Abstract
The organic molecule tenoxicam and similar derivatives, piroxicam and isoxicam have been studied by quantum chemical theory (DFT), FT-Raman and FT-IR. By FMOs energies the charge transfer inside the molecules are obtained. The UV-Vis spectra of the compounds are simulated to study the electronic transition in the target molecules. By using natural bond orbital (NBO), charge delocalization analyzes arising from hyper conjugative interactions and the stability of the molecules are obtained. First order hyperpolarizability of piroxicam is higher than that of isoxicam and tenoxicam. The reactive areas are thoroughly studied by MEP. Prediction of Activity Spectra gives activities, anti-inflammatory, CYP2C9 substrate and gout treatment. Docked ligands form a stable complex with the receptors.
Keywords: Organic chemistry, Theoretical chemistry, Pharmaceutical chemistry, DFT, MEP, FT-IR, FT-Raman, Molecular docking
1. Introduction
Oxicams are enolcarboxamides that exhibit number of pharmacological properties and effective for postoperative pain, arthritis, degenerative joint diseases and osteoarthritis [1]. Tenoxicam is a nonsteroidal anti-inflammatory drug that is a part of the oxicam family and it can be used as an effective analgesic and antipyretic agent [2]. Piroxicam possess multifunctional activity including chemoprevention and its photochemical properties are sensitive to medium [3, 4]. Tamasi et al. [5] reported the synthesis and DFT studies of oxicam complexes. By giving the mol files of tenoxicam, piroxicam and isoxicam in the software it predicts different biological activities. Literature survey shows that there is no detailed study done on the molecules both quantum chemical and experimental spectroscopic studies which are very essential for micro level function of any organic compounds. The structural and physio-chemical properties of the compounds can be found out by spectroscopic and quantum computational tools like Density Functional Theory. These structural and physio-chemical properties can be used to establish relationships between these properties and biological activity of the compound [6]. Due to a large number of applications of NLO materials in optoelectronic technology, the molecules have been analyzed for their hyperpolarizability [7]. Several properties like highest occupied molecular orbital, lowest unoccupied molecular orbital energies, various chemical descriptors, molecular electrostatic potential analysis are carried out to provide information about charge transfer within the molecules. The spectral analysis of tenoxicam, piroxicam and isoxicam are performed and compared with theoretical values. The redistribution of electron density are investigated.
2. Calculation
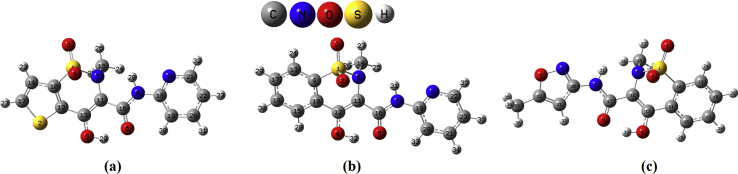
All calculations are performed using the Gaussian09 software package [8]. DFT method was employed using B3LYP functional and cc-pVDZ (5D, 7F) basis set. Results from frequency calculations after scaling were used to get the IR spectral data, which is compared with the experimental spectral vibrations [9]. By using the TD-DFT method the electronic properties of the molecules (Fig. 1) determined using CAM-B3LYP functional and cc-pVDZ basis set. The spectral data are obtained from Bio-Rad Laboratories, Inc. SpectraBase [10].
Fig. 1.
Optimized geometry of (a) tenoxicam, (b) piroxicam and (c) isoxicam.
3. Results and discussions
3.1. Natural bond orbital analysis
NBO analysis provides information about various hyper conjugative interactions and intermolecular charge transfer between bonding and antibonding orbitals. In the current work the analysis has been done using DFT method at B3LYP/cc-pVDZ (5D, 7F) level. The stabilization energy forms an important characteristic in this analysis and higher this energy, greater will be the interaction between the electron donors and hence greater the extent of conjugation. Intra molecular interactions are very much important in predicting the stability and reactivity of the target molecules [11]. To study intra and inter-molecular non-bonded interactions the NBO is the efficient method for organic and bio-molecular compounds [12]. Based on the second order perturbation theory the important donor-acceptor interactions are calculated. The important interactions are: For tenoxicam: The strong interactions are N8→π*(O6-C16), N8→π*(C18-C19), O6→ σ*(N8-C16), O5→π*(C11-C13), O4→ σ*(S1-C10), O4→ σ*(S1-O3), O3→ σ*(S1-C10), O3→ σ*(S1-O4), S2→π*(C14-C17), S2→π*(C10-C12), C18-C19→π*(C20-C22), C18-C19→π*(N9-C21), C11-C13→π*(O6-C16) with energies 79.05, 38.52, 21.56, 48.55, 22.48, 16.29,20.19, 17.93, 21.35, 26.13, 22.73, 17.71, 26.08 kcal/mol. For piroxicam: N7→π*(O5-C16), N7→π*(C19-C20), O5→ σ*(N7-C16), O4→π*(C11-C12), O3→ σ*(S1-N6), O3→ σ*(S1-C9), O2→σ*(S1-N6), O2→ σ*(S1-C9), C19-C20→π*(N8-C22), C19-C20→π*(C21-C23), C11-C12→π*(O5-C16) with energies, 79.26, 38.71, 21.48, 48.24, 34.03, 19.38, 33.32, 22.57, 17.71, 22.81, 25.94 kcal/mol and for isoxicam: N8→π*(O5-C17), N8→π*(N9-C20), O6→π*(N9-C20), O6→π*(C21-C22), O5→ σ*(N8-C17), O4→π*(C12-C13), O3→ σ*(S1-N7), O2→ σ*(S1-N7), O2→ σ*(S1-O3), O2→ σ*(S1-C10), C21-C22→π*(N9-C20), C12-C13→π*(O5-C17), C10-C14→π*(C11-C16) with energies, 74.85, 46.86, 16.12, 35.11, 22.01, 48.41, 33.52, 34.25, 18.74, 19.44, 28.36, 26.52, 19.66 kcal/mol. The delocalization energies are very high and hence the molecules are stable enough to show desired medicinal properties.
