Abstract
Background
Despite increasing numbers of human immunodeficiency virus (HIV)–infected South Africans receiving antiretroviral therapy (ART), tuberculosis (TB) remains the leading cause of mortality. Approximately 25% of patients treated for TB have microbiologically unconfirmed diagnoses. We assessed whether elevated Kaposi’s sarcoma–associated herpesvirus (KSHV) viral load (VL) contributes to mortality in hospitalized HIV-infected patients investigated for TB.
Methods
Six hundred eighty-two HIV-infected patients admitted to Khayelitsha Hospital, South Africa, were recruited, investigated for TB, and followed for 12 weeks. KSHV serostatus, peripheral blood KSHV-VL, and KSHV-associated clinical correlates were evaluated.
Results
Median CD4 count was 62 (range, 0–526) cells/μL; KSHV seropositivity was 30.7% (95% confidence interval [CI], 27%–34%); 5.8% had detectable KSHV-VL (median, 199.1 [range, 13.4–2.2 × 106] copies/106 cells); 22% died. Elevated KSHV-VL was associated with mortality (adjusted odds ratio, 6.5 [95% CI, 1.3–32.4]) in patients without TB or other microbiologically confirmed coinfections (n = 159). Six patients had “possible KSHV-inflammatory cytokine syndrome” (KICS): 5 died, representing significantly worse survival (P < .0001), and 1 patient was diagnosed with KSHV-associated multicentric Castleman disease at autopsy.
Conclusions
Given the association of mortality with elevated KSHV-VL in critically ill HIV-infected patients with suspected but not microbiologically confirmed TB, KSHV-VL and KICS criteria may guide diagnostic and therapeutic evaluation.
Keywords: HIV, tuberculosis, Kaposi’s sarcoma, Kaposi’s sarcoma–associated herpesvirus, MCD, KICS, mortality, epidemiology, South Africa
AIDS-related deaths have declined from an estimated 1.9 million in 2005 to 1.0 million in 2016, due to global scale-up of antiretroviral therapy (ART). Of those, 730 000 occurred in sub-Saharan Africa (SSA) [1]. Although ART scale-up has led to a global shift in the proportion of deaths from communicable diseases toward chronic noncommunicable conditions [2, 3], in SSA, tuberculosis (TB) remains the leading cause of mortality among human immunodeficiency virus (HIV)–infected individuals, resulting in a third of all AIDS-related deaths [4, 5].
The high burden of suspected TB in South Africa has led to overdiagnosis and overtreatment, and associated delay in diagnosis of cancers such as lymphoma and lung cancer given their overlapping clinical findings [6, 7]. Symptoms of Kaposi’s sarcoma–associated herpesvirus (KSHV, or human herpesvirus 8)–associated diseases may also mimic TB. KSHV is the etiological agent of Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman disease (MCD), which primarily occur in HIV-infected patients [8–10]. KS is the commonest AIDS-related malignancy worldwide and of particular significance in SSA where KSHV seroprevalence is elevated. KS incidence in HIV-infected individuals on ART in SSA is estimated to be 286 per 100 000 person-years [11]. In Africa, there were an estimated 32 446 new cases and 17 659 deaths in 2018 [12]. KS often presents with cutaneous disease, but advanced visceral disease with limited or no cutaneous involvement may occur. In contrast, KSHV-MCD is a rare (although most certainly underreported [13]) B-cell lymphoproliferative disorder associated with KSHV lytic activation and interleukin 6 (IL-6)– and interleukin 10–associated inflammatory syndromes. A recently described KSHV inflammatory cytokine syndrome (KICS) is also associated with KSHV lytic activation and similar cytokine dysregulation. KICS has only been described in 2 clinicopathologic series of 6 [14] and 10 [15] HIV/KSHV-coinfected patients in the United States. Most also had KS, and 2 had PEL, and it has been proposed that KICS contributes to the inflammatory symptoms seen in some patients with severe KS or PEL [15]. KICS in the absence of a KSHV-associated malignancy has also been reported [14, 15].
KSHV has latent and lytic phases characterized by distinct viral gene expression [16, 17]. In KS and PEL, KSHV expresses a limited number of latent phase genes [18, 19], and KS patients generally do not have elevated KSHV viral load (VL) in the blood [14, 20]. In contrast, lytically active KSHV in MCD and KICS expresses a broader range of genes that contribute to pathogenesis [8, 14]. KSHV-VL is elevated in MCD patients [14], and both MCD and KICS are characterized by overproduction of host IL-6, KSHV-encoded viral (v)IL-6, and other cytokines, giving rise to inflammatory symptoms such as fever, wasting, hypoalbuminemia, cytopenia, hyponatremia, and elevated C-reactive protein (CRP) [14]. Untreated KSHV-MCD and KICS have a high mortality [15]. KSHV-MCD diagnosis requires histologic confirmation, whereas KICS is a proposed clinical diagnosis requiring exclusion of KSHV-MCD [15] and other serious intercurrent infections. Rituximab is a highly effective therapy for KSHV-MCD, while management of KICS is directed at treating associated malignancies. A working case definition of KICS has been proposed [15] that may serve as a surveillance tool for individuals with HIV/KSHV coinfection who are at high risk of mortality.
Few cases of MCD and no cases of KICS have been reported from SSA despite the high HIV/KSHV prevalence [13, 15, 21]. KS is an independent risk factor for death in HIV-infected people, and a broader range of KSHV-associated diseases with lytic syndromes may play an unrecognized role in HIV-associated morbidity and mortality in SSA. We hypothesized that KSHV may contribute to clinical features and mortality in hospitalized HIV-associated TB patients in South Africa, and/or be an underrecognized cause of disease in the setting of culture-negative TB.
MATERIALS AND METHODS
Study Design
We conducted a retrospective analysis of an existing hospitalized HIV-associated TB cohort (n = 682) in South Africa. The primary objective was to evaluate whether elevated KSHV-VL, defined as >100 copies/106 cells, predicted 12-week mortality in the entire cohort, or in a subset that was culture negative for TB. Secondarily, we evaluated associations of KSHV-VL and serologic assays with clinical features in the cohort, as well as the use of clinical parameters that define KICS to predict mortality.
Due to the retrospective nature of this study, no prospective sample size calculation was performed.
