Abstract
Purpose
Fractures of the humerus account for 5%–8% of all fractures. Nonunion is found with an incidence of up to 15%, depending on the location of the fracture. In case of a manifest nonunion the surgeon faces a challenging problem and has to conceive a therapy based on the underlying pathology. The aim of this study was to describe our treatment concepts for this entity and present our results of the last five years.
Methods
Twenty-six patients were treated for nonunion of the humerus between January 2013 and December 2017. Their charts were reviewed retrospectively and demographic data, pathology, surgical treatment and outcome were assessed.
Results
The most frequent location for a nonunion was the humeral shaft, with the most common trauma mechanism being multiple falls. Most often atrophic nonunion (n = 14), followed by hypertrophic and infection-caused nonunion (each n = 4), were found. Our treatment concept could be applied in 19 patients, of which in 90% of those who were available for follow-up consolidation could be achieved.
Conclusion
Humeral nonunion is a heterogeneous entity that has to be analyzed precisely and be treated correspondingly. We therefore present a treatment concept based on the underlying pathology.
Keywords: Humerus, Nonunion, Delayed union, Humeral fractures
Introduction
Fractures of the humerus are a common injury accounting for 5%–8% of all fractures.1 In general, they have a good tendency to heal. However nonunion is found with an incidence up to 15%.2 This differs depending on the location of the fracture. The highest is found in proximal humerus fractures.3
Smoking, alcohol abuse, diabetes mellitus and age, unstable primary osteosynthesis, open fractures, vessel injury and infection are common risk factors for developing a nonunion.
Different systems have been proposed for the classification of nonunions with that by Weber and Cech (1973) the most commonly used and which is orientated on the surgical treatment of nonunions.4 It differentiates hypertrophic nonunion which is biologically active and vital from non-viable and biologically inactive nonunion. Those are commonly caused by insufficient blood-supply and can turn into atrophic nonunion.5
In the case of a nonunion the surgeon faces a challenging problem. Depending on the trauma mechanism, the patient's constitution, comorbidities and previous treatment he has to deal with critical soft-tissue, infection, and poor quality of the bone or large bone-defects. After analysis of the underlying problem, the surgeon must decide which operative strategy is appropriate. Different techniques, such as fixed-angle locking plates, intramedullary nailing, allografts, external fixator and combinations are available.
When looking at the literature, no significant difference can be found between intramedullary nailing (IM) and open reduction and internal fixation (ORIF) concerning the incidence of nonunion in primary therapy of humeral fractures.6, 7, 8, 9, 10 In the case of nonunion treatment, the literature shows better results for open revision and locking plating compared to intramedullary nailing.11, 12
The aim of this retrospective study was to describe our treatment concepts for this entity and present the results of patients treated for nonunion of the humerus in our level one trauma center within the last five years.
Methods
Twenty-six patients were treated for nonunion of humeral fractures between January 2013 and December 2017 in our level one trauma center. Patients were identified by searching our institute's database for ICD-code M84.12. Patients treated for nonunion of the humerus (proximal, shaft, distal) following conservative or operative treatment between 18 and 85 years were included. Patients with pathological fractures and immunocompromised patients were excluded.
Patients' charts were reviewed retrospectively in terms of demographic and social information (gender, age, comorbidities). Fractures were classified with regard to their location and as proposed by the AO foundation (AO Trauma Deutschland, Berlin, Germany), respectively after Worland et al. in the case of periprosthetic fractures.13
Nonunion was defined as lack of boney bridging of a fracture after 6–12 months and delayed union was defined as fracture healing after four but within six months.14
The diagnosis was based on radiological and clinical findings and classified as described by Weber and Cech4 as hypertrophic atrophic and avital. Furthermore, infection triggered and defect nonunion were differentiated.
Patients' history was analyzed with regard to the initial fracture treatment, number of operations, revision strategy, time to union and achievement of union. Union was evaluated in conventional radiographs and was defined as appearance of bridge callus or bridging of the cortex with at least partial obliteration of the fracture site observed on anteroposterior and lateral radiographs.15
Therapy costs were registered from our institute's data base.
Data were analyzed with the statistical framework R (version 3.5.1) with continuous variables are presented as medians and median absolute deviation (MAD). The Wilcoxon-Mann-Whitney-U-test was used for comparisons between two groups. Pearson's chi-square-test was used to analyze the independence of two variables. A p value < 0.05 is considered statistically significant. Patient information was anonymized prior to analysis.
Results
Fourteen (54%) patients treated for nonunion of the humerus were female and 12 (46%) were male. The median age was 59 years (median = 59, MAD = 15.26). Analyzing comorbidities diabetes mellitus type II (n = 4), arterial hypertension (n = 8), hypothyroidism (n = 4) as well as depression (n = 3) were most common. Seven patients had no comorbidities (Table 1).
Table 1.
Demographic characteristics, fracture classification, initial trauma, comorbidities and costs of nonunion-treatment.
