Summary
In the European Union/European Economic Area (EU/EEA) approximately 9 million people are chronically infected with hepatitis B virus (HBV) or hepatitis C virus (HCV), and many are undiagnosed. Targeted active case finding initiatives are needed. Iatrogenic transmission of HBV/HCV is relevant in Europe but people at risk of infection are often overlooked. This study aimed to identify groups at increased risk of HBV/HCV infection due to iatrogenic transmission, including healthcare workers, and to estimate incidence and prevalence. PubMed and Embase were systematically searched in February 2017 using strings combining terms for HBV/HCV, occurrence and population subgroups. All retrieved publications were screened and included articles were quality assessed. A predefined set of variables were extracted, and detailed summary tables were developed per population group of interest, virus and outcome. Thirty-eight articles were included, two reported on HBV, 22 on HCV and 16 on both, contributing 70 estimates of prevalence or incidence among: haemodialysis recipients, diabetes patients, recipients of substances of human origin, recipients of medical/dental procedures and healthcare workers. Estimates varied widely from 0.4% to 11.7% for HBV and from 0.7% to over 90% for HCV with most being higher than in the general population. Despite the limited number of studies retrieved, mostly old and focused on populations with multiple risk factors, our findings highlight the importance of considering population groups at higher risk for HBV/HCV iatrogenic transmission as target groups for active case finding in the EU/EEA. Test offers should be guided by individual risk assessment alongside local epidemiological data and local context.
Keywords: Hepatitis B, Hepatitis C, Healthcare workers, Iatrogenic transmission, European Union, European Economic area
Introduction
In the European Union/European Economic Area (EU/EEA) approximately 4.7 million people have a chronic hepatitis B virus (HBV) infection and 3.9 million are chronically infected with hepatitis C virus (HCV), according to recent prevalence estimates [1]. Due to the silent nature of these diseases, chronic infections may go unnoticed for years, if not decades. As a result, a considerable proportion of people who are chronically infected may not be aware of their health status and may not be linked to appropriate care. Estimates of the undiagnosed fraction for chronic hepatitis B (CHB) and chronic hepatitis C (CHC) infections are available for some EU/EEA countries ranging from 20% to 78% for HCV and from 45% to 55% for HBV [2]. Recent modelling across Europe suggests that the overall undiagnosed fraction was greater than 60% in the EU/EEA for HCV, and 16% in the wider European region for HBV [3], [4]. In order to achieve the goals for elimination set by the European Regional Action Plan [5], scaling up of targeted testing initiatives is needed.
The major population groups in the EU/EEA at increased risk for HBV and HCV transmission or at high prevalence of chronic infection include people who inject drugs (PWID), migrants, people in prison, people living with HIV (PLHIV) and men who have sex with men (MSM) [6], [7], [8]. However, identifying and targeting additional population groups with active case finding initiatives may be needed depending upon the local epidemiological situation. Individuals at risk of iatrogenic transmission (i.e. resulting from the activity of a healthcare provider or institution) of HBV and HCV in the past are one such group. In particular, individuals who have been recipients of substances of human origin (SOHO) before the introduction of adequate safety measures, such as screening for HBV and HCV, are of major concern in many EU/EEA countries [9], [10], [11]. Despite the considerable improvements in blood supply safety, injection safety and infection control in healthcare settings, iatrogenic HBV and HCV transmission is still an issue in some EU/EEA countries with evidence of current transmission as reported to the European Hepatitis B and C surveillance system [12], [13]. In addition, iatrogenic transmission is frequently implicated in outbreaks reported in the medical literature [11], [14], [15].
The scope of this study was to identify specific population groups at increased risk or with a high burden of hepatitis B and C due to iatrogenic transmission, including healthcare workers (HCWs). With this aim, a systematic review was conducted to retrieve data on the prevalence or incidence of HBV and HCV infections among these population subgroups in EU/EEA countries. This study was conducted as part of a larger project to develop a European testing guidance for HBV and HCV coordinated by the European Centre for Disease Prevention and Control (ECDC).
Methods
Existing national and supranational HBV and HCV testing guidance from EU/EEA countries was reviewed to identify population groups considered to be at risk for HBV and HCV transmission or at high burden of infection and it was complemented with inputs from the authors [2]. The following groups at risk of iatrogenic transmission were included: haemodialysis recipients; diabetes patients; recipients of SOHO, including organs; blood and plasma; and people who have received medical or dental interventions. In consideration of their risk from occupational exposures, HCWs were also included in the target population groups.
To address the following research question: ‘Which population subgroups have a higher risk of acquiring HBV or HCV, and/or have a higher burden of disease?’, a search of PubMed and Embase was conducted on the 14 February 2017 for records published since 1 January 2005 using search strings combining terms for HBV and HCV with terms for occurrence (e.g. incidence or prevalence) and population subgroups. A detailed description of the strategy used and strings can be found in Appendix A: Supplementary Data. No language limitations were applied. A geographical search string was added to limit the searches to studies from EU/EEA countries. For the search in Embase, publication type was limited to reviews, articles and articles in press. Original research articles were included in the review, and the reference lists of relevant systematic reviews retrieved in the literature search were checked manually for additional original articles. The search results were transferred to an EndNote library and duplicate records were removed using the built-in EndNote option, followed by a manual round.
All publications retrieved during the search were screened by title and abstract using a set of pre-determined inclusion/exclusion criteria. Publications were included if they reported on HBV/HCV incidence or prevalence among people at risk of iatrogenic infection in EU/EEA countries and HCWs, irrespective of the year of data collection. Publications reporting single-site data or outbreak reports were excluded (refer to Appendix A: Supplementary Data for the full list). Two reviewers performed the selection. An iterative approach to refine the inclusion and exclusion criteria was adopted and a random sample of 5% of the records was screened in duplicate, followed by an assessment of consistency and a review of the criteria. After reaching a level of concordance higher than 95%, the rest of the retrieved publications were split between the two reviewers. The full text of selected articles was subsequently screened by two reviewers, of which a random sample of 20% were screened in duplicate to assess for consistency. When more than 95% concordance was achieved, the remaining publications were split between the two reviewers. For both screening steps, in cases of uncertainty about inclusion or exclusion that were not resolved after discussion, articles were included. Peer-reviewed literature identified through other sources (e.g. hand search or suggested by the project team) were subject to the same inclusion and exclusion criteria.