Study Cohort
HIV-infected adults presenting with clinical syndromes compatible with pulmonary or extrapulmonary TB were recruited at Khayelitsha Hospital, Cape Town, South Africa, from January 2014 to October 2016 in the context of a study entitled “Defining Interventions to Reduce Mortality in Severe HIV-Associated Tuberculosis” (University of Cape Town [UCT] Human Research Ethics Committee [HREC]/Ref: 057/2013). Emergency room and medical ward patients were screened, eligible patients were enrolled, and written consent was obtained. Eligible patients with a depressed level of consciousness were enrolled and followed up daily until they regained capacity to consent. If a patient died prior to providing consent, we obtained approval from UCT HREC to use the patient’s data.
Clinical details, including physical examination with evaluation of skin and oral mucosa, and samples were collected at enrollment. CD4 cell count, HIV-VL, CRP, full blood and differential count, and renal and liver function tests were performed by the National Health Laboratory Services, as well as serum cryptococcal antigen lateral flow assays (IMMY). Citrate whole blood and plasma were stored at –80°C for KSHV-VL and immunologic assays. The standardized TB diagnostic workup included sputum induction if required. TB blood culture in Myco/Flytic bottles (Becton Dickinson Biosciences), sputum Xpert MTB/RIF assay, sputum TB culture, urine lipoarabinomannan (LAM), and urine Xpert MTB/RIF on concentrated urine were performed during enrollment. Bacterial blood cultures were performed in all patients who had not received intravenous antibiotics prior to presentation to hospital. Patients were followed for 12 weeks to ascertain vital status.
Definition of Patient Groups
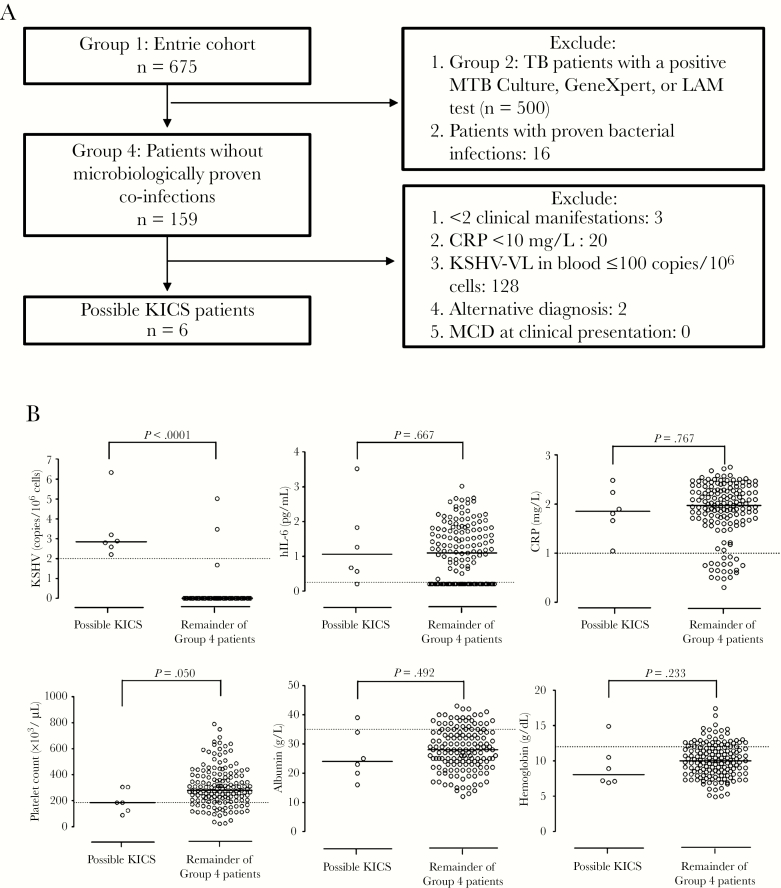
Patients were grouped into 4 overlapping categories based on the presence or absence of microbiologically confirmed infections. Group 1 (n = 675) consisted of the total patient cohort analyzed; group 2 (n = 500) included all patients with microbiologically confirmed TB (Mycobacterium tuberculosis on culture or GeneXpert on any clinical sample or urine LAM positive); group 3 (n = 175) included the remainder of the total patient cohort without microbiologically confirmed TB; group 4 (n = 159) consisted of group 3 patients without another microbiologically confirmed infection (eg, bacterial bloodstream infection or Cryptococcus species), although this group included some patients who were treated for TB despite negative microbiology. These groups are not mutually exclusive.
Definition of “Possible KICS”
We evaluated group 4 patients for KICS. The working case definition of KICS requires at least 2 clinical manifestations from at least 2 of 3 categories [15]: (1) symptoms (including fever, fatigue, edema, cachexia, respiratory symptoms, gastrointestinal disturbance, arthralgia and myalgia, altered mental state, and neuropathy); (2) laboratory abnormalities (anemia, thrombocytopenia, hypoalbuminemia, and hyponatremia); and (3) radiographic abnormalities (lymphadenopathy, splenomegaly, hepatomegaly, and body cavity effusions), together with evidence of systemic inflammation (elevated CRP [>10 mg/L]), evidence of KSHV lytic activity (elevated [>100 copies/106 cells] KSHV-VL in peripheral blood), and exclusion of MCD. As this analysis was done retrospectively, MCD could not be excluded for all patients, hence the designation “possible KICS” patients.
KSHV and IL-6 Assays
KSHV assays were performed for all patients. Cryopreserved plasma was tested by enzyme-linked immunosorbent assay (ELISA) for antibodies against latency-associated nuclear antigen (open reading frame [ORF] 73) and a lytic structural glycoprotein (K8.1), following established specifications [22]. Participants were considered KSHV seropositive if antibodies to either antigen were detected [22]. Plasma IL-6 was measured using the Human IL-6 SimpleStep ELISA kit (Abcam), with a minimum detectable dose of 1.6 pg/mL (reference median for IL-6 in well HIV-infected patients, 1.80 pg/mL [interquartile range, 1.20–2.89 pg/mL]) [23]).