| No. | Age | Sex | Fracture | Trauma causes | Comorbidities | Costs (in €) |
|---|---|---|---|---|---|---|
| 1 | 60 | F | AO 12A | Multiple falls | Asthma bronchiale | 8566.00 |
| 2 | 53 | M | B3 (Worland) | Fall | Hypertension, shoulder prosthesis | 8719.00 |
| 3 | 75 | F | AO 11C | Fall | Hypertension, rheumatoid arthritis, thyroidectomy, stroke, diabetes mellitus II, Giullain-Barre | 8762.00 |
| 4 | 55 | F | AO 11A | Fall | Depression | 8762.00 |
| 5 | 82 | M | AO 11A | Fall | Poliomyelitis (paraparesis), gait disorder, spinal stenosis, diabetes II chronic gastritis | 9761.00 |
| 6 | 20 | M | AO13C | Fall, luxation fracture | None | 7534.00 |
| 7 | 70 | F | AO 12A, AO 13C | Multiple falls | Stroke, hypertension, plexus brachialis-lesion, radialis paresis | 9651.00 |
| 8 | 78 | F | AO 11B | Fall | Hypertension | 4904.00 |
| 9 | 22 | M | AO 13C | Polytrauma | Polytrauma: splenic rupture, renal rupture, pancreatic rupture, liver rupture, sternal fracture, pneumothorax, lumbar fracture, acl-rupture, tossy–III– injury | 6305.00 |
| 10 | 70 | M | AO 12A | Fall | Stroke | 6541.00 |
| 11 | 26 | M | Gunshot, distal shaft defect | Gunshot | None | 6107.00 |
| 12 | 47 | M | AO 12A | Motorbike crash | None | 10448.00 |
| 13 | 77 | F | AO 12B | Fall | Hypothyreosis | 5177.00 |
| 14 | 51 | M | AO 12A | Fall | None | 5352.00 |
| 15 | 63 | F | AO 11A | Fall | Diabetes mellitus II, hypothyreosis, hypertension | 5141.00 |
| 16 | 51 | M | AO 11A | Fall | None | 4488.00 |
| 17 | 58 | F | AO 12C | Car crash | Rheumatoid arthritis | 2212.00 |
| 18 | 18 | F | AO 13C | Horse kick, osteotomy | None | 5247.00 |
| 19 | 68 | F | AO 12A | Fall | COPD, depression, reflux oesophagitis | 5246.00 |
| 20 | 29 | M | Gunshot, defect | Gunshot | None | 7847.00 |
| 21 | 75 | F | AO 13C | Fall | Hypothyreosis, hypertension | 3127.00 |
| 22 | 65 | F | AO 12A | Fall | Hypertension, atrial fibrillation | 6684.00 |
| 23 | 48 | F | AO 12B | Fall, iatrogenic | Mammary-carcinoma, depression, glaucoma | 6684.00 |
| 24 | 55 | F | AO 12A | Fall | Hepatitis C, former IVDA, hypertension, chron. alcohol abuse | 4970.00 |
| 25 | 73 | M | AO 13A | Fall | Bronchial carcinoma, heart failure NYHA 4, pulmonary hypertension, anemia, COPD, hypertension, femoralis stent, gastric ulcera, chronic pancreatitis, reflux oesophagitis | 14382.00 |
| 26 | 70 | M | Periprosthetic | Fall | Diabetes mellitus II, hypercholesterinaemia, myocardial infarct | 5246.00 |
F: female; M: male.
Initial accident causes were fall, and multiple falls in 16 patients. Traffic accidents were indicated two cases (n = 1 car, n = 1 motorcycle), an osteotomy following improperly healed fracture after horse kick in one case, gunshot injuries in two cases and one patient suffered from polytrauma (Table 1).
Analyzing the fracture site, seven subcapital humeral fractures, 12 shaft-fractures, six distal humeral fractures and two periprosthetic humeral fractures (n = 1 shoulder, n = 1 elbow-prosthesis) were found. Detailed classification is listed in Table 1.
In the case of the 14 atrophic nonunions, ten fractures were initially stabilized by plates ± cerclage (n = 5 shaft, n = 2 distal, n = 3 subcapital). Three were fixed by intramedullary nailing (n = 3 shaft) and one subcapital fracture was treated conservatively. Three of the four hypertrophic fractures were initially stabilized by intramedullary nailing (n = 1 subcapital, n = 2 shaft) and one shaft-fracture was fixed by a plate. In three cases an infection occurred after plating (n = 3 distal) and, in one case, after a conservative therapy (chronic infection). One gunshot-fracture was initially stabilized by an intramedullary nail (shaft) and one with elastic stable intramedullary nail (ESIN) and K-wires (distal + prox. radius). In one case, an atrophic nonunion occurred due to a broken fixed-angle plate (subcapital) and, in one patient, a conservatively treated fracture of the shaft dislocated (Table 2).
Table 2.
Classification and site of nonunion, initial and revision treatment, substitute, surgical interventions, and outcome.
| No. | Nonunion | Fracture site | Initial treatment | Revision | Substitute | No. of operations | Union | Time to union (month) |
|---|---|---|---|---|---|---|---|---|
| 1 | Atrophic | Shaft | Plate → im-nail + cerclage | Fixed-angle plate | Allograft, RIA | 1 | Yes | 12 |
| 2 | Atrophic | Shaft | Plate + cerclage | Fixed-angle plate | Allograft, RIA, strut graft | 1 | Yes | 8 |
| 3 | Atrophic | Subcapital | Conservative | Prothesis (Global FX) | Iliac crest, spongiosa | 1 | Prothesis | |
| 4 | Hypertrophic | Subcapital | Im-nail | Prothesis (Epoca), inverse prothesis, revision | Iliac crest, spongiosa | 4 | Prothesis | |
| 5 | Infection | Subcapital | Conservative | Resection humeral head | None | 5 | Resection | |
| 6 | Infection | Distal | Double plating | Prothesis (tornier) | 15 | Prothesis | ||
| 7 | Atrophic | Shaft + distal | Im-nailing | Fixed angle plate, prothesis (Lattitude) | None | 3 | No folow-up | |
| 8 | Atrophic | Subcapital | Plating | Fixed-angle plate | Iliac crest, spongiosa | 1 | Progressive consolidation after 12 weeks | |
| 9 | Infection | Distal | Double plating → DJD + free latissimus flap | Fixed-angle plate | Iliac crest, spongiosa | 5 | No folow-up | |
| 10 | Atrophic | Shaft | Plating | Fixed-angle plate | RIA, nano-bone | 2 | None (8 months) | |
| 11 | Defect | Distal shaft + proximal radius | ESIN + K-wires | Fixed-angle plate | Spongiosa | 1 | Yes | 3 |
| 12 | Hypertrophic | Shaft | Plating | Fixed-angle plate, external fixator, fibula-transfer, plating and screw | RIA, fibula-transfer, spongiosa | 5 | Yes | 14 |
| 13 | Atrophic | Shaft | Plating | None | Iliac crest, spongiosa | 1 | Yes | 6 |
| 14 | Hypertrophic | Shaft | Im-nail | Bigger im-nail | Reaming | 1 | No follow-up | |