3.2. Electronic spectra and NLO properties
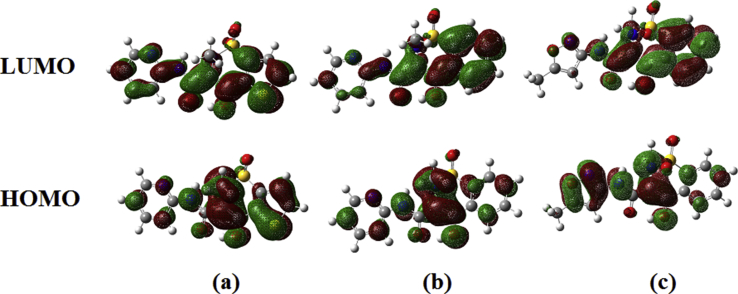
The 3D diagrams of HOMO and LUMO are shown in Fig. 2. HOMO represents the donating nature of an electron and LUMO represent accepting nature of electrons [13]. HOMO and LUMO energy are -7.837, -4.931 for tenoxicam, -8.004, -5.315 for piroxicam and -7.867, -5.268 for isoxicam. The band gap energy is 2.908 for tenoxicam, 2.689 for piroxicam and 2.599 for isoxicam explains the ultimate transfer of charge happening within the molecule and shows the biological activity. The values of chemical descriptors are given in Table 1. Due to the low value of HOMO-LUMO energy gap [14] these compounds have high softness nature. The low value of the electrophilicity index suggests the biological activity of the compounds. Nonlinear optical studies are an important part in the present world of researchers as NLO active materials find applications in telecommunication, potential applications in modern communication technology, optical signal processing and data storage [15]. Molecular based nonlinear optical behavior (NLO) materials have current attention and great importance because they involve new technical phenomena owing to the emerging application in electronic devices [16]. First order hyperpolarizability of piroxicam (9.232×10−30 esu) > isoxicam (9.112×10−30 esu) > tenoxicam (7.756×10−30 esu) which are 71, 70 and 60 times that of urea while the second order values are -18.132×10−37, -18.336×10−37 and -19.060×10−37 for tenoxicam, piroxicam and isoxicam [17]. These values show that the title compounds are an important class of compounds in the rank of NLO materials [18]. Electronic transitions in a molecule usually happen in the UV and Visible region of the electromagnetic spectra. Being a time dependent phenomena, original DFT treatment could not explain this phenomenon which involves a change in the electric field of the radiations. For that time dependent density functional theory, known as TDDFT is used to simulate the electronic spectra of the compounds. Long range corrected density functional- CAM-B3LYP is used in this study with the generic 6-31G(d) basis set in methanol solvent cage as provided in the PCM solvation model [19]. In the case of tenoxicam, the DOS spectra show no unusual overlap in the frontier molecular orbitals. Simulated UV spectrum shows two strong excitations at 336.76 nm and 263.69 nm with oscillator strength 0.7602 and 0.103 respectively. The former may be due to the pi to antibonding pi orbital transitions and it is found that HOMO to LUMO transition contributes 75% to it followed by HOMOI-1 to LUMO (21%). The second transition can be attributed to the lone pair to antibonding orbital interactions, hence of low intensity. Data shows that this transition is due to HOMO-LUMO (14%), HOMO-1 to LUMO (60%) and HOMO-3 to LUMO (11%). For the compound pyroxicam, the one dominant transition was at 310 nm with oscillator strength 0.8658 due to the HOMO-1 to LUMO (21%) and HOMO to LUMO (74%), which is due to the pi to pi antibonding transition. There are other two less intense transitions too at 265.48 and 253.00 nm originating from the antibonding orbitals. Isoxicam shows an intense peak at 304.81 nm with intensity 0.6978 and can be attributed to HOMO to LUMO (91%) and HOMO-1 to LUMO (4%). There is another low intense transition originating from the lone pairs at 262.10 nm with oscillator strength of 0.0262.
Fig. 2.
HOMO-LUMO plots of (a) tenoxicam, (b) piroxicam and (c) isoxicam.
Table 1.
Chemical descriptors.
| Compound | HOMO | LUMO | I = -EHOMO | A = -ELUMO | Gap | η=(I-A)/2 | μ = -(I + A)/2 | ω = μ2/2η |
|---|---|---|---|---|---|---|---|---|
| Tenoxicam | -7.837 | -4.931 | 7.837 | 4.931 | 2.908 | 1.454 | -6.384 | 14.015 |
| Piroxicam | -8.004 | -5.315 | 8.004 | 5.315 | 2.689 | 1.345 | -6.660 | 16.489 |
| Isoxicam | -7.867 | -5.268 | 7.867 | 5.268 | 2.599 | 1.300 | -6.568 | 16.592 |
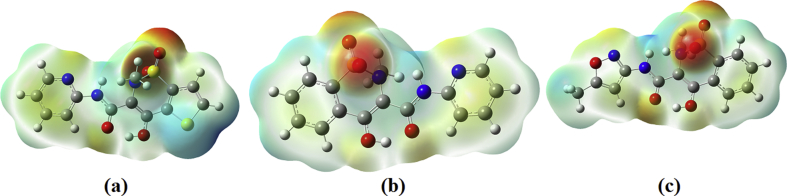
3.3. Molecular electrostatic potential
MEPs map of the title compounds are shown in Fig. 3 [20]. The various surfaces of the molecule are having different electrostatic potentials and are in different colors. The negative spots are represented by red, blue is the regions of the positive and the green gives zero potential. From the diagram we can see that the negative portions are near the oxygen atoms and the N atom in the ring for all the compounds. The positive areas are around the NH groups. In this molecule, the negative regions attract proton from the amino acids or protein. These active sites are evidence of the biological activity of the title molecules.
Fig. 3.
MEP plots of (a) tenoxicam, (b) piroxicam and (c) isoxicam.
3.4. IR and Raman spectra
Bands (Table 2) at 3390 (IR), 3411 (DFT) for tenoxicam, 3400 (IR), 3409 (DFT) for piroxicam and 3290 (IR), 3300 (Raman), 3417 (DFT) for isoxicam are assigned as the NH stretching modes [21]. The υC=O is assigned at 1610 (IR), 1605 (Raman), 1614 (DFT) for tenoxicam, 1640 (IR), 1615 (Raman), 1617 (DFT) for piroxicam and 1630 (IR), 1624 (DFT) for isoxicam [21]. The downshift of these NH and C=O modes are due to strong hyper conjugative interactions as given by NBO analysis. The C=C stretching modes are assigned at 1422 (IR), 1500, 1429 (Raman), 1501, 1422 (DFT) for tenoxicam, 1600 (IR), 1600 (Raman), 1598 (DFT) for piroxicam and at 1608, 1600 (IR), 1603 (Raman), 1606, 1590 (DFT) for isoxicam [21]. The SO2 stretching modes are assigned nearly at around 1251 (IR) and 1250 (DFT) for all the three molecules [21]. The CS stretching mode are observed at 785, 653 (tenoxicam), 631 (piroxicam), 632 (isoxicam) in IR, 668, 645 (tenoxicam), 640 (piroxicam and isoxicam) in Raman spectrum [21]. All the experimentally observed bands are identified as assigned.
Table 2.
