Abstract
Background
Development of a cytomegalovirus (CMV) vaccine is a high priority. However, the ability of antibodies to protect against CMV infection is not well characterized. Studies of maternal antibodies in infants offer the potential to identify humoral correlates of protection against postnatal acquisition.
Methods
This hypothesis-generating study analyzed 29 Ugandan mother-infant pairs that were followed weekly for CMV acquisition. Seventeen mothers and no infants were infected with human immunodeficiency virus (HIV). We evaluated the association between CMV-specific immunoglobulin G (IgG) responses in mothers at the time of delivery and their infants’ CMV status at 6 months of age. We also assessed levels of CMV-specific IgG in infants at 6 weeks of age. CMV-specific IgG responses in the mother-infant pairs were then analyzed on the basis of perinatal HIV exposure.
Results
We found similar levels of multiple CMV glycoprotein–specific IgG binding specificities and functions in mothers and infants, irrespective of perinatal HIV exposure or infant CMV status at 6 months of age. However, the glycoprotein B–specific IgG titer, measured by 2 distinct assays, was higher in infants without CMV infection and was moderately associated with delayed CMV acquisition.
Conclusions
These data suggest that high levels of glycoprotein B–specific IgG may contribute to the partial protection against postnatal CMV infection afforded by maternal antibodies, and they support the continued inclusion of glycoprotein B antigens in CMV vaccine candidates.
Keywords: Cytomegalovirus, immune correlates, vaccine
Congenital cytomegalovirus (CMV) infection occurs in 1 in 150 live births, and approximately 15% of infected infants develop permanent neurologic sequelae [1, 2]. Owing to the high disease burden of congenital CMV, in 2000 the National Academy of Medicine declared development of a CMV vaccine a tier 1 priority [3]. However, a poor understanding of immune correlates that protect against CMV transmission has hindered vaccine development. Because young children shed high levels of virus in saliva and urine following postnatal infection, they are major sources of transmission to pregnant women [4, 5].
Antibody may be capable of protecting against CMV infection, based on findings of early phase clinical trials of CMV vaccines, including those based on the viral glycoprotein B (gB) [6]. Additionally, high avidity and functional CMV-specific immunoglobulin G (IgG) is transferred to the fetus, achieving levels higher than those in maternal plasma at delivery [7]. This passive immunity may explain why only 40% of breastfeeding infants born to CMV-seropositive mothers are infected postnatally despite near uniform exposure to CMV in breast milk [8]. Thus, investigations of the protective role of passively acquired maternal IgG in infants may elucidate targets to interrupt CMV transmission between mothers and infants.
In this hypothesis-generating study, we used a unique birth cohort of Ugandan infants and their CMV-seropositive mothers [9] to conduct a comprehensive analysis of the relationship between maternal CMV glycoprotein–specific IgG specificities and functions and the risk of postnatal infection. Since T cells are not transferred from the mother to fetus in significant numbers, this approach investigates the role of IgG in postnatal transmission risk independent of T-cell immunity. While our data did not demonstrate major differences in CMV-specific IgG binding or function between mother-infant pairs with or without postnatal acquisition at 6 months of age, we identified a potential association with CMV gB–specific IgG and delayed infant postnatal CMV acquisition. The data presented in this study support continued development of a vaccine that uses gB as an immunogen in a future, likely multitarget CMV vaccine.
MATERIALS AND METHODS
Study Population
Plasma samples were collected as part of a previously described cohort of 32 mother-infant pairs [9]; maternal plasma was obtained at delivery, and infant plasma specimens were obtained at approximately 6 weeks of age. All subjects provided informed consent as approved by the human subject’s protection committees in Kampala, Uganda; Seattle, Washington; and Vancouver, Canada. Weekly oral swab specimens were collected for viral quantitative polymerase chain reaction (qPCR) testing, and the timing of postnatal CMV infection in infants was defined by the onset of persistent viral shedding in saliva and/or detection of viremia [9]. Three mother-infant pairs were excluded, with 2 excluded because congenital CMV was confirmed by saliva qPCR in the first week of life and 1 excluded because they were followed up for <6 months.
Cell Culture
Human retinal pigment epithelial cells (ARPE-19, ATCC CRL-2302) were grown in Dulbecco’s modified Eagle’s medium with high glucose (Gibco 1195065), supplemented with 10% fetal calf serum (FCS), 2mM L-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin. Human lung fibroblasts (MRC-5, ATCC CCL-171) were grown in minimal essential medium (Gibco 11095080), supplemented with 10% FCS, 50 U/mL penicillin, and 50 μg/mL streptomycin. Human monocyte cells (THP-1, ATCC TIB-202) were grown in Roswell Park Memorial Institute 1640 medium (Gibco 11875119), supplemented with 10% FCS.
