Abstract
Introduction
To construct a prognostic model based on amyloid positron emission tomography (PET) to predict clinical progression in individual patients with mild cognitive impairment (MCI).
Methods
We included 411 MCI patients from the Alzheimer's Disease Neuroimaging Initiative. Prognostic models were constructed with Cox regression with demographics, magnetic resonance imaging, and/or amyloid PET to predict progression to Alzheimer's disease dementia. The models were validated in the Amsterdam Dementia Cohort.
Results
The combined model (Harrell's C = 0.82 [0.78–0.86]) was significantly superior to demographics (β = 0.100, P < .001), magnetic resonance imaging (β = 0.037, P = .011), and PET only models (β = 0.053, P = .003).The models can be used to calculate individualized risk, for example, a female MCI patient (age = 60, APOE ε4 positive, Mini-Mental State Examination = 25, hippocampal volume = 5.8 cm3, amyloid PET positive) has 35% (19–57) risk in one year and 85% (64–97) risk in three years. Model performances in the Amsterdam Dementia Cohort were reasonable.
Discussion
The present study facilitates the interpretation of an amyloid PET result in the context of a patient's own characteristics and clinical assessment.
Keywords: Biomarkers, Amyloid positron emission tomography, MCI, Progression, Alzheimer's disease
Highlights
-
•
Our models facilitate amyloid PET–based prognosis in light of patient characteristics.
-
•
Amyloid PET is the key player in our prognostic models.
-
•
Our models are easy to use and can guide clinical decision making.
1. Background
Introduction of amyloid positron emission tomography (PET) tracers allowed visualization of fibrillary amyloid-β plaques during life and had a tremendous impact on the field of Alzheimer's disease (AD) research [1]. The recent US Food and Drug Administration and European Medicines Agency approvals of fluorine-labeled tracers enable the implementation of amyloid PET imaging in clinical practice [2]. Guidelines for appropriate use of amyloid PET have been published to guide clinicians in its use [3]. In these appropriate use criteria, a longstanding and unexplained mild cognitive impairment (MCI) is proposed as an indication for amyloid PET. Moreover, recently published research guidelines incorporate amyloid PET evidence to support AD as underlying etiology [4].
To assess the clinical utility of amyloid PET, multiple studies investigated the diagnostic value of amyloid PET and demonstrated that amyloid PET contributes to changes in etiological diagnosis, increases diagnostic confidence, and affects patient management [5], [6], [7], [8]. In addition, in predementia stages, amyloid PET positivity has repeatedly been associated with an increased risk of dementia, showing its prognostic value [9], [10]. Nonetheless, the predictive value may vary with patient characteristics, and therefore, the translation to an individual patient remains challenging. For instance, age and APOE ε4 are the two most important risk factors for AD, and both have been strongly associated with an increased risk of amyloid deposition [11], [12]. Findings on the effect of other factors, such as gender and cognitive status, on amyloid deposition have been inconsistent [9], [13], [14].
Ideally, the meaning of an amyloid PET result should be interpreted in the context of a patient's entire clinical assessment. As it is quite unlikely that a clinician would order amyloid PET without first ordering magnetic resonnance imaging (MRI), the interpretation of an amyloid PET should not only take into account demographics and cognitive screening but also MRI results. However, studies investigating the impact of patient characteristics and MRI on the predictive ability of amyloid PET are lacking, hampering the interpretation of PET results for the individual patient [7], [15].
Studies such as IDEAS [16] and AMYPAD [17] are now evaluating the clinical utility of amyloid PET into clinical routine on a large scale. As these studies are implementing amyloid PET in the diagnostic workup, the importance to translate results to the individual patient becomes more imminent. But in this light, one important question remains largely unanswered to date: how should the professional interpret amyloid PET findings in conjunction with other information gathered during the diagnostic process such as patient characteristics and MRI findings, and especially in patients who are not yet demented, what does this mean for the prognosis. The purpose of the present study was to construct a prognostic model for clinical progression based on amyloid PET taking into account patients' demographic and clinical characteristics, to allow risk estimations in individual MCI patients.
2. Methods
2.1. Patients
We selected 411 MCI patients from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). Insititutional review boardapproval for the ADNI protocol was obtained at each participating site. All patients gave written informed consent.
Inclusion criteria for the present study were a baseline diagnosis of MCI, availability of amyloid PET, and at least 6 months of clinical follow-up. A detailed description of the methods can be found in the Supplementary Material. According to the routine protocol, clinical assessment consisted of a clinical interview, Mini-Mental State Examination scoring (MMSE) and neurologic and neuropsychological assessment. MRI was performed at baseline, and PET scans took place within 2 weeks after the screening/baseline visit. Baseline diagnosis of MCI was made in accordance with the criteria of Petersen et al. [18].
Patients were monitored longitudinally at 3- to 12-month intervals up to 8 years for possible changes in diagnosis. The criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association for probable AD were used to diagnose patients with mild AD dementia [19].
2.2. APOE ε4 genotyping
Genotyping of APOE was performed at baseline using DNA extracted by Cogenics from a 3 mL aliquot of EDTA blood. APOE ε4 positivity, defined as having ≥1 ε4 allele, was used as predictor. APOE genotype was available for all patients.