DNA was extracted from peripheral blood mononuclear cells (PBMCs) with plasma removed using the QIAamp DNA Blood Mini kit (Qiagen). DNA concentration was adjusted to 25 ng/μL, with 10 μL used per PCR reaction (total volume 50 μL) to detect KSHV DNA using 100 pmole K6 gene region forward and reverse primers, 5 pmole FAM/TAMRA labeled probe [24], and 2X Universal Master Mix (Applied Biosystems). KSHV DNA was quantified against a K6-plasmid standard curve on a LightCycler 480II System (Roche) as follows: 2 minutes at 50°C; 8 minutes at 95°C; and 45 cycles of 15 seconds at 95°C and 1 minute at 60°C. Cellular equivalents were determined using a quantitative assay for human endogenous retrovirus 3 [25]. Samples were tested in triplicate, averaged, and reported as viral DNA copies per million cells.
Postmortem Histology
After obtaining consent from the family, an excisional cervical lymph node biopsy was performed 2 days postmortem on a patient with possible KICS. Tissue was fixed in 10% formal saline for 48 hours, processed overnight in a Tissue-Tek Vacuum Infiltration Processor (Sakura Finetek), and embedded in paraffin. Tissue sections were cut at 4 µm thickness and stained with hematoxylin and eosin and ORF73 immunoperoxidase (Cell Marque) and the Benchmark XT automated staining platform with the Ventana ultraView Universal DAB Detection kit (Roche Diagnostics). Immunostaining for kappa and lambda light chains was also performed. Photomicrographs were obtained with an Olympus SC30 3.3 megapixel USB digital color camera attached to an Olympus BX41 microscope using analySIS getIT 5.1 digital imaging software (Olympus Soft Imaging Solutions).
Statistical Analysis
KSHV-VL was treated both as a categorical variable (elevated >100 copies/106 cells vs ≤100 copies/106 cells or nondetectable) and a continuous variable and assessed for association with mortality using the χ2, Fisher exact, or Wilcoxon rank-sum test, as appropriate. The relationship between KSHV-VL and mortality was assessed by binomial logistic regression, controlling for age, sex, CD4 cell count, and ART status. Linearity of the continuous variables with respect to the logit of the dependent variable was confirmed via the Box–Tidwell procedure [26], and studentized residuals with values <2.5 standard deviations were accepted.
To compare “possible KICS” patients to the remainder of the cohort, associations of categorical variables (sex, receiving ART, KSHV seropositivity, presence of skin KS) and continuous variables (age, weight, HIV-VL, CD4 cell count, KSHV-VL, K8.1 optical density [OD], ORF73 OD, IL-6, CRP, hemoglobin, white cell count, platelet count, albumin, and sodium) were assessed by Fisher exact or Wilcoxon rank-sum test, respectively. To assess the independent associations of KSHV seropositivity or KSHV antibody levels (OD) with mortality, binomial logistic regression or multiple linear regression was performed, respectively. Continuous variables were transformed, where appropriate, to approximate normal distributions. Survival analysis was performed using Kaplan–Meier method and log-rank sum test. P values are 2-tailed and considered significant if <.05. Statistical testing was performed using SPSS version 25 (IBM Corp, 2017). Performance characteristics of KICS criteria for predicting death were calculated in R using a confusion matrix.
RESULTS
Assessment of Clinical Parameters
Among all of the patients recruited (n = 682), 7 were excluded (2 withdrew, 1 was HIV-negative, and 4 had no blood samples stored). Six hundred seventy-five patients were included in this analysis; 12 (1.8%) were lost to follow-up, and 146 (22%) were confirmed dead by 12 weeks of follow-up. Median CD4 count was 62 cells/μL (range, 0–526 cells/μL) (Table 1). Ten patients had a clinical diagnosis of cutaneous or oral KS at enrollment (4 in group 2, and 6 in group 3, of which 5 were in group 4). Two hundred seven of 675 patients (30.7% [95% confidence interval {CI}, 27%–34%]) were KSHV seropositive, of whom 39 (5.8%) showed detectable VL in the blood (median, 199.1 [range, 13.4–2.2 × 106] copies/106 cells) (Table 1). Plasma IL-6 was detected in 559 (82.8%) of all patients, with a median concentration of 42.3 pg/mL (IQR, 9.8–103.8 pg/mL), being highly elevated compared to a reference population of HIV-infected patients [23]. In binomial logistic regression including age, sex, CD4 cell count, hemoglobin levels, and ART status, only CD4 count was significantly associated with KSHV seropositivity (P = .001; adjusted odds ratio [aOR], 1.2 [95% CI, 1.1–1.3]; Supplementary Table 1A) whereas sex (male) was associated with elevated KSHV-VL (P = .029; aOR, 2.4 [95% CI, 1.1–5.1]; Supplementary Table 1B) and defaulted ART status was associated with lower KSHV-VL (P = .042, aOR, .3 [95% CI, .1–1.0]; Supplementary Table 1B). Anemia was associated with higher K8.1 antibody levels (P = .022; unstandardized coefficient, –0.033) when adjusted for age, sex, CD4 cell count, and ART status, but not with anti-ORF73 titers (Supplementary Table 2).
Table 1.
Baseline Demographic and Clinical Characteristics of the Study Cohort (n = 675)
| Characteristic | No. (%)* |
| Sex | |
| Male | 320 (47.4) |
| Female | 355 (52.6) |
| Age, y, median (range) | 36.1 (18.5–80.8) |
| Weight, kg, median (range) | 53 (30–104) |
| Receiving ART | 252 (37.3) |
| HIV-VL, copies/mL, median (range) | 104 252 (29–1 × 107) |
| CD4 count, cells/μL, median (range) | 62 (0–526) |
| Patients with anemia | 622 (92.1) |
| Hemoglobin, g/dL, median (range) | 8.9 (3.9–17.4) |
| Patients with detectable IL-6 | 559 (82.8) |
| IL-6 concentration, pg/mL, median (IQR) | 42.3 (9.8–103.8) |
| TB/infection category | |
| Patients with proven TB | 500 (74.1) |
| Patients with an alternate microbiologically proven OI | 16 (2.4) |
| Patients without microbiologically proven TB or other coinfections | 159 (23.5) |
| KSHV status in patients | |
| Seropositive | 207 (30.7) |
| Seropositive with detectable VL | 39 (5.8) |
| Seropositive with detectable VL, copies/106 cells, median (range) | 199.05 (13.4–2.2 × 106) |
| Seropositive with elevated VL (>100 copies/106 cells) | 29 (4.3) |
| Seronegative | 468 (69.3) |
| Seronegative with detectable VL | 4 (0.6) |
| Seronegative with detectable VL, copies/106 cells, median (range) | 1084.29 (141.4–57 054) |
| Seronegative with elevated VL (>100 copies/106 cells) | 4 (0.6) |
| Skin or oral Kaposi’s sarcoma | 10 (1.5) |
| Confirmed vital status at 12 wk | |
| Lost to follow-up | 12 (1.8) |
| Survived | 517 (76.6) |
| Died | 146 (21.6) |
*Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IL-6, interleukin 6; IQR, interquartile range; KSHV, Kaposi’s sarcoma–associated herpesvirus; OI, opportunistic infection; TB, tuberculosis; VL, viral load.