Vibrational assignments.
| 2.1: Tenoxicam | |||||
|---|---|---|---|---|---|
| B3LYP/CC-pVDZ (5D, 7F) |
IR |
Raman |
Assignmentsa |
||
| υ(cm−1) | IRI | RA | υ(cm−1) | υ(cm−1) | - |
| 3411 | 61.99 | 188.9 | 3390 | - | υNH |
| 3132 | 4.19 | 49.90 | 3145 | 3140 | υCH |
| 3109 | 3.53 | 82.61 | 3105 | 3110 | υCH |
| 3084 | 16.36 | 311.8 | 3088 | 3085 | υCH |
| 2990 | 13.91 | 62.94 | 3000 | - | υCH3 |
| 2902 | 31.62 | 73.61 | 2915 | 2920 | υCH3 |
| 1614 | 137.2 | 371.6 | 1610 | 1605 | υC=O |
| 1566 | 142.5 | 303.6 | 1563 | - | υRingI |
| 1501 | 105.7 | 1950.4 | - | 1500 | υC=CRingII |
| 1499 | 368.0 | 34.43 | 1495 | - | δNH |
| 1422 | 18.88 | 562.4 | 1422 | 1424 | υC=CRingIII |
| 1406 | 2.99 | 12.86 | - | 1404 | δCH3 |
| 1381 | 2.03 | 241.3 | - | 1380 | δOH |
| 1364 | 6.11 | 127.2 | 1370 | - | δCH3 |
| 1344 | 152.0 | 627.7 | 1345 | 1347 | υRingIII |
| 1315 | 78.31 | 80.18 | 1320 | - | δCH |
| 1292 | 92.89 | 74.80 | 1296 | 1290 | υRingI |
| 1253 | 176.2 | 5.31 | 1251 | - | υSO2 |
| 1247 | 27.41 | 126.2 | - | 1249 | υCN |
| 1201 | 58.53 | 299.0 | 1200 | 1200 | υCN |
| 1186 | 79.13 | 141.0 | - | 1188 | υCN |
| 1186 | 79.13 | 141.0 | - | 1188 | υCN |
| 1141 | 31.75 | 18.56 | 1148 | 1147 | δCH |
| 1130 | 26.31 | 47.27 | 1138 | - | δCH3 |
| 1102 | 9.25 | 72.35 | 1100 | 1100 | υCC |
| 1082 | 7.35 | 5.65 | 1085 | - | δCH3 |
| 1046 | 15.54 | 48.98 | 1048 | 1050 | δCH |
| 1009 | 39.54 | 3.08 | 995 | 1005 | υCN |
| 978 | 0.28 | 0.56 | 976 | - | γCH |
| 905 | 83.51 | 0.71 | 903 | - | γOH |
| 895 | 14.22 | 2.80 | - | 893 | δC=O |
| 870 | 6.93 | 27.68 | - | 871 | γCH |
| 865 | 2.01 | 2.35 | 858 | - | γCH |
| 839 | 10.64 | 11.24 | 840 | 840 | υRingI |
| 781 | 19.91 | 4.33 | 785 | - | υCS |
| 769 | 38.51 | 0.77 | - | 770 | τRingI |
| 763 | 16.96 | 14.04 | 760 | 755 | υSN |
| 735 | 40.39 | 8.93 | 737 | - | τRingI |
| 703 | 38.57 | 2.00 | 703 | 700 | γNH |
| 662 | 11.20 | 6.26 | - | 668 | υCS |
| 656 | 7.09 | 18.92 | 653 | 645 | υCS |
| 588 | 79.05 | 7.45 | 590 | 580 | δRingI |
| 531 | 16.05 | 3.06 | 535 | 536 | δSO2 |
| 513 | 35.12 | 6.20 | - | 508 | δSO2 |
| 480 | 17.16 | 1.48 | 478 | 478 | τRingII |
| 455 | 3.03 | 1.97 | 448 | 452 | τRingIII |
| 406 | 2.37 | 0.78 | 403 | 404 | τRingI |
| 305 | 2.51 | 11.22 | - | 303 | τRingIII |
| 246 | 1.65 | 0.96 | - | 250 | δRingIII |
| 171 | 2.90 | 2.94 | - | 173 | τCH3 |
| 2.2: Piroxicam | |||||
|---|---|---|---|---|---|
| B3LYP/CC-pVDZ (5D, 7F) |
IR |
Raman |
Assignmentsb |
||
| υ(cm−1) | IRI | RA | υ(cm−1) | υ(cm−1) | - |
| 3409 | 68.10 | 200.6 | 3400 | - | υNH |
| 3098 | 2.69 | 157.6 | - | 3100 | υCHRingIII |
| 3084 | 16.55 | 311.8 | 3084 | - | υCHRingI |
| 3062 | 9.34 | 120.9 | - | 3061 | υCHRingI |
| 3036 | 23.86 | 151.6 | 3030 | 3038 | υCHRingI |
| 2988 | 14.68 | 69.53 | 2950 | 2965 | υCH3 |
| 2900 | 31.75 | 72.08 | 2900 | - | υCH3 |
| 1617 | 167.8 | 141.0 | 1640 | 1615 | υC=O |
| 1598 | 76.25 | 596.6 | 1600 | 1600 | υC=C |
| 1579 | 57.84 | 599.6 | 1578 | 1585 | υRingI |
| 1566 | 89.17 | 119.3 | - | 1563 | υRingI |
| 1545 | 44.02 | 131.4 | 1540 | 1547 | υRingIII |
| 1501 | 13.91 | 149.2 | - | 1498 | δNH |
| 1445 | 23.91 | 114.6 | 1447 | 1443 | υRingIII |
| 1409 | 299.2 | 2.94 | 1410 | - | υRingI |
| 1406 | 2.74 | 31.88 | - | 1405 | δCH3 |
| 1364 | 9.45 | 112.9 | - | 1363 | δCH3 |
| 1344 | 139.8 | 620.8 | 1348 | 1334 | υCO |
| 1305 | 17.95 | 24.83 | 1304 | 1303 | υRingIII |
| 1292 | 81.25 | 4.40 | 1290 | - | υRingI |
| 1269 | 10.86 | 13.99 | - | 1272 | δCHRingI |
| 1250 | 176.4 | 10.55 | 1250 | - | υSO2 |
| 1247 | 7.99 | 202.3 | - | 1245 | υCN |
| 1211 | 52.10 | 222.5 | 1215 | 1210 | υCN |
| 1185 | 34.03 | 74.70 | 1180 | 1187 | δNH |
| 1138 | 40.10 | 88.75 | 1145 | 1140 | δCHRingIII |
| 1117 | 6.93 | 12.80 | 1120 | - | δCHRingI |
| 1101 | 81.74 | 132.2 | 1100 | 1100 | δCHRingIII |
| 1071 | 26.20 | 11.67 | - | 1072 | δCHRingI |
| 1065 | 76.29 | 7.29 | 1065 | 1059 | δCHRingII |
| 1028 | 5.04 | 29.65 | 1033 | - | δCHRingI |