Relevant data were extracted from included articles and recorded in the data extraction file in Microsoft Excel. As articles may report more than one prevalence estimate, the unit for data extraction was ‘study’. One study reports HBV or HCV prevalence in a defined population group, in a certain geographic area, over a discrete period of time. A predefined set of variables including study characteristics, sampling, laboratory testing, study population details and outcomes were identified, and data were extracted for each study (Appendix A: Supplementary Data). The complete list of variables is provided in the Appendix A: Supplementary Data.
The quality of all included articles was assessed. As none of the included articles concerned studies with designs that can be critically appraised using standard checklists such as those available from SIGN [16] a list was compiled of relevant aspects from standard checklists, which were answered with ‘yes’ or ‘no’ per study. A complete list of questions is available in Appendix A: Supplementary Data. Using the checklist, it was not possible to calculate an overall quality score for studies, therefore, all relevant articles were included regardless of their quality and their limitations (checklist items answered with ‘no’), if any, in data extraction tables. However, articles were excluded when the methods and/or results provided an insufficient level of detail making it not possible to accurately extract data.
A set of detailed summary tables was developed per population group of interest, virus and outcome (Appendix A: Supplementary Data). The summary tables contain the following information: study reference, country, study period, sampling approach, study design, population subgroup, study population and sample size, results (prevalence/incidence), critical appraisal and comments. Within each table, findings are ordered by country, year of publication and multiple risk category. For HBV, prevalence is defined as HBsAg positivity. For HCV, prevalence is defined as anti-HCV antibody positivity. Positivity to HCV-RNA is also presented in summary tables, when available. The prevalence estimates obtained are presented in synthetic tables in the results section, alongside prevalence estimates in the general population [1] for each country for which data could be retrieved.
Results
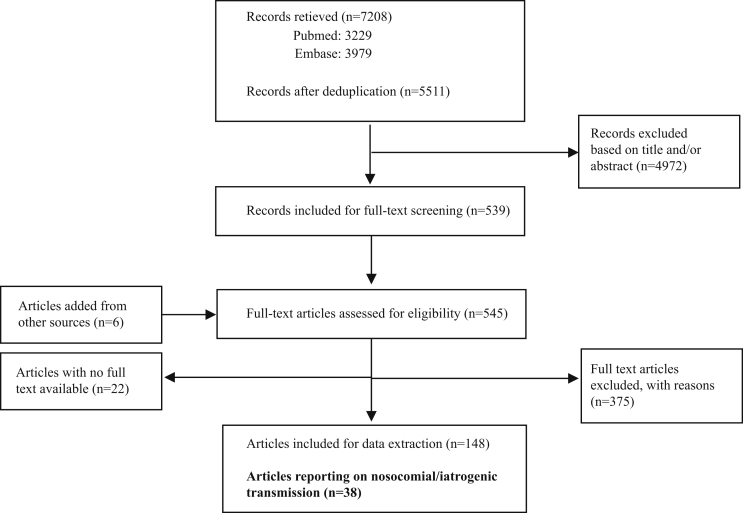
The main literature search identified 5511 unique publications, of which 539 were selected based on title and abstract. Six additional articles were found in reference lists of systematic reviews or were known to authors. The full text selection of 523 articles (22 were not available in full text) yielded 38 articles eligible for inclusion and reporting on prevalence or incidence of HBV and HCV among the population groups at risk of iatrogenic transmission (Figure 1). Of these, four reported on HBV, 23 on HCV and 11 on both, contributing a total of 70 studies reporting estimates of prevalence or incidence. The identified subgroups at risk included haemodialysis recipients (seven studies for HBV, 21 for HCV), HCW (four studies for HBV, 11 for HCV), diabetes mellitus patients (three studies for HBV, eight for HCV), recipients of SOHO (four studies for HBV, eight for HCV) and recipients of (unspecified) medical or dental procedures (four studies for HCV). There were estimates of prevalence identified from seven countries for HBV and 12 countries for HCV. More detailed information on each included study can be found in the summary tables in Appendix A: Supplementary Data. Data on HBV and HCV prevalence (17 HBV prevalence estimates and 44 HCV prevalence estimates) are presented in Table I, Table II, respectively.
Figure 1.
PRISMA flow diagram.
Table I.
Hepatitis B virus prevalence in population groups at risk of iatrogenic transmission by European Union/European Economic Area (EU/EEA) country
| Haemodialysis recipients |
Recipients of SOHO |
Diabetes patients |
Healthcare workers |
General population |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Prevalence (%) | Ref. | N | Prevalence (%) | Ref. | N | Prevalence (%) | Ref. | N | Prevalence (%) | Ref. | Prevalence (%)* | |
| France | 1b | 5.9 | [23] | 1 | 0.7 | [27] | 0.7–0.8 | ||||||
| Greece | 1 | 5.5 | [32] | 1c | 2 | [22] | 2.9–3.3 | ||||||
| Italy | 1 | 1.9 | [43] | 1d | 4 | [21] | 1 | 1.6 | [25] | 0.7–0.8 | |||
| Lithuania | 1 | 11.7 | [17] | 0.6 | |||||||||
| Poland | 1 | 9.8 | [24] | 3e | 0.6–1.2 | [28], [29], [30] | 0.5 | ||||||
| Romania | 2 | 7.9–9.5 | [18], [19] | 1 | 2.2 | [18] | 4.4 | ||||||
| Spain | 1a | 7.8 | [20] | 1 | 0.4 | [26] | 0.1–0.8 | ||||||
| EU/EEA wide | 6 | 1.9–11.7 | 4 | 2–9.8 | 3 | 0.4–1.6 | 4 | 0.6–2.2 | 0.9 | ||||
* Prevalence estimates were derived from a previously published systematic review (Hofstraat et al. Epidemiol Infect 2017; 14:2873–85).
N, number of included studies.
HIV+ haemodialysis recipients.
HIV+ haemophiliacs.
Cardiac surgery patients who received blood units.
Patients with inherited bleeding disorders treated before 1986.
Includes one study in which sample includes administrative workers (prevalence 0.6%).
Table II.
