachyzoites from RH strain of T. gondii were attained primarily in the intraperitoneal serial passages of Balb/c mice from the Toxoplasmosis Research Center (TRC) in Mazandaran University of Medical Sciences, Sari, Iran. Intraperitoneal inoculation was performed with 1 × 106 of the tachyzoites in male Balb/c mice (6 week-old, 18–20 g weight) the mice were housed in cages under standard laboratory conditions such as 20–25 °C, 60 ± 10% humidity, light (12 h per day), given drinking water, and regular diet in the animal center of Shahid Chamran University of Ahvaz, Ahvaz, Iran. After 48–72 h, tachyzoites harvested from peritoneal exudates of infected mice, there after ascitic fluid was centrifuged at 200 g for 10 min at room temperature to remove cells and debris. The supernatant, which contained the tachyzoites, was collected and centrifuged at 1000 g for 10 min. The tachyzoites got diluted with sterile phosphate-buffered saline (PBS) PH: 7.4, containing 100 IU/ml of penicillin and 100 μg/mL of streptomycin. The number of tachyzoites was counted by hemacytometer under optical microscope. (Kalani et al. 2016; Martins-Duarte et al. 2006).
Venom preparation
The adult scorpion species of H. lepturus were collected from Khuzestan province, Iran and transported alive to Razi Vaccine and Serum Research Institute, Ahvaz, Khuzestan, Iran. Thereafter scorpions kept into the glass aquarium tanks until extracted venom, the venom was extracted by means of electrical stimulation (6-v) of the last post abdominal segment (telson). Venom was lyophilized by Freeze dryer at −75 °C. 10 mg of Crude venom was dissolved in 10 ml deionized water then centrifuged at 1300 g for 5 min at 4 °C to remove the unsolved material. The supernatant filtrated through a 0.45 μm filter and was stored at −20 °C until further study. The concentration of the purified venom and dilutions were determined using spectrophotometer respecting to the Bradford method (Bradford 1976).
LD50 determination
For LD50 determination, of the venom were injected intravenously (IV) to albino mice (average weight 18–20 g). Following treatment with venom solution, animals were monitored for 24 h, then, LD50 was calculated. LD50 was determined using the Spearman-Kaerber method (World Health Organization: WHO, 1981).
Cell culture
Vero cells, kidney fibroblast from African green monkey (ATCC No. CCL-81), were applied for in vitro assays. Cells were suspended in RPMI supplemented with 10% inactivated FBS, 100 units/mL of penicillin, and 100 μg/mL of streptomycin then maintained in cell culture flasks in a 37 °C incubator with 5% CO2.
Cellular viability in Vero cells
Cytotoxicity of H. lepturus total venom was measured on Vero cell using a colorimetric assay, according to mitochondrial oxidation of the MTT reagent (Methylthiazol tetrazolium 98%) sigma (5 mg/ml). Vero cells were cultured at concentrations of 1 × 105 cells/mL suspended in RPMI medium with 10% FBS in 96-well plates after that cells were exposed to serial dilutions of H. lepturus venom 100, 90, 70, 50, 20 μl (1 mg/mL). PBS was used as negative control. The incubated conditions were: 5% CO2, and 37 °C for 24 h. the next day, cellular viability in the presence of H. lepturus total venom was determined by MTT assay. Briefly, MTT solution was added to the 96-well plates and then incubated for 4 h at 37 °C in 5% CO2 atmosphere thereafter 200 μl of DMSO was added to acquire the formazan crystals, after 30 min the optical density was evaluated at 570 nm wavelength in a plate reader.
Tachyzoite viability
The RH strain of T. gondii viability assay was don based on Castanheira et al. (2015). tachyzoites (1 × 106/mL) were treated with diverse volumes (100, 90, 70, 50, 20 μl) of H. lepturus venom (1 mg/mL). After 0, 30 min and 2 h tachyzoites were dyed by trypan blue. The number of viable tachyzoites was counted using hemacytometer according to presented a clear cytoplasm.