2.3. MRI
MRI was available for 89% (n = 366) of the patients. The number of patients at risk did not differ between patients with MRI and the total sample (Supplementary Fig. 1). MRI was acquired as described online (adni.loni.usc.edu/data-samples/mri/). Brain MRI was performed on 1.5 T (n = 6) and 3.0 T (n = 360) MRI systems. Hippocampal volume (HCV) and whole brain volume were estimated with Freesurfer version 4.4 (n = 6) and Freesurfer version 5.1 (n = 360).
2.4. Amyloid PET
[11C] PIB (n = 6) and [18F] florbetapir (n = 405) image data were acquired (adni.loni.usc.edu/data-samples/pet/) and processed as described online (adni.loni.usc.edu/methods/pet-analysis/) [14]. In brief, global cortical [11C] PIB PET and [18F] florbetapir retention was estimated by the mean of the standardized uptake values (SUV) from cortical gray matter (lateral and medial frontal, anterior and posterior cingulate, and lateral parietal and lateral temporal) regions relative to the uptake in gray and white matter cerebellum, creating an SUV ratio (SUVr). Patients were categorized as either PET positive or negative using a cut-off value (SUVr) of 1.50 for [11C] PIB and 1.10 for [18F] florbetapir [20], [21], [22], [23], [24].
2.5. Statistical analysis
We used STATA 14 SE for statistical analyses. Prognostic models were constructed using Cox proportional hazard analysis. Clinical endpoint was clinical progression to AD-dementia. Patients progressing to non-AD dementia were censored at progression. For purpose of comparison, we first constructed (1) a demographics only model based on patient characteristic age, sex, MMSE score, and APOE ε4 and (2) an MRI model based on patient characteristics, HCV (cm3), and normalized whole brain volume (NWBV, cm3). Next, (3) we based the PET model on patient characteristics and amyloid PET result (positive/negative). Finally, (4) we constructed a combined model based on amyloid PET, HCV, NWBV, and patient characteristics. In all models, main effects and two-way interactions between all possible combinations of variables were included in the model via a backward selection procedure if the P value ≤ .10. For comparison, we constructed the models without APOE ε4 as a potential predictor in an additional set of analyses. For all models, we estimated Harrell's C statistic to assess the prognostic accuracy of the model. We tested differences in model fit using the somersd package combined with lincom command in STATA [25]. Based on the obtained prognostic models, we calculated one- and three-year cumulative progression probabilities with 95 percent confidence intervals (CIs) using the STATA ‘survci’ command [26]. Probabilities were read from the survival function for specific combinations of patient characteristics and PET result. As an example, we provide probabilities of progression (95% CI) for patients with either a positive or negative PET scan, APOE ε4 positive or negative, man or woman, and 80th or 20th percentile of HCV, NWBV, with good (MMSE = 30) or impaired (MMSE = 25) cognition, and low (60) age or high (80) age. Based on these examples, we report for each model the lowest and highest probabilities of progression. Moreover, to appreciate the added value of biomarkers in a more practical way, we present the progression probabilities of an example case of a woman, APOE ε4 positive patient, aged 62, with an MMSE of 25.
2.6. Validation
We performed an external validation on data from the clinical Amsterdam dementia cohort (ADC) (n = 107) [27], [28]. Demographic characteristics can be found in Supplementary Table 1. During the baseline visit, all patients received a one-day standardized baseline clinical assessment. Clinical diagnosis of MCI was made by consensus during a multidisciplinary meeting [29], [30]. The standardized annual follow-up included a follow-up visit with the neurologist and neuropsychologist, after which the diagnosis was reevaluated. AD diagnosis was based on international diagnostics or research consensus criteria [19], [31], [32].
MRI was performed on 1.0, 1.5, and 3.0 T MRI systems and according to a standardized protocol [27], [28]. Left and right HCVs (mL) were estimated using FMRIBs Integrated registration and segmentation tool (FSL FIRST) [33]. NWBV (mL) were estimated with Structural Image Evaluation using Normalization of Atrophy Cross-sectional (SIENAX) [34]. Amyloid PET was performed on different scanners (Gemini TF PET-CT, Ingenuity TF PET-CT, and Ingenuity PET/MRI system; Philips Medical Systems, Best, the Netherlands) using different PET tracers ([11C] PiB [n = 42], [18F] flutemetamol [n = 10], [18F] florbetaben [n = 54], and [18F] florbetapir [n = 1]). An experienced nuclear medicine physician visually rated the scans as positive or negative.
The established models were fitted to amyloid PET biomarker values and patient characteristics. Regression on the prognostic index was performed, and Harrell's C statistic was calculated to assess prognostic performance of these models in the ADC.
3. Results
3.1. Demographic and clinical characteristics
Mean age of the group of MCI patients was 72 ± 7, and n = 184 (45%) were women; average MMSE was 28 ± 2; n = 199 (48%) were APOE ε4 positive. A positive amyloid PET scan was found in n = 231 (56%), and over 3 ± 2 years, n = 100 (24%) progressed to AD-dementia (Table 1).
Table 1.