Virus Production
BadrUL131-Y4 is a green fluorescent protein–tagged, UL131-repaired strain of AD169 (a kind gift from Tom Shenk [10]). ARPE-19 cells were infected with BadrUL131-Y4 at a multiplicity of infection of 0.01. Virus was harvested when >95% of cells had cytopathic effect, on approximately day 21 of infection. Cells were harvested and sonicated for 5 seconds 3 times and then added to the supernatant from the culture flasks. The virus stock was concentrated on a 20% sucrose cushion at 50 000 ×g (20 000 rpm) in a Beckman SW28 rotor for 1.5 hours. The virus pellet was resuspended in 10 mL of ARPE-19 growth medium adjusted to 0.2 M sucrose. Concentrated BadrUL131-Y4 stock was titered on ARPE-19 cells by the limiting-dilution technique in 96-well plates as described previously [11]. AD169r (with UL131 repaired) stock was a kind gift from Merck [12].
IgG Binding and Avidity Enzyme-Linked Immunosorbent Assays (ELISAs)
ELISAs were performed as previously described [13]. Briefly, 384-well ELISA plates (Corning 3700) were coated overnight at 4oC with 20 ng of gB or 1250 plaque-forming units of AD169r. The 50% effective dilution (ED50) and area under the curve (AUC) were determined using nonlinear regression in GraphPad Prism. The avidity index was determined as previously described [13], using a plasma dilution of 1:2000 for gB antigen and 1:90 of AD169r antigen.
Binding-Antibody Multiplex Assay (BAMA)
IgG binding to CMV antigens involved in cell entry (gB, gH/gL, and gH/gL/UL128/UL130/UL131 [also known as the pentameric complex {PC}]) was measured using BAMA as previously described [13, 14]. gB antigen was a kind gift from Sanofi, and gH/gL and PC antigens were kind gifts from Andrea Carfi (Novartis). Plasma were diluted 1:500 before mixing with conjugated beads. Total IgG was measured using phycoerythrin (PE)–conjugated anti-IgG, and IgG3 was measured with biotin-conjugated anti-IgG3 followed by PE-conjugated streptavidin. Data were acquired on a Bio-Plex 200 system (Bio-Rad) to determine the median fluorescence intensity (MFI).
Neutralization Assays
Plasma were serially diluted 3-fold in cell growth medium then mixed with 20 000 plaque-forming units of virus per well for 1 hour at 37oC with 5% CO2. A total of 50 μL of the plasma/virus mix per well was added in duplicate to black-walled, clear-bottomed, 384-well plates (Corning 3764) seeded with either ARPE-19 or MRC5 cells in a volume of 40 μL. Plates were incubated for 48 hours at 37oC with 5% CO2, fixed with 3.7% formaldehyde, subjected to nuclear staining with DRAQ5 (ThermoFischer Scientific 62251), and imaged using a Cellomics fluorescent reader to determine the percentage of infected cells per well. The 50% infectious dilution (ID50) was determined using nonlinear regression in GraphPad Prism.
Antibody-Dependent Cellular Phagocytosis (ADCP)
Whole-virion phagocytosis of BadrUL131-Y4 was performed as previously described [14]. Briefly, virus was concentrated, conjugated to AF647 NHS ester (Invitrogen), and quenched with 1 M Tris-HCL (pH 8.0). Conjugated virus was mixed 1:1 with plasma diluted 1:10 in duplicate for 2 hours at 37oC with 5% CO2. Then, 25 000 THP-1 cells were added to each well, spinoculated for 1 hour at 1200 ×g at 4oC, and incubated for 1 hour at 37oC with 5% CO2. Cells were then fixed with DPBS plus 1% formalin. AF647-positive cells were quantified using an LSR Fortessa (BD Biosciences). Data analysis was performed with FlowJo software.
Natural Killer (NK) Cell Degranulation Assay
NK cell degranulation was performed as previously described [14]. Briefly, ARPE cells were infected with BadrUL131-Y4 at a multiplicity of infection of 1 for 48 hours as target cells. Primary human NK cells were isolated from peripheral blood mononuclear cells by depletion of magnetically labeled cells, using the Human NK Cell Isolation Kit (Miltenyi Biotech). Plasma samples were diluted 1:25; added to cells in the presence of brefeldin A, monensin, and CD107a-FITC (BD Biosciences, clone H4A3); and incubated for 6 hours in 37oC with 5% CO2. Cells were stained with Live/Dead Aqua Dead Cell Stain followed by CD56-PE/Cy7 (BD Biosciences, clone NCAM16.2), CD16-PacBlue (BD 271 Biosciences, clone 3G8), and CD69-BV785 (BioLegend, clone FN50). Cells were fixed and permeabilized, followed by intracellular staining for interferon γ (IFN-γ)–BV711 (BioLegend, clone 4S.B3) and tumor necrosis factor α (TNF-α)–BV650 (BD Biosciences, clone Mab11). Flow cytometry was performed using the LSR Fortessa platform (BD biosciences), and data were analyzed with FlowJo software.