| 1011 | 5.14 | 45.75 | - | 1008 | υRingIII |
| 980 | 0.53 | 0.59 | 985 | - | γCHRingIII |
| 978 | 0.29 | 0.53 | 977 | - | γCHRingI |
| 943 | 0.65 | 1.16 | 942 | 942 | γCHRingI |
| 873 | 0.72 | 4.59 | - | 875 | γCHRingIII |
| 844 | 11.16 | 8.81 | 842 | 842 | δRingI |
| 778 | 19.45 | 10.84 | - | 777 | τRingII |
| 751 | 16.41 | 10.12 | 750 | 750 | τRingIII |
| 735 | 4.03 | 4.68 | 738 | - | τRingI |
| 703 | 27.83 | 3.56 | 698 | 702 | δRingIII |
| 642 | 1.31 | 3.32 | - | 640 | υCS |
| 635 | 2.26 | 9.99 | 631 | - | υCS |
| 592 | 6.43 | 10.96 | - | 590 | δRingII |
| 552 | 5.93 | 7.71 | 553 | 550 | δRingI |
| 522 | 35.66 | 5.43 | 523 | 523 | τRingII |
| 441 | 9.47 | 1.26 | 445 | 446 | τRingIII |
| 413 | 4.79 | 2.93 | 415 | - | τRingIII |
| 406 | 1.98 | 0.74 | 405 | - | τRingI |
| 372 | 2.14 | 2.23 | - | 373 | τRingII |
| 292 | 9.25 | 3.30 | - | 290 | τRingIII |
| 245 | 1.46 | 0.90 | - | 248 | τRingI |
| 201 | 0.18 | 0.74 | - | 200 | τSO2 |
| 167 | 1.88 | 2.52 | - | 170 | τCH3 |
| 2.3: Isoxicam | |||||
|---|---|---|---|---|---|
| B3LYP/CC-pVDZ (5D, 7F) |
IR |
Raman |
Assignmentsc |
||
| υ(cm−1) | IRI | RA | υ(cm−1) | υ(cm−1) | - |
| 3417 | 75.13 | 140.7 | 3290 | 3300 | υNH |
| 3187 | 6.20 | 26.83 | 3180 | 3180 | υCHRingI |
| 3077 | 8.03 | 215.5 | 3075 | 3078 | υCHRingIII |
| 3024 | 5.34 | 7.67 | 3010 | - | υCH3 |
| 2988 | 14.04 | 70.57 | - | 3000 | υCH3 |
| 2984 | 6.43 | 144.4 | - | 2984 | υCH3 |
| 2924 | 18.09 | 333.1 | 2945 | 2935 | υCH3 |
| 1624 | 166.4 | 4.56 | 1630 | - | υC=O |
| 1606 | 72.67 | 48.99 | 1608 | 1603 | υC=C |
| 1590 | 405.8 | 7.93 | 1600 | - | υC=C |
| 1579 | 2.75 | 316.0 | 1580 | 1570 | υRingIII |
| 1546 | 24.68 | 33.18 | 1548 | 1548 | υRingIII |
| 1448 | 161.2 | 2.32 | 1450 | - | δNH |
| 1446 | 44.78 | 187.9 | - | 1443 | υRingIII |
| 1430 | 394.5 | 1.80 | 1429 | - | υC=N |
| 1381 | 33.06 | 9.08 | 1380 | - | δOH |
| 1365 | 6.22 | 73.39 | - | 1368 | δCH3 |
| 1343 | 21.96 | 172.3 | 1347 | 1347 | δCH3 |
| 1337 | 128.3 | 5.21 | 1332 | - | υCO |
| 1305 | 28.65 | 26.13 | 1298 | 1300 | υRingIII |
| 1251 | 7.46 | 24.56 | - | 1252 | υCO |
| 1250 | 139.5 | 7.16 | 1250 | - | υSO2 |
| 1223 | 33.45 | 5.17 | 1225 | - | υCN |
| 1190 | 14.90 | 8.44 | 1200 | 1188 | υCN |
| 1140 | 40.88 | 93.06 | 1145 | 1145 | δCH3 |
| 1116 | 12.25 | 23.28 | 1119 | 1120 | δCH3 |
| 1067 | 96.69 | 7.89 | 1066 | 1070 | δCHRingIII |
| 1028 | 7.01 | 1.96 | - | 1035 | δCHRingI |
| 1016 | 5.61 | 6.85 | - | 1018 | δCHRingIII |
| 1011 | 11.88 | 5.75 | 1010 | - | υRingIII |
| 1001 | 7.32 | 11.65 | - | 1000 | δCH3 |
| 983 | 3.01 | 14.98 | - | 985 | δCH3 |
| 966 | 0.48 | 1.93 | 960 | - | δRingI |
| 949 | 0.73 | 0.59 | 946 | - | γCHRingIII |
| 908 | 76.85 | 0.99 | 910 | 910 | γOH |
| 894 | 51.44 | 2.41 | 893 | 892 | υNO |
| 813 | 11.99 | 6.31 | 812 | 816 | δRingII |
| 795 | 21.01 | 0.87 | 793 | 793 | γCHRingI |
| 775 | 15.58 | 2.02 | - | 778 | γCHRingIII |
| 753 | 30.39 | 8.11 | 752 | 752 | γCHRingIII |
| 740 | 16.42 | 4.18 | 740 | - | τRingIII |
| 722 | 14.50 | 1.23 | - | 723 | τRingIII |
| 694 | 55.72 | 4.92 | 703 | 700 | δRingI |
| 655 | 48.79 | 1.50 | 653 | 658 | γNH |
| 641 | 1.35 | 3.49 | - | 640 | υCS |
| 633 | 5.02 | 4.30 | 632 | - | υCS |
| 563 | 1.49 | 6.70 | 558 | - | δRingII |
| 545 | 32.26 | 1.47 | 544 | - | τRingIII |
| 523 | 41.26 | 6.16 | 515 | 525 | τSO2 |
| 441 | 7.86 | 1.31 | 447 | 440 | τRingII |
| 422 | 4.05 | 5.81 | 425 | 423 | τRingIII |
| 397 | 5.00 | 8.06 | 400 | 400 | τRingI |
| 370 | 11.34 | 2.96 | - | 372 | δRingII |
| 353 | 4.66 | 4.21 | - | 348 | δRingI |
| 306 | 4.54 | 3.99 | - | 304 | τRingII |
| 276 | 5.02 | 1.30 | - | 275 | τRingIII |
| 244 | 1.30 | 3.02 | - | 247 | δRingIII |
| 203 | 0.08 | 0.79 | - | 200 | τCH3 |
| 177 | 2.88 | 2.65 | - | 175 | τCH3 |
υ-stretching; δ-in-plane deformation; γ-out-of-plane deformation; τ-torsion;; IRI-IR intensity(KM/Mole); RA-Raman activity(Ǻ4/amu); RingI-pyridine ring; RingII-Ring having SO2; RingIII-Five member ring.