Demographic characteristics of MCI patients
| Characteristics | Total (n = 411) | MCI-AD (n = 100) | MCI-stable (n = 311) |
|---|---|---|---|
| Age | 71 ± 7 | 73 ± 7 | 71 ± 8 |
| Sex, F (%) | 184 (45) | 42 (42) | 142 (46) |
| MMSE, median (IQR) | 28 (27–29) | 27 (26–29) | 29 (28–30) |
| APOE ε4 carrier | 282 (41%) | 71 (71%) | 128 (41%) |
| Amyloid PET positive | 199 (48%) | 88 (88%) | 144 (46%) |
| HCV (cm3) | 7.0 ± 1.1 | 6.4 ± 1.1 | 7.2 ± 1.1 |
| NWBV (cm3) | 1059 ± 105 | 1049 ± 110 | 1062 ± 104 |
| FU time, years | 3 ± 1 | 2 ± 1 | 3 ± 1 |
NOTE. Data are mean ± standard deviation, unless otherwise specified.
Abbreviations: AD, Alzheimer's disease; APOE, Apolipoprotein E; FU, follow-up; HCV, hippocampal volume (sum); IQR, interquartile range; MCI, mild cognition impairment; MMSE, Mini-Mental State Examination; NWBV, normalized whole brain volume; PET, positron emission tomography.
3.2. Demographic model
Cox proportional hazards analysis revealed that age, MMSE, and APOE ε4 were retained in the demographic only model. The Harrell's C statistic was 0.73 (0.68–0.77) (Table 2). Probabilities of progression ranged from 1% (0–2) in one year and 5% (3–9) in three years in young (age 60) APOE ε4 negative patients with a high MMSE score (30) to 23% (15–34) in one year and 62% (49–76) in three years in older (age 80) APOE ε4 positive patients with a lower MMSE score (25).
Table 2.
Regression coefficients of the final models
| Models | Coefficient | Standard error | P value | Harrell's C |
|---|---|---|---|---|
| Demographics only (n = 411) | ||||
| Age | 0.03 | 0.01 | .025 | |
| MMSE | −0.23 | 0.05 | <.001 | 0.73 (0.68–0.77) |
| APOEε4 | 1.12 | 0.23 | <.001 | |
| MRI model (n = 366) | ||||
| HCV | −0.66 | 0.10 | <.001 | |
| Sex | 0.39 | 0.22 | .081 | |
| MMSE | −0.17 | 0.06 | .004 | 0.78 (0.73–0.83) |
| APOEε4 | 1.16 | 0.24 | <.001 | |
| PET model (n = 411) | ||||
| PET | 1.63 | 0.32 | <.001 | |
| MMSE | −0.20 | 0.05 | <.001 | 0.77 (0.72–0.81) |
| APOEε4 | 0.54 | 0.23 | .0420 | |
| Combined model (n = 366) | ||||
| PET | 1.75 | 0.37 | <.001 | |
| HCV | −0.76 | 0.11 | <.001 | |
| Age | −0.04 | 0.02 | .018 | |
| Sex | 0.49 | 0.22 | .030 | 0.82 (0.78–0.86) |
| MMSE | −0.14 | 0.06 | .018 | |
| APOE ε4 | 0.50 | 0.25 | .046 |
NOTE. Models were constructed with Cox proportional hazards analysis, outcome: progression to AD-dementia. No interactions retained in the models. Including APOE ε4 count instead of APOE ε4 presence (yes/no) yielded similar results (data not shown).
MRI versus demographics only (β = 0.063 [0.019], P = .001).
PET versus demographic only (β = 0.048 [0.017], P = .007).
PET versus MRI (β = −0.015 [0.024], NS).
Combined versus demographics only (β = 0.100, P < .001).
Combined versus MRI (β = 0.037, P = .011).
Combined versus PET (β = 0.053, P = .003).
Abbreviations: APOE, Apolipoprotein E; HCV, hippocampal volume (cm3); MRI, magnetic resonance imaging; MMSE, Mini-Mental State Examination; PET, positron emission tomography.
3.3. MRI model
The MRI model contained HCV, sex, MMSE, and APOE ε4 as predictors; none of the interaction terms contributed significantly to the model. The Harrell's C statistic was 0.78 (0.73–0.83), and MRI model had a better model fit than the demographic only model (β = 0.063 [0.019], P = .001, Table 2).
Probabilities of progression ranged from 1% (0-2) in one year and 3% (2–6) in three years in men, APOE ε4 negative patients with a high MMSE score (30) and high HCV (8 cm3, 80th percentile) to 21% (13–32) in one year and 63% (47–77) in three years in women, APOE ε4 positive patients with a low MMSE score (25) and low HCV (5.8 cm3, 20th percentile).
3.4. PET model
In the PET model, only main effects of amyloid PET result, MMSE, and APOE ε4 are retained in the model. Harrell's C statistic was 0.77 (0.73–0.83) (Table 2), and the fit of this model was better than the model with demographic information only (β = 0.048 [0.017], P = .007) but comparable with the MRI model (β = -0.015 [0.024], not significant). Probabilities of progression ranged from 0% (0–2) in one year and 3% (2–7) in three years in PET negative, APOE ε4 negative patients with a high MMSE score (30) to 20% (13–29) in one year and 58% (46–70) in three years in PET positive, APOE ε4 positive patients with a low MMSE score (25).