Statistical Analysis
GraphPad Prism was used to calculate the AUC, ED50, and ID50 via nonlinear regression for ELISAs and neutralization assays. The following assay data underwent log10 transformation before analysis: gB ELISA ED50, AD169r ELISA AUC, and neutralization ID50. P values determined with Wilcoxon rank sum tests were used to compare CMV-specific IgG levels between mothers of CMV-infected or uninfected infants, infants with or without CMV infection by 6 months of age, mothers with or without HIV infection, and infants with or without HIV exposure. P values were then adjusted using the Benjamini-Hochberg procedure to control for the false-discovery rate (FDR). Data were grouped by infant CMV infection status at 6 months of age and maternal HIV infection status, with each outcome undergoing its own correction. Cox-hazard regression modeling was used to determine whether CMV-specific IgG is associated with a later time to postnatal CMV acquisition, followed again by FDR correction. Correlations in CMV-specific IgG levels between mother-infant pairs were calculated using nonparametric Spearman rank correlation, followed by FDR correction.
RESULTS
CMV-Specific IgG Responses in Plasma of Mothers Are Not Associated With Postnatal CMV Infection in Their Infants by 6 Months of Age
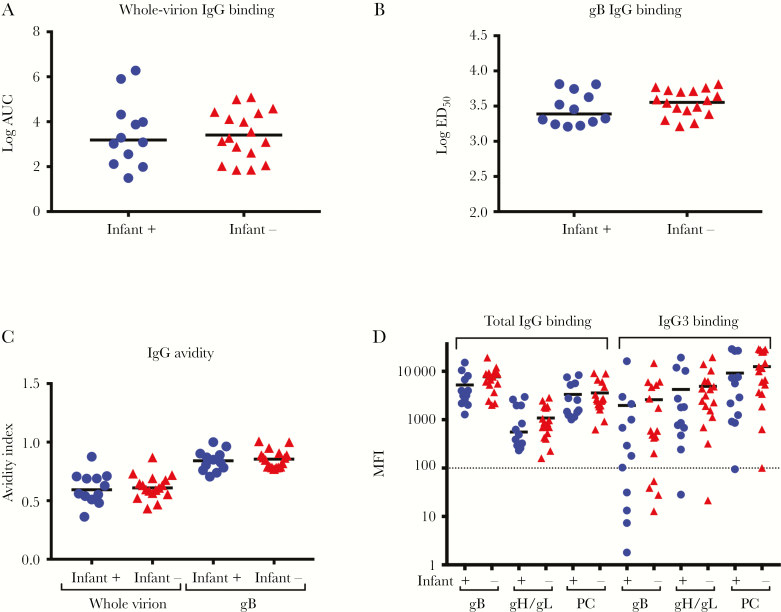
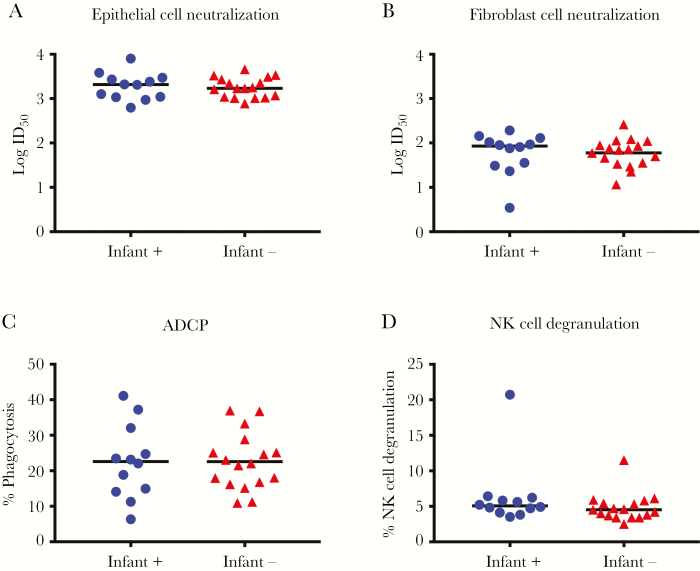
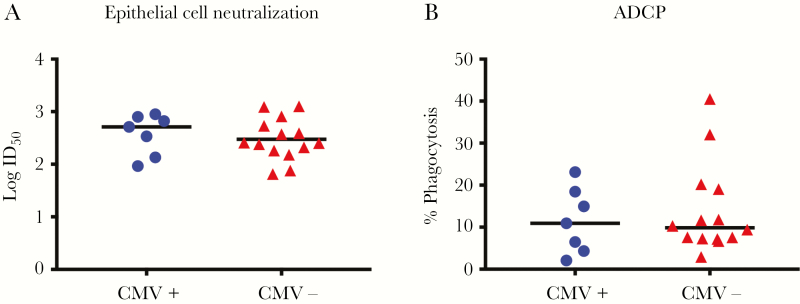
CMV-specific IgG binding and function were assessed in plasma specimens collected at delivery from mothers of Ugandan infants who were followed weekly for postnatal CMV acquisition (Supplementary Table 1). Similar CMV-specific IgG binding and avidity to whole AD169r virions (Figures 1A and 1C) and soluble gB (Figures 1B and 1C) were found in mothers whose infants who did and those who did not acquire CMV by 6 months of age. We further assessed IgG binding to glycoproteins required for epithelial and fibroblast cell entry, using BAMA. The magnitude of the binding of total IgG to gB, gH/gL, and the PC was similar between mothers of infants who did and those who did not acquire CMV by 6 months of age (Figure 1D). Additionally, plasma CMV neutralizing activity was similar in both epithelial cells (Figure 2A) and fibroblast cells (Figure 2B). Nonneutralizing functional CMV-specific IgG responses, including ADCP (Figure 2C) and NK cell degranulation (Figure 2D), were also similar between mothers of infants who did and those who did not acquire CMV by 6 months of age. Nonneutralizing functional IgG responses are effectively mediated by IgG3 via interaction with FcδRIII expressed on effector cells [15]. Therefore, we assessed levels of IgG3 binding to gB, gH/gL, and the PC (Figure 1D), which were also similar between groups. Since half of the mothers in each group were HIV infected, we reanalyzed the CMV-specific IgG binding and functional assay data after stratification by maternal HIV infection status (Supplementary Table 3). However, plasma IgG binding (Supplementary Figure 2) and functional IgG responses (Supplementary Figure 3) were also similar between HIV-infected and uninfected mothers.