υ-stretching; δ-in-plane deformation; γ-out-of-plane deformation; τ-torsion;; IRI-IR intensity(KM/Mole); RA-Raman activity(Ǻ4/amu); RingI-pyridine ring; RingII-Ring having SO2; RingIII-Phenyl ring.
υ-stretching; δ-in-plane deformation; γ-out-of-plane deformation; τ-torsion;; IRI-IR intensity(KM/Mole); RA-Raman activity(Ǻ4/amu); RingI- Five member ring; RingII-Ring having SO2; RingIII-Phenyl ring.
3.5. Molecular docking
PASS (Prediction of Activity Spectra) [22] gives (Table 3) activities, anti-inflammatory, CYP2C9 substrate and gout treatment (activity values 0.934, 0.904 and 0.898. Receptors, 3DY9, 4NZ2 and 2AYR were obtained from the protein data bank website. PatchDock Server is used for docking purpose [23, 24, 25, 26].
Table 3.
PASS prediction for the activity spectrum. Pa represents probability to be active and Pi represents probability to be inactive.
| Pa | Pi | Activity |
|---|---|---|
| 0.934 | 0.004 | Antiinflammatory |
| 0.904 | 0.004 | CYP2C9 substrate |
| 0.898 | 0.002 | Gout treatment |
| 0.862 | 0.005 | Analgesic |
| 0.853 | 0.005 | Antiarthritic |
| 0.794 | 0.010 | CYP2C substrate |
| 0.774 | 0.004 | Non-steroidal antiinflammatory agent |
| 0.759 | 0.003 | Peroxidase substrate |
| 0.729 | 0.005 | Analgesic, non-opioid |
| 0.708 | 0.004 | CYP2C9 inhibitor |
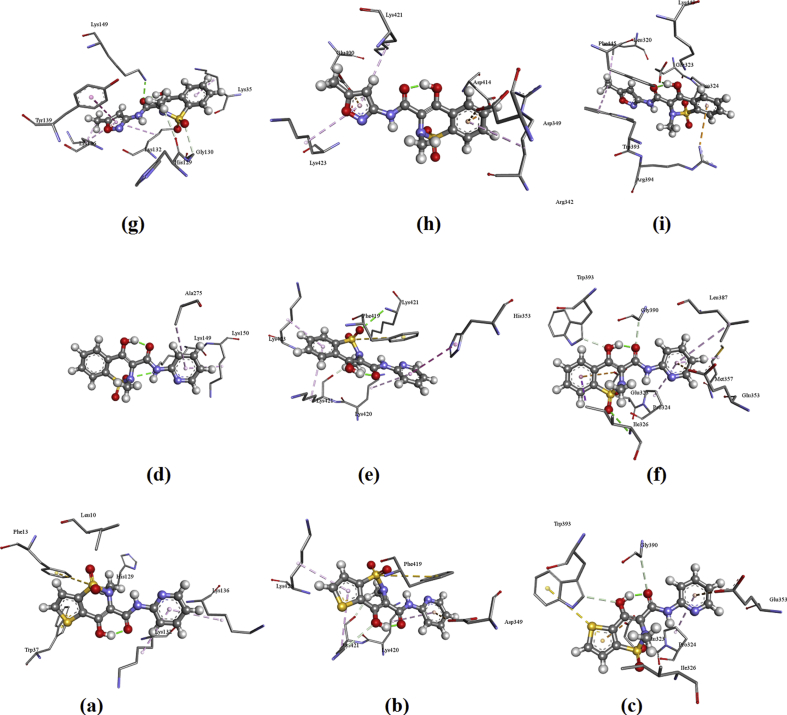
For the protein 3DY9: the amino acid interactions are: Amino acid His129 forms H-bond with methylene while Phe13 has π-sulfur interaction with SO2 group. Lys132, Lys136 having π-alkyl interaction with pyridine ring and Trp37 shows two π-sulfur interaction with sulphur atom of the thiophene ring for tenoxicam; Lys149 forms H-bond with SO2 and Lys150, Ala275 having π-alkyl bond with pyridine ring for piroxicam and Lys149, Gly130, His129 forms H-bond with carbonyl group, SO2 group, methyl group respectively while Tys139, Lys136, Lys132 shows π-π-T shaped, alkyl, π-alkyl interaction respectively with the ligand for isoxicam.
For the protein 4NZ2: The residues of Lys421 forms H-bond with methylene and OH while Asp349 shows π-anion interaction with pyridine ring. Lys420, Lys421, Lys423 having π-alkyl interaction with pyridine and phenyl ring where as Phe419 shows π-sulfur interaction with SO2 group for tenoxicam; Lys421 forms H-bond with SO2 and methyl group as well as π-alkyl interaction with pyridine ring. Lys150, Ala275 having π-alkyl interaction with phenyl ring. His353, Lys420 shows π-π-T shaped, π-alkyl interactions respectively with pyridine whereas Phe419 has a π-sulfur interaction with SO2 group for piroxicam and Amino acids Asp414, Asp349 forms π-anion interaction and Arg342 shows π-alkyl interaction with phenyl ring. Lys423, Lys421 shows π-alkyl interaction respectively with the isoxicam.
For the protein 2AYR: The residues of amino acid Gly390, Trp393 forms H-bond with C=O and OH while Glu323, Glu353 shows π-anion interaction with thiophene ring and pyridine. Trp393 forms π-sulfur interaction with sulphur atom and Pro324 having π-alkyl interaction with SO2 group for tenoxicam; Amino acids Ile326, Gly390, Trp393 forms H-bond with sulphur atom, C=O, OH group respectively while Glu323, Glu353 shows π-anion interaction with pyridine. Pro324, Met357 having π-alkyl interaction with pyridine ring whereas Ile326, Lys35 gives π-sigma, π-alkyl interactions respectively with phenyl ring for piroxicam and Amino acids Glu323 forms H-bond with OH while Arg394 has π-cation interaction with phenyl ring. Leu320, Trp393 having π-alkyl with methyl and Pro324 forms π-alkyl interaction with SO2 group for isoxicam.
The plot of docked ligand with receptors is shown in Fig. 4 and the docked ligand at the active site of receptors are given in Fig. 5. The docked ligands form a stable complex (Fig. 5) with these receptors with lowest ten minimum conformation of Patch Dock Energy values are tabulated in Table 4. From atomic contact energy value of isoxicam is high in comparison with that tenoxicam and piroxicam and hence isoxicam forms more stable complex with 3DY9, 4NZ2 and 2AYR. For tenoxicam, isoxicam and piroxicam atomic contact energy is high for the protein 2AYR and has high affinity in comparison with other two proteins. The results show that the molecules have inhibitory activity against these receptors.
Fig. 4.