| 15 | Material failure | Subcapital | Fixed-angle plate | Fixed-angle plate | Iliac crest, spongiosa | 2 | No follow-up | |
| 16 | Atrophic | Subcapital | Fixed-angle plate | Fixed-angle plate | Iliac crest, spongiosa | 2 | No follow-up | |
| 17 | Atrophic | Shaft | Im-nail | Fixed-angle plate/suralis | RIA | 2 | Yes | 11 |
| 18 | Atrophic | Distal | Plating | Fixed-angle plate | Iliac crest, spongiosa | 2 | Yes | 12 |
| 19 | Atrophic | Subcapital | Plating + cerclage | Fixed-angle plate | Iliac crest, spongiosa | 1 | No follow-up | |
| 20 | Defect | Shaft | Im-nail | Im-nail | RIA + allograft (ChronOs) | 1 | No follow-up | |
| 21 | Atrophic | Distal | Plating + tension band wiring | Fixed-angle plate | Spongiosa | 3 | Not yet | |
| 22 | Dislocation | Shaft | Conservative | Fixed-angle plate | None | 1 | No follow-up | |
| 23 | Atrophic | Shaft | Im-nail | Fixed-angle plate | None | 1 | Partially | 3 |
| 24 | Hypertrophic | Shaft | Im-nail | Fixed-angle plate | RIA | 2 | No follow-up | |
| 25 | Infection | Distal | Plating | External fixator | Curettage | 4 | Partially | |
| 26 | Atrophic | Shaft | Plating + cerclage | Fixed-angle plate | Allograft, strut graft | 1 | No follow-up |
Twelve of the 14 atrophic nonunions received fixed-angle locking plate in revision, one received an anatomic shoulder prothesis (after initially conservative treatment) and one was changed to fixed-angle locking plate (shaft) and elbow prosthesis.
In the case of the four hypertrophic nonunions, one was treated by an inverse shoulder prothesis (after intramedullary nailing), one was reamed and changed to a bigger nail, one underwent osteosynthesis by fixed-angle locking plate and one fixed-angle locking plate plus free fibula-transfer. One of the four patients with infection received elbow prosthesis after infection control (15 operations), one was stabilized by fixed-angle locking plates (distal) and one by an external fixator. In one case the humeral head had to be resected.
One of the two gunshot injuries was treated by an intramedullary nail plus spongiosa and allograft (reamer/irrigator/aspirator (RIA) + beta-tricalcium phosphate (chronOs®, Synthes GmbH, Eimattstr., Oberdorf, Swiss) and one with fixed-angle locked plates plus spongiosa.
In the case of the conservatively treated shaft fracture, that was secondarily dislocated, a fixed-angle locked plate was used for stabilization (Table 2).
There was no statistically significant dependency between the use of an intramedullary nail or a plate and the occurrence of an atrophic, or hypertrophic nonunion.
In the case of an infection as reason for nonunion, patients required significantly more operative interventions compared to other patients in order to control the infection (p = 0.002, infection: median = 5, MAD = 3.86, no infection: median 1, MAD = 0.84).
Three of the 26 patients received an endoprosthesis and one underwent resection of the humeral head. Ten patients got lost for follow-up. Of the remaining 12 patients seven (58%) reached complete consolidation, two (17%) reached partial consolidation and three (25%) did not show consolidation within the period of observation. The mean time to achieve complete consolidation was 11 months (median = 11, MAD = 3.22). Of the seven consolidated nonunions five were atrophic, one resulted from defect and one was hypertrophic (Table 2).
The median age of patients that achieved union was 53 years (median = 53, MAD = 15.5), while the median age of patients that did not achieve union was 75 years (median = 75, MAD = 2.89).
The costs of therapy including surgical interventions in our center accounted for 6432.00€ per patient (median = 6432.00, MAD = 2001.69, mean = 6840.81) (Table 1).
Discussion
Results of the last five years
Hypertension (31%), diabetes mellitus II (15%), and hypothyroidism (15%) were the most common comorbidities among our patients who suffered from humeral nonunions. Besides smoking, alcohol abuse and nonsteroid anti-inflammatory drugs (NSAID)-therapy, these comorbidities count as risk factors for developing delayed or nonunion.3, 16, 17
Rheumatoid arthritis (8%) and depression (12%) were stated to be further promoting conditions17, 18 and were found among our patients as well. Contrary to the data of Hernandez et al., who found older patients to have lower odds for fracture healing complications than younger,17 we observed a trend for older patients to develop an irregular healing process. Some authors observed a higher rate in younger and middle-aged patients to develop nonunion compared to older patients.19, 20 Van Wunnik et al. showed that even though older patients might have a decreased bone quality, osteoporosis has now impact on the occurrence of nonunion and the measurement of bone mineral density (BMD) has no clinical value21 to identify osteoporosis as a risk factor for nonunion. However, once a nonunion has actually occurred, older patients have an increased risk for persisting nonunion.22 This is in line with the results in our study, with the mean age of all patients being 59 years but 75 years in patients with persisting nonunion.
Due to the low sample number no statistical evaluation could be performed in our study.
Low sample size is identified as one of the limitations of this study, in addition to which no clinically evaluated outcome scores were used and only 12 patients could be followed up. Patients were very inhomogeneous and due to different underlying pathologies, varying locations and initial treatment concepts, “humeral nonunion” is a heterogeneous entity itself. In this study, we aimed to describe our treatment concepts for different manifestations of humeral nonunion depending on their underlying pathology and present our results of the last five years.
