Abstract
Purpose:
We retrospectively assessed the incidence of cataracts in patients with retinoblastoma (Rb) treated with either lens-sparing radiation therapy (LSRT) or whole-eye radiation therapy (WERT). A secondary aim of this study was to model the dose-response risk of cataract.
Methods and Materials:
We reviewed 65 patients with Rb treated with radiation therapy (RT) at Children’s Hospital, Los Angeles from 1997 to 2015. Eyes that were enucleated before RT or lacked follow-up eye examinations were excluded. All patients underwent computed tomography simulation, and mean lens dose data were collected. Follow-up ophthalmologic examinations and intraocular lens implant history were reviewed for cataracts. The primary event-free survival (EFS) outcome was cataract development. Eyes without cataracts were censored on the last date of eye examination or post-RT enucleation, if applicable. Kaplan-Meier estimates were used to compare EFS outcomes, and dose response was projected with Cox regression and logistic regression models.
Results:
Sixty-one patients (94 eyes) were analyzed with a median follow-up of 51.8 months. For eyes treated with WERT, cataracts developed in 71.7% versus 35.3% for LSRT. Median EFS for WERT and LSRT were 20.8 and 67.9 months, respectively. Compared with WERT, a significant EFS benefit was demonstrated for LSRT (P <.001). Mean lens dose had a significant effect on cataracts in both Cox regression and logistic regression models (P <.01). The mean lens dose of 7 Gy was projected to have a 5-year cataract incidence of 20% and 25% with the logistic and Cox regression models, respectively.
Conclusions:
We report the first clinical data demonstrating significantly improved EFS in patients with Rb treated with LSRT. Through lens dose-response modeling, we validate a mean lens dose threshold of 7 Gy to keep cataract risk below 25%. Although RT is used less often for Rb owing to advances in chemotherapy delivery options, these findings are relevant for refining lens dose constraints, particularly in children who have received radiation dose near the orbit.
Summary
We report the first clinical data demonstrating a significantly lower cataract formation rate in patients with retinoblastoma treated with lens-sparing radiation therapy in the modern era. Through dose-response modeling, we validate a mean lens dose threshold of 7 Gy to keep the cataract risk below 25%. Although radiation therapy is used significantly less often for retinoblastoma owing to advances in chemotherapy delivery options, these findings are relevant for refining lens dose constraints.
Introduction
Retinoblastoma is the most common intraocular tumor in children, with an annual incidence of approximately 300 cases per year in the United States.1–3 In the current era, the estimated 5-year survival rate is approximately 98 in developed countries.4 Treatment has traditionally included radiation therapy (RT); however, its use has declined because of potential late toxicities, which include cataract formation,5–7 impaired orbital bone growth,8 and, most concerning, significant risk of secondary malignancies.1,9,10
The current treatment paradigm for Rb includes intravenous or intra-arterial chemotherapy, focal therapies (eg, cryotherapy, laser therapy, thermotherapy, plaque brachytherapy). External beam RT can be reserved for chemotherapy-resistant cases, recurrent seeding, and salvage.11 The use of this treatment modality as salvage is also decreasing in the intravitreal-chemotherapy era,12 resulting in reservation of RT for the most critical clinical situations, such as active tumor that is recalcitrant to other therapies in the only remaining eye. In this clinical scenario, cataract development can affect not only the vision of a monocular child but also prevent the ocular oncologist from adequately monitoring the tumor for recurrence. Thus, in the few eyes that require RT for Rb, lens-sparing radiation therapy (LSRT) techniques are of critical clinical importance. Over the past few decades, LSRT have been investigated and improved; the rationale behind such efforts is that the disease control that whole-eye radiation therapy (WERT) effectively provides can still be harnessed while mitigating dose to the lens with the intent of reducing visual impairment and cataract development.13 Such LSRT techniques have evolved from D-shaped lateral fields to include contemporary modalities such as proton therapy and intensity modulated radiation therapy (IMRT).
Here, we report long-term cataract outcomes in a large single-institution experience of patients with Rb irradiated with LSRT and WERT. This study is the first to provide cataract incidence for patients with Rb after treatment with lens-sparing IMRT and is also the first to present lens dose-response data for cataract formation in the modern RT era.
Methods and Materials
Study design and patient selection
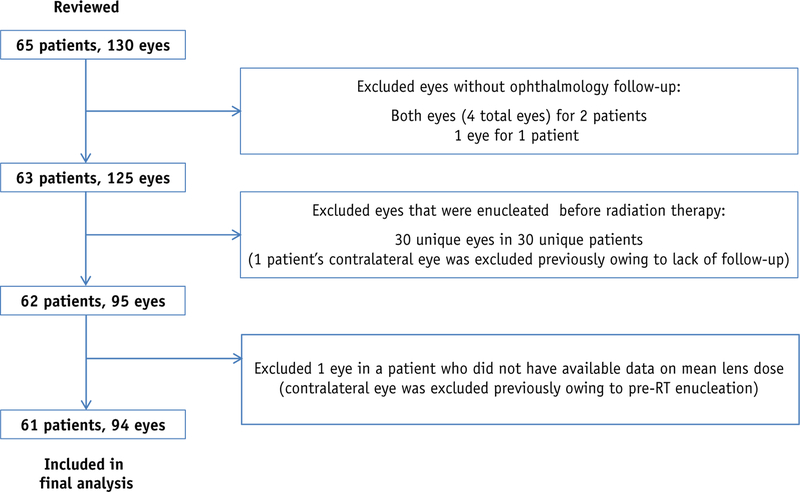
After receiving institutional review board approval, we retrospectively reviewed our institutional experience from October 1997 to February 2015, and we identified 65 patients with Rb (130 total eyes) treated with either WERT or LSRT after target volumes were determined with the ocular oncologist. Nontarget fellow eyes that were not the intended target for treatment, but received collateral dose, were also reviewed for analysis. Patients and eyes that did not have ophthalmology follow-up examination in our institution after RT were excluded. Also, eyes that were enucleated before RT or had cataracts before RT were excluded (Fig. 1).