Elevated KSHV-VL Is Associated With Mortality in Patients With Microbiologically Unconfirmed TB
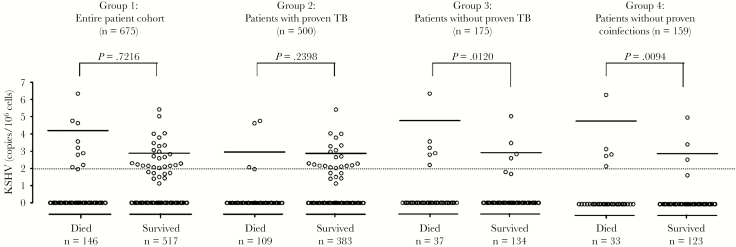
Group 1 (n = 675) was assessed for an association between elevated KSHV-VL (ie, >100 copies/106 cells) and 12-week mortality. We identified 33 patients with elevated KSHV-VL, of whom 9 (27.3%) died before 12 weeks, compared with 137 of 630 (21.7%) patients with VL ≤100 copies/106 cells. This difference was not statistically significant (Table 2, Figure 1). Patients with proven TB (n = 500, group 2) also showed no significant association of KSHV-VL with mortality (Table 2, Figure 1). However, in patients without proven TB (n = 175, group 3) and particularly in patients without proven TB or other coinfections (n = 159, group 4), elevated KSHV-VL was detected at a higher frequency (P = .011; odds ratio, 7.1 [95% CI, 1.6–31.7]; Table 2). Importantly, KSHV-VL was significantly higher in patients who died than those who survived 12 weeks (P = .0094; Figure 1), and overall 5 of 8 (62.5%) of group 4 patients with elevated KSHV-VL died, compared with 28 of 148 (18.9%) with low or nondetectable KSHV-VL. Binomial logistic regression revealed a statistically significant association of age, CD4 cell count, and elevated KSHV-VL with death among group 4 patients (Table 3). The aOR for death given elevated KSHV-VL was 6.5 (95% CI, 1.3–32.4). No significant relationship between KSHV seropositivity and mortality was noted (data not shown).
Table 2.
Association of Kaposi’s Sarcoma–Associated Herpesvirus Viral Load With 12-Week Mortality
| Group | KSHV Viral Load | Died, No. (%) | Survived, No. (%) | OR (95% CI) | P Valuea |
|---|---|---|---|---|---|
| Group 1: Entire patient cohort (n = 675) | >100 copies/106 cells | 9 (1.3) | 24 (3.6) | 1.3 (.6–3.0) | .455b |
| ≤100 copies/106 cells or nondetectable | 137 (20.3) | 493 (73.0) | |||
| Group 2: Patients with microbiologically proven TB (n = 500) | >100 copies/106 cells | 3 (0.4) | 20 (3.0) | 0.5 (.2–1.7) | .281b |
| ≤100 copies/106 cells or nondetectable | 106 (15.7) | 363 (53.8) | |||
| Group 3: Patients without proven TB (n = 175) | >100 copies/106 cells | 6 (0.9) | 4 (0.6) | 6.3 (1.7–23.7) | .008c |
| ≤100 copies/106 cells or nondetectable | 31 (4.6) | 130 (19.3) | |||
| Group 4: Patients without microbiologically confirmed infections (n = 159) | >100 copies/106 cells | 5 (0.7) | 3 (0.4) | 7.1 (1.6–31.7) | .011c |
| ≤100 copies/106 cells or nondetectable | 28 (4.1) | 120 (17.8) |
Frequency of mortality among patients with elevated KSHV viral load (>100 copies/106 cells) vs those with undetectable KSHV viral load or viral load ≤100 copies/106 cells. Twelve patients of the total cohort were lost to follow-up.
Abbreviations: CI, confidence interval; KSHV, Kaposi’s sarcoma–associated herpesvirus; OR, odds ratio; TB, tuberculosis.
a P value refers to the frequency of elevated KSHV viral load (categorical variable) in patients with confirmed vital status.
bχ2 test for association if expected cell frequencies were >5.
cFisher exact test.
Figure 1.
Twelve-week mortality vs Kaposi’s sarcoma–associated herpesvirus (KSHV) viral load in the entire patient cohort (group 1, n = 675); patients with proven tuberculosis (TB) (group 2, n = 500); patients without proven TB (group 3, n = 175); and patients without microbiologically proven coinfections (group 4, n = 159). Twelve patients of the total cohort were lost to follow-up. P value is by Wilcoxon rank-sum test, assessing the association of the level of KSHV viral load (continuous variable) with mortality of patients with confirmed vital status at the end of the 12-week study period. Data are log transformed. The dotted line indicates elevated KSHV viral load (>100 copies/106 cells), and the solid lines indicate the median.
Table 3.
Logistic Regression of Factors Assessed for Association With Confirmed Mortality at the End of the 12-Week Study Period Based on Elevated Kaposi’s Sarcoma–Associated Herpesvirus Viral Load Among Group 4 Patients (n = 159, Including 3 Patients Lost to Follow-up)
| Characteristic | Unadjusted OR | 95% CI for Unadjusted OR | 95% CI for Adjusted OR | P Value | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | Adjusted OR | Lower | Upper | |||
| Elevated KSHV-VLa | 7.143 | 1.611 | 31.671 | 6.467 | 1.290 | 32.406 | .023 |
| Sexb | 0.949 | .433 | 2.082 | 0.477 | .187 | 1.214 | .120 |
| Agec | 1.051 | 1.014 | 1.088 | 1.067 | 1.022 | 1.113 | .003 |
| CD4 cell countd | 0.892 | .742 | 1.072 | 0.777 | .619 | .975 | .029 |
| ART statuse | .980 | ||||||
| Defaulted | 0.790 | .285 | 2.190 | 1.067 | .346 | 3.290 | .910 |
| On ART | 0.967 | .395 | 2.368 | 1.105 | .417 | 2.930 | .840 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; KSHV, Kaposi’s sarcoma–associated herpesvirus; OR, odds ratio; VL, viral load.
aKSHV-VL is for elevated (>100 copies/106 cells) compared to nonelevated (≤100 copies/106 cells or nondetectable).
bSex is for males compared to females.
cAge is scaled per 1 year.
dCD4 count is scaled per 50 cells/µL.
eFor ART status, naive is the reference category.
