Highlights
-
•
Capnocytophaga belong to the oral microbiota.
-
•
Capnocytophaga bacteremia is uncommon.
-
•
Capnocytophaga identification has evolved with the advent of MALDI TOF-MS.
-
•
Capnocytophaga treatment is uncomplicated, although further insights into drug resistance development has been reported and need to be considered in the treatment.
-
•
Capnocytophaga treatment may be complicated by drug resistance development.
Keywords: Capnocytophaga, febrile neutropenia, Microbiota, Hematopoietic stem cell transplantation
Abstract
Capnocytophaga species belong to the oral microbiota but are not a common cause of infection in febrile neutropenia. Nevertheless, neutropenia can cause bacteremia associated with mucositis, with lower rates of mortality. While empirical therapy with beta-lactams is generally effective, there is concern about the emergence of bacterial resistance. We present a case of a febrile neutropenic patient with mucositis presenting with C. sputigena bacteremia.
Introduction
The main source of bacteremia in febrile neutropenia (FN) is bacterial translocation from the normal intraluminal flora. To date, gram-negative bacilli such as Enterobacteriaceae are the most frequently causative microorganisms, while anaerobes and fastidious gram-negatives are less frequent [1]. Capnocytophaga spp. belongs to the microbiota of the healthy oral cavity as well as in patients receiving chemotherapy [2]. Infections due to Capnocytophaga are associated with mucositis, gingival bleeding or periodontal disease [3], but not regularly isolated in infections associated with neutropenia.
Capnocytophaga sputigena, belongs to the genus Capnocytophaga, family Flavobacteriaceae, is a gram-negative bacillus and a facultative anaerobic fermenter. There are 9 Capnocytophaga species described that inhabit exclusively in the oral cavity of mammals. The following species are present in human oral microbiota: C. gingivalis, C. granulosa, C. haemolytica, C. leadbetteri, C. ochracea, C. sputigena, C. genospecies AHN8471; while C. canimorsus and C. cynodegmi are present in the oral microbiota of dogs and cats [4]. All species have been reported as pathogens in humans.
Present in the healthy gingival sulcus and supra gingival dental plaque, overgrowth of Capnocytophaga spp. is associated with periodontal disease in healthy and diabetic adults [5]. Surveillance studies within pediatric population, report that Capnocytophaga spp. are present in the dental plaque of cancer patients undergoing chemotherapy, and in the microbiome of those who undergo hematopoietic stem cell transplantation (HSCT) [2].
We present the case of a patient with relapsed lymphoma/leukemia after HSCT, presenting with C. sputigena bacteremia in a patient with febrile neutropenia.
Case report
A 17-year-old male, with lymphoma/lymphoblastic leukemia, underwent 5 cycles of HyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) without complications. The patient received an haploidentical HSCT donated by his father, with a granulocytic graft at day +17. He developed no infections during this period of neutropenia. At day +57, he presented to the emergency room with pleuritic chest pain. The PET-CT showed a relapse of the index disease and salvage chemotherapy was then started with FLAG-IDA regimen (fludarabine, cytarabine, idarubicin and Granulocyte-colony stimulating factor). On the 9th day of neutropenia, the patient developed grade III mucositis (Fig. 1) and 48 h later presented with temperature up to 38.5 °C. At this point, treatment with piperacilin tazobactam was initiated. After about 20 h of incubation of the peripheral blood cultures, a gram-negative bacillus growth was observed. However, the initial identification with VITEK-2 was not obtained. Despite the rapid onset of antimicrobial treatment, the patient persisted with fever and clinical deterioration. Thus, after 48 h the antibiotic was changed to meropenem, resulting in clinical improvement and fever resolution. With the use of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI TOF-MS) the final identification was Capnocytophaga sputigena. Bacteremia resolved. Finally, a control PET-CT was performed, but progression of the lymphoma was observed during treatment, the patient received palliative management but died 3 months later.
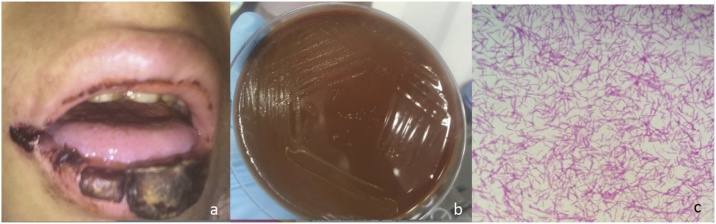
Fig. 1.