Treatment concepts
Giannoudis et al. developed the diamond concept stating required conditions for fracture healing. Besides the presence of growth factors and osteogenic cells, an osteoconductive scaffold and mechanical stability as well as sufficient vascular support are essential factors to support fracture healing.23
A key factor for development of particularly atrophic nonunion is an inadequate angiogenesis caused by the initial trauma or subsequent to surgical interventions.16
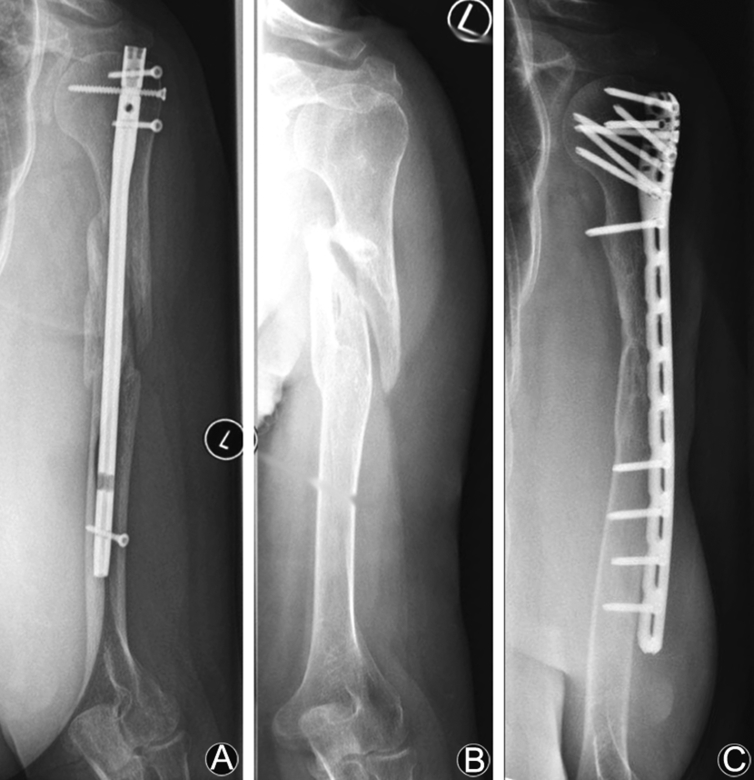
In the case of insufficient reduction and fixation of the fracture, the risk of irregular healing process is increased significantly5 (Fig. 1). Drosos et al.24 found a significantly prolonged time to union in tibial shaft fractures after static locked intramedullary-nailing in presence of a fracture gap >3 mm.
Fig. 1.
Fracture of the humeral shaft after car crash. Initially treated with locked intramedullary nail in Turkey. (A) Postoperative lesion of nervus radialis caused by the distal locking screw with complete loss of function of nervus radialis. (B) Removement of the nail and interposition of nervus suralis three months after initial accident. (C) Nonunion and revision with RIA and locking plate 16 months after suralis-interposition. After one year complete consolidation, active wrist extension 60°, no sensorial deficiency.
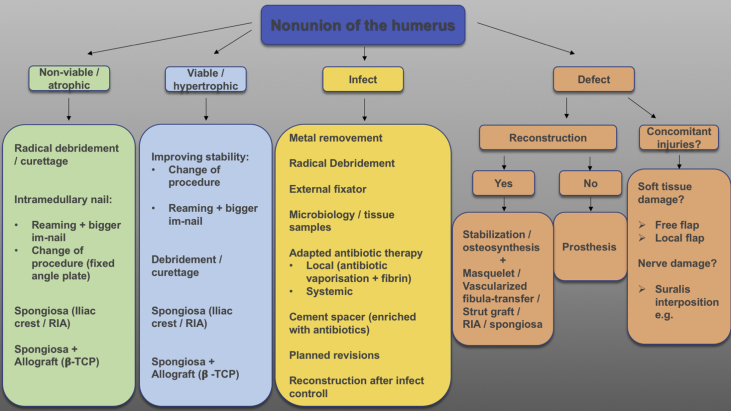
In order to treat nonunion successfully, the underlying and most often multifactorial pathology has to be analyzed exactly and therapy must aim at restoring the missing factors (Fig. 2).
Fig. 2.
Treatment algorithm for nonunions of the humerus.
Non-viable/atrophic
The underlying cause of non-viable, atrophic nonunion might be found in a lack of osteogenic cells and growth factors at the fracture site, caused by reduced vascularity at the defect gap.25 Extensive soft-tissue stripping by the initial injury or subsequent to surgery is suggested to cause osteonecrosis.16 Therefore, non-viable, atrophic fragments and fibrous scar tissue in the fracture gap should be removed radically.
If osteosynthesis was initially performed by an intramedullary nail, reaming and exchange to a bigger sized nail might be considered. Reaming debris contains a rich amount of osteoblast-like cells, growth factors and viable osteoprogenitor cells.26, 27, 28 Due to the transport of mesenchymal stem cells (MSC) into the intramedullary space, an “internal bone grafting” can be achieved by the reaming procedure.5
To prevent excessive heat during reaming procedure, a sharp drill bit should be used.28 However, as a reaction to the destroyed endostal circulation, it is reported, that subsequent to the reaming procedure, the periosteal blood flow is increased and supports bone formation.29
Another important aspect of exchange nailing is the improved biomechanical stability that can be achieved by using a bigger nail. The diameter of the new nail should be at least 1 mm bigger than previously.28, 29
There are no significant differences regarding the incidence of nonunion if intramedullary nailing or respectively dynamic compression plating of humeral fractures was performed initially.7, 8, 9, 10 However, in the case of a once manifested nonunion, locking compression plates seem to bring out better results concerning fracture consolidation.11, 12
In line with Rupp et al. we therefore recommend compression plating in combination with bone grafting (Fig. 1) to ensure sufficient debridement of devitalized tissue and defect filling.