Fig. 1.
Patient and eye selection flow diagram.
Four patients in the final cohort received multiple courses of radiation to 1 of their respective eyes (4 eyes). Three patients (3 eyes) received brachytherapy before either WERT (2 eyes) or LSRT (1 eye). For this study, the clinical courses for these eyes were followed from the completion date of their external beam RT course to either cataract or censorship. One patient (1 eye) was reirradiated with WERT after an initial LSRT course. This eye was enucleated shortly after reirradiation. For analysis, the clinical course of this eye was followed from the initial LSRT completion date to the enucleation date.
Mean lens dose data were collected from the Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA). Lens dose data were collected for both target and nontarget fellow eyes. Clinical data related to patients and/or specific eyes, such as patient age, sex, enucleation history, intraocular lens (IOL) implant history, cataract history, stage (International Intraocular Retinoblastoma Classification group),14 and RT course history, were collected from our electronic medical record systems, Cerner (Kansas City, MO) or ARIA (Varian Medical Systems), or from scanned paper charts for patients treated before implementation of the electronic medical record systems.
Although it is not the focus of this report, our lens-sparing IMRT technique used multiple noncoplanar IMRT beams and most closely resembles the IMRT method described by Reisner et al.15 Figures E1 through (available online at https://doi.org/10.1016/j.ijrobp.2018.12.004) illustrate the beam arrangement for one of our patients.
Outcomes and analyses
The primary outcome of this study was post-RT cataract formation clinically diagnosed by ophthalmologists specializing in Rb at our institution. Kaplan-Meier curves and estimates were generated as a function of event-free survival (EFS), which was defined from completion of RT to cataract diagnosis. Eyes in which cataracts did not develop were censored on the last ophthalmologic examination date or on the date of enucleation, if applicable. Treatment groups (WERT, LSRT, and nontarget fellow eyes) were compared with the log-rank test. Univariate Cox regression analyses were performed to assess the effects of mean lens dose and treatment group on EFS. Cumulative cataract incidences at discrete mean lens doses were modeled from the Cox regression analysis. Eyes with a minimum follow-up of 5 years were included in a binomial logistic regression to model the mean lens dose response. Statistical analysis was performed using SPSS for Windows software (version 20.0, IBM Inc, Armonk, NY).
Results
Patient and eye characteristics
Ninety-four eyes of 61 patients were included in the final analysis (Fig. 1). Sixty eyes (63.8%) were treated with WERT, 17 (18.1%) were treated with LSRT, and 17(18.1%) were nontarget eyes. The median age at diagnosis was 10.0 months (range 0.8–31.8 months) with a median time to initiation of RT of 8.8 months from diagnosis (range, 3.2–91.8 months). Fifty-seven patients had bilateral Rb (93.4%), and 4 had unilateral disease (6.6%). There were 25 female patients (41.0%) and 36 male patients (59.0%). Fifteen of the 17 lens-sparing eyes were treated with IMRT; lateral fields were used for the other 2 eyes. Tables 1 and 2 summarize the characteristics of our eye cohort.
Table 1.
Eye characteristics (N Z 94)
| Characteristics | Median (range) or no. eyes (%) |
|---|---|
| Whole-eye radiation therapy prescribed dose (Gy) | 36.00 (25.20–45.00) |
| LSRT prescribed dose (Gy) | 36.00 (26.00–50.00) |
| Whole-eye radiation therapy lens dose (Gy)* | 37.53 (25.20–46.94) |
| LSRT lens dose (Gy)* | 9.93 (4.68–26.04) |
| Nontarget eyes lens dose (Gy)* | 0.36 (0.08–6.24) |
| Lens dose for eyes with cataracts (Gy)* | 37.36 (3.60–46.94) |
| Lens dose for eyes without cataracts (Gy)* | 8.80 (0.08–43.13) |
| No. of fractions | 18 (13–28) |
| Patient age at start of follow-up (mo) | 21.1 (9.8–135.4) |
| International Intraocular Retinoblastoma Classification | |
| Group A | 5 (5.32) |
| Group B | 10 (10.64) |
| Group C | 7 (7.45) |
| Group D | 45 (47.87) |
| Group E | 22 (23.40) |
| No disease | 4 (4.26) |
| Rb of unknown stage | 1 (1.06) |
| Radiation therapy techniques for treated eyes (n = 77) | |
| En face electrons | 6 (7.79) |
| IMRT | 67 (87.01) |
| Lateral fields | 2 (2.60) |
| VMAT | 2 (2.60) |
| Treatment group | |
| Whole-eye radiation | 60 (63.83) |
| LSRT | 17 (18.09) |
| Nontarget eyes | 17 (18.09) |
| Cataract formation status | |
| No cataract | 44 (46.81) |
| Cataract | 50(53.19) |
| Post—radiation therapy enucleation | |
| Not enucleated | 58 (61.70) |
| Enucleated | 36 (38.30) |
| Intraocular lens (eyes with cataract formation, n = 50) | |
| No intraocular lens | 18 (36.00) |
| Intraocular lens | 32 (