Identification and Contribution of Possible KICS to Mortality
We next evaluated group 4 for KICS as TB and other microbiologically proven infections as alternate cause of clinical presentation had already been excluded in this group as per KICS definition [15]. We identified 6 “possible KICS” patients with the caveat of not excluding MCD at clinical presentation (Figure 2A). Compared to others in group 4, “possible KICS” subjects were older (Table 4), had lower platelet counts (Table 5, Figure 2B), higher K8.1 and ORF73 antibody levels (Table 5), and, by definition, elevated KSHV-VL (Table 5, Figure 2B). HIV-VL and CD4 counts did not differ significantly between “possible KICS” and the remainder of group 4 patients (Table 4); neither did IL-6 and CRP although being markedly elevated (Table 5, Figure 2B), nor did hemoglobin and albumin levels although being abnormally low [15] (Table 5, Figure 2B).
Figure 2.
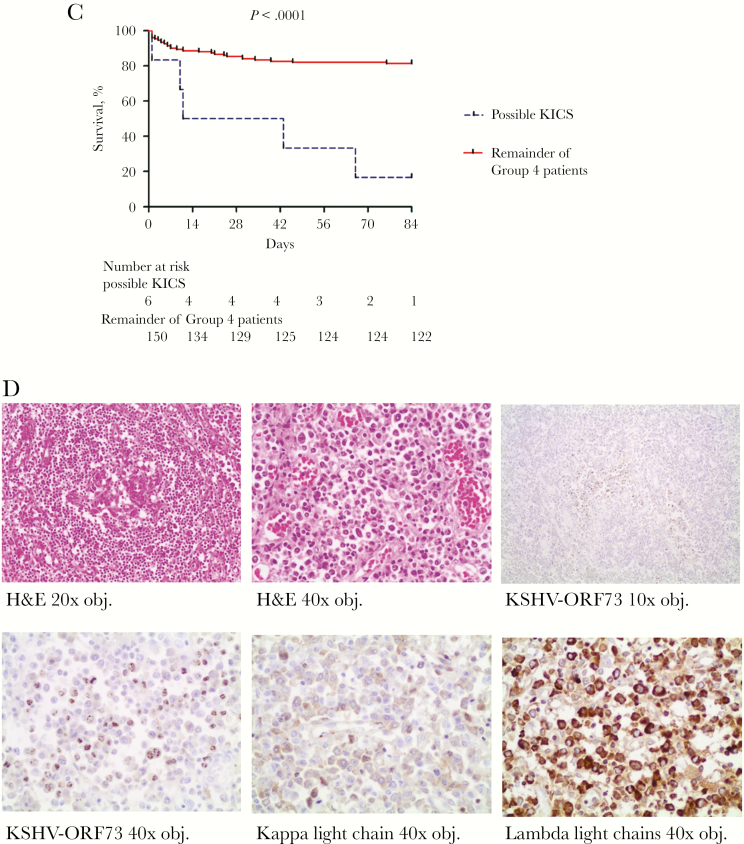
Identification and contribution of possible Kaposi’s sarcoma–associated herpesvirus (KSHV) inflammatory cytokine syndrome (KICS) to mortality in a cohort of critically ill patients investigated for tuberculosis (TB). A, Schematic flowchart showing the diagnosis of “possible KICS” by exclusion in the entire cohort. Patients were excluded if they had microbiologically proven TB or other bacterial or fungal infections, and those who remained were further evaluated according to the criteria previously described in the KICS working case definition [15]. Two patients were excluded on the basis of alternative diagnoses (TB meningitis and community-acquired pneumonia, respectively). As this analysis was done retrospectively, multicentric Castleman disease could not be excluded at clinical presentation, hence the designation “possible KICS” patients. B, Selected KICS-defining parameters (KSHV viral load [VL], interleukin 6 [IL-6] level, C-reactive protein [CRP] level, platelet count, albumin, hemoglobin) in “possible KICS” patients (n = 6) compared to the remainder of group 4 patients (n = 153). The dotted lines mark abnormal levels (KSHV-VL >100 copies/106 cells; human IL-6 >1.8 pg/mL [23]; CRP >10 mg/L; platelet count <186 ×103 cells/µL; albumin <35 g/L; and hemoglobin <12 g/dL), and the solid lines indicate the median. P values are by Wilcoxon rank-sum test. Data are log transformed where necessary. C, Overall confirmed survival at end of the 12-week study period in “possible KICS” patients (n = 6) compared to the remainder of group 4 patients (n = 153, including 3 patients who were lost to follow-up). P value is by log-rank test. D, Histopathological assessment of postmortem lymph node biopsies taken from a “possible KICS” patient with the highest KSHV-VL of the entire patient cohort. Top panel, from left to right: hematoxylin and eosin (H&E) stain showing a regressed germinal center with sheets of plasma cells in the mantle zone among prominent capillaries (20× objective magnification); H&E stain showing an infiltrate of numerous benign plasma cells (40× objective magnification); immunohistochemical stain of KSHV open reading frame (ORF) 73 showing aggregates of KSHV-positive cells in the lymph node staining brown (10× objective magnification). Bottom panel, left to right: immunohistochemical stain showing brown granular nuclear KSHV-ORF73 positivity among the numerous background plasma cells (40× objective magnification); immunohistochemistry for kappa light chains demonstrate that few of the plasma cells in an area of ORF73-positive cells are kappa restricted cells (40× objective magnification); immunohistochemistry of lambda light chains in the area of ORF73-positive cells demonstrate that a large number of the plasma cells are lambda-restricted cells (40× objective magnification). Abbreviations: CRP, C-reactive protein; H&E, hematoxylin and eosin; hIL-6, human interleukin 6; KICS, Kaposi’s sarcoma–associated herpesvirus inflammatory cytokine syndrome; KSHV, Kaposi’s sarcoma–associated herpesvirus; LAM, lipoarabinomannan; MCD, multicentric Castleman disease; MTB, Mycobacterium tuberculosis; ORF, open reading frame; TB, tuberculosis; VL, viral load.