Figure 1.
Maternal cytomegalovirus (CMV)–specific immunoglobulin G (IgG) binding responses are similar between women whose infants did and those whose infants did not acquire CMV by 6 months of age. CMV-specific IgG binding responses of mothers whose infants were CMV infected (circles) or CMV uninfected (triangles) at 6 months of age. A and B, Whole-virion binding (A) and glycoprotein B (gB) binding (B) determined by enzyme-linked immunosorbent assay. C, Avidity of binding responses to whole virions (left) and gB (right). D, Total IgG binding (left) and IgG3 binding (right) to CMV antigens required for entry into epithelial cells. Dashed line indicates the limit of detection. The median of each data set is indicated with a line. AUC, area under the curve; ED50, 50% effective dilution; MFI, median fluorescence intensity; PC, pentameric complex.
Figure 2.
Maternal cytomegalovirus (CMV)–specific functional immunoglobulin G (IgG) responses are similar between women whose infants did and those whose infants did not acquire CMV by 6 months of age. CMV-specific IgG binding responses of mothers whose infants were CMV infected (circles) or CMV uninfected (triangles) at six months of age. A and B, Neutralization of virus entry into ARPE-19 epithelial cells (A) and MRC5 fibroblast cells (B). C and D, Nonneutralizing functional CMV-specific IgG responses: antibody-dependent cellular phagocytosis (ADCP; C) and natural killer (NK) cell degranulation (D). The median of each data set is indicated with a line. ID50, 50% infectious dilution.
CMV-Specific Plasma IgG Levels Are Strongly Correlated Within Mother-Infant Pairs, Irrespective of Infant CMV or Maternal HIV Infection Status
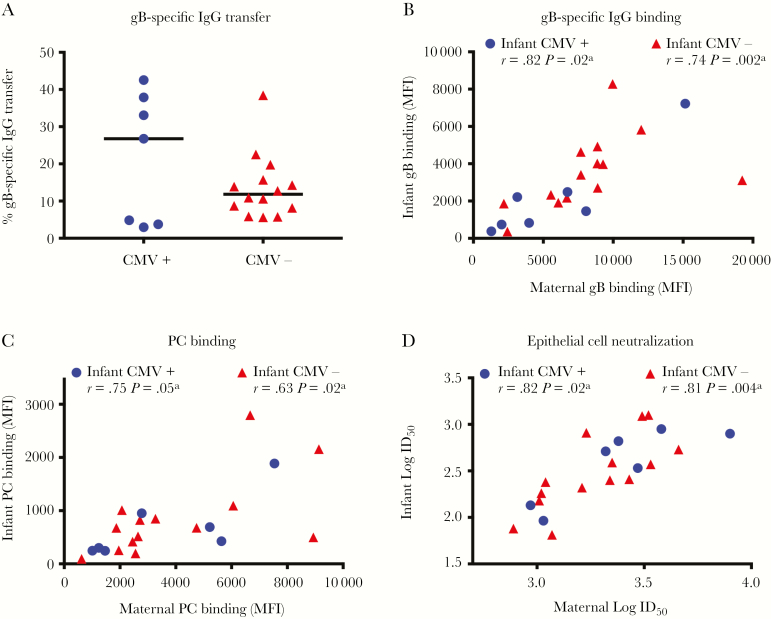
High-potency, neutralizing, CMV-specific IgG is transferred via the placenta to the fetus [7, 16]. Furthermore, gB-specific IgG mediates up to half of the CMV-neutralizing response [17]. Therefore, we calculated the placental transfer efficiency of gB-specific IgG, using the IgG concentration in maternal and infant plasma specimens, determined by ELISA. The transfer efficiency of gB-specific IgG was similar between mother-infant pairs irrespective of infant CMV status (Figure 3A). However, correlations of binding and functional CMV-specific IgG responses between mother-infant pairs were stronger when the infant was uninfected with CMV at 6 months of age (Figure 3B–D, Supplementary Figure 1, and Supplementary Table 2).
Figure 3.