Hepatitis C virus prevalence in population groups at risk of iatrogenic transmission by European Union/European Economic Area (EU/EEA) country
| Haemodialysis recipients |
Recipients of medical/dental procedures |
Recipients of SOHO |
Diabetes patients |
Healthcare workers |
General population |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Prevalence (%) | Ref. | N | Prevalence (%) | Ref. | N | Prevalence (%) | Ref. | N | Prevalence (%) | Ref. | N | Prevalence (%) | Ref. | Prevalence* (%) | |
| France | 3 | 7.7–16.3 | [31], [44], [45] | 1a | 1.4–3.2 | [27] | 2d | 8.2–47.1 | [23], [27] | 3g | 2.1–8.2 | [27] | 0.8 | |||
| Germany | 2 | 3.6–5.2 | [46], [47] | 1b | 11.3 | [48] | 0.4 | |||||||||
| Greece | 2 | 24–29 | [32], [33] | 2e | 2–54.2 | [22], [49] | 2.2 | |||||||||
| Italy | 2 | 15.1–18.8 | [34], [35] | 1c | 8.9 | [50] | 4f | 35.5–95.4 | [21], [51] | 1 | 5.9 | [25] | 2h | 3–6.4 | [39], [40] | 0.4–5.9 |
| Netherlands | 1i | 1.4 | [52] | 0.1 | ||||||||||||
| Poland | 7j | 0.8–1.7 | [28], [29], [30], [38], [53], [54] | 0.7 | ||||||||||||
| Lithuania | 1 | 12.5 | [17] | 1.5 | ||||||||||||
| Romania | 2 | 27.3–39.3 | [18], [19] | 2 | 4.5–7.7 | [55] | 1 | 1.07 | [18] | 3.2 | ||||||
| Spain | 3k | 5.7–53.1 | [20], [56] | 1 | 2.4 | [26] | 0.2–1.1 | |||||||||
| Sweden | 1 | 0.7 | [37] | 0.06 | ||||||||||||
| UK | 1 | 3.3 | [42] | 0.04 | ||||||||||||
| Multi-countryl | 1 | 6.7 | [36] | |||||||||||||
| EU/EEA wide | 17 | 3.3–39.3 | 3 | 8.9–11.3 | 8 | 2–95.4 | 8 | 0.7–9.2 | 11 | 0.8–6.4 | 1.1 | |||||
*Prevalence estimates were derived from a previously published systematic review (Hofstraat et al. Epidemiol Infect 2017; 14:2873–85).
N, number of included studies.
Diabetes patients who had surgery, endoscopy or other invasive procedures.
Persons who received cardiac surgery as infants before 1991.
Family members of hepatitis C virus positive (HCV+) persons who have had dental procedures.
Includes one study in diabetes patients who had blood transfusions (prevalence: 8.2%) and one study in HIV-positive recipients of blood transfusions (prevalence: 47.1%).
Includes one study in cardiac surgery patients who received blood units (prevalence: 2%).
Includes one study in patients with inherited bleeding disorders treated before 1986 (prevalence: 95.4%).
Includes one study in diabetes patients who had blood transfusions (prevalence: 8.2%) and one study in diabetes patients who had medical procedures.
Includes one study on healthcare workers (HCWs) with high risk of exposure (prevalence: 3%).
Exposure prone procedures-performing HCW.
Includes one study in which the sample includes administrative personnel (prevalence: 1.7%).
Includes one study in which the sample was composed of HIV-positive individuals.
The study covered the following countries: Belgium, Italy, France, Germany, Spain, Sweden and UK.
Prevalence of HBV ranged from 1.9% in one Italian study to 11.7% in one study from Lithuania among haemodialysis recipients, from 2% in one study in Greece to 9.8% in a study from Poland among recipients of SOHO, from 0.4% in a Spanish study to 1.6% in one study from Italy among diabetes patients and from 0.6% in a Polish study to 2.2% in one study from Romania among HCWs.
The six studies on haemodialysis patients were conducted in the period 2001–2011 and present data from five EU/EEA countries, with the highest prevalence rates reported in studies conducted in Lithuania and Romania [17], [18], [19]. One study from Spain [20] reports relatively high prevalence of HBV (7.8%) among a sample of haemodialysis patients living with HIV. All four studies reporting on SOHO recipients were performed in the early 2000s, some sampled patients hospitalized before 1991 [21], [22] and some sampled HIV- or HCV-infected individuals [23], [24]. Of the three studies on diabetes patients, two were conducted in the second half of the 2000s among outpatients [25], [26], and the third was an older study conducted among hospitalized patients only [27]. Finally, of the studies on HCW, three were conducted in Poland in the years 2008–2009, yielding similar estimates [28], [29], [30], while the last one [18] reports higher prevalence (2.1%) among HCWs with occupational exposure in Romania.
Prevalence of HCV ranged from 3.3% in one UK study to 39.3% in one study from Romania among haemodialysis recipients, from 8.9% in one Italian study to 11.3% in one study from Germany among recipients of medical or dental procedures, from 2% in one Italian study to 95.4% in another study from Italy among recipients of SOHO, from 0.7% in one study from Sweden to 9.2% in one study from France among diabetes patients and from 0.8% in one study from Poland to 6.4% in one Italian study among HCWs. Of the 17 studies on HCV prevalence among haemodialysis patients, those reporting estimates in the upper range were either conducted in the late 1990s and early 2000s (12.5–29%) [17], [31], [32], [33], [34], [35], or reported data from Romania (27.3–39.3%) [18], [19]. The highest reported estimate (53.1%) is derived from a study conducted among HIV-infected patients in Spain [20]. Finally, a prospective, multicentre, cohort study provides an overall estimate of 6.7% HCV prevalence among haemodialysis patients from across seven EU/EEA countries, namely Belgium, Italy, France, Germany, Spain, Sweden and UK [36].
HCV prevalence among SOHO recipients was only reported among patients sampled in the early 2000s or before, most of whom presented with overlapping risks such as being transfused before 1991, hospitalized or presenting with comorbidities. All the included studies registered very high estimates. Conversely, the included studies on diabetic patients were conducted over more than a decade (1996–2011) sampling both inpatients and outpatients, and yielded comparable results ranging from 2.1 to 9.2%, with the notable exception of one study from Sweden reporting a prevalence as low as 0.8% in 1998 [37]. All three studies on recipients of medical or dental procedures sampled populations groups with multiple risk factors such as being a household member of an HCV-positive patient, and reported high prevalence rates. HCV prevalence estimates among HCW was reported in 11 studies. Of these, seven were conducted in Poland in the period 2005–2010, presenting comparable results (0.8–1.7%), with one large nationwide study reporting an anti-HCV antibody prevalence of 1.4% [38]. In contrast, two Italian single-centre studies conducted between 1999 and 2009 reported higher prevalence rates up to 6.4% [39], [40].
Only one study from the UK reported on HBV incidence in these at-risk populations. According to this prospective study that was conducted over a period of six months, HBV incidence was reportedly 0% in haemodialysis recipients [41].