Table 4.
Baseline Characteristics of Patients With Kaposi’s Sarcoma–Associated Herpesvirus Inflammatory Cytokine Syndrome (n = 6) and All Other Group 4 Patients (n = 153)
| Characteristic | Possible KICS Subjects (n = 6) |
Remainder of Patients Without Microbiologically Proven Coinfection (n = 153) | P Value |
|---|---|---|---|
| Confirmed death | 5 (83.3) | 28 (18.3) | .002 |
| Male sex | 4 (67) | 61 (39.9) | .227 |
| Age, y, median (range) | 47 (26–74) | 38 (19–81) | .049 |
| Weight, kg, median (range) | 58 (35–71) | 53.0 (30–99) | .912 |
| Receiving ART | 3 (50) | 63 (41.2) | .290 |
| HIV-VL, copies/mL, median (range) | 81 298 (29–3 507 840) | 70 363 (29–7 934 692) | .978 |
| CD4 count, cells/μL, median (range) | 106.5 (10–328) | 97 (2–519) | .818 |
| KSHV seropositive | 6 (100) | 55 (35.9) | .003 |
| Skin KS | 1 (16.7) | 4 (2.6) | .177 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; KICS, Kaposi’s sarcoma–associated herpesvirus inflammatory cytokine syndrome; KS, Kaposi’s sarcoma; KSHV, Kaposi’s sarcoma–associated herpesvirus; VL, viral load.
Table 5.
Selected Laboratory Abnormalities in Patients With Kaposi’s Sarcoma–Associated Herpesvirus Inflammatory Cytokine Syndrome (n = 6) and All Other Group 4 Patients (n = 153)
| Characteristic | Possible KICS Subjects (n = 6) | Remainder of Patients Without Microbiologically Proven Coinfection (n = 153) | P Value | ||
|---|---|---|---|---|---|
| Abnormala, No. (%) | Median (Range) | Abnormala, No. (%) | Median (Range) | ||
| KSHV-VL, copies/106 cells | 6 (100) | 699.6 (160.7–2 165 641) | 2 (1) | 1.00 (1.00–104 664.39) | < .0001 |
| K8.1 OD | 6 (100) | 2.1 (1.3–2.3) | 46 (30) | 0.48 (0.10–2.57) | .001 |
| ORF73 OD | 4 (67) | 3.3 (0.32–7.4) | 33 (22) | 0.24 (0.03–8.14) | .003 |
| IL-6, pg/mL | 5 (83) | 11.5 (1.6–3 307.4) | 97 (63) | 12.5 (1.6–1 045.4) | .978 |
| CRP, mg/L | 6 (100) | 71.4 (11.1–304) | 131 (85) | 94.0 (0.38–564.9) | .767 |
| Hemoglobin, g/dL | 5 (83) | 8.1 (6.9–14.9) | 130 (85) | 10 (4.9–17.4) | .231 |
| White cell count, ×103/µL | 1 (17) | 7.6 (3.5–21.6) | 25 (16.3) | 6.8 (1.0–43.2) | .783 |
| Platelet count, ×103/µL | 4 (67) | 185 (89.0–305) | 29 (19) | 280 (23.0–789) | .050 |
| Albumin, g/L | 5 (83) | 24.0 (16.0–39.0) | 118 (77) | 28.0 (12.0–43.0) | .489 |
| Sodium, mEq/L | 5 (83) | 132 (125–138) | 119 (78) | 131 (113–150) | .692 |
P values are by Fisher exact test or Wilcoxon rank-sum test, as appropriate.
Abbreviations: CRP, C-reactive protein; IL-6, interleukin 6; KICS, Kaposi’s sarcoma–associated herpesvirus inflammatory cytokine syndrome; KSHV, Kaposi’s sarcoma–associated herpesvirus; OD, optical density; ORF, open reading frame; VL, viral load.
a“Abnormal” refers to elevated KSHV-VL, >100 copies/106 cells; positive K8.1 and ORF73, OD values >1 (normalized cutoff); elevated IL-6, >1.8 pg/mL; elevated CRP, >10 mg/L; low hemoglobin, <12 g/dL (female) or <13 g/dL (male); low white cell count <3.9 ×103/µL; low platelet count, <186 ×103/µL; low albumin, <35 g/L; and low sodium, <135 mEq/L.
Finally, 12-week mortality among “possible KICS” patients was 83% (5/6) and thereby significantly higher compared to 18% for the remainder of the group 4 patients. Median time to death in “possible KICS” patients was 11 days (95% CI, 0–51 days) (Figure 2C), supporting previous reports of markedly elevated risk of death in KICS subjects [15]. KICS criteria identified all patients with elevated KSHV-VL in group 4 (Table 2) who died within 12 weeks. Moreover, KICS criteria were found to be specific for predicting death in this cohort, with the following characteristics: sensitivity 0.15, specificity 0.99, positive predictive value 0.83, and negative predictive value 0.81.
Post Hoc Description of the Identified “Possible KICS” Patients
Of the 6 “possible KICS” patients, 1 was diagnosed with KSHV-associated MCD at autopsy. This patient displayed the highest KSHV-VL of the entire cohort (2 165 642 copies/106 cells) and was positive for K8.1 but not ORF73, suggesting a highly lytically active KSHV infection. In the absence of any clinical measure of KICS or KSHV on presentation, this patient was empirically treated for TB for 6 months with no improvement prior to admission. He presented with further deterioration after completion of TB-treatment and died on the day of enrollment. His CD4 count was 328 cells/μL, and HIV-VL was undetectable. The patient had evidence of systemic inflammation (CRP, 304 mg/L) and cytokine activation (IL-6, 3307 pg/mL), as well as severe anemia (hemoglobin, 6.9 g/dL), thrombocytopenia (platelet count, 124 ×103 cells/μL), hypoalbuminemia (albumin, 16 g/L), hyponatremia (sodium, 125 mEq/L), lymphadenopathy, and hepatomegaly. KICS-associated symptoms included respiratory symptoms (cough), weight loss, nausea, body pain, and weakness. Histologic examination of lymph nodes was consistent with KSHV-associated MCD (Figure 2D). There was no evidence of PEL. Two other “possible KICS” patients’ deaths were retrospectively likely attributable to KICS in the setting of KS [15]. One had biopsy-confirmed KS and was restarted on ART but died before assessment for chemotherapy. One patient, after deterioration on empiric anti-TB therapy, had skin KS confirmed on biopsy and features of lung KS on computed tomographic scan 2 weeks prior to death. The other “possible KICS” patients did not have identified KSHV-associated malignancies despite elevated VL assessed retrospectively. One was started on TB treatment empirically, deteriorated on treatment, and was diagnosed with an invasive keratinizing moderately differentiated squamous cell carcinoma during evaluation of an upper gastrointestinal bleed. The other patient suffered from chronic renal failure due to urethral stricture and was admitted with an episode of acute kidney injury. The patient was treated for suspected bacterial sepsis but died within the study period. The sixth patient was treated for suspected bacterial meningitis, improved, and survived the follow-up period.