Either spongiosa from the iliac crest or femur (RIA, reamer/irrigator/aspirator) or a bone graft substitute (e.g. β-tricalciumphosphate, chronOs®, Synthes GmbH, Eimattstr., Oberdorf, Swiss) in combination with autologous spongiosa can be used. Harvest procedure and tissue site have significant influence on the quality of autologous bone graft as we evaluated in previous studies. Spongiosa acquired by RIA contains significantly higher levels of CD34 + progenitor cells, early endothelial progenitor cells (EPC) and MSC when compared to aspirates from iliac crest.27 Also, calcium deposition is significantly increased in femur-derived MSCs compared to iliac-crest derived MSC.30
As a future perspective bone marrow mononuclear cells (BMC) seeded on a bone graft substitute might be an alternative to autologous spongiosa. BMC have been shown to have beneficial effects on bone healing in several preclinical studies.31, 32 They can be harvested from iliac crest and do not have to be cultured for expansion. Currently these preclinical findings are transferred to clinic in our multicenter phase IIa clinical study (EudraCT-Nr.: 2015-001820-51).33
Viable/hypertrophic
Development of hypertrophic nonunion is associated to insufficient stability.16 Therefore, surgical intervention must aim to improve stability, e.g. by exchange nailing or locked angle plating. Fibrous scar tissue or possible interposed tissue must be debrided. If necessary, spongiosa, with or without allograft has to be implanted.
A non-operative option for stimulation of bone healing is low-intensity pulsed ultrasound (LIPUS). Interestingly, hypertrophic nonunions benefit more than atrophic nonunions by this procedure. The reason for that remains unclear, especially as LIPUS does not address mechanical stability.34
One suggested means of action is stimulation of COX2-production and subsequently expression of osteogenic genes that stimulate mineralization and enhance endochondral ossification.35
In their analysis, Zura et al. found a healing rate of 86.2% in 767 chronic nonunions when treated with LIPUS.36
Some authors recommend to not apply ultrasound therapy because of lack of evidence and questionable functional benefit.37, 38 However, LIPUS might be considered as an helpful option additionally to surgery as no harmful side-effects are known, it is easy to use, allows self-administration at home and many publications report a benefit for healing of nonunions36, 39, 40 (Fig. 3).
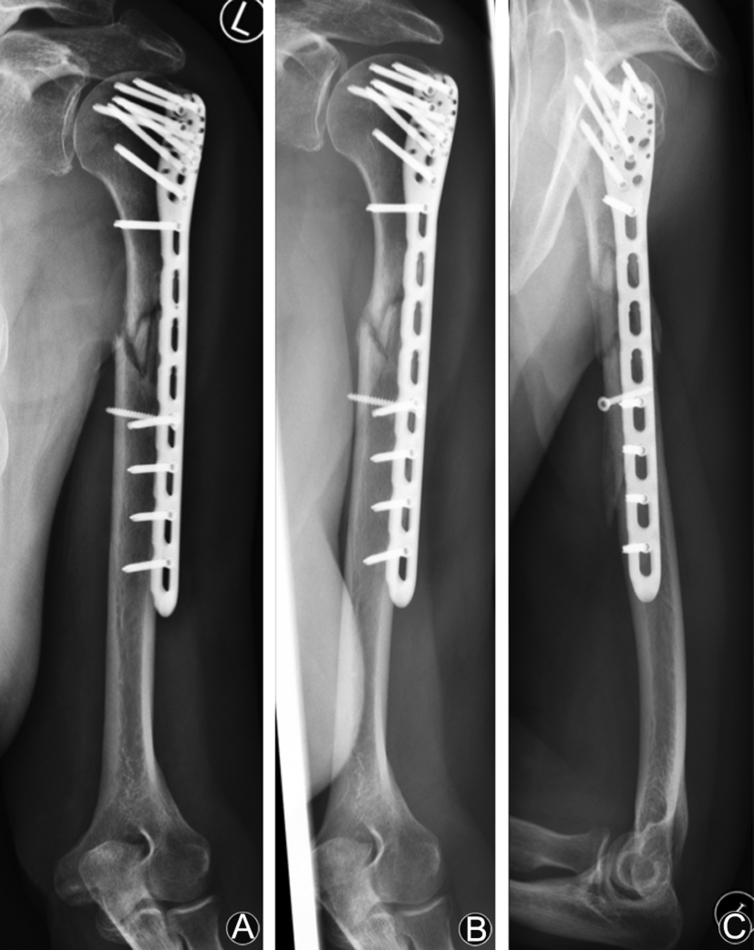
Fig. 3.
A 53-year old female patient with multifragmentary shaft-fracture (AO type 12C3) and open reduction and fixation with PHILOS. (A) After 12 weeks no signs of consolidation. (B and C) After 18 weeks and 4 weeks of low-intensity pulsed ultrasound (LIPUS) distinctly progredient consolidation and boney bridging.
Infection
Infection is stated to be the cause of nonunion in up to 38% of the cases.16 Mills et al.16 reported the importance of taking multiple tissue samples in every surgical revision of nonunion as they found entirely unexpected infections in 5% of their patients.
In the case of an infect-triggered nonunion, radical debridement is required. Implanted metal must be removed and stabilization has to be achieved by an external fixator if necessary. Multiple tissue samples (at least three samples) must be taken for microbiological analysis. Adapted antibiotic treatment should be conducted systemically and locally. For topical application, we recommend the local fixation of antibiotics by fibrin spray as described by Janko et al.41
In presence of an infection, significantly more revisions were needed in order to achieve bone healing (p = 0.002). Reconstruction of the defect can be planed after control of infection.
Defect
In the case of nonunion due to an osseous defect, e.g. subsequent to a gunshot injury or after multiple debridements, it has to be evaluated as to whether reconstruction is possible or an endoprosthetic management is required. Concomitant injuries have to be taken into account and the consolidation of soft tissue must be achieved, if necessary, by a free or local flap, before further reconstruction.42 Neurological status has to be assessed and in the presence of nerve damage, reconstruction has to be considered (e.g. interposition of nervus suralis)43, 44 (Fig. 4).