(a) Lips swelling and ulcerative oral mucositis with sloughing and necrosis on day +9 of neutropenia. (b) Colonial and microscopic findings of Capnocytophaga sputigena. Gray-colored non-hemolytic colonies on a blood agar plate after 48 h of incubation at 35 °C with 5% CO2. (c) Thin, spindle-shaped, gram-negative bacilli from smear preparations of the blood agar plate (Gram stain, ×100).
Discussion
Capnocytophaga species are found between 50 and 80% of patients with mucositis and periodontal disease [6]. However, the isolation of Capnocytophaga spp. in bacteremia related to FN in patients receiving chemotherapy is not frequent. The incidence of Capnocytophaga bacteremia varies between 1.3 and 3%, being an uncommon of all bacteremia in neutropenia [6].
To date, 124 cases of Capnocytophaga bacteremia in patients with neutropenia have been reported in the literature; however, the species was identified only in 43% of them. The most frequent species is C. ochracea, found in 72% of the cases, followed by C. sputigena in 22%, C. gingivalis in 3.7% and C. canimorsus in 1.8%. In 82% of the cases, these microorganisms were associated with severe mucositis, gingivitis, periodontitis or glossitis. There was a case of dog-transmitted C. canimorsus bacteremia after HSCT in a patient with functional asplenia and in other cases it was isolated in polymicrobial bacteremia. Conversely, in the remaining cases, the source is not described [6]. Similarly, this microorganism has been isolated in the bloodstream of patients with hematological malignancy with a rate of mortality of 7.8%. Including the present case, Capnocytophaga bacteremia occurs in 16% of patients that underwent HSCT (17 cases). The overall attributable mortality was 5.8%. In immunocompetent patients or in patients with underlying conditions other than hematological, Capnocytophaga species are also associated with infections such as chorioamnionitis, neonatal sepsis, empyema, pneumonia, pulmonary abscess, endocarditis, iliopsoas abscess, osteomyelitis, spondylitis, endophthalmitis and central nervous system infections.
The traditional identification of Capnocytophaga species was based on morphological and physiological characteristics, and biochemical tests. However, with the advent of molecular techniques and the availability of MALDI TOF-MS, the identification of the species is faster compared to VITEK-2, as demonstrated in our case. Susceptibility studies described that penicillin, clindamycin, cephalosporins, quinolones, linezolid, tetracyclines, imipenem, and their combinations with beta-lactamase inhibitors, have shown activity against this microorganism. On the contrary, most of the strains have shown resistance to polymyxin, fosfomycin and trimethoprim [7]. Although beta-lactams have traditionally been used as part of the empirical therapy for FN, strains of the oral microbiota with beta-lactamase production are increasing. Likewise, strains of Capnocytophaga spp. and C. gingivalis resistant to quinolones are found in up to 50% of cases probably related with the use of quinolones as prophylactics antibiotics in HSCT.
In bacteremia associated with FN, strains of C. ochracea with beta-lactamases and other multi-resistant strains have been detected. However, the presence of enzymes has not been genetically characterized. In clinical isolates, beta-lactamases have been found in 26% of the cases. Of these, extended-spectrum beta-lactamases such as TEM-17 encoded by plasmids have been characterized. Another extended-spectrum beta-lactamase reported from a clinical isolate in the strain C. sputigena is CSP-1. Similarly, chromosomal beta-lactamases of the class A, group 2E in the Ambler's classification such as CfxA3 are found in C. ochracea and C. sputigena [8]. CfxA2 beta-lactamases, located in mobile elements, facilitate horizontal transfer and dissemination, and confer resistance to cephalosporins, including third generation cephalosporins. Both CfxA3 and CfxA2 are responsible for the 80% of resistance to beta-lactams of the Capnocytophaga spp.
Several attempts to characterize resistance to fluoroquinolones have been carried out. Mutations of the DNA gyrase A, the main molecular target in Capnocytophaga, causes an increase in the minimum inhibitory concentrations (MIC) of ciprofloxacin to >1 mg/L. However, mutations in parC and parE do not alter this susceptibility, suggesting the presence of other mechanisms leading to antimicrobial resistance, such as membrane impermeability, efflux pumps, molecular target protection and enzymatic modification [9].