DISCUSSION
South Africa has one of the highest global rates of both HIV and TB [27]. Improved diagnostics for treatable diseases that mimic TB are needed to limit unnecessary empiric TB treatment. Utilizing a large, well-characterized patient cohort presenting to a Cape Town hospital with suspected TB, we retrospectively evaluated KSHV as a contributor to mortality.
KSHV seroprevalence is high in SSA with variable regional prevalence [28]. We found 30.7% (95% CI, 27%–34%) KSHV seroprevalence in Cape Town, which is in agreement with other South African estimates of 30%–40% from Soweto, Johannesburg, and Kwa-Zulu Natal. Of KSHV-seropositive patients, 18.8% (5.8% of the entire cohort) showed detectable virus in the blood, suggestive of poor immune control of KSHV [29].
Focusing on the 33 (5%) patients with elevated (>100 copies/106 cells) KSHV-VL, we found no higher mortality within the context of either the entire patient cohort or the cohort with confirmed TB, suggesting that KSHV does not play a significant role in potentiating TB mortality. However, in patients with neither microbiologically proven TB nor alternative coinfection, elevated PBMC-associated KSHV-VL was associated with 6.5 higher odds of mortality when adjusted for age, sex, CD4 cell count, and ART status. In contrast, KSHV seropositivity alone was not associated with mortality (data not shown), suggesting that it is the burden of KSHV that contributes to the observed association [8, 14]. Similarly, elevated plasma KSHV-VL, a marker of circulating tumour DNA, has been noted as a risk factor for death in people with established KS [30]. Although a strong association between elevated KSHV-VL and mortality among microbiologically unconfirmed TB patients was identified, additional factors such as KSHV-associated malignancies, functional immune dysregulation (cytokine syndromes), or other pathological processes (eg, coinfections or other cancers) likely also contribute to death.
We further investigated whether application of KICS criteria [15] identified patients with a high mortality and found 6 “possible KICS” patients, of whom 5 died, with a median survival of 11 days. Three had identified untreated KSHV-associated malignancies. Although “possible KICS” patients had elevated IL-6 and CRP, these were not distinguishing features compared to other patients with TB or other critical illnesses. This suggests that CRP is a less useful screening tool than KSHV-VL in this population.
To our knowledge, this is the first systematic evaluation of KICS in South Africa and has implications for other countries with high prevalence of HIV/KSHV coinfection. A study from Uganda reports that 3 in every 10 patients with HIV-associated lymphoma had a possible misdiagnosis and were treated for TB before a final diagnosis of lymphoma was made, and our data suggest that KSHV-associated diseases are important to include in the differential diagnosis of suspected TB [6].
A limitation of our study was that only 1 of the 6 “possible KICS” patients had a pathological examination of a lymph node biopsy. This was performed following death and demonstrated KSHV-MCD. Another had possible pulmonary KS diagnosed based on chest radiograph and confirmed cutaneous KS. It is possible that the other patients also had undiagnosed KSHV-associated diseases such as KS or MCD. Although we have not definitively established that “possible KICS” patients died of KSHV-associated malignancies in most cases, our study demonstrates that elevated KSHV-VL in the peripheral blood represents a significant parameter associated with mortality. Our data support that patients meeting KICS criteria should be evaluated for KSHV-MCD, visceral KS, or PEL, particularly in settings with oncology capacity to manage these treatable KSHV-associated malignancies.
Another important finding was that the majority of the entire patient cohort was anemic, which was significantly associated with elevated antibody levels to the KSHV lytic antigen K8.1 in KSHV-seropositive group 4 patients. This is consistent with results from a recent study from Uganda, which reported a link between elevated KSHV serumpositivity and anemia in the setting of malaria. Additional studies are required to evaluate this association. For example, anemia may lead to reactivation of KSHV through relative tissue hypoxia [31], or KSHV reactivation may lead to anemia or chronic inflammation mediated through IL-6.
In sum, our data suggest that elevated KSHV-VL should be considered as an important pathology in HIV-infected patients investigated for TB, and that for those meeting other KICS criteria, evaluation for KSHV-VL should be considered. Increasing implementation of PCR-based TB diagnostics should facilitate more rapid TB diagnostic workup, thereby facilitating selection of patients for whom KSHV testing may be indicated. In selected patients, KSHV-VL has a strong prognostic value. KSHV-VL has been linked to an increased risk of KSHV-associated malignancies [32]; therefore, HIV-infected patients with elevated KSHV-VL should be evaluated for KSHV-related malignancies and treated appropriately. KSHV-associated malignancies are treatable, and earlier diagnosis may improve survival. Given its high mortality, evaluation of therapeutic strategies for KICS and KICS-like syndromes are urgently needed.
SUPPLEMENTARY DATA
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors particularly thank all participants in this study. The authors thank Amy Ward and Saskia Janssen for their work on the clinical study.
Disclaimer. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The opinions, findings, and conclusions expressed in this manuscript reflect those of the authors alone.
Financial support. This research was funded by grants from the Cancer Association of South Africa to G. S.; the South African Medical Research Council (SA-MRC) to A. A. K. and C. S. (National Health Scholars Programme); the National Research Foundation (NRF) of South Africa to G. S. and M. J. B.; the University of Cape Town to G. S.; and in part by the Intramural Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (contract number HHSN261200800001E to D. W.). D. B. was funded by a Wellcome Trust research training fellowship (number 105165/Z/14/A). G. M. was supported by the Wellcome Trust (grant numbers 098316 and 203135/Z/16/Z), the South African Research Chairs Initiative of the Department of Science and Technology and NRF of South Africa (grant number 64787), NRF incentive funding (UID: 85858), and the South African Medical Research Council through its TB and HIV Collaborating Centres Programme with funds received from the National Department of Health (RFA number SA-MRC–RFA-CC: TB/HIV/AIDS-01-2014).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Fifth Workshop on Emerging Issues in Oncogenic Virus Research, San Pietro, Bevagna, Italy, 30 May–3 June 2018; and Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 4–7 March 2019.