Effects of H. lepturus venom on intracellular T. gondii in vitro
Vero cells were seeded in 96-well plates (1 × 105 cells/well). After all of the cells were reached to 85% confluency were infected with the RH strain of T. gondii tachyzoites (1 × 106). In order to eliminate extracellular parasites the RPMI medium was changed After 24 h thereafter exposed to the different volumes (100, 90, 70, 50, 20 μl) of H. lepturus venom (1 mg/mL) and incubated for 24 h at 37 °C in 5% CO2. Pyrimethamine and RPMI 1640 were considered as positive and negative control, respectively then T. gondii viabilities were measured by using a colorimetric MTT assay. The experimental protocol applied in this research was approved by the Shahid Chamran University of Ahvaz Ethics Committee (Permit number EE/97.24.3.49892/SCU.ac.ir).
Statistical analysis
The 50% inhibitory concentrations (IC50s) and 50% cytotoxic concentrations (CC50s) were performed by Graph Pad Prism 8.0 software. Differences between treatments and controls were analyzed by one-way ANOVA test using SPSS-16. The percent of viable cells and tachyzoites in relation to controls and also selectivity indices were calculated by the equations below:
Results
LD50 determination and protein concentrations
The LD50 amounts of H. lepturus venom were determined 6.3 mg/kg by IV injection in albino mice. The amount of protein were 1.26, 1.13, 0.88, 0.63 and 0.25 mg in 100, 90, 70, 50 and 20 μl previously.
Cellular viability
In order to achieve toxic concentrations, The Vero cells were exposed to the different concentration of H. lepturus venom (1 mg/mL), the results of MTT assay revealed that there are significant differences between various concentrations and pyrimethamine (P < 0.05). Diverse of venom concentrations, show slight changes according to control and have less cytotoxicity compared with drug (Fig. 1).