Data on HCV incidence (nine estimates) are presented in Table III. Incidence of HCV ranged from 0 to 6.2 cases per 100 person-years among haemodialysis recipients, and was 0 cases per 100 person-years in two studies in Italy among medical or dental procedure recipients and HCWs. With the exception of one study from the UK [41], the studies among haemodialysis patients were either conducted between 1993 and 2005, with the highest value (6.2%) registered in a Greek study sampling patients in the period 1993–1995 [33], or among patients returning to the country of residence in Europe after a period of dialysis in countries of intermediate/high endemicity [42].
Table III.
Hepatitis C virus (HCV) incidence in population groups at risk of nosocomial and iatrogenic transmission by European Union/European Economic Area (EU/EEA country) (cases per 100 person-years)
| Haemodialysis recipients |
Recipients of medical/dental procedures |
Healthcare workers |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Incidence | Ref. | N | Incidence | Ref. | N | Incidence | Ref. | |
| France | 2 | 0–0.4 | [31], [44] | ||||||
| Germany | 1 | 0 | [47] | ||||||
| Greece | 1 | 6.2 | [33] | ||||||
| Italy | 1 | 2.5 | [34] | 1a | 0 | [57] | 1 | 0 | [40] |
| UK | 2 | 0–2.6 | [41], [42] | ||||||
| EU/EEA | 7 | 2.4–6.2 | 1 | 0 | 1 | 0 | |||
N, number of included studies.
Endoscopy patients.
Discussion
A systematic review was performed to assess the extent of the burden of HBV and HCV infection among population subgroups at risk of iatrogenic transmission in the EU/EEA. This is the first attempt to describe viral hepatitis epidemiology among these population groups at European level. This study is part of a larger endeavour to assess the risk for HBV or HCV transmission and of HBV or HCV infection in selected population groups in the EU/EEA.
The epidemiology of hepatitis B and C across Europe varies markedly both within and between countries, and over time [1], [58], [59], [60]. The two epidemics have evolved and epidemiological parameters including prevalence, incidence and genotype distribution have dramatically changed over the last 15 years. Several key factors have contributed towards these changes including the introduction of routine childhood and adolescent vaccination against HBV, significant changes in patterns of intravenous drug use and immigration of people from intermediate- and high-endemicity countries as well as improvements in blood transfusion safety and advancement in healthcare standards including infection control [8], [60]. Blood safety has rapidly progressed for HCV following the identification of the virus in 1989 and the production of screening tests to protect the blood supply introduced in most European countries during the early 1990s [61]. These blood tests are highly sensitive and post transfusion hepatitis C very seldom occurs as, in accordance with EU standards, EU/EEA countries have robust systems in place for the quality control of blood safety [62]. However iatrogenic transmission is still identified as a source of newly diagnosed HBV and HCV infections in the EU/EEA [12], [13].
In the face of the limited coverage and challenges of acute HCV surveillance in the EU/EEA, in 2016 iatrogenic transmission accounted for 22.5% of all notifications for which data on the report of transmission were available [12]. Many acute and chronic HCV cases attributed to iatrogenic transmission are reported by Italy and Romania [12]. This is reflected in the findings from this study, whereby prevalence among patients or recipients of medical procedures from these two countries are generally higher as compared to others. Our findings indicate a high prevalence of HCV infection in all the identified population groups with history of iatrogenic risk across the EU/EEA, namely haemodialysis recipients, recipients of SOHO, medical and dental procedures and diabetic patients. A time-gradient was observed, with earlier studies reporting higher HCV prevalence estimates and few of the retrieved studies conducted in the current decade, with many presenting data collected in the late 1990s or early 2000s focusing on patients who received medical, dental procedures or SOHO before the widespread introduction of screening tests, thus our findings may be an overestimate of the true prevalence. Unfortunately, it was not possible to investigate in detail how changes in national patient safety regulations might have affected the burden of disease at local level. Still, considering the recommendation of WHO to promote active case finding for HCV among population subgroups with prevalence above the 2% threshold [63], the groups identified in our study, haemodialysis recipients, recipients of SOHO and diabetic patients, should be targeted for testing in the EU/EEA. High range estimates were detected in those studies sampling patients with overlapping risks such as HIV infection [20], [23], thus underlying the importance of performing individual risk assessment to identify patients who might benefit from testing for HCV.
Of note, very few studies targeting recipients of medical or dental interventions were retrieved, and all of them were conducted among populations with overlapping risks, such as household members of HCV-infected patients or diabetes patients. The detected prevalence estimates are likely to be overestimated, and it is difficult to draw any firm conclusions from the available data.
Possibly due to the challenges in investigating incidence, studies reporting on HCV new infections among population groups at risk of iatrogenic transmission are few with limited geographic coverage and largely conducted more than a decade ago, posing some concerns on the applicability of the findings to the present context. Older studies from France, Greece, Italy and the UK present evidence of HCV transmission among haemodialysis patients, while more recent ones detected no incident cases [41], [44], [47], and one Italian study specifically referred to no case of seroconversion occurring after 1986 [21]. This is very likely due to an improvement in the application of infection-control procedures [45], [64]. Still evidence of iatrogenic transmission in haemodialysis units is reported in the literature, albeit linked to outbreaks [14]. Suboptimal infection control standards have been implicated in transmission events in a number of settings, including long-term care facilities and other healthcare facilities [11], [15]. In countries with a sizeable proportion of migrants from non-EU countries, a further element of concern are dialysis patients travelling back on their holidays to high endemicity countries and receiving dialysis in facilities where infection control may be suboptimal, as reported in one study from the UK [42]. These findings highlight the need for strict infection control measures in services accessed by patients returning from low- and middle-income countries.
Iatrogenic transmission is also identified as an ongoing source of acute HBV infections in the EU/EEA as it accounts for 18.6% of all new cases and for 40.4% of chronic cases reported to the ECDC in 2016, mostly by Italy and Romania [13]. Despite fewer studies retrieved as compared to HCV, our findings suggest a high prevalence of HBV among haemodialysis patients, well above the WHO threshold of 2% [63] in all countries with available data. While all the studies were performed during the past decade, and infection-control standards as well as vaccine coverage may have improved since then [64], HBV vaccination for patients receiving haemodialysis is essential [65]. According to a European review of HBV vaccination policies among risk groups, 70% of the countries reported targeted vaccination for haemodialysis patients, including all countries with at least one estimate included in the present review, with the sole exception of Romania [66]. However, data on coverage among the target population was hardly available, hampering the possibility of assessing the impact of such intervention on the burden of HBV. Furthermore, higher HBV prevalence estimates among haemodialysis patients were reported from those countries with higher prevalence among the general population, thus reflecting the underlying epidemiological profile [1], with the exception of one study from Spain [20] conducted among HIV-positive patients. As a comparison, the few studies on diabetes patients report prevalence estimates comparable to those observed for the general population in the corresponding countries. Still, the risk of transmission among this population group continues as indicated by the numerous HBV outbreaks linked to blood glucose monitoring [15]. Finally, in our findings, HBV prevalence data among recipients of SOHO are largely influenced by the choice of the study population, which is characterized, in all included studies, by presence of multiple risk factors (e.g. co-infection with HIV/HCV), preventing any reliable conclusion from being drawn.