3.5. Combined model
The combined model consisted of amyloid PET, HCV, age, sex, MMSE, and APOE ε4, while none of the interaction terms added prognostic value. The Harrell's C statistic was 0.82 (0.78–0.86) (Table 2), and the fit of this model was better than each of the other models (demographics only β = 0.100, P < .001; MRI β = 0.037, P = .011; PET model β = 0.053, P = .003). Probabilities of progression ranged from 0% (0–1) in one year and 0.5% (0–2) in three years in older (age 80) men, APOE ε4 negative patients with a high MMSE (30), high HCV (8 cm3), and a negative amyloid PET, to 35% (19–57) in one year and 85% (64–97) in three years in young (age 60) women, APOE ε4 positive patients with a low MMSE (25), low HCV (5.8 cm3) and a positive amyloid PET.
To appreciate the added value of amyloid PET in a more practical way, we present the progression probabilities of an example case in Box 1.
Box 1. Example case.
Female patient, aged 62, MMSE of 25, and APOE ε4 positive.
Based on demographic and basic clinical information only, this patient would have a probability to progress to AD-dementia of 13% (8–23) in one year and 42% (28–58) in three years. These progression probabilities are elevated in comparison with the baseline risk of 24% in 3 ± 2 years in this cohort.
If an MRI was performed and this patient would have a high HCV (8 cm3), her progression risk would drop to 5% (3–10) in one year and 21% (12–34) in three years. On the other hand, if she would have a low HCV (5.8 cm3), her risk would increase to 21% (13–32) in one year and 63% (47–77) in three years.
Taking into account the result of the amyloid PET scan but without looking at the information from the MRI, a negative amyloid PET would result in a decreased risk of 4% (2–9) in one year and 15% (8–29) in three years. In case of a positive amyloid PET, this risk would increase to 20% (13–29) in one year and 58% (46–70) in three years.
Combining information from the MRI and amyloid PET, the risk of progression would decrease in case of a negative amyloid PET; high HCV (8 cm3): one year 1% (0–4), three year 6% (2–14); low HCV (5.8 cm3): one year 7% (3–15), three year 26% (12–51). Progression probabilities would increase in case of a low HCV (5.8 cm3) and a positive amyloid PET to 33% (19%–54%) in one year and 83% (62%–96%) in three years.
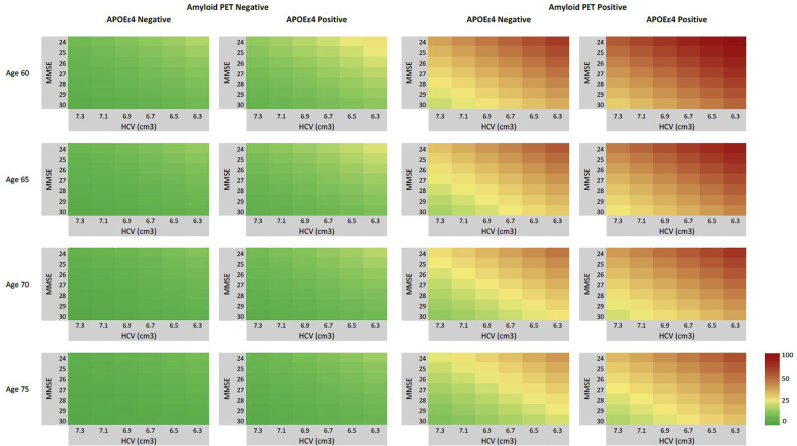
Using the combined model, we calculated three-year risk for all MCI patients. Fig. 1 provides isographs that visualize these probabilities. These illustrate that younger age, lower HCV, lower MMSE, and APOE ε4 positivity each somewhat contribute to a higher probability of AD-dementia. The prognostic value of amyloid PET stands out however: MCI patients with a negative amyloid PET scan, regardless of APOE status or values on the other variables have low prognostic values, illustrating the high negative predictive value of amyloid PET. A positive amyloid PET predisposes for a higher risk of AD-dementia, especially in younger patients (age < 65).
Fig. 1.
Probability isographs for three-year progression to AD-dementia; Probability of progression within three years based on the combined model. Risk is low (green-yellow) in amyloid PET negative patients. Risk increases with lower HCV, younger age, and lower MMSE. In this combined model, amyloid PET clearly has the strongest contribution to prognosis as high probabilities can be found in amyloid PET positive patients (orange-red).
In an additional set of analysis, we constructed the models without APOE ε4 as a potential predictor, as in clinical practice APOE e4 is not often used. Model performances were similar to the models with APOE ε4 (Supplementary Table 2). Of note, including APOE ε4 count also resulted in similar model performances as the models with APOE ε4 presence and patient with one and two ε4 alleles were equally at risk.
3.6. External validation
External validation in the ADC showed comparable prognostic performances: demographics only model, Harrell's C = 0.66; MRI model, Harrell's C = 0.66; the PET model, Harrell's C = 0.81; and the combined model, Harrell's C = 0.76.