Fig. 1.
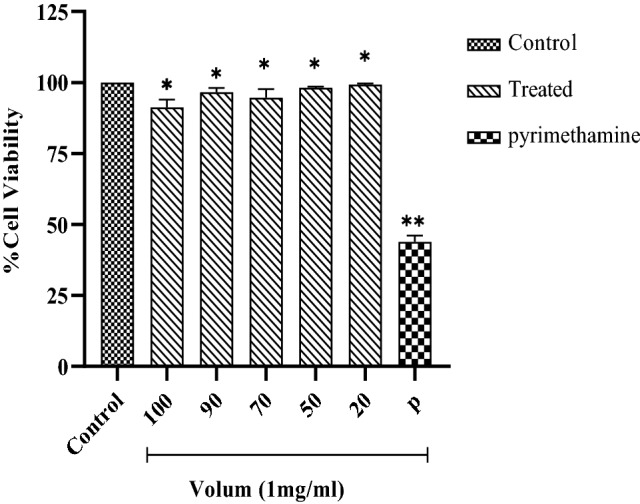
In vitro effects of H. lepturus venom on Vero cells
Tachyzoites viability
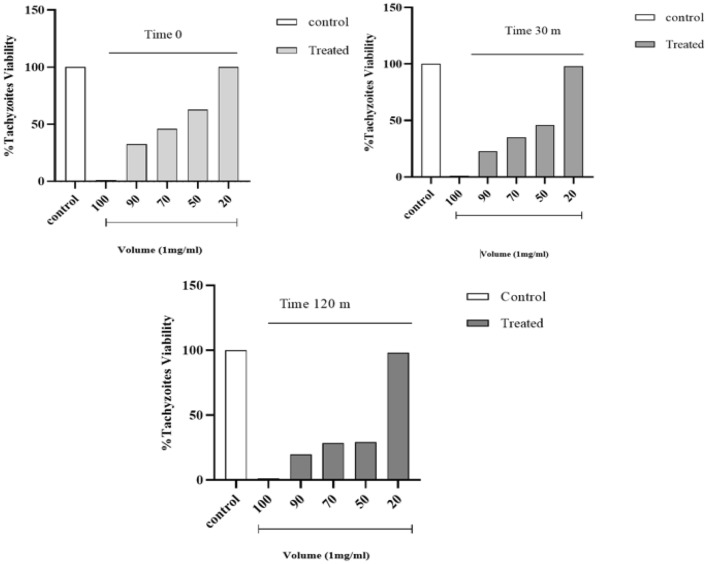
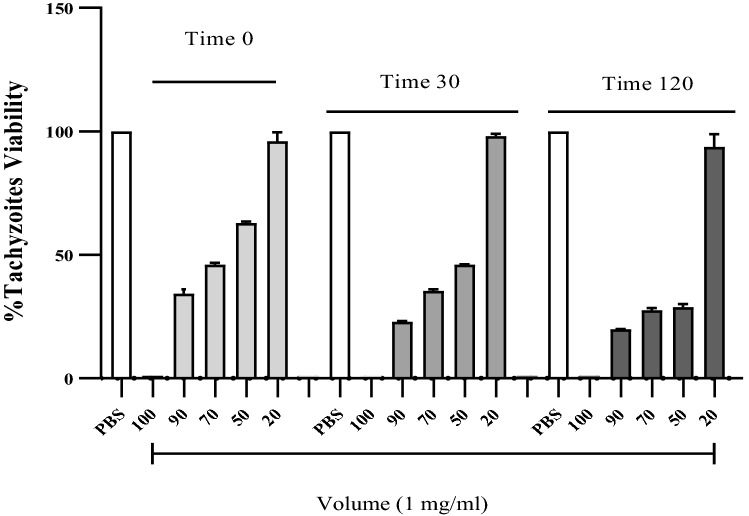
Tachyzoites were treated with H. lepturus venom, after different time tachyzoites were dyed by trypan blue. H. lepturus venom reduced tachyzoites viability at all the concentrations, meanwhile, there are no considerable differences between the treatment with 20 μl of venom and control (PBS). Figure 2. The results, as shown in Fig. 3, indicate that there is significant reduction in treated groups at the different time (P < 0.05).
Fig. 2.
Tachyzoites viability in different time
Fig. 3.
Effect of H. lepturus venom on T. gondii tachyzoites in different groups and time
Effects of H. lepturus venom on intracellular T. gondii in Vitro
The results obtained from anti-Toxoplasma effects of each concentrations from H. lepturus venom by using MTT assay are presented in Fig. 4. Statistical tests revealed significant differences are found between diverse concentrations, also, this result exhibited a significant difference between treated with H. lepturus venom and treated with Pyrimethamine (P < 0.05).
Fig. 4.
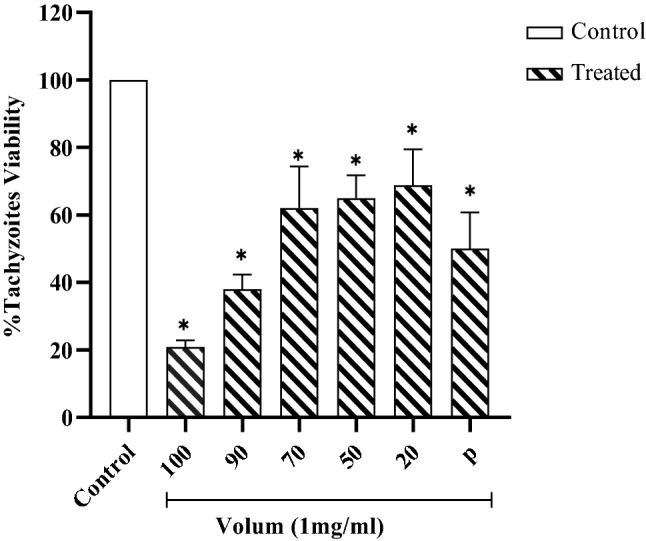
Effects of H. lepturus venom on intracellular T. gondii in Vitro
Table 1 presents the CC50, IC50 and SI in uninfected Vero cells and infected cells, in the current experiment, comparing H. lepturus venom with Pyrimethamine showed that H. lepturus venom has an anti-T. gondii activity with an IC50 of 39.06 and SI of 1.855.