The impact of childhood HBV vaccination programmes, well-established in the EU/EEA region [67], have been largely unaccounted for in the included studies, although it may be inferred from the lower prevalence seen for HBV as compared to HCV. Data on vaccination programmes and their coverage should be considered alongside prevalence when assessing the needs for and developing targeted testing initiatives at country level.
Finally, few studies were identified on HBV and HCV among HCWs, and from a limited number of countries in the region. Despite the heterogeneity in the sampling approaches and the composition of the study populations, often including a mixture of different cadres, the resulting estimates may be suggestive of a prevalence comparable to that of general population from the corresponding country [1]. While occupational transmission is a risk for HCWs [68], adherence to universal precautions remains the primary means of preventing occupational exposure [63], [64]. Alongside effective infection control procedures and use of safer devices, HBV vaccination is a mainstay of such a prevention approach, and as such it is recommended for HCWs [65], [69]. However, HBV or HCV chronically infected HCWs, especially those performing exposure-prone procedures, require occupational management in line with national policy [70], [71]. HBV and HCV prevalence among HCWs may be influenced by additional factors, such as the underlying prevalence in the population of origin, especially in countries where the proportion of foreign-born migrants among the healthcare workforce is substantial.
Our study has some limitations. Neither a meta-analysis nor pooling of the retrieved prevalence estimates was performed due to the limited data comparability resulting from the large degree of heterogeneity between studies and population groups sampled. For example, study populations differed in the proportion who received any medical treatment before infection control/blood-safety measures to prevent HBV/HCV exposure became routine practice in the country. In addition, for HBV prevalence studies, the vaccinated proportion of a population varied between studies and was often not mentioned. The diagnostic methods were an additional source of heterogeneity, as the laboratory tests used, including for confirmatory tests, varied across the studies. Finally, many estimates dated back in time and were based on local, rather than national data derived from small studies with sub-optimal sampling methods and are probably not generalizable to the wider population. Differences between HBV/HCV prevalence or incidence estimates reported in the included studies can at least partly be explained by these factors, and for this reason direct comparisons were deemed not possible. Information on other potentially relevant factors such as the infection control standards in place or any significant change in policy or practice over time was not extracted. Lastly, while this study focused on a pre-defined list of populations potentially at high risk of HBV/HCV or with high burden of diseases, it is recognized that other patient groups, such as surgical or intensive care unit patients, may have high prevalence levels and may have been missed in our search strategy.
In conclusion, our findings indicate high levels of HBV and HCV infection among specific groups known to be at risk of iatrogenic transmission of blood-borne viruses, including haemodialysis recipients, recipients of SOHO, medical and dental procedures and diabetic patients. Whilst some evidence of declining trends in estimates of prevalence among these groups was found, the findings suggest transmission of these infections in healthcare settings across EU/EEA countries, which reinforces the importance of maintaining infection-control measures. Estimates of prevalence found among these groups were generally higher for HCV than HBV, reflecting the impact and relevance of HBV vaccination as a key prevention strategy. Differences between countries may reflect many factors including the impact of differing prevention strategies as well as the epidemiology in the broader population, and this is a possible area for future research. Whilst our findings indicated that the prevalence of HBV and HCV in HCWs was comparable to that of the general population from the corresponding country, occupational transmission is a risk for HCWs and this group needs close assessment, management and education in accordance with national occupational workforce guidelines. Our results also suggest a need for countries to consider groups at higher risk for iatrogenic transmission of HBV and HCV as target groups for active case finding in the EU/EEA. In the effort to reduce the undiagnosed fraction for HBV and HCV in Europe, hospitalization history and history of receiving SOHO or other invasive procedures could be considered as relevant information to collect during patient consultation in order to promote testing for viral hepatitis.
Acknowledgements
The authors would like to thank Andrew Amato Gauci for his valuable input to the manuscript and the ECDC library for their technical support with the systematic review search. The authors would also like to acknowledge all the ECDC colleagues who helped with translating and screening the retrieved records published in languages other than English.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jhin.2019.03.004.