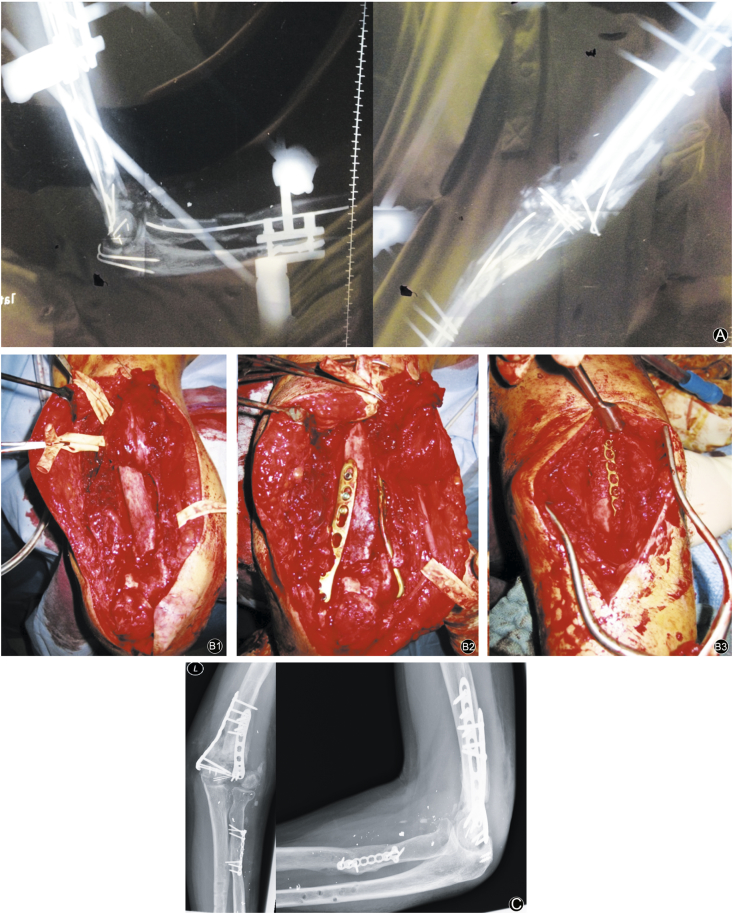
Fig. 4.
A patient with gunshot injury of left elbow and forearm in Lebanon. (A) Extensive destruction of supracondylar humerus, olecranon and proximal radius with loss of function of radial and ulnar nerve. Primary treatment with external fixator, ESIN and K-wires. Humeral and radial nonunion after 11 months. (B) Revision humerus with removal of initial osteosynthesis, neurolysis (kinking of nervus radialis), debridement, cancellous bone from iliac crest, double plating (locked, radial and ulnar). Revision of the proximal radius with debridement, internal reduction and defect filling with cancellous bone from iliac crest. (B1: intraoperative situs, B2: radial and ulnar locking plate humerus, B3: radial defect filled with cancellous bone and stabilized with plate). (C) After 12 weeks consolidation radius, progredient consolidation humerus, range of motion 0°/5°/45° ext/flex, 30°/0°/15° pro-/supination. Active wrist extension above horizontal level.
If reconstruction is possible, different options are available. Firstly, stabilization must be achieved, either by fixed-angle locking plate, nail or external fixator, such as a ring fixator (Ilizarov).
Secondly, reconstruction of the osseous defect follows. While defects up to 2 cm can be treated with debridement, shortening and local application of bone graft (RIA, iliac crest, allograft), larger defects might be treated with a two-step procedure as described by Masquelet et al.22, 45
Fibula grafts represent a further option, especially after multiple operations with bone loss due to previous implants, scalloping around screws or osteoporotic patients. They can be implanted as intramedullary strut or in sandwich technique, vascularized or non-vascularized46 (Fig. 5).
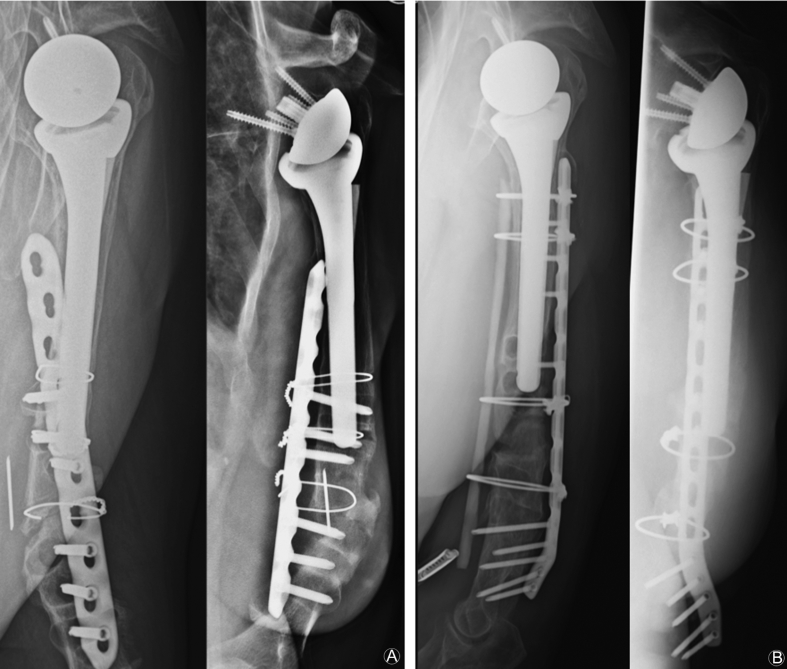
Fig. 5.
(A) Nonunion after periprosthetic fracture with open reduction and internal fixation with locking plate and cerclage. (B) Revision after 17 months with metal removal, debridement, defect-filling with RIA, allogenic strut graft in sandwich-technique and fixation with locking plate and cerclages.