Maternal cytomegalovirus (CMV)–specific immunoglobulin G (IgG) responses correlate with levels of IgG placentally transferred to infants. A, Glycoprotein B (gB)–binding IgG transfer efficiency as determined by the ratio of mother to infant gB IgG concentration, based on binding determined by enzyme-linked immunosorbent assay, are not different between groups. B–D, gB total IgG binding (B), pentameric complex (PC) binding (C), and epithelial cell neutralization CMV-specific IgG responses (D) were correlated for mother-infant pairs with CMV-infected infants (circles) and those with CMV-uninfected infants (triangles). Maternal responses at delivery and infant’s responses at 6 weeks of age were analyzed using nonparametric Spearman rank correlations. ID50, 50% infectious dilution; MFI, median fluorescence intensity. aStatistically significant.
HIV-exposed, uninfected infants typically have lower levels of placentally transferred IgG as compared to their unexposed counterparts [18], and these infants also have higher reported rates of congenital CMV infection [19, 20]. Although levels of several CMV-specific IgG specificities generally had stronger correlations within HIV-infected/exposed mother-infant pairs as compared to HIV-uninfected/unexposed pairs (Supplementary Figures 4B–D and 5 and Supplementary Table 4), the gB-specific IgG transfer efficiency was similar irrespective of maternal HIV status (Supplementary Figure 4A).
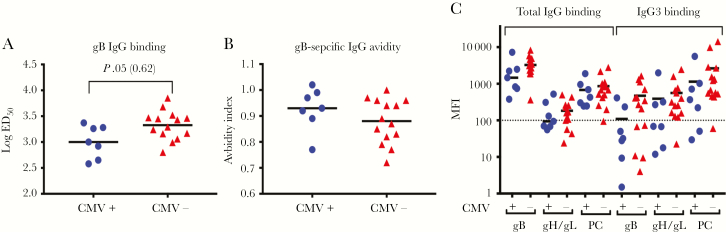
Infant CMV-Specific IgG Responses at 6 Weeks of Age Trended Higher in Infants Who Remained Uninfected at 6 Months of Age
Infant CMV-specific IgG levels were determined in plasma specimens collected at approximately 6 weeks of age (Supplementary Table 1). The gB-specific IgG binding ED50 trended higher in infants who were uninfected at 6 months of age (P = .05; Figure 4A) but was not statistically significant after correction for multiple comparisons (adjusted P = .62). The avidity of gB-specific IgG was similar between infants with and those without CMV infection at 6 months of age (Figure 4B). Total IgG binding to gB and gH/gL, as well as IgG3 binding to gB and PC, also showed a trend toward higher levels in infants uninfected by 6 months of age (Figure 4C). Yet epithelial cell neutralization (Figure 5A) and ADCP (Figure 5B) levels were similar between infants with and those without infection at 6 months of age. Both CMV-specific binding (Supplementary Figure 6) and functional (Supplementary Figure 7) IgG levels were similar between HIV-exposed and unexposed infants (Supplementary Table 3).
Figure 4.
Infant cytomegalovirus (CMV)–specific immunoglobulin G (IgG) binding responses are similar at 6 weeks of age between infants who did and those who did not acquire CMV by 6 months of age. CMV-specific IgG levels in infants who were CMV infected by 6 months (circles) and CMV-uninfected infants at 6 months (triangles). A and B, gB-specific IgG binding (A) and avidity index (B) determined by enzyme-linked immunosorbent assay. C, Total IgG (left) and IgG3 (right) binding to CMV antigens required for entry into epithelial cells. Dashed line indicates the limit of detection. The median of each data set is indicated with a line. ED50, dilution effective dilution; MFI, median fluorescence intensity; PC, pentameric complex.
Figure 5.
Infant cytomegalovirus (CMV)–specific functional immunoglobulin G (IgG) levels are similar at 6 weeks of age between infants who did and those who did not acquire CMV by 6 months of age. CMV-specific IgG levels in infants who were CMV infected by 6 months (circles) and CMV-uninfected infants at 6 months (triangles). A and B, Functional CMV-specific IgG responses: neutralization of virus entry into ARPE-19 epithelial cells (A) and antibody-dependent cellular phagocytosis (ADCP; B). The median of each data set is indicated with a line. ID50, 50% infectious dilution.