The interactive plot of docked ligands (a) tenoxicam with 3DY9 (b) tenoxicam with 4NZ2 (c) tenoxicam with 2AYR (d) piroxicam with 3DY9 (e) piroxicam with 4NZ2 (f) piroxicam with 2AYR and (g) isoxicam with 3DY9 (h) isoxicam with 4NZ2 (i) isoxicam with 2AYR.
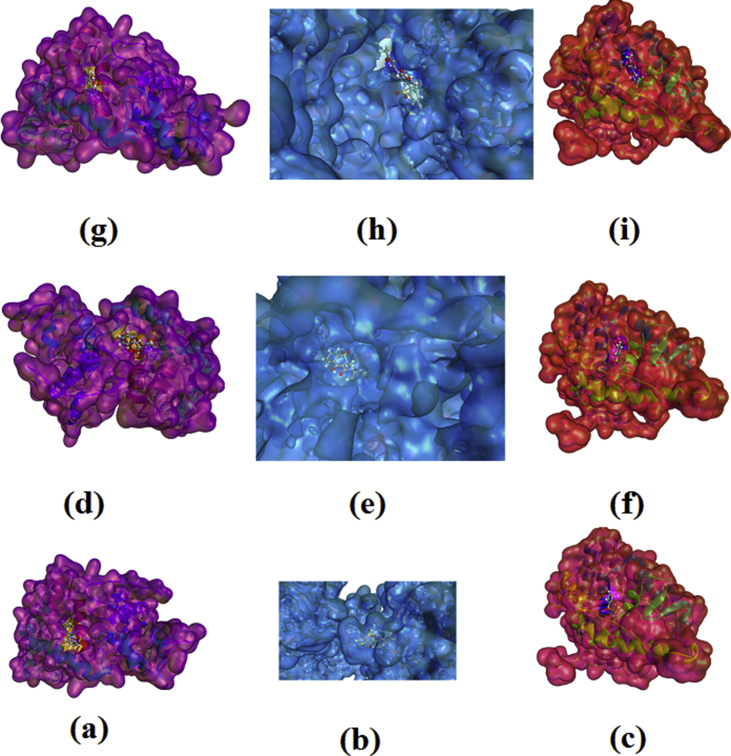
Fig. 5.
The docked ligands (a) tenoxicam with 3DY9 (b) tenoxicam with 4NZ2 (c) tenoxicam with 2AYR (d) piroxicam with 3DY9 (e) piroxicam with 4NZ2 (f) piroxicam with 2AYR and (g) isoxicam with 3DY9 (h) isoxicam with 4NZ2 (i) isoxicam with 2AYR at the active sites of proteins.
Table 4.
The top ten conformation of the complex candidate of ligands.
| No. | Global |
Attractive |
Repulsive |
Atomic Contact |
|---|---|---|---|---|
| Energy | Vdw | Vdw | Energy | |
| 4.1: Tenoxicam with 3DY9 | ||||
| 1 | -24.54 | -10.31 | 4.74 | -10.59 |
| 2 | -23.13 | -9.64 | 3.26 | -9.97 |
| 3 | -20.96 | -8.23 | 3.68 | -9.86 |
| 4 | -19.95 | -8.81 | 3.95 | -8.85 |
| 5 | -19.00 | -9.76 | 4.36 | -7.27 |
| 6 | -18.42 | -8.52 | 3.30 | -6.46 |
| 7 | -18.03 | -10.12 | 4.77 | -6.69 |
| 8 | -17.80 | -8.15 | 1.05 | -5.16 |
| 9 | -17.65 | -6.41 | 1.11 | -6.73 |
| 10 | -16.05 | -11.04 | 6.24 | -4.37 |
| 4.2: Tenoxicam with 4NZ2 | ||||
| 1 | -34.66 | -16.32 | 12.43 | -13.55 |
| 2 | -31.94 | -17.60 | 9.76 | -8.63 |
| 3 | -31.87 | -15.12 | 3.04 | -81.0 |
| 4 | -31.68 | -14.01 | 4.05 | -10.74 |
| 5 | -31.34 | -15.29 | 7.15 | -10.20 |
| 6 | -31.01 | -13.51 | 2.10 | -8.87 |
| 7 | -30.53 | -13.98 | 9.73 | -12.44 |
| 8 | -29.49 | -15.72 | 4.58 | -6.27 |
| 9 | -29.32 | -12.55 | 2.09 | -9.18 |
| 10 | -28.73 | -18.44 | 5.89 | -6.08 |
| 4.3: Tenoxicam with 2AYR | ||||
| 1 | -41.31 | -17.01 | 4.57 | -14.33 |
| 2 | -40.33 | -15.82 | 3.69 | -12.34 |
| 3 | -37.62 | -15.70 | 5.96 | -12.74 |
| 4 | -37.42 | -15.77 | 4.36 | -13.93 |
| 5 | -36.79 | -14.47 | 4.89 | -12.86 |
| 6 | -36.31 | -14.68 | 7.37 | -14.24 |
| 7 | -35.81 | -16.05 | 6.72 | -12.25 |
| 8 | -33.94 | -13.08 | 3.54 | -11.40 |
| 9 | -33.89 | -14.92 | 2.07 | -10.81 |
| 10 | -33.11 | -16.88 | 7.67 | -11.98 |
| 4.4: Piroxicam with 3DY9 | ||||
| 1 | -21.21 | -9.13 | 1.14 | -6.21 |
| 2 | -19.67 | -9.40 | 1.72 | -6.40 |
| 3 | -19.37 | -8.70 | 2.61 | -8.16 |
| 4 | -19.32 | -9.50 | 1.49 | -5.22 |
| 5 | -19.26 | -9.60 | 4.33 | -7.93 |
| 6 | -18.28 | -8.85 | 4.22 | -7.68 |
| 7 | -16.16 | -6.83 | 2.99 | -6.25 |
| 8 | -16.08 | -11.30 | 3.95 | -3.14 |
| 9 | -15.74 | -11.86 | 10.39 | -5.40 |
| 10 | -14.54 | -7.95 | 3.79 | -6.54 |
| 4.5: Piroxicam with 4NZ2 | ||||
| 1 | -31.03 | -15.28 | 3.95 | -7.96 |
| 2 | -30.98 | -12.00 | 1.99 | -9.53 |
| 3 | -30.62 | -15.97 | 2.28 | -6.32 |
| 4 | -30.06 | -19.11 | 4.45 | -5.50 |
| 5 | -30.05 | -16.21 | 8.99 | -10.29 |
| 6 | -29.95 | -16.89 | 4.80 | -5.55 |
| 7 | -29.60 | -13.91 | 2.61 | -8.22 |
| 8 | -29.33 | -14.44 | 5.50 | -9.38 |
| 9 | -28.70 | -16.15 | 3.84 | -5.78 |
| 10 | -28.66 | -15.18 | 4.96 | -7.65 |
| 4.6: Piroxicam with 2AYR | ||||
| 1 | -37.85 | -17.33 | 5.90 | -12.41 |
| 2 | -36.08 | -16.48 | 4.57 | -10.83 |
| 3 | -35.96 | -17.16 | 4.34 | -11.19 |
| 4 | -34.04 | -14.50 | 4.95 | -11.94 |
| 5 | -33.99 | -15.73 | 5.94 | -12.53 |
| 6 | -33.99 | -15.11 | 6.47 | -11.55 |
| 7 | -33.51 | -3.65 | 3.54 | -11.09 |
| 8 | -33.12 | -15.10 | 2.21 | -7.97 |
| 9 | -31.94 | -14.23 | 1.24 | -7.53 |
| 10 | -31.02 | -12.45 | 1.87 | -10.00 |
| 4.7: Isoxicam with 3DY9 | ||||
| 1 | -26.00 | -11.53 | 3.63 | -10.59 |
| 2 | -25.72 | -13.30 | 3.88 | -8.13 |
| 3 | -23.43 | -8.48 | 1.41 | -8.64 |
| 4 | -19.41 | -11.68 | 5.27 | -5.59 |
| 5 | -19.35 | -9.10 | 2.36 | -7.89 |
| 6 | -19.18 | -10.49 | 3.90 | -6.91 |
| 7 | -19.07 | -8.18 | 3.38 | -7.26 |
| 8 | -18.81 | -8.40 | 4.97 | -8.39 |
| 9 | -18.60 | -12.28 | 7.80 | -5.68 |
| 10 | -18.36 | -7.93 | 0.54 | -7.12 |
| 4.8: Isoxicam with 4NZ2 | ||||
| 1 | -37.12 | -16.16 | 2.35 | -10.90 |
| 2 | -34.72 | -13.37 | 2.18 | -12.16 |
| 3 | -33.12 | -12.59 | 0.90 | -9.56 |
| 4 | -32.08 | -15.24 | 5.98 | -9.06 |
| 5 | -31.53 | -15.55 | 4.47 | -8.39 |
| 6 | -29.81 | -16.07 | 2.44 | -5.55 |
| 7 | -29.54 | -13.01 | 0.78 | -8.32 |
| 8 | -28.60 | -16.09 | 8.45 | -8.67 |
| 9 | -27.16 | -14.20 | 5.24 | -8.46 |
| 10 | -27.05 | -12.26 | 2.32 | -8.50 |
| 4.9: Isoxicam with 2AYR | ||||
| 1 | -42.31 | -19.70 | 5.02 | -12.99 |
| 2 | -38.46 | -16.62 | 2.34 | -9.76 |
| 3 | -34.63 | -14.96 | 2.06 | -9.48 |
| 4 | -34.52 | -15.48 | 3.75 | -10.61 |
| 5 | -33.93 | -14.41 | 1.86 | -9.98 |
| 6 | -33.62 | -15.27 | 3.42 | -11.22 |
| 7 | -30.98 | -14.96 | 7.86 | -9.62 |
| 8 | -30.94 | -12.87 | 5.08 | -12.59 |
| 9 | -29.64 | -11.61 | 3.41 | -10.35 |
| 10 | -29.61 | -15.41 | 4.42 | -9.28 |
4. Conclusion