Of the patients included in this study, 19 were treated following the concept as shown in Fig. 2. Of these patients, we were able to follow up eleven. One of those did not achieve healing after eight months, while the other ten patients showed consolidation of the nonunion. The key to successful treatment of a nonunion is the detailed analysis of the underlying pathology which then might be addressed as proposed in Fig. 2.
Conclusion
Humeral nonunion is a heterogeneous entity that has to be analyzed for its underlying pathology and be treated correspondingly.
Tissue samples should be taken in every revision for nonunion in order to exclude an infection as trigger. Consequent debridement, as well as stable fixation, followed by adequate defect filling by implantation of cancellous bone, with or without allograft, are essential requirements for bone healing. In some cases, more excessive methods, such as the induced membrane technique or free fibula transplants become necessary. BMC-enriched allografts are a future perspective to support bone formation.
Funding
There has been no source of support/funding, either pharmaceutical or industry support. No funding from any organization has been received.
Ethical statement
This retrospective study was approved by the ethic committee of the Johann Wolfgang Goethe-University Frankfurt (404/18).
Conflicts of interest
Maximilian Leiblein, René Verboket, Ingo Marzi, Nils Wagner, Christoph Nau declare that they have no conflict of interest.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Volgas D.A., Stannard J.P., Alonso J.E. Nonunions of the humerus. Clin Orthop Relat Res. 2004;419:46–50. doi: 10.1097/00003086-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Wenzl M.E., Porté Thomas, Fuchs Stefan. Verfahren zur rekonstruktion und osteosynthese von pseudarthrosen des humerus. Trauma Berufskrankh. 2003;5:s86–s91. [Google Scholar]
- 3.Badman B.L., Mighell M., Kalandiak S.P. Proximal humeral nonunions treated with fixed-angle locked plating and an intramedullary strut allograft. J Orthop Trauma. 2009;23:173–179. doi: 10.1097/BOT.0b013e31819b0bdc. [DOI] [PubMed] [Google Scholar]
- 4.Weber B.G., Cech O. first ed. Hans Huber; Bern: 1973. Pseudarthrosen: Pathophysiologie, Biomechanik, Therapie, Ergebnisse; pp. 42–44. [Google Scholar]
- 5.Rupp M., Biehl C., Budak M. Diaphyseal long bone nonunions - types, aetiology, economics, and treatment recommendations. Int Orthop. 2018;42:247–258. doi: 10.1007/s00264-017-3734-5. [DOI] [PubMed] [Google Scholar]
- 6.Rommens P.M., Kuechle R., Bord T. Humeral nailing revisited. Injury. 2008;39:1319–1328. doi: 10.1016/j.injury.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Changulani M. Comparison of the use of the humerus intramedullary nail and dynamic compression plate for the management of diaphyseal fractures of the humerus: reply to Subasi and Cebesoy. Int Orthop. 2007;32:141. doi: 10.1007/s00264-006-0200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denies E., Nijs S., Sermon A. Operative treatment of humeral shaft fractures. Comparison of plating and intramedullary nailing. Acta Orthop Belg. 2010;76:735–742. [PubMed] [Google Scholar]
- 9.Wali M.G.R., Baba A.N., Latoo I.A. Internal fixation of shaft humerus fractures by dynamic compression plate or interlocking intramedullary nail: a prospective, randomised study. Strategies Trauma Limb Reconstr. 2014;9:133–140. doi: 10.1007/s11751-014-0204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu G.D., Zhang Q.G., Ou S. Meta-analysis of the outcomes of intramedullary nailing and plate fixation of humeral shaft fractures. Int J Surg. 2013;11:864–868. doi: 10.1016/j.ijsu.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Kumar M.N., Ravindranath V.P., Ravishankar M. Outcome of locking compression plates in humeral shaft nonunions. Indian J Orthop. 2013;47:150–155. doi: 10.4103/0019-5413.108899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilyas I., Younge D.A. Locked intramedullary nailing for difficult nonunions of the humeral diaphysis. Int Orthop. 2003;27:278–281. doi: 10.1007/s00264-003-0470-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worland R.L., Kim D.Y., Arredondo J. Periprosthetic humeral fractures: management and classification. J Shoulder Elb Surg. 1999;8:590–594. doi: 10.1016/s1058-2746(99)90095-2. [DOI] [PubMed] [Google Scholar]
- 14.Kaminski A., Muhr G. Pseudarthrosen. Orthopädie und Unfallchirurgie. 2014;9:463–480. [Google Scholar]
- 15.Ayotunde O.A., Sunday O.K., Oluwatoyin A. Results of surgical treatment of nonunion of humeral shaft fracture with dynamic compression plate and cancellous bone grafting. Acta Ortopédica Bras. 2012;20:223–225. doi: 10.1590/S1413-78522012000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills L., Tsang J., Hopper G. The multifactorial aetiology of fracture nonunion and the importance of searching for latent infection. Bone Joint Res. 2016;5:512–519. doi: 10.1302/2046-3758.510.BJR-2016-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez R.K., Do T.P., Critchlow C.W. Patient-related risk factors for fracture-healing complications in the United Kingdom general practice research database. Acta Orthop. 2012;83:653–660. doi: 10.3109/17453674.2012.747054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie C., Wang Z., Liu X. The effect of depression on fracture healing and osteoblast differentiation in rats. Neuropsychiatric Dis Treat. 2018;14:1705–1713. doi: 10.2147/NDT.S168653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zura R., Braid-Forbes M.J., Jeray K. Bone fracture nonunion rate decreases with increasing age: a prospective inception cohort study. Bone. 2017;95:26–32. doi: 10.1016/j.bone.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Mills L.A., Aitken S.A., Simpson A.H.R.W. The risk of non-union per fracture: current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017;88:434–439. doi: 10.1080/17453674.2017.1321351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Wunnik B.P., Weijers P.H., van Helden S.H. Osteoporosis is not a risk factor for the development of nonunion: a cohort nested case-control study. Injury. 2011;42:1491–1494. doi: 10.1016/j.injury.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Miska M., Findeisen S., Tanner M. Treatment of nonunions in fractures of the humeral shaft according to the Diamond Concept. Bone Joint J. 2016;98-B:81–87. doi: 10.1302/0301-620X.98B1.35682. [DOI] [PubMed] [Google Scholar]