4. Discussion
In this study, we constructed prognostic models that provide personalized progression probabilities to optimize the interpretation of amyloid PET in individual MCI patients. We showed how prognostic information for an individual MCI patient can be extracted from amyloid PET. Reasoning that amyloid PET will always be made after structural neuroimaging, we also constructed a model including both HCV and amyloid PET. In this combined model, amyloid PET has the strongest contribution to prognosis.
Former studies have repeatedly demonstrated on a group level that the predictive value of amyloid PET is largely attributable to a high negative predictive value [10], [24], [35], [36]. These former studies cannot directly be translated to the individuals, however, as the interpretation of amyloid PET should take into account the background of a patient's own characteristics. In particular, age is a well-known risk factor for developing AD and is associated with increased amyloid deposition [9], [11], [37]. In the present study, we extended on these former findings as we showed that positive predictive value of amyloid PET appears somewhat stronger in younger and somewhat less strong in older patients, illustrating the value of our individualized prediction model that allows to interpret the amyloid PET in the context of an individual's own characteristics.
Another factor that is closely linked to amyloid positivity is the presence of APOE ε4. We therefore reasoned that for amyloid PET to be of use, it should add predictive value over APOE ε4 positivity. As a result, the demographic model, which also included APOE ε4, had rather high performance to start with. Nonetheless, amyloid PET clearly added prognostic value. Excluding APOE ε4 from the models did not decrease the prognostic performances, illustrating that amyloid PET can also be safely interpreted without knowledge of APOE ε4. This finding is in agreement with those observed in earlier studies and suggests that the predictive effect of APOE ε4 is indirect [38], [39], [40].
Only a few former studies investigated the incremental value of MRI and PET biomarkers on predictive accuracy. While some studies showed an increase in predictive accuracy or faster decline in patients with reduced HCV on MRI and a positive amyloid PET [10], [35], [41], [42], only one study concluded the opposite [43]. In the present study, combining MRI and amyloid PET biomarkers increased prognostic performance compared to a model with either MRI or amyloid PET alone. In a former study, we constructed individualized prognostic models based on MRI and cerebrospinal fluid. This study also showed that biomarkers should best be combined [44].
Although amyloid PET has been approved for diagnostic use, it is currently not part of standard diagnostic assessment. Outside specialized centers, amyloid PET is only limitedly available, making its clinical availability less than that of amyloid measured in cerebrospinal fluid. In this light, the current models with PET biomarkers may be valuable outside the clinic, for example, to select patients for trial enrollment.
But the fact that amyloid PET did not find its way to clinical practice may also be due to the lack of information on how to implement amyloid PET and what test results mean for an individual patient. As the field is shifting toward a biological definition of Alzheimer's disease, in which evidence of amyloid is the key player, the need for information on how to implement amyloid PET becomes more pressing. To pave the way for effective and efficient implementation of amyloid PET, the present study is of high importance. The recent IDEAS paper showed that amyloid greatly impacts patient management [16], and we add to this by showing that amyloid PET in MCI patients has prognostic value. Moreover, our models allow a clinician to appreciate how a positive or negative result could contribute to the probability estimation, depending on the specific constellation of other (demographic) variables. Such models have very practical value; they can be used to interpret the results of diagnostic tests already performed.
It is also conceivable that a clinician would use the models before actually ordering the diagnostic test. This might be even a more realistic scenario, as amyloid PET is not part of standard diagnostic assessment. Best case/worst case scenarios could be calculated to appreciate the added value of amyloid PET in comparison with MRI only. In this light, the models would serve as a decision modeling tool, which could even promote shared decision making and could help to manage expectations of the outcome of the test. With the new biological definition of AD, it would also be interesting to see how the positive or negative result of an amyloid PET impacts clinical prognosis in cognitively normal individuals, for example, with subjective cognitive decline.
A recent review shows that only few studies have addressed potential risks of disclosure to nondemented memory clinic patients [45]. The most important argument against the disclosure of results was theoretical of nature and focused on the principle of “do no harm” [45], [46]. Nonetheless, based on the few available empirical studies, disclosure of both positive and negative PET results had low risk of psychological harm [8], [46], [47], [48]. On the other hand, important arguments in favor of disclosure are the autonomy of a patient and that it enhances future decision making. Moreover, patients and caregivers become more and more assertive in their need for information. Many, but not all, patients and caregivers who are referred to a memory clinic want information on the likely course of their disease [48], [49]. Therefore, it is of importance that before embarking on testing, realistic expectations are set with regard to how the results of the test can help to determine the patients' need to learn more about the cause of their complaints, how certain the prognosis will become/how uncertain it will remain after testing, and how the results could affect clinical management [50].
By definition, predictions are estimates and as a result, have a degree of uncertainty. Therefore, giving a range in which the prediction falls would be more appropriate than only a point estimate. It is not customary to accompany predictions with CIs, yet intervals allow researchers and clinicians to easily assess the precision of the prediction. For that reason, our model provides CIs as well. Owing to the strong negative predictive value of amyloid PET, the low risks have small CIs which illustrate that these risks can be estimated accurately. On the contrary, higher risks widen the CIs and show less secure estimates of progression, requiring a more cautious interpretation. How to communicate these uncertainties with patients and their caregivers is subject of current research in the Alzheimer's disease biomarkers in daily practiceproject.