The spectroscopic analysis of tenoxicam, piroxicam and isoxicam are reported. The theoretical normal modes of vibrations based on DFT theory has been investigated and compared with the experimental values. NBO analysis was carried out on the molecule to find the interactions and found that these compounds are highly stable due to hyperconjugative interactions. Simulated electronic spectra show that there is an intense peak in red shift region due to pi to anti-bonding π-electron transition and a weak peak due to lone pair to anti-bonding orbital transition. The lower value of HOMO and LUMO energy gap describes the stability and biological activity of the tenoxicam, piroxicam and isoxicam compounds. Reactive sites were obtained from MEP, which indicates that there are enough sites for nucleophilic and electrophilic interaction in the molecules, which is very important to show biological activities. Finally the molecular docking shows the ligands have good pharmacological properties with the proteins. From atomic contact energy and global energy values more stable complex are identified.
Declarations
Author contribution statement
Y. Shyma Mary, Y. Sheena Mary, K.S. Resmi, Renjith Thomas: Conceived and designed the analysis; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ho J., Coote M.L., Franco-Perez M., Gomez-Balderas R. First principles prediction of the pK(a)s of anti-inflammatory oxicams. J. Phys. Chem. A. 2010;114:11992–12003. doi: 10.1021/jp107890p. [DOI] [PubMed] [Google Scholar]
- 2.Al-Rashdi A.A., Naggar A.H., Farghaly O.A., Khouda M.M., Shafter M.M. Potentiometric and conductometric determination of metal complexes of tenoxicam in different dosage forms. Int. J. Pharm. Phytopharmacol. Res. 2018;8:13–22. [Google Scholar]
- 3.Ritland S.R., Gendler S.J. Chemoprevention of intestinal adenomas in the Apcmin mouse by piroxicam, kinetics, strain effects and resistance to chemosuppression. Carcinogenesis. 1999;20:51–58. doi: 10.1093/carcin/20.1.51. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee R., Chakraborty H., Sarkar M. Photophysical studies of oxicam group NSAIDs: piroxicam, meloxicam and tenoxicam. Spectrochim. Acta. 2003;59:1213–1222. doi: 10.1016/s1386-1425(02)00300-1. [DOI] [PubMed] [Google Scholar]
- 5.Tamasi G., Bernini C., Corbini G., Owens N.F., Messori L., Scaletti F., Massai L., Giudice P.L., Cini R. Synthesis, spectroscopic and DFT structural characterization of two novel ruthenium(III) oxicam complexes. In vivo evaluation of anti-inflammatory and gastric damaging activities. J. Inorg. Biochem. 2014;134:25–35. doi: 10.1016/j.jinorgbio.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Thomas R., Hossain M., Mary Y.S., Resmi K.S., Armaković S., Armaković S.J., Nanda A.K., Ranjan V.K., Vijayakumar G., Van Alsenoy C. Spectroscopic analysis and molecular docking of imidazole derivatives and investigation of its reactive properties by DFT and molecular dynamics simulations. J. Mol. Struct. 2018;1156:336–347. [Google Scholar]
- 7.Mary Y.S., Panicker C.Y., Anto P.L., Sapnakumari M., Narayana B., Sarojini B.K. Molecular structure, FT-IR, NBO, HOMO and LUMO, MEP and first order hyperpolarizability of (2E)-1-(2,4-dichlorophenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one by HF and density functional methods. Spectrochim. Acta. 2015;135:81–92. doi: 10.1016/j.saa.2014.06.140. [DOI] [PubMed] [Google Scholar]
- 8.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., Nakatsuji H., Caricato M., Li X., Hratchian H.P., Izmaylov A.F., Bloino J., Zheng G., Sonnenberg J.L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J.A., Jr., Peralta J.E., Ogliaro F., Bearpark M., Heyd J.J., Brothers E., Kudin K.N., Staroverov V.N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J.C., Iyengar S.S., Tomasi J., Cossi M., Rega N., Millam J.M., Klene M., Knox J.E., Cross J.B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R.E., Yazyev O., Austin A.J., Cammi R., Pomelli C., Ochterski J.W., Martin R.L., Morokuma K., Zakrzewski V.G., Voth G.A., Salvador P., Dannenberg J.J., Dapprich S., Daniels A.D., Farkas O., Foresman J.B., Ortiz J.V., Cioslowski J., Fox D.J. Gaussian, Inc.; Wallingford CT: 2010. Gaussian 09, Revision B.01. [Google Scholar]
- 9.Mary Y.S., Miniyar P.B., Mary Y.S., Resmi K.S., Panicker C.Y., Armakovic S., Armakovic S.J., Thomas R., Sureshkumar B. Synthesis and spectroscopic study of three new oxadiazole derivatives with detailed computational evaluation of their reactivity and pharmaceutical Potential. J. Mol. Struct. 2018;1173:469–480. [Google Scholar]
- 10.Bio-Rad Laboratories, Inc. SpectraBase, http://spectrabase.com/.