- 23.Giannoudis P.V., Einhorn T.A., Marsh D. Fracture healing: the diamond concept. Injury. 2007;38(Suppl 4):S3–S6. doi: 10.1016/s0020-1383(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 24.Drosos G.I., Bishay M., Karnezis I.A. Factors affecting fracture healing after intramedullary nailing of the tibial diaphysis for closed and grade I open fractures. J Bone Joint Surg Br. 2006;88:227–231. doi: 10.1302/0301-620X.88B2.16456. [DOI] [PubMed] [Google Scholar]
- 25.Moghaddam A., Ermisch C., Schmidmaier G. Non-union current treatment concept. Int J Med Educ. 2016;3:e4546. [Google Scholar]
- 26.Wenisch S., Trinkaus K., Hild A. Human reaming debris: a source of multipotent stem cells. Bone. 2005;36:74–83. doi: 10.1016/j.bone.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Henrich D., Seebach C., Sterlepper E. RIA reamings and hip aspirate: a comparative evaluation of osteoprogenitor and endothelial progenitor cells. Injury. 2010;41(Suppl 2):S62–S68. doi: 10.1016/S0020-1383(10)70012-7. [DOI] [PubMed] [Google Scholar]
- 28.Hierholzer C., Friederichs J., Glowalla C. Reamed intramedullary exchange nailing in the operative treatment of aseptic tibial shaft nonunion. Int Orthop. 2017;41:1647–1653. doi: 10.1007/s00264-016-3317-x. [DOI] [PubMed] [Google Scholar]
- 29.Brinker M.R., O'Connor D.P. Exchange nailing of ununited fractures. J Bone Joint Surg Am. 2007;89:177–188. doi: 10.2106/JBJS.F.00742. [DOI] [PubMed] [Google Scholar]
- 30.Henrich D., Nau C., Kraft S.B. Effect of the harvest procedure and tissue site on the osteogenic function of and gene expression in human mesenchymal stem cells. Int J Mol Med. 2016;37:976–988. doi: 10.3892/ijmm.2016.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seebach C., Henrich D., Schaible A. Cell-based therapy by implanted human bone marrow-derived mononuclear cells improved bone healing of large bone defects in rats. Tissue Eng Part A. 2015;21:1565–1578. doi: 10.1089/ten.TEA.2014.0410. [DOI] [PubMed] [Google Scholar]
- 32.Seebach C., Henrich D., Meier S. Safety and feasibility of cell-based therapy of autologous bone marrow-derived mononuclear cells in plate-stabilized proximal humeral fractures in humans. J Transl Med. 2016;14:314. doi: 10.1186/s12967-016-1066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verboket R., Leiblein M., Seebach C. Autologous cell-based therapy for treatment of large bone defects: from bench to bedside. Eur J Trauma Emerg Surg. 2018;44:649–665. doi: 10.1007/s00068-018-0906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leighton R., Watson J.T., Giannoudis P. Healing of fracture nonunions treated with low-intensity pulsed ultrasound (LIPUS): a systematic review and meta-analysis. Injury. 2017;48:1339–1347. doi: 10.1016/j.injury.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Harrison A., Lin S., Pounder N. Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics. 2016;70:45–52. doi: 10.1016/j.ultras.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Zura R., Rocca Della G.J., Mehta S. Treatment of chronic (>1 year) fracture nonunion: heal rate in a cohort of 767 patients treated with low-intensity pulsed ultrasound (LIPUS) Injury. 2015;46:2036–2041. doi: 10.1016/j.injury.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 37.Poolman R.W., Agoritsas T., Siemieniuk R.A. Low intensity pulsed ultrasound (LIPUS) for bone healing: a clinical practice guideline. BMJ. 2017;356:j576. doi: 10.1136/bmj.j576. [DOI] [PubMed] [Google Scholar]
- 38.Schandelmaier S., Kaushal A., Lytvyn L. Low intensity pulsed ultrasound for bone healing: systematic review of randomized controlled trials. BMJ. 2017;356:j656. doi: 10.1136/bmj.j656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe Y., Matsushita T., Bhandari M. Ultrasound for fracture healing: current evidence. J Orthop Trauma. 2010;24(Suppl 1):S56–S61. doi: 10.1097/BOT.0b013e3181d2efaf. [DOI] [PubMed] [Google Scholar]
- 40.Romano C.L., Romano D., Logoluso N. Low-intensity pulsed ultrasound for the treatment of bone delayed union or nonunion: a review. Ultrasound Med Biol. 2009;35:529–536. doi: 10.1016/j.ultrasmedbio.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 41.Janko M., Nau C., Marzi I. Local fixation of antibiotics by fibrin spray : in bone defects with soft tissue involvement. Chirurg. 2017;88:166–174. doi: 10.1007/s00104-016-0320-0. [DOI] [PubMed] [Google Scholar]
- 42.Lowenberg D.W., Feibel R.J., Louie K.W. Combined muscle flap and Ilizarov reconstruction for bone and soft tissue defects. Clin Orthop Relat Res. 1996;332:37–51. doi: 10.1097/00003086-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Grinsell D., Keating C.P. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fannouch G., Alfaisali S., Bobseit A. Masquelet technique for Reconstruction of extensive bone defect of tibia after gunshot injury in a child: case report. MOJ Orthop Rheumato. 2018;10:00382. [Google Scholar]
- 45.Masquelet A.C., Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin N Am. 2010;41:27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 46.Kashayi-Chowdojirao S., Vallurupalli A., Chilakamarri V.K. Role of autologous non-vascularised intramedullary fibular strut graft in humeral shaft nonunions following failed plating. J Clin Orthop Trauma. 2017;8:S21–S30. doi: 10.1016/j.jcot.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]