One of the limitations is that ADNI represents a trial population, including primarily amnestic MCI patients, rather than a clinical heterogeneous sample of MCI patients. Constructing models for amyloid PET in ADNI might result in an overestimating of the effect of amyloid PET. Therefore, we validated the prognostic models in an external cohort. In the ADC, the models containing HCV performed less, whereas models based on PET performed comparably well (i.e., no overestimation). The ADC cohort is rather young, which may have led to a somewhat lower performance of the MRI model, yet again showing the added value of amyloid PET in this younger population. In addition, the validation sample is rather small, resulting in an underrepresentation of amyloid PET positivity in stable MCI patients. Moreover, a variety of different tracers was used in the ADC, and the ADC used visual rating to interpret the PET imaging data. Despite the use of different techniques, the prognostic model still performed reasonably well in the ADC, confirming external generalizability. Nonetheless, the models deserve further validation in larger and different datasets.
Current guidelines and expert opinions report that amyloid PET alone cannot predict the trajectory of progression for an individual patient [7], [50]. Furthermore, these guidelines underline the meaning of an amyloid PET result should be interpreted in the context of a patient's entire clinical assessment, without actually detailing how that should be done. Our present study addresses this ardent clinical need. Of note, we show that even when taking patient characteristics and clinical assessment into account, amyloid PET clearly is the key player in prognostic models helping patients to understand what they can expect of the future. Our models are easy to use, and we provide a calculator upon request. We intend these models to serve as input for a web-based tool to support clinicians in integrating biomarkers in their daily clinical practice. The present study takes the first steps toward precision medicine based on amyloid PET.
Research in context.
-
1.
Systematic review: We searched PubMed for literature on amyloid PET and biomarker based prognostic models that predict clinical progression in patients with mild cognitive impairment (MCI). We also search for relevant publications in the reference lists of articles. Studies demonstrated the predictive value of amyloid PET in MCI, which seems largely attributable to a high negative predictive value. As the interpretation of amyloid PET should take into account the background of a patient's own characteristics, results from these former studies cannot directly be translated to the individual.
-
2.
Interpretation: Our study takes the first steps in a precision medicine approach for MCI patients with amyloid PET. With our externally validated models, probabilities of clinical progression within one and three years with accompanying confidence intervals can be calculated.
-
3.
Future directions: Future research should aim to further study the use of personalized prediction based on amyloid PET in larger cohorts of MCI patients.
Acknowledgments
We would like to thank S. Ingala for her help with calculating brain volumes in the ADC. Research of the Alzheimer center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. The Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds. W.M.vd.F. is recipient of a grant by Stichting LSH-TKI (ABIDE-communication: LSHM16025, cofunded by Life-MI and Alzheimer Nederland) and ZonMW-Memorabel (ABIDE; project no 733050201), a project in the context of the Dutch Deltaplan Dementia.
Footnotes
Disclosures: Dr. Scheltens has acquired grant support (for the institution) from GE Healthcare, Danone Research, Piramal, and MERCK. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Lilly, GE Healthcare, Novartis, Sanofi, Nutricia, Probiodrug, Biogen, Roche, Avraham, and EIP Pharma. Dr. van Berckel receives grant support from ZonMW, ISAO, CTMM, Janssen Pharmaceuticals, GE, and AVID radiopharmaceuticals. In addition, he is a trainer for GE and Piramal. Dr. van der Flier performs contract research for Biogen MA Inc. Research programs of W.M.v.d.F. have been funded by ZonMW, Pasman stichting, NWO, EU-FP7, EU-JPND, Alzheimer Nederland, Cardiovasculair Onderzoek Nederland, stichting Dioraphte, Gieskes-Strijbis fonds, Boehringer Ingelheim, Piramal Neuroimaging, Roche BV, Janssen Stellar, and Combinostics. W.M.vd.F. is recipient of a grant by Stichting LSH-TKI (ABIDE-communication: LSHM16025) and ZonMW-Memorabel (ABIDE; project no 733050201), a project in the context of the Dutch Deltaplan Dementia. All funding is paid to her institution. van Maurik, van der Kall, de Wilde, Dr. Bouwman, and Dr. Berkhof report no disclosures.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2019.04.011.