gB-Specific IgG Response in Infants Is Moderately Associated With Delayed Postnatal CMV Acquisition
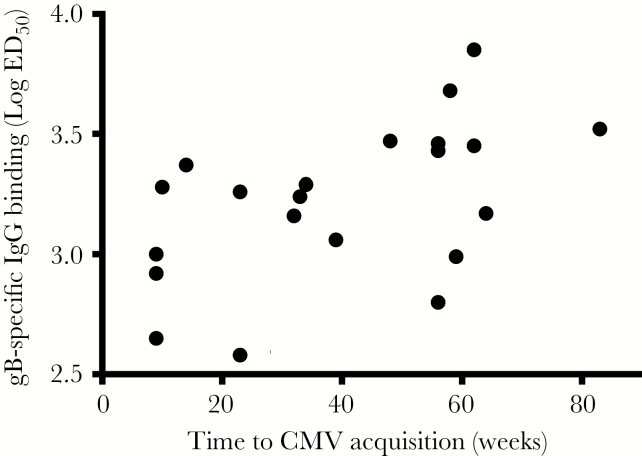
Finally, Cox regression modeling was used to determine whether CMV-specific IgG levels in the mother or infant were associated with delayed postnatal CMV acquisition. Interestingly, the magnitude of the gB-specific IgG log ED50 in infants had a moderate association with delayed postnatal CMV acquisition, with a significant hazard ratio of 0.09, indicating that a 0.1 log increase in the CMV-specific IgG log ID50 is associated with a 21% decreased risk of CMV acquisition (P = .01; Figure 6 and Supplementary Table 5). However, this observation did not reach statistical significance after correction for multiple comparisons (adjusted P = .35).
Figure 6.
High glycoprotein B (gB)–specific immunoglobulin G (IgG) binding in infant plasma moderately associates with delayed cytomegalovirus (CMV) acquisition. Scatterplot comparing gB log 50% effective dilution (ED50) as determined by enzyme-linked immunosorbent assay and week of postnatal CMV acquisition. The Cox hazard ratio between infant gB binding in log ED50 and week of infant CMV acquisition is 0.09 (P = .01; adjusted P = .35).
DISCUSSION
It is predicted that CMV-specific antibodies offer partial protection against virus acquisition [21], yet the specificity and function of potentially protective antibodies against CMV is not defined. Vaccine development has primarily focused on gB because this protein is the main viral fusion protein, the dominant target of the IgG response, and the target of up to half of the fibroblast-neutralizing response [17]. In fact, a phase 2 trial of a gB-subunit vaccine demonstrated 50% protection in women of childbearing age [22]. However, the gB-subunit vaccine–elicited IgG-mediated neutralization activity in epithelial cells was 15-fold lower than that in CMV-seropositive subjects [11, 14]. Furthermore, gB-subunit vaccine–elicited IgG responses only weakly neutralized the autologous vaccine virus strain and not heterologous CMV strains. These vaccine-elicited IgG responses also demonstrated poor NK cell degranulation but mediated ADCP similar to that in seropositive controls [14]. One strategy for improving the low-level functional antiviral response elicited by vaccination is to add the PC to CMV vaccine immunogens, as this complex is required for entry into epithelial but not fibroblast cells [23]. Indeed, isolated human monoclonal antibodies against the PC mediate high-potency neutralization in epithelial cells [24]. The data in this study support continued development of a multicomponent CMV vaccine, including gB, likely in combination with other glycoproteins, such as PC and gH/gL, since uninfected infants trended to have higher IgG binding to these antigens.
This hypothesis-generating immune correlate study did not find strong differences in levels of binding and functional CMV-specific IgG in mother-infant pairs irrespective of infant CMV infection status by 6 months of age (Supplementary Table 1). However, infants who remained uninfected by 6 months of age showed a trend toward higher gB-specific IgG binding in 2 distinct assays: ELISA (Figure 4A) and BAMA (Figure 4C). Both of these assays measure antigen-specific antibody binding, but because of differences in antigen presentation (antigen coated on plate vs antigen conjugated to bead), binding results in these assays were described as being both concordant and discordant in previous studies with various HIV vaccine antigens [25–27]. Additionally, the gB-specific IgG ELISA is quantitative, with a 12-point dilution curve used to determine the log ED50, whereas BAMA is a semiquantitative single dilution of both total IgG and IgG3 gB binding. gB-specific IgG binding by ELISA (measured as the log ED50) was significantly higher in uninfected infants before correction for multiple comparisons (P = .05; adjusted P = .62; Figure 4A). Additionally, uninfected infants had a trend toward higher gB-specific total IgG and IgG3 binding, as measured by BAMA (total IgG, P = .09 and adjusted P = .62; IgG3, P = .08 and adjusted 0.62; Figure 4C). Moreover, delayed postnatal CMV acquisition was moderately associated with a higher gB-specific IgG log ED50 in infant plasma (hazard ratio, 0.09; P = .01; adjusted P = .36; Figure 6 and Supplementary Table 5). Not surprisingly, given the small sample size and large number of tests performed, this association did not remain statistically significant after correction for multiple comparisons. However, the consistent trends between assays, given the small sample size of 7 infected and 12 uninfected infants, strengthen the validity of the results.
The potential for an antibody response to be associated with a delay in postnatal acquisition is clinically relevant because exposure to high-level CMV shedding in saliva and urine by infants and toddlers is a major reservoir of maternal infection. Moreover, a temporary decrease in susceptibility to CMV acquisition during pregnancy through enhancement of maternal CMV-specific antibody responses would be relevant for reducing the rate of congenital CMV infection. Therefore, a maternal vaccine that could either delay maternal virus acquisition until after pregnancy and/or delay infant postnatal acquisition would be predicted to reduce congenital CMV transmission. Furthermore, these data are consistent with the partial protection observed in the gB-subunit vaccine trials [22] and support a role for gB-specific IgG in preventing CMV acquisition. We also found a trend toward higher levels of total IgG against gH/gL and IgG3 against PC in infants who remained uninfected, suggesting that these antibodies may also contribute to delayed CMV acquisition.