- 11.Mary Y.S., Panicker C.Y., Yamuna T.S., Siddegowda M.S., Yathirajan H.S., Al-Saadi A.A., Van Alsenoy C. Theoretical investigations on the molecular structure, vibrational spectral, HOMO-LUMO and NBO analysis of 9-[3-(dimethylamino) propyl]-2-trifluoromethyl-9H-thioxanthen-9-ol. Spectrochim. Acta. 2014;132:491–501. doi: 10.1016/j.saa.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Thomas R., Mary Y.S., Resmi K.S., Narayana B., Sarojini B.K., Armakovic S., Armakovic S.J., Vijayakumar G., Van Alsenoy C., Mohan B.J. Synthesis and spectroscopic study of two new pyrazole derivatives with detailed computational evaluation of their reactivity and pharmaceutical potential. J. Mol. Struct. 2019;1181:599–612. [Google Scholar]
- 13.Thomas R., Mary Y.S., Resmi K.S., Narayana B., Sarojini B.K., Vijayakumar G., Van Alsenoy C. Two neoteric pyrazole compounds as potential anti-cancer agents: synthesis, electronic structure, physic-chemical properties and docking analysis. J. Mol. Struct. 2019;1181:455–466. [Google Scholar]
- 14.Shafieyoon P., Mehdipour E., Mary Y.S. Synthesis, characterization and biological investigation of glycine based sulfonamide derivative and its complex: vibration assignment, HOMO-LUMO analysis, MEP and molecular docking. J. Mol. Struct. 2019;1181:244–252. [Google Scholar]
- 15.Kumar C.S.C., Panicker C.Y., Fun H.K., Mary Y.S., Harikumar B., Chandraju S., Quah C.K., Ooi C.W. FT-IR, molecular structure, first order hyeprpolarizability, HOMO and LUMO analysis, MEP and NBO analysis of 2-(4-chlorophneyl)-2-oxoethyl 3-nitrobenzoate. Spectrochim. Acta. 2014;126:208–219. doi: 10.1016/j.saa.2014.01.145. [DOI] [PubMed] [Google Scholar]
- 16.Zhang T., Wei X., Zuo Y., Chao J. An efficient measure to improve the NLO performance by point charge electric field. Optik. 2019;182:295–302. [Google Scholar]
- 17.El-Azab A.S., Mary Y.S., Panicker C.Y., Abdel-Aziz A.A.M., El-Sherbeny M.A., Van Alsenoy C. DFT and experimental (FT-IR and FT-Raman) investigation of vibrational spectroscopy and molecular docking studies of 2-(4-oxo-3-phenethyl-3,4-dihydroquinazolin-2-ylthio)-N-(3,4,5-trimethoxyphenyl)acetamide. J. Mol. Struct. 2016;1113:133–145. [Google Scholar]
- 18.Rani P.R.K., Mary Y.S., Fernandez A., Priya S.A., Mary Y.S., Thomas R. Single crystal XRD, DFT investigations and molecular docking study of 2-((1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)amino)naphthalene-1,4-dione as a potential anti-cancer lead molecule, Comput. Biol. Chem. 2019;78:153–164. doi: 10.1016/j.compbiolchem.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Haruna K., Kumar V.S., Mary Y.S., Popoola S.A., Thomas R., Roxy M.S., Al-Saadi A.A. Conformational profile, vibrational assignments, NLO properties and molecular docking of biologically active herbicide 1,1-dimethyl-3-phenylurea. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mary Y.S., Panicker C.Y., Sapnakumari M., Narayana B., Sarojini B.K., Al-Saadi A.A., Van Alsenoy C., War J.A., Fun H.K. Molecular structure, FT-IR, Vibrational assignments, HOMO-LUMO analysis and molecular docking study of 1-[5-(4-Bromophenyl)-3-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl]ethanone. Spectrochim. Acta. 2015;136:473–482. doi: 10.1016/j.saa.2014.09.060. [DOI] [PubMed] [Google Scholar]
- 21.Roeges N.P.G. John Wiley and Sons Inc.; New York: 1994. A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures. [Google Scholar]
- 22.Lagunin A., Stepanchikova A., Filimonov D., Poroikov V. PASS: prediction of activity spectra for biologically active substances. Bioinformatics. 2000;16:747–748. doi: 10.1093/bioinformatics/16.8.747. [DOI] [PubMed] [Google Scholar]
- 23.Duhovny D., Nussinov R., Wolfson H.J. Efficient unbound docking of rigid molecules. In: Gusfield, editor. Proceedings of the 2'nd Workshop on Algorithms in Bioinformatics(WABI) Rome, Italy, Lecture Notes in Computer Science 2452. Springer Verlag; 2002. pp. 185–200. [Google Scholar]
- 24.Zhang C., Vasmatzis G., Cornette J.L., DeLisi C. Determination of atomic desolvation energies from the structures of crystallized proteins. J. Mol. Biol. 1997;267:707–726. doi: 10.1006/jmbi.1996.0859. [DOI] [PubMed] [Google Scholar]
- 25.Chen R., Mintseris J., Janin J., Weng Z. A protein–protein docking benchmark. Proteins. 2003;52:88–91. doi: 10.1002/prot.10390. [DOI] [PubMed] [Google Scholar]
- 26.Connolly M.L. Analytical molecular surface calculation. J. Appl. Crystallogr. 1983;16:548–558. [Google Scholar]