Supplementary Data
References
- 1.Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 2.Yeo J.M., Waddell B., Khan Z., Pal S. A systematic review and meta-analysis of (18)F-labeled amyloid imaging in Alzheimer's disease. Alzheimers Dement (Amst) 2015;1:5–13. doi: 10.1016/j.dadm.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson K.A., Minoshima S., Bohnen N.I., Donohoe K.J., Foster N.L., Herscovitch P., Alzheimer's Association. Society of Nuclear Medicine and Molecular Imaging. Amyloid Imaging Taskforce Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers Dement. 2013;9 doi: 10.1016/j.jalz.2013.01.002. e-1-e-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zwan M.D., Bouwman F.H., Konijnenberg E., van der Flier W.M., Lammertsma A.A., Verhey F.R. Diagnostic impact of [(18)F]flutemetamol PET in early-onset dementia. Alzheimers Res Ther. 2017;9:2. doi: 10.1186/s13195-016-0228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boccardi M., Altomare D., Ferrari C., Festari C., Guerra U.P., Paghera B., Incremental Diagnostic Value of Amyloid PETWFWG Assessment of the incremental diagnostic value of florbetapir F 18 imaging in patients with cognitive impairment: the incremental diagnostic value of amyloid PET with [18F]-Florbetapir (INDIA-FBP) Study. JAMA Neurol. 2016;73:1417–1424. doi: 10.1001/jamaneurol.2016.3751. [DOI] [PubMed] [Google Scholar]
- 7.Chiotis K., Saint-Aubert L., Boccardi M., Gietl A., Picco A., Varrone A., Geneva Task Force for the Roadmap of Alzheimer's Biomarkers Clinical validity of increased cortical uptake of amyloid ligands on PET as a biomarker for Alzheimer's disease in the context of a structured 5-phase development framework. Neurobiol Aging. 2017;52:214–227. doi: 10.1016/j.neurobiolaging.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 8.de Wilde A., van der Flier W.M., Pelkmans W., Bouwman F., Verwer J., Groot C. Association of amyloid positron emission tomography With changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE Project. JAMA Neurol. 2018;75:1062–1070. doi: 10.1001/jamaneurol.2018.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen W.J., Ossenkoppele R., Knol D.L., Tijms B.M., Scheltens P., Verhey F.R., Amyloid Biomarker Study Group Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trzepacz P.T., Yu P., Sun J., Schuh K., Case M., Witte M.M. Comparison of neuroimaging modalities for the prediction of conversion from mild cognitive impairment to Alzheimer's dementia. Neurobiol Aging. 2014;35:143–151. doi: 10.1016/j.neurobiolaging.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Jack C.R., Jr., Wiste H.J., Weigand S.D., Rocca W.A., Knopman D.S., Mielke M.M. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol. 2014;13:997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris J.C., Roe C.M., Xiong C., Fagan A.M., Goate A.M., Holtzman D.M. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheinin N.M., Wikman K., Jula A., Perola M., Vahlberg T., Rokka J. Cortical (1)(1)C-PIB uptake is associated with age, APOE genotype, and gender in “healthy aging”. J Alzheimers Dis. 2014;41:193–202. doi: 10.3233/JAD-132783. [DOI] [PubMed] [Google Scholar]
- 14.Landau S.M., Mintun M.A., Joshi A.D., Koeppe R.A., Petersen R.C., Aisen P.S., Alzheimer's Disease Neuroimaging Initiative (ADNI) Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisoni G.B., Boccardi M., Barkhof F., Blennow K., Cappa S., Chiotis K. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurol. 2017;16:661–676. doi: 10.1016/S1474-4422(17)30159-X. [DOI] [PubMed] [Google Scholar]
- 16.Rabinovici G.D., Gatsonis C., Apgar C., Chaudhary K., Gareen I., Hanna L. Association of Amyloid Positron Emission Tomography With Subsequent Change in Clinical Management Among Medicare Beneficiaries With Mild Cognitive Impairment or Dementia. JAMA. 2019;321:1286–1294. doi: 10.1001/jama.2019.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisoni G.B., Barkhof F., Altomare D., Berkhof J., Boccardi M., Canzoneri E. AMYPAD Diagnostic and Patient Management Study: Rationale and design. Alzheimers Dement. 2018;15:388–399. doi: 10.1016/j.jalz.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Petersen R.C., Aisen P.S., Beckett L.A., Donohue M.C., Gamst A.C., Harvey D.J. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Rowe C.C., Ng S., Ackermann U., Gong S.J., Pike K., Savage G. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 21.Bourgeat P., Chetelat G., Villemagne V.L., Fripp J., Raniga P., Pike K. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology. 2010;74:121–127. doi: 10.1212/WNL.0b013e3181c918b5. [DOI] [PubMed] [Google Scholar]
- 22.Jack C.R., Jr., Lowe V.J., Senjem M.L., Weigand S.D., Kemp B.J., Shiung M.M. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi A.D., Pontecorvo M.J., Clark C.M., Carpenter A.P., Jennings D.L., Sadowsky C.H. Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer's disease and cognitively normal subjects. J Nucl Med. 2012;53:378–384. doi: 10.2967/jnumed.111.090340. [DOI] [PubMed] [Google Scholar]
- 24.Koivunen J., Scheinin N., Virta J.R., Aalto S., Vahlberg T., Nagren K. Amyloid PET imaging in patients with mild cognitive impairment: a 2-year follow-up study. Neurology. 2011;76:1085–1090. doi: 10.1212/WNL.0b013e318212015e. [DOI] [PubMed] [Google Scholar]