However, these data also suggest that plasma CMV-specific IgG alone will not provide adequate protection from mucosal virus acquisition. Importantly, mucosal immunity, specifically breast milk IgG and IgA, may play a vital role in preventing of infant infection [28]. The role of neutralizing versus nonneutralizing antibodies in breast milk and saliva is unclear. Unfortunately, this cohort did not have breast milk or saliva samples available for IgG and IgA assessment. Interestingly, both mother and infant total IgG PC-specific IgG binding and epithelial cell neutralizing activity in plasma were similar between groups, although these responses were previously associated with a decreased risk of congenital CMV infection [29]. Yet this lack of difference in PC-specific IgG binding in plasma between infected and uninfected infants highlights the importance of future studies of mucosal antibody responses, which are relevant to the route of CMV acquisition. Additionally, this study did not address the role of NK cell responses or CMV-specific T-cell responses in transmission, which have both been shown to play a major role in CMV control [30].
We also investigated the impact of perinatal HIV exposure on postnatal CMV acquisition. Similar CMV-specific IgG levels in mothers and infants were measured irrespective of maternal HIV status. However, correlations of maternal-infant IgG levels were stronger in pairs with HIV-unexposed infants. These data suggest that HIV-unexposed infants received more-consistent levels of CMV-specific IgG during pregnancy. This potential lack of consistent CMV-specific IgG placental transfer may play a role in higher rates of congenital CMV in HIV-exposed infants [19, 20].
As mentioned above, this study was limited by the small number of mother-infant pairs, which affected our power to detect significant differences in IgG binding and function. Given the large number of assays completed owing to the comprehensive nature of this study, we cannot predict whether a larger sample size would yield similar results. Nevertheless, we identified a trend toward higher gB-specific IgG binding in 2 distinct assays (ELISA and BAMA) and a moderate association of delayed CMV acquisition in infants with high-magnitude gB-specific IgG log ED50 (hazard ratio, 0.09; P = .01; adjusted P = .35).
Our study was also limited by sample collection constraints. Specifically, the earliest available infant plasma samples were collected at 6 weeks of age, when some waning of maternal IgG has already occurred. Without samples collected from infants at the time of delivery, our analysis likely substantially underestimates the efficiency of placental IgG transfer, as we would expect CMV-specific IgG levels in cord blood or infant plasma at birth to be equal to or higher than levels in maternal plasma at delivery [7, 16]. Thus, measurement of infant CMV-specific IgG levels at 6 weeks of age may have limited the accuracy of our analysis of the efficiency of placental IgG transfer. The lack of cord or birth plasma sampling may also have reduced our ability to detect differences in protective IgG levels, if CMV qPCR overestimated the week of infection and some infants with early infection developed active CMV-specific responses before 6 weeks of age. Finally, our study was limited by the lack of breast milk or infant saliva, in which the role of mucosal IgG and IgA could have been assessed. Nevertheless, our findings are valuable because we were able to evaluate the role of multiple CMV-specific IgG specificities and functions in protection against CMV acquisition, independent of other adaptive CMV-specific immune responses.
Since infants shedding CMV are a major source of transmission to pregnant women, a better understanding of humoral immune protection against virus acquisition in infants will aid CMV vaccine design. Our findings indicate that that passively transferred gB-specific maternal IgG may contribute to delayed postnatal infection. These data support the continued inclusion of the gB antigen in a multitarget CMV vaccine candidate. Importantly, humoral immunity from natural CMV infection is incomplete; infants are infected from breast milk in the first months of life despite maternal antibody, and reinfection among seropositive individuals is common. Therefore, a highly effective CMV vaccine should aim to induce protection that exceeds that of natural immunity.
SUPPLEMENTARY DATA
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Tom Shenk, for the kind gift of BadrUL131-Y4 virus; Merck, for the kind gift of AD169r virus; Sanofi, for the kind gift of gB antigen; Andrea Carfi, for the kind gifts of gH/gL and PC antigens; and R. Whitney Edwards, for technical assistance with NK-cell degranulation assays.
Financial support. This work was supported by the Pediatric Infectious Diseases Society (fellowship to F. M. S., funded by the National CMV Foundation), the Thrasher Foundation (early career award to F. M. S.), the Derfner Research Foundation (to F. M. S.), and the National Institutes of Health (Loan Repayment Program for Pediatric Research to F. M. S. and grants 1R21-AI136556 and 4DP2-HD075699 to S. R. P.).