References
- 1. Joint United Nations Programme on HIV/AIDS. UNAIDS Data 2017. http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf Accessed 15 July 2018. [PubMed]
- 2. Farahani M, Mulinder H, Farahani A, Marlink R. Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: a systematic review and meta-analysis. Int J STD AIDS 2017; 28:636–50. [DOI] [PubMed] [Google Scholar]
- 3. Blumenthal MJ, Ujma S, Katz AA, Schäfer G. The role of type 2 diabetes for the development of pathogen-associated cancers in the face of the HIV/AIDS epidemic. Front Microbiol 2017; 8:2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cox JA, Kiggundu D, Elpert L, Meintjes G, Colebunders R, Alamo S. Temporal trends in death causes in adults attending an urban HIV clinic in Uganda: a retrospective chart review. BMJ Open 2016; 6:e008718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015; 29:1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buyego P, Nakiyingi L, Ddungu H, et al. . Possible misdiagnosis of HIV associated lymphoma as tuberculosis among patients attending Uganda Cancer Institute. AIDS Res Ther 2017; 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Masamba LPL, Jere Y, Brown ERS, Gorman DR. Tuberculosis diagnosis delaying treatment of cancer: experience from a new oncology unit in Blantyre, Malawi. J Glob Oncol 2016; 2:26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oksenhendler E, Carcelain G, Aoki Y, et al. . High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood 2000; 96:2069–73. [PubMed] [Google Scholar]
- 9. Nador RG, Cesarman E, Chadburn A, et al. . Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood 1996; 88:645–56. [PubMed] [Google Scholar]
- 10. Soulier J, Grollet L, Oksenhendler E, et al. . Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995; 86:1276–80. [PubMed] [Google Scholar]
- 11. Semeere A, Wenger M, Busakhala N, et al. . A prospective ascertainment of cancer incidence in sub-Saharan Africa: the case of Kaposi sarcoma. Cancer Med 2016; 5:914–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424. [DOI] [PubMed] [Google Scholar]
- 13. Gopal S, Liomba NG, Montgomery ND, et al. . Characteristics and survival for HIV-associated multicentric Castleman disease in Malawi. J Int AIDS Soc 2015; 18:20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uldrick TS, Wang V, O’Mahony D, et al. . An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without multicentric Castleman disease. Clin Infect Dis 2010; 51:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polizzotto MN, Uldrick TS, Wyvill KM, et al. . Clinical features and outcomes of patients with symptomatic Kaposi sarcoma herpesvirus (KSHV)-associated inflammation: prospective characterization of KSHV inflammatory cytokine syndrome (KICS). Clin Infect Dis 2016; 62:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Della Bella S, Taddeo A, Calabrò ML, et al. . Peripheral blood endothelial progenitors as potential reservoirs of Kaposi’s sarcoma-associated herpesvirus. PLoS One 2008; 3:e1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monini P, Colombini S, Stürzl M, et al. . Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi’s sarcoma. Blood 1999; 93:4044–58. [PubMed] [Google Scholar]
- 18. Parravicini C, Chandran B, Corbellino M, et al. . Differential viral protein expression in Kaposi’s sarcoma-associated herpesvirus-infected diseases: Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. Am J Pathol 2000; 156:743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Staskus KA, Sun R, Miller G, et al. . Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. J Virol 1999; 73:4181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blumenthal MJ, Schutz C, Meintjes G, et al. . EPHA2 sequence variants are associated with susceptibility to Kaposi’s sarcoma-associated herpesvirus infection and Kaposi’s sarcoma prevalence in HIV-infected patients. Cancer Epidemiol 2018; 56:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sitas F, Newton R. Kaposi’s sarcoma in South Africa. J Natl Cancer Inst Monogr 2001:1–4. [DOI] [PubMed] [Google Scholar]
- 22. Mbisa GL, Miley W, Gamache CJ, et al. . Detection of antibodies to Kaposi’s sarcoma-associated herpesvirus: a new approach using K8.1 ELISA and a newly developed recombinant LANA ELISA. J Immunol Methods 2010; 356:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borges AH, O’Connor JL, Phillips AN, et al. . Factors associated with plasma IL-6 levels during HIV infection. J Infec Dis 2015; 212:585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Sanjosé S, Marshall V, Solà J, et al. . Prevalence of Kaposi’s sarcoma-associated herpesvirus infection in sex workers and women from the general population in Spain. Int J Cancer 2002; 98:155–8. [DOI] [PubMed] [Google Scholar]
- 25. Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV-3 real time PCR assay. J Virol Methods 2001; 91:109–17. [DOI] [PubMed] [Google Scholar]
- 26. Box GEP, Tidwell PW. Transformation of the independent variables. Technometrics 1962; 4:531–50. [Google Scholar]
- 27. Abdool Karim SS, Churchyard GJ, Karim QA, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet 2009; 374:921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dedicoat M, Newton R. Review of the distribution of Kaposi’s sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi’s sarcoma. Br J Cancer 2003; 88:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maskew M, Macphail AP, Whitby D, Egger M, Wallis CL, Fox MP. Prevalence and predictors of Kaposi sarcoma herpes virus seropositivity: a cross-sectional analysis of HIV-infected adults initiating ART in Johannesburg, South Africa. Infect Agent Cancer 2011; 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Letang E, Lewis JJ, Bower M, et al. . Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS 2013; 27:1603–13. [DOI] [PubMed] [Google Scholar]
- 31. Nalwoga A, Cose S, Nash S, et al. . Relationship between anemia, malaria coinfection, and Kaposi sarcoma-associated herpesvirus seropositivity in a population-based study in rural Uganda. J Infect Dis 2018; 218:1061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Campbell TB, Borok M, Gwanzura L, et al. . Relationship of human herpesvirus 8 peripheral blood virus load and Kaposi’s sarcoma clinical stage. AIDS 2000; 14:2109–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.