Author contributions
L.T., E.D., L.M., I.V. and E.B. contributed to the design of the project and the development of the study protocol, L.T. coordinated the study. L.M., U.P., L.T. performed the systematic review, including data collection and data analysis. All authors contributed to data interpretation, manuscript drafting and review. L.T. drafted the first version of the manuscript.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding sources
This work was supported by the European Centre for Disease Prevention and Control (framework contract number ECDC/2016/027) and is part of a wider undertaking to develop European guidance on integrated testing for HBV, HCV and HIV.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Hofstraat S.H.I., Falla A.M., Duffell E.F., Hahne S.J.M., Amato-Gauci A.J., Veldhuijzen I.K. Current prevalence of chronic hepatitis B and C virus infection in the general population, blood donors and pregnant women in the EU/EEA: a systematic review. Epidemiol Infect. 2017;14:2873–2885. doi: 10.1017/S0950268817001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control . 2017. Hepatitis B and C testing activities, needs and priorities in the EU/EEA. Stockholm, Sweden.https://ecdc.europa.eu/sites/portal/files/documents/HepatitisBC-testing-in-EU-May2017.pdf Available at: [last accessed October 2018] [Google Scholar]
- 3.Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(5):325–336. doi: 10.1016/S2468-1253(17)30045-6. 2017/04/12. [DOI] [PubMed] [Google Scholar]
- 4.Polaris Observatory Collaborators. Razavi-Shearer D., Gamkrelidze I., Nguyen M.H., Chen D.-S., Van Damme P., Abbas Z. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . vol. 10. Aug. 2016. http://www.euro.who.int/__data/assets/pdf_file/0008/357236/Hepatitis-9789289052870-eng.pdf (Action plan for the health sector response to viral hepatitis in the WHO European Region). Available at: [last accessed September 2018] [Google Scholar]
- 6.Platt L., Easterbrook P., Gower E., McDonald B., Sabin K., McGowan C. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808. doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- 7.Falla A.M., Hofstraat S.H.I., Duffell E., Hahné S.J.M., Tavoschi L., Veldhuijzen I.K. Hepatitis B/C in the countries of the EU/EEA: a systematic review of the prevalence among at-risk groups. BMC Infect Dis. 2018;18(1):79. doi: 10.1186/s12879-018-2988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control . 2016. Epidemiological assessment of hepatitis B and C among migrants in the EU/EEA. Stockholm, Sweden.https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/epidemiological-assessment-hepatitis-B-and-C-among-migrants-EU-EEA.pdf Available at: [last accessed September 2018] [Google Scholar]
- 9.Zanetti A.R., Tanzi E., Romanò L., Mele A. Epidemiology and prevention of hepatitis type C in Italy. Res Virol. 1995;146(4):253–259. doi: 10.1016/0923-2516(96)80568-1. [DOI] [PubMed] [Google Scholar]
- 10.Spada E., Mele A., Mariano A., Zuccaro O., Tosti M.E. Risk factors for and incidence of acute hepatitis C after the achievement of blood supply safety in Italy: Results from the national surveillance system. J Med Virol. 2013;85(3):433–440. doi: 10.1002/jmv.23485. [DOI] [PubMed] [Google Scholar]
- 11.Stępień M., Zakrzewska K., Rosińska M. Significant proportion of acute hepatitis B in Poland in 2010–2014 attributed to hospital transmission: combining surveillance and public registries data. BMC Inf Dis. 2018;18(1):164. doi: 10.1186/s12879-018-3063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control . 2017. Annual epidemiological report for 2015 – hepatitis C. Stockholm.https://www.ecdc.europa.eu/sites/portal/files/documents/AER_for_2015-hepatitis-C.pdf Available at: [last accessed October 2019] [Google Scholar]
- 13.European Centre for Disease Prevention and Control (ECDC) 2017. Annual epidemiological report for 2015 - hepatitis B. Stockholm.https://www.ecdc.europa.eu/sites/portal/files/documents/AER_for_2015-hepatitis-B.pdf Available at: [last accessed October 2018] [Google Scholar]
- 14.Senatore S., Galli C., Conti A., Faccini M., Cantoni S., Ciconali G. Hepatitis C virus outbreak in a haemodialysis unit: learning from failures. J Hosp Infect. 2016;94(3):249–252. doi: 10.1016/j.jhin.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Duffell E.F., Milne L.M., Seng C., Young Y., Xavier S., King S. Five hepatitis B outbreaks in care homes in the UK associated with deficiencies in infection control practice in blood glucose monitoring. Epidemiol Infect. 2011;139(3):327–335. doi: 10.1017/S0950268810001007. [DOI] [PubMed] [Google Scholar]
- 16.Scottish Intercollegiate Guidelines Network. Critical appraisal notes and checklists. Available at: http://www.sign.ac.uk/checklists-and-notes.html [last accessed August 2018].
- 17.Kuzminskis V., Žiginskiene E., Arune B.,I. A registry of haemodialysis patients and the progress of haemodialysis services in Lithuania. Nephrol Dial Transplant. 2005;20(12):2623–2628. doi: 10.1093/ndt/gfi170. [DOI] [PubMed] [Google Scholar]
- 18.Voiculescu M., Iliescu L., Ionescu C., Micu L., Ismail G., Zilisteanu D. A cross-sectional epidemiological study of HBV, HCV, HDV and HEV prevalence in the SubCarpathian and South-Eastern regions of Romania. J Gastrointest Liver Dis. 2010;19(1):43–48. doi: 10.1007/s11749-009-0177-3. [DOI] [PubMed] [Google Scholar]
- 19.Schiller A., Timar R., Siriopol D., Timar B., Bob F., Schiller O. Hepatitis B and C virus infection in the hemodialysis population from three romanian regions. Nephron. 2015;129(3):202–208. doi: 10.1159/000371450. [DOI] [PubMed] [Google Scholar]
- 20.Saracho R., Martin Escobar E., Comas Farnes J., Arcos E., Mazuecos Blanca A., Gentil Govantes M.A. Clinical evolution of chronic renal patients with HIV infection in replacement therapy. Nefrologia. 2015;35(5):457–464. doi: 10.1016/j.nefro.2015.06.027. 2015/09/28. [DOI] [PubMed] [Google Scholar]
- 21.Tagliaferri A., Rivolta G.F., Biasoli C., Valdre L., Rodorigo G., D’Inca M. A web-based registry of inherited bleeding disorders in the region of Emilia-Romagna: results at three and a half years. Haemophilia. 2008;14(2):343–354. doi: 10.1111/j.1365-2516.2007.01623.x. [DOI] [PubMed] [Google Scholar]