Potential conflicts of interest. S. G. has received research funding and consults from Merck, GSK, VBI Vaccines, and Meridian Bioscience. S. R. P. is a consultant for Merck, Pfizer, Sanofi, and Moderna Vaccines. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: St Jude PIDS Pediatric Infectious Diseases Research Conference, Memphis, Tennessee, 9–10 March 2018 [abstract 19155]; Pediatric Academic Societies Meeting, Toronto, Canada, 5–8 May 2018 [abstract 4455]; National Institute of Allergy and Infectious Diseases/Infectious Diseases Society of America Infectious Diseases Research Careers Meeting, Bethesda, Maryland, 7–9 June 2018; CMV Public Health and Policy Conference, Burlington, Vermont, 23–25 September 2018; IDWeek, San Francisco, California, 3–7 October 2018 [abstract 116].
References
- 1. Schleiss M. Progress in cytomegalovirus vaccine development. Herpes 2005; 12:66–75. [PubMed] [Google Scholar]
- 2. Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis 2013; 57(Suppl 4):S178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stratton KR, DS, Lawrence RS, ed. Vaccines for the 21st century: a tool for decision making. Washington DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 4. Mayer BT, Matrajt L, Casper C, et al. Dynamics of persistent oral cytomegalovirus shedding during primary infection in Ugandan infants. J Infect Dis 2016; 214:1735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krause PR, Bialek SR, Boppana SB, et al. Priorities for CMV vaccine development. Vaccine 2013; 32:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plotkin S. The history of vaccination against cytomegalovirus. Med Microbiol Immunol 2015; 204:247–54. [DOI] [PubMed] [Google Scholar]
- 7. Nozawa N, Fang-Hoover J, Tabata T, Maidji E, Pereira L. Cytomegalovirus-specific, high-avidity IgG with neutralizing activity in maternal circulation enriched in the fetal bloodstream. J Clin Virol 2009; 46(Suppl 4):S58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luck S, Sharland M. Postnatal cytomegalovirus: innocent bystander or hidden problem? Arch Dis Child Fetal Neonatal Ed 2009; 94:F58–64. [DOI] [PubMed] [Google Scholar]
- 9. Gantt S, Orem J, Krantz EM, et al. Prospective characterization of the risk factors for transmission and symptoms of primary human herpesvirus infections among Ugandan infants. J Infect Dis 2016; 214:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang D, Shenk T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol 2005; 79:10330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cui X, Meza BP, Adler SP, McVoy MA. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine 2008; 26:5760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Li F, Freed DC, et al. Quantitative analysis of neutralizing antibody response to human cytomegalovirus in natural infection. Vaccine 2011; 29:9075–80. [DOI] [PubMed] [Google Scholar]
- 13. Bialas KM, Westreich D, Cisneros de la Rosa E, et al. Maternal antibody responses and nonprimary congenital cytomegalovirus infection of HIV-1-exposed infants. J Infect Dis 2016; 214:1916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nelson CS, Huffman T, Jenks JA, et al. HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc Natl Acad Sci U S A 2018; 115:6267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014; 5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mussi-Pinhata MM, Pinto PC, Yamamoto AY, et al. Placental transfer of naturally acquired, maternal cytomegalovirus antibodies in term and preterm neonates. J Med Virol 2003; 69:232–9. [DOI] [PubMed] [Google Scholar]
- 17. Marshall GS, Rabalais GP, Stout GG, Waldeyer SL. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J Infect Dis 1992; 165:381–4. [DOI] [PubMed] [Google Scholar]
- 18. Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012; 2012:985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adachi K, Xu J, Ank B, et al. Congenital CMV and HIV perinatal transmission. Pediatr Infect Dis J 2018; 37(10):1016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duryea EL, Sánchez PJ, Sheffield JS, et al. Maternal human immunodeficiency virus infection and congenital transmission of cytomegalovirus. Pediatr Infect Dis J 2010; 29:915–8. [DOI] [PubMed] [Google Scholar]
- 21. Adler SP, Nigro G. Prevention of maternal-fetal transmission of cytomegalovirus. Clin Infect Dis 2013; 57(Suppl 4):S189–92. [DOI] [PubMed] [Google Scholar]
- 22. Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009; 360:1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryckman BJ, Rainish BL, Chase MC, et al. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol 2008; 82:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Macagno A, Bernasconi NL, Vanzetta F, et al. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol 2010; 84:1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martinez DR, Vandergrift N, Douglas AO, et al. Maternal binding and neutralizing IgG responses targeting the C-terminal region of the V3 loop are predictive of reduced peripartum HIV-1 transmission risk. J Virol 2017; 91(9): e02422–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pollara J, McGuire E, Fouda GG, et al. Association of HIV-1 envelope-specific breast milk IgA responses with reduced risk of postnatal mother-to-child transmission of HIV-1. J Virol 2015; 89:9952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zolla-Pazner S, deCamp A, Gilbert PB, et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 2014; 9:e87572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ehlinger EP, Webster EM, Kang HH, et al. Maternal cytomegalovirus-specific immune responses and symptomatic postnatal cytomegalovirus transmission in very low-birth-weight preterm infants. J Infect Dis 2011; 204:1672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lilleri D, Kabanova A, Revello MG, et al. Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection. PLoS One 2013; 8:e59863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jackson SE, Mason GM, Wills MR. Human cytomegalovirus immunity and immune evasion. Virus Res 2011; 157:151–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.