- 25.Newson R.B. Comparing the predictive powers of survival models using Harrell's C or Somers. D Stata J. 2010;10:339–358. [Google Scholar]
- 26.Cefalu M. Pointwise confidence intervals for the covariate-adjusted survivor function in the Cox model. Stata J. 2011;11:64–81. [Google Scholar]
- 27.van der Flier W.M., Pijnenburg Y.A., Prins N., Lemstra A.W., Bouwman F.H., Teunissen C.E. Optimizing patient care and research: the Amsterdam Dementia Cohort. J Alzheimers Dis. 2014;41:313–327. doi: 10.3233/JAD-132306. [DOI] [PubMed] [Google Scholar]
- 28.van der Flier W.M., Scheltens P. Amsterdam dementia cohort: performing research to optimize care. J Alzheimers Dis. 2018;62:1091–1111. doi: 10.3233/JAD-170850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 30.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois B., Feldman H.H., Jacova C., Dekosky S.T., Barberger-Gateau P., Cummings J. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 32.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patenaude B., Smith S.M., Kennedy D.N., Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith S.M., Zhang Y., Jenkinson M., Chen J., Matthews P.M., Federico A. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 35.Rowe C.C., Bourgeat P., Ellis K.A., Brown B., Lim Y.Y., Mulligan R. Predicting Alzheimer disease with beta-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann Neurol. 2013;74:905–913. doi: 10.1002/ana.24040. [DOI] [PubMed] [Google Scholar]
- 36.Wolk D.A., Price J.C., Saxton J.A., Snitz B.E., James J.A., Lopez O.L. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowe C.C., Ellis K.A., Rimajova M., Bourgeat P., Pike K.E., Jones G. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Hollands S., Lim Y.Y., Laws S.M., Villemagne V.L., Pietrzak R.H., Harrington K. APOEvarepsilon4 genotype, amyloid, and clinical disease progression in cognitively normal older adults. J Alzheimers Dis. 2017;57:411–422. doi: 10.3233/JAD-161019. [DOI] [PubMed] [Google Scholar]
- 39.Yu J.T., Tan L., Hardy J. Apolipoprotein E in Alzheimer's disease: an update. Annu Rev Neurosci. 2014;37:79–100. doi: 10.1146/annurev-neuro-071013-014300. [DOI] [PubMed] [Google Scholar]
- 40.Fleisher A.S., Chen K., Liu X., Ayutyanont N., Roontiva A., Thiyyagura P. Apolipoprotein E epsilon4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging. 2013;34:1–12. doi: 10.1016/j.neurobiolaging.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Jack C.R., Jr., Wiste H.J., Vemuri P., Weigand S.D., Senjem M.L., Zeng G. Alzheimer's Disease Neuroimaging I. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thurfjell L., Lotjonen J., Lundqvist R., Koikkalainen J., Soininen H., Waldemar G. Combination of biomarkers: PET [18F]flutemetamol imaging and structural MRI in dementia and mild cognitive impairment. Neurodegener Dis. 2012;10:246–249. doi: 10.1159/000335381. [DOI] [PubMed] [Google Scholar]
- 43.Ong K.T., Villemagne V.L., Bahar-Fuchs A., Lamb F., Langdon N., Catafau A.M. Abeta imaging with 18F-florbetaben in prodromal Alzheimer's disease: a prospective outcome study. J Neurol Neurosurg Psychiatry. 2015;86:431–436. doi: 10.1136/jnnp-2014-308094. [DOI] [PubMed] [Google Scholar]
- 44.van Maurik I.S., Zwan M.D., Tijms B.M., Bouwman F.H., Teunissen C.E., Scheltens P., Alzheimer's Disease Neuroimaging Initiative Interpreting Biomarker Results in Individual Patients With Mild Cognitive Impairment in the Alzheimer's Biomarkers in Daily Practice (ABIDE) Project. JAMA Neurol. 2017;74:1481–1491. doi: 10.1001/jamaneurol.2017.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Wilde A., van Buchem M.M., Otten R.H.J., Bouwman F., Stephens A., Barkhof F. Disclosure of amyloid positron emission tomography results to individuals without dementia: a systematic review. Alzheimers Res Ther. 2018;10:72. doi: 10.1186/s13195-018-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grill J.D., Johnson D.K., Burns J.M. Should we disclose amyloid imaging results to cognitively normal individuals? Neurodegener Dis Manag. 2013;3:43–51. doi: 10.2217/nmt.12.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts J.S., Dunn L.B., Rabinovici G.D. Amyloid imaging, risk disclosure and Alzheimer's disease: ethical and practical issues. Neurodegener Dis Manag. 2013;3:219–229. doi: 10.2217/nmt.13.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grill J.D., Cox C.G., Kremen S., Mendez M.F., Teng E., Shapira J. Patient and caregiver reactions to clinical amyloid imaging. Alzheimers Dement. 2017;13:924–932. doi: 10.1016/j.jalz.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunneman M., Pel-Littel R., Bouwman F.H., Gillissen F., Schoonenboom N.S.M., Claus J.J. Patients' and caregivers' views on conversations and shared decision making in diagnostic testing for Alzheimer's disease: the ABIDE project. Alzheimers Dement (N Y) 2017;3:314–322. doi: 10.1016/j.trci.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grill J.D., Apostolova L.G., Bullain S., Burns J.M., Cox C.G., Dick M. Communicating mild cognitive impairment diagnoses with and without amyloid imaging. Alzheimers Res Ther. 2017;9:35. doi: 10.1186/s13195-017-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.