- 22.Zervou E.K., Georgiadou S.P., Liapi G.K., Karabini F., Giogiakas V., Zisiadis K. Markers of hepatitis viruses and human T-lymphotropic virus types I/II in patients who have undergone open-heart surgery: evidence of increased risk for exposure to HBV and HEV. Eur J Intern Med. 2005;16(6):424–428. doi: 10.1016/j.ejim.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Larsen C., Pialoux G., Salmon D., Antona D., Le Strat Y., Piroth L. Prevalence of hepatitis C and hepatitis B infection in the HIV-infected population of France, 2004. Euro Surveill. 2008;13(22) [PubMed] [Google Scholar]
- 24.Kucharska M., Inglot M., Szymczak A., Rymer W., Zalewska M., Malyszczak K. Co-infection of the hepatitis C virus with other blood-borne and hepatotropic viruses among hemophilia patients in Poland. Hepat Mon. 2016;16(9) doi: 10.5812/hepatmon.35658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soverini V., Persico M., Bugianesi E., Forlani G., Salamone F., Massarone M. HBV and HCV infection in type 2 diabetes mellitus: A survey in three diabetes units in different Italian areas. Acta Diabeto. 2011;48(4):337–343. doi: 10.1007/s00592-011-0293-x. [DOI] [PubMed] [Google Scholar]
- 26.Esparza-Martín N., Hernández-Betancor A., Suria-González S., Batista-García F., Braillard-Pocard P., Sánchez-Santana A.Y. Serology for Hepatitis B and C, HIV and syphilis in the initial evaluation of diabetes patients referred for an external Nephrology consultation. Nefrologia. 2013;33(1):124–127. doi: 10.3265/Nefrologia.pre2012.Jul.11331. [DOI] [PubMed] [Google Scholar]
- 27.Cadranel J.F., Di Martino V., Lambrey G., Mourlhon C., Nalet B., Anciaux M.L. Prevalence of hepatitis C infection and risk factors in hospitalized diabetic patients: results of a cross-sectional study. Eur J Gastroenterol Hepatol. 2008;20(9):829–836. doi: 10.1097/MEG.0b013e3282fc73a1. [DOI] [PubMed] [Google Scholar]
- 28.Rybacki M., Piekarska A., Wiszniewska M., Walusiak-Skorupa J. Hepatitis B and C infection: is it a problem in Polish healthcare workers? Int J Occup Med Environ Health. 2013;26(3):430–439. doi: 10.2478/s13382-013-0088-0. [DOI] [PubMed] [Google Scholar]
- 29.Slusarczyk J., Malkowski P., Bobilewicz D., Juszczyk G. Cross-sectional, anonymous screening for asymptomatic HCV infection, immunity to HBV, and occult HBV infection among health care workers in Warsaw, Poland. Przegl Epidemiol. 2012;66(3):445–451. [PubMed] [Google Scholar]
- 30.Ganczak M., Szych Z., Szczeniowski A., Dmytrzyk-Danilow G. [Attitudes of medical specialists toward HBV, HCV or HIV infected surgical staff and a sero-survey among staff members] Med Pr. 2013;64(5):639–647. doi: 10.13075/mp.5893.2013.0060. [DOI] [PubMed] [Google Scholar]
- 31.Izopet J., Sandres-Sauné K., Kamar N., Salama G., Dubois M., Pasquier C. Incidence of HCV infection in French hemodialysis units: A prospective study. J Med Virol. 2005;77(1):70–76. doi: 10.1002/jmv.20415. [DOI] [PubMed] [Google Scholar]
- 32.Mina P., Georgiadou S.P., Rizos C., Dalekos G.N., Rigopoulou E.I. Prevalence of occult hepatitis B virus infection in haemodialysis patients from central Greece. World J Gastroenterol. 2010;16(2):225–231. doi: 10.3748/wjg.v16.i2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sypsa V., Psichogiou M., Katsoulidou A., Skoutelis G., Moutafis S., Hadjiconstantinou V. Incidence and patterns of hepatitis C virus seroconversion in a cohort of hemodialysis patients. Am J Kidney Dis. 2005;45(2):334–343. doi: 10.1053/j.ajkd.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Di Napoli A., Pezzotti P., Di Lallo D., Petrosillo N., Trivelloni C., Di Giulio S. Epidemiology of hepatitis C virus among long-term dialysis patients: a 9-year study in an Italian region. Am J Kidney Dis. 2006;48(4):629–637. doi: 10.1053/j.ajkd.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Fabrizi F., De Vecchi A.F., Como G., Lunghi G., Martin P. De novo HCV infection among dialysis patients: A prospective study by HCV core antigen ELISA assay. Aliment Pharmacol Ther. 2005;21(7):861–869. doi: 10.1111/j.1365-2036.2005.02416.x. [DOI] [PubMed] [Google Scholar]
- 36.Goodkin D.A., Bieber B., Jadoul M., Martin P., Kanda E., Pisoni R.L. Mortality, Hospitalization, and Quality of Life among Patients with Hepatitis C Infection on Hemodialysis. Clin J Am Soc Nephrol. 2017;12(2):287–297. doi: 10.2215/CJN.07940716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sjöberg K., Widell A., Verbaan H. Prevalence of hepatitis C in Swedish diabetics is low and comparable to that in health care workers. Eur J Gastroenterol Hepatol. 2008;20(2):135–138. doi: 10.1097/MEG.0b013e3282f476f5. [DOI] [PubMed] [Google Scholar]
- 38.Flisiak R., Halota W., Horban A., Juszczyk J., Pawlowska M., Simon K. Prevalence and risk factors of HCV infection in Poland. Eur J Gastroenterol Hepatol. 2011;23(12):1213–1217. doi: 10.1097/MEG.0b013e32834d173c. [DOI] [PubMed] [Google Scholar]
- 39.Montella M., Crispo A., Grimaldi M., Ruffolo P., Ronga D., Izzo F. An assessment of hepatitis C virus infection among health-care workers of the National Cancer Institute of Naples. Southern Italy. Eur J Public Health. 2005;15(5):467–469. doi: 10.1093/eurpub/cki032. [DOI] [PubMed] [Google Scholar]
- 40.Marconi A., Candido S., Talamini R., Libra M., Nicoletti F., Spandidos D.A. Prevalence of hepatitis C virus infection among health-care workers: A 10-year survey. Mol Med Rep. 2010;3(4):561–564. doi: 10.3892/mmr_00000297. [DOI] [PubMed] [Google Scholar]
- 41.Corbett R.W., Prout V., Haynes D., Edwards C., Frankel A.H. Problems associated with hemodialysis and travel. J Travel Med. 2014;21(4):255–259. doi: 10.1111/jtm.12121. [DOI] [PubMed] [Google Scholar]
- 42.Ghafur A., Raza M., Labbett W., Chawla A., Smith C., Ngui S.L. Travel-associated acquisition of hepatitis C virus infection in patients receiving haemodialysis. Nephrol Dial Transplant. 2007;22(9):2640–2644. doi: 10.1093/ndt/gfm202. [DOI] [PubMed] [Google Scholar]
- 43.Fabrizi F., Messa P.G., Lunghi G., Aucella F., Bisegna S., Mangano S. Occult hepatitis B virus infection in dialysis patients: a multicentre survey. Aliment Pharmacol Ther. 2005;21(11):1341–1347. doi: 10.1111/j.1365-2036.2005.02501.x. [DOI] [PubMed] [Google Scholar]
- 44.Ayzac L., Béruard M., Girard R., Hannoun J., Kuentz F., Marc J.M. Dialin: Infection surveillance network for haemodialysis patients. First results. Nephrol Ther. 2009;5(1):41–51. doi: 10.1016/j.nephro.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Saune K., Kamar N., Miedouge M., Weclawiak H., Dubois M., Izopet J. Decreased prevalence and incidence of HCV markers in haemodialysis units: a multicentric French survey. Nephrol Dial Transpl. 2011;26(7):2309–2316. doi: 10.1093/ndt/gfq696. [DOI] [PubMed] [Google Scholar]