Given the potential emergence of antimicrobial resistance, in patients that do not respond to empirical therapy, a shift to carbapenem should be considered, until a susceptibility test is performed. In our case, we were not able to obtain a susceptibility tests do to failure to grow this microorganism in conventional culture media. Nevertheless, as the patient presented with clinical deterioration that only responded to meropenem, we suspect that the isolated strain could exhibit some extended-spectrum beta-lactamases.
In summary, Capnocytophaga spp. bacteremia is uncommon in FN, but it might be present in patients with mucositis. Capnocytophaga identification has evolved with the advent of MALDI TOF-MS. Treatment is uncomplicated, although further insights into drug resistance development has been reported and need to be considered in the treatment.
Author contributions
All authors participated in data collection, analysis and drafted the manuscript. All authors read and approved the final manuscript
Funding
This research did not receive specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Ethical approval
This is a clinical case and no data that allow patient identification are included, so ethical approval is not necessary.
Conflict of interest
The author declares having no conflict of interests.
References
- 1.Gudiol C., Bodro M., Simonetti A. Changing etiology, clinical features, antimicrobial resistance, and outcomes of bloodstream infection in neutropenic cancer patients. Clin Microbiol Infect. 2012;3:1–5. doi: 10.1111/j.1469-0691.2012.03879.x. [DOI] [PubMed] [Google Scholar]
- 2.Sixou J.L., Aubry-Leuliette a., De Medeiros-Battista O. Capnocytophaga in the dental plaque of immunocompromised children with cancer. Int J Paediatr Dent. 2006;16(2):75–80. doi: 10.1111/j.1365-263X.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- 3.Bilgrami S., Bergstrom S.K., Peterson D.E. Capnocytophaga bacteremia in a patient with Hodgkin’s disease following bone marrow transplantation: case report and review. Clin Infect Dis. 1992;14(5):1045–1049. doi: 10.1093/clinids/14.5.1045. [DOI] [PubMed] [Google Scholar]
- 4.Frandsen E.V.G., Poulsen K., Könönen E. Diversity of Capnocytophaga species in children and description of Capnocytophaga leadbetterisp. nov. and Capnocytophaga genospecies AHN8471. Int J Syst Evol Microbiol. 2008;58(2):324–336. doi: 10.1099/ijs.0.65373-0. [DOI] [PubMed] [Google Scholar]
- 5.Socransky S.S., Holt S.C., Leadbetter E.R. Capnocytophaga: new genus of Gram-negative gliding bacteria. III. Physiological characterization. Arch Microbiol. 1979;122(1):29–33. doi: 10.1007/BF00408042. [DOI] [PubMed] [Google Scholar]
- 6.Martino R., Ramila E., Capdevila J. Bacteremia caused by Capnocytophaga species in patients with neutropenia and cancer: results of a multicenter study. Clin Infect Dis. 2001;33(4) doi: 10.1086/322649. E20–2. [DOI] [PubMed] [Google Scholar]
- 7.Ehrmann E., Jolivet-Gougeon A., Bonnaure-Mallet M. Role of DNA gyrase and topoisomerase IV mutations in fluoroquinolone resistance of Capnocytophaga spp. clinical isolates and laboratory mutants. J Antimicrob Chemother. 2017;(Apr 26) doi: 10.1093/jac/dkx119. doi:10.93/jac/dkx119 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Jolivet-Gougeon A., Tamanai-shacoori Z., Desbordes L. Genetic analysis of an ambler class a extended-spectrum beta-lactamase from Capnocytophaga ochracea genetic analysis of an ambler class a extended-spectrum beta-lactamase from Capnocytophaga ochracea. J Clin Microbiol. 2004;42(2):2–5. doi: 10.1128/JCM.42.2.888-890.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roscoe D.L., Zemcov S.J.V., Thornber D. Antimicrobial susceptibilities and ß-lactamase characterization of Capnocytophaga species. Antimicrob Agent Chemother. 1992;36(10):2197–2200. doi: 10.1128/aac.36.10.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]