Table 1.
In vitro inhibition of T. gondii by scorpion venom and evaluate of CC50, IC50 and SI in uninfected Vero cells and infected cells
| Venom/drug μg/mL | CC50 μg/mL | IC50 μg/mL | SI |
|---|---|---|---|
| H. lepturus venom | 72.46 | 39.06 | 1.855 |
| Pyrimethamine | 54.29 | 66.32 | 0.81 |
CC50, half cytotoxic concentration
IC50, half maximal inhibitory concentration
SI selectivity index
Discussion
The treatment of toxoplasmosis is done with the combination of pyrimethamine and sulfadiazine drugs. Pyrimethamine is dihydrofolate reductase enzyme inhibitors, which is considered as a key enzyme of the folate synthesis pathway also Sulfadiazine has an inhibitory effect on the tic drugs have an effect on toxoplasmosis, but the fact remains that the synthetic drugs have a serious side and toxic effects on host, when viewed from this perspective, these facts become much more significant. For instance Pyrimethamine which is a main anti-toxoplasma drug, can lead to suppressive effects of bone marrow and teratogenic effects in early in pregnancy (Mirzaalizadeh et al. 2018; Zaware et al. 2013) In immunocompromised patient, especially HIV positive people, cancer patient, and organ transplant recipients, toxoplasma is give rise to encephalitis and serious health problems (Di Cristina et al. 2008). What is more, it can lead to teratogenic in pregnant woman (Wiengcharoen et al. 2007). Thus, these days the achievement of optimal treatment with minimal side effect is a research priorities for Toxoplasma infections. To date, many studies have been done on the therapeutic potential of natural products (Aspinall et al. 2002), that have demonstrated natural venoms have a vast variety an antimicrobial activities against parasites, bacteria, viruses and fungi (Bahar and Ren 2013). Scorpion venoms possess of polypeptides and enzymes that specifically acts on ion channel and enzymatic activities, moreover these peptides have an antimicrobial function (Perumal et al. 2017). H. lepturus venom consist AMPs, in regard to antimicrobial activities, this venom is similar to other scorpions venom, thus what is important is applying these peptides to treat parasitic infections. In this research, we manifested H. lepturus venom anti toxoplasma potential by using MTT assay. Recently, researchers have shown an increased interest in treat parasitic disease by scorpion venom, by way of example two linear cationic antimalarial peptides reported from Mesobuthus eupeus scorpion. Interestingly, these peptides have no toxicity effect on mammalian cells (Du et al. 2010). Effect of Tityus discrepans venom indicated that has a significant reduce on Leishmania mexicana as it was lead to 50% reduction on growth of promastigotes (Borges et al. 2006). Moreover in vitro studies have shown that some scorpion venom have Anti-malarial and anti-Trypanosoma activity (Perumal et al. 2017). Del Rio-Portilla et al. (2016) examined the 2 peptides, HgeD and Hge36 from Hoffmannihadrurus gertschi on cysticerci of Taenia crassiceps and throphozoites of Entamoeba hystolytica. They perceived these parasites have been a considerable decrease while both peptides had a minimal effects on lymphocytes. In another major study, Xu et al. (2008) and El-Asmar et al. (1980) realized scorpion venom have a cytotoxity effect on Ancylostoma caninum and Shistosoma mansoni cercariae, respectively. Mesobuthus epeus venom against the protoscolices of Echinococcus granulosus was first demonstrated experimentally by (Jafari et al. 2018). They revealed M. epeus venom fraction killed all protoscolices during 30 min furthermore, molecular weights of this fraction were under 10 kDa. One of the issues that emerge from this finding is that all of the concentrations were lower than LD50. Taken together, these findings suggest a role for Hemiscorpius lepturus venom in anti-T. gondii activity.
Acknowledgements
The authors wish to thank vice-chancellor for research of the Shahid Chamran University of Ahvaz for a research grant to this project. The authors are also grateful to Professor Marie-Laure Dardé (Head of Biological Resource Center for Toxoplasma, Limoges University, France) and Professor Ahmad Daryani (Medical Parasitology Department of Medical Parasitology, School of Medicine) Toxoplasmosis Research Center (TRC), Mazandaran University of Medical Sciences, Sari, Iran for kindly supplying the reference strains for Type I (RH strain), Type II (PRU strain), and Type III (VEG strain), Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization (AREEO).
Author’s contribution
LKR: The student which undertook all of the project process as her Ph. D project. HH: Devise the plan and superintendence of process. MHRJ: Evaluation of the potency of venom. HJ: Preparation of scorpions and venom. HNV: Data analysis and venom purification. MRSA: Cell culture.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmadpour E, Sarvi S, Hashemi Soteh MB, Sharif M, Rahimi MT, Valadan R, Tehrani M, Khalilian A, Montazeri M, Daryani A. Evaluation of the immune response in BALB/c mice induced by a novel DNA vaccine expressing GRA 14 against Toxoplasmagondii. Parasite Immunol. 2017;39(4):12419. doi: 10.1111/pim.12419. [DOI] [PubMed] [Google Scholar]
- Araujo FG, Remington JS. Recent advances in the search for new drugs for treatment of toxoplasmosis. Int J Antimicrob Agents. 1992;1:153–164. doi: 10.1016/0924-8579(92)90002-9. [DOI] [PubMed] [Google Scholar]
- Aspinall TV, Joynson DH, Guy E, Hyde JE, Sims PF. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J Infect Dis. 2002;185(11):1637–1643. doi: 10.1086/340577. [DOI] [PubMed] [Google Scholar]
- Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals. 2013;6(12):1543–1575. doi: 10.3390/ph6121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos LM, Júnior RJO, Silva DAO, Mineo JR, Vieira CU, Teixeira DNS, Homsi-Brandeburgo MI, Rodrigues VM, Hamaguchi A. Toxoplasma gondii: effects of neuwiedase, a metalloproteinase from Bothrops neuwiedi snake venom, on the invasion and replication of human fibroblasts in vitro. Exp Parasitol. 2008;120(4):391–396. doi: 10.1016/j.exppara.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Borges A, Silva S, den Camp HJO, Velasco E, Alvarez M, Alfonzo MJ, Jorquera A, De Sousa L, Delgado O. In vitro leishmanicidal activity of Tityus discrepans scorpion venom. Parasitol Res. 2006;99(2):167–173. doi: 10.1007/s00436-006-0133-z. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castanheira LE, de Souza DLN, Silva RJ, Barbosa B, Mineo JR, Tudini KA, Rodrigues R, Ferro EV, Rodrigues VM. Insights into anti-parasitism induced by a C-type lectin from Bothrops pauloensis venom on Toxoplasma gondii. Int J Biol Macromol. 2015;74:568–574. doi: 10.1016/j.ijbiomac.2014.11.035. [DOI] [PubMed] [Google Scholar]
- Conde R, Zamudio FZ, Rodtiguez MH, Possani LD. Scorpine, an antimalarial and anti-bacterial agent purified from scorpion venom. Fed Eur Biochem. 2000;471:165–168. doi: 10.1016/S0014-5793(00)01384-3. [DOI] [PubMed] [Google Scholar]
- Dehghani R, Kamiabi F, Kassiri H, Hashemi A, Mohammadzadeh N, Gharagazloo F. Research article a study on litter size in several important medical scorpions species (Arachnida: Scorpionida) IR Iran J Entomol. 2018;15(3):155–160. doi: 10.3923/je.2018.155.160. [DOI] [Google Scholar]
- Del Rio-Portilla F, Flores-Solis D, Toledano Y, Rodríguez-Lima O, Cano-Sánchez P, Ernestina Ramírez-Cordero B, Landa A, de la Vega Rodríguez, Ricardo C. Solution structure and anti-parasitic activity of scorpine-like peptides from Hoffmannihadrurus gertschi. FEBS Lett. 2016;590(14):2286–2296. doi: 10.1002/1873-3468.12255. [DOI] [PubMed] [Google Scholar]
- Di Cristina M, Marocco D, Galizi R, Proietti C, Spaccapelo R, Crisanti A. Temporal and spatial distribution of Toxoplasma gondii differentiation into bradyzoites and tissue cyst formation in vivo. Infect Immun. 2008;76(8):3491–3501. doi: 10.1128/IAI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Gao B, Xu J, Carmen Rodriguez M, Lanz-Mendoza H, Hernández-Rivas R, Zhu S. Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus. Biochimie. 2010;92:350–359. doi: 10.1016/j.biochi.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Dubey JP. The history of Toxoplasma gondii—the first 100 years. J Eukaryot Microbiol. 2008;55:467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP. The evolution of the knowledge of cat and dog coccidia. Parasitol. 2009;136:1469–1475. doi: 10.1017/S003118200900585X. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasmosis of animals and humans. 2. Boca Raton: CRC Press; 2010. [Google Scholar]
- Dubey JP, Beattie CP. Toxoplasmosis of animals and man. 2. Boca Raton: CRC Press; 2010. [Google Scholar]
- El-Asmar MF, Swelam N, Abdel Aal TM, Ghoneim Kh, Hodhod SS. Factor(s) in the venom of scorpions toxic to Schistosoma mansoni (intestinal belharzia) cercariae. Toxicon. 1980;18(5–6):711–715. doi: 10.1016/0041-0101(80)90106-3. [DOI] [PubMed] [Google Scholar]
- Hassan F (1984) Production of scorpion antivenom. In: Tu AT (Ed), Handbook of toxins. Insects poisons, allergens and other invertebrate venoms, vol. 2. Marcel Dekker, New York 577–605
- Heidarpour M, Ennaifer E, Ahari H, Srairi-Abid N, Borchani L, Khalili G, Amini H, Anvar AA, Boubaker S, El-Ayeb M, Shahbazzadeh D. Histopathological changes induced by hemiscorpius lepturus scorpion venom in mice. Toxicon. 2012;59(3):373–378. doi: 10.1016/j.toxicon.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Jafari H, Nemati M, Haddad Molayan P, Khaleghi Rostamkolaie L, Hamidinejat H (2018) Scolicidal activity of Mesobuthus epeus venom against the protoscolices of Echinococcus granulosus. 10.22092/ARI.2018.121416.1213 [DOI] [PubMed]
- Kalani H, Daryani A, Sharif M, Ahmadpour E, Alizadeh A, Nasrolahei M, Sarvi S, Kalani F, Faridnia R. Comparison of eight cell-free media for maintenance of Toxoplasma gondii tachyzoites. Iran J Parasitol. 2016;11(1):104. [PMC free article] [PubMed] [Google Scholar]
- Kazemi-Lomedasht F, Khalaj V, Bagheri KP, Behdani M, Shahbazzadeh D. The first report on transcriptome analysis of the venom gland of Iranian scorpion, Hemiscorpius lepturus. Toxicon. 2017;125:123–130. doi: 10.1016/j.toxicon.2016.11.261. [DOI] [PubMed] [Google Scholar]
- Kim S, Fouts AE, Boothroyd JC. Toxoplasma gondii dysregulates IFN-γ- inducible gene expression in human fibroblasts: insights from a genome—wide transcriptional profiling. J Immuonol. 2007;178(8):5154–5165. doi: 10.4049/jimmunol.178.8.5154. [DOI] [PubMed] [Google Scholar]
- Lass A, Pietkiewicz H, Modzelewska E, Dumètre A, Szostakowska B, Myjak P. Detection of Toxoplasma gondii oocysts in environmental soil samples using molecular methods. Eur J Clin Microbiol Infect Dis. 2009;28(6):599–605. doi: 10.1007/s10096-008-0681-5. [DOI] [PubMed] [Google Scholar]
- Martins-Duarte ES, Urbina JA, de Souza W, Vommaro RC. Antiproliferative activities of two novel quinuclidine inhibitors against Toxoplasma gondii tachyzoites in vitro. J Antimicrob Chemother. 2006;58(1):59–65. doi: 10.1093/jac/dkl180. [DOI] [PubMed] [Google Scholar]
- Miller CM, Boulter NR, Smith NC. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol. 2009;39(1):23–39. doi: 10.1016/j.ijpara.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Mirzaalizadeh B, Sharif M, Daryani A, Ebrahimzadeh MA, Zargari M, Sarvi S, Mehrzadi S, Rahimi MT, Mirabediny Z, Golpour M, Montazeri M. Effects of aloe vera and eucalyptus methanolic extracts on experimental toxoplasmosis in vitro and in vivo. Exp Parasitol. 2018;192:6–11. doi: 10.1016/j.exppara.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Montazeri M, Sharif M, Sarvi S, Mehrzadi S, Ahmadpour E, Daryani A. A systematic review of in vitro and in vivo activities of anti-Toxoplasma drugs and compounds (2006 to 2016) Front Microbiol. 2017;8:25. doi: 10.3389/fmicb.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal SR, Stiles BG, Franco OL, Sethi G, Lim LHK. Animal venoms as antimicrobial agents. Biochem Pharmacol. 2017;134:127–138. doi: 10.1016/j.bcp.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Schmidt DR, Hogh B, Andersen O, Hansen SH, Dalhoff K, Petersen E. Treatment of infants with congenital toxoplasmosis: tolerability and plasma concentrations of sulfadiazine and pyrimethamine. Eur J Pediatr. 2006;165:19–25. doi: 10.1007/s00431-005-1665-4. [DOI] [PubMed] [Google Scholar]
- Tempone AG, Melhem MSC, Prado FO, Motoie G, Hiramoto RM, Antoniazzi MM, Haddad CFB, Jared C. Amphibian secretions for drug discovery studies: a search for new antiparasitic and antifungal compounds. Lett Drug Des Discov. 2007;4:67–73. doi: 10.2174/157018007778992856. [DOI] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12):1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiengcharoen J, O Hanley R, Armstrong T, Best W, Sukthana Y, Thompson RA. Novel drug compounds against Neospora caninum and Toxoplasma gondii in vitro. Southeast Asian J Trop Med Public Health. 2007;38:15. [Google Scholar]
- Xu ZM, Li ZS, Wen MX, Peng RY, Sun L, Wu XY, Zhou LX, Tao YP, Yang L. In vitro effect of medicinal scorpion on the larvae of Ancylostoma caninum. Chinese J Parasitol & Parasitic Dis. 2008;26(5):387–391. [PubMed] [Google Scholar]
- Zaware N, Sharma H, Yang J, Devambatla RKV, Queener SF, Anderson KS, Gangjee A. Discovery of potent and selective inhibitors of Toxoplasma gondii thymidylate synthase for opportunistic infections. ACS Med Chem Lett. 2013;4(12):1148–1151. doi: 10.1021/ml400208v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang NZ, Wang M, Xu Y, Petersen E, Zhu XQ. Recent advances in developing vaccines against Toxoplasma gondii: an update. Expert Rev Vaccines. 2015;14:1609–1621. doi: 10.1586/14760584.2015.1098539. [DOI] [PubMed] [Google Scholar]