- 46.Baid-Agrawal S., Schindler R., Reinke P., Staedtler A., Rimpler S., Malik B. Prevalence of occult hepatitis C infection in chronic hemodialysis and kidney transplant patients. J Hepatol. 2014;60(5):928–933. doi: 10.1016/j.jhep.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Ross R.S., Viazov S., Clauberg R., Wolters B., Fengler I., Eveld K. Lack of de novo hepatitis C virus infections and absence of nosocomial transmissions of GB virus C in a large cohort of German haemodialysis patients. J Viral Hepat. 2009;16(4):230–238. doi: 10.1111/j.1365-2893.2008.01068.x. [DOI] [PubMed] [Google Scholar]
- 48.Vogt M., Klostermann B., Braun S., Busch R., Hess J., Frosner G. Prevalence and clinical role of GBV-C infection after cardiac surgery in childhood: a study on 414 patients. J Infect. 2006;53(1):43–48. doi: 10.1016/j.jinf.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 49.Christofidou M., Jelastopulu E., Economides G., Spillopoulou I., Siagris D., Labropoulou-Karatza C. Epidemiology of chronic hepatitis C virus infection in high risk groups. Hepat Mon. 2008;8(1):11–16. [Google Scholar]
- 50.La Torre G., Miele L., Mannocci A., Chiaradia G., Berloco F., Gabrieli M.L. Correlates of HCV seropositivity among familial contacts of HCV positive patients. BMC Public Health. 2006;6:237. doi: 10.1186/1471-2458-6-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Federici A.B., Santagostino E., Rumi M.G., Russo A., Mancuso M.E., Soffredini R. The natural history of hepatitis C virus infection in Italian patients with von Willebrand’s disease: A cohort study. Haematologica. 2006;91(4):503–508. [PubMed] [Google Scholar]
- 52.Zaaijer H.L., Appelman P., Frijstein G. Hepatitis C virus infection among transmission-prone medical personnel. Eur J Clin Microbiol Infect Dis. 2012;31(7):1473–1477. doi: 10.1007/s10096-011-1466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ganczak M., Bohatyrewicz A., Korzen M., Karakiewicz B. The comparison of sharps injuries reported by doctors versus nurses from surgical wards in the context of the prevalence of HBV, HCV and HIV infections. Pol Przegl Chir. 2012;84(4):190–195. doi: 10.2478/v10035-012-0031-2. [DOI] [PubMed] [Google Scholar]
- 54.Zagozdzon P., Parszuto J., Raj A., Calus-Kania D., Korczak A., Ejsmont J. [Prevalence and risk factors of chronic hepatitis C virus infection among health-care workers in Pomeranian voivodeship] Przegl Epidemiol. 2009;63(1):39–43. [PubMed] [Google Scholar]
- 55.Sporea I., Sirli R., Hogea C., Sink A.A., Serban V. Diabetes mellitus and chronic HCV infection. Rom J Intern Med. 2009;47(2):141–147. [PubMed] [Google Scholar]
- 56.Gallego E., Lopez A., Perez J., Llamas F., Lorenzo I., Lopez E. Effect of isolation measures on the incidence and prevalence of hepatitis C virus infection in hemodialysis. Nephron Clin Pr. 2006;104(1):c1–c6. doi: 10.1159/000093252. [DOI] [PubMed] [Google Scholar]
- 57.Ciancio A., Manzini P., Castagno F., D’Antico S., Reynaudo P., Coucourde L. Digestive endoscopy is not a major risk factor for transmitting hepatitis C virus. Ann Intern Med. 2005;142(11):903–909. doi: 10.7326/0003-4819-142-11-200506070-00008. [DOI] [PubMed] [Google Scholar]
- 58.Cornberg M., Razavi H.A., Alberti A., Bernasconi E., Buti M., Cooper C. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31(Suppl 2):30–60. doi: 10.1111/j.1478-3231.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 59.Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 60.Esteban J.I., Sauleda S., Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48(1):148–162. doi: 10.1016/j.jhep.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 61.Houghton M. Hepatitis C: The next 25years. Antiviral Res. 2014;110:77–78. doi: 10.1016/j.antiviral.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 62.Lieshout-Krikke R.W., Domanovic D., De Kort W., Mayr W., Liumbruno G.M., Pupella S. Selection strategies for newly registered blood donors in European countries. Blood Transfus. 2017;15(6):495–501. doi: 10.2450/2016.0107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Health Organization . WHO; Geneva: 2016. Hepatitis B and C testing. [Google Scholar]
- 64.Vanholder R., Canaud B., Fluck R., Jadoul M., Labriola L., Marti-Monros A. Diagnosis, prevention and treatment of haemodialysis catheter-related bloodstream infections (CRBSI): a position statement of European Renal Best Practice (ERBP) Clin Kidney J. 2010;3(3):234–246. doi: 10.1093/ndtplus/sfq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.World Health Organization Hepatitis B vaccines: WHO position paper – July 2017. Relev Epidemiol Hebd. 2017;92(27):369–392. [Google Scholar]
- 66.European Centre for Disease Prevention and Control . 2010. Surveillance and prevention of hepatitis B and C in Europe. Stockholm.https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/101012_TER_HepBandC_survey.pdf Available at: [last accessed October 2018] [Google Scholar]
- 67.Miglietta A., Quinten C., Lopalco P.L., Duffell E. Impact of hepatitis B vaccination on acute hepatitis B epidemiology in European Union/European Economic Area countries, 2006 to 2014. Euro Surveill. 2018;23(6) doi: 10.2807/1560-7917.ES.2018.23.6.17-00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rice B.D., Tomkins S.E., Ncube F.M. Sharp truth: health care workers remain at risk of bloodborne infection. Occup Med. 2015;65(3):210–214. doi: 10.1093/occmed/kqu206. [DOI] [PubMed] [Google Scholar]
- 69.De Schryver A., Claesen B., Meheus A., van Sprundel M., Francois G. European survey of hepatitis B vaccination policies for healthcare workers. Eur J Public Health. 2011;21(3):338–343. doi: 10.1093/eurpub/ckq122. [DOI] [PubMed] [Google Scholar]
- 70.Raven S.F.H., De Heus B., Wong A., Zaaijer H.L., Van Steenbergen J.E. Fluctuation of viremia in hepatitis B virus-infected healthcare workers performing exposure-prone procedures in the Netherlands. Infect Control Hosp Epidemiol. 2016;37(6):655–660. doi: 10.1017/ice.2016.49. [DOI] [PubMed] [Google Scholar]
- 71.Public Health England . 2014. The management of HIV infected healthcare workers who perform exposure prone procedures: updated guidance, january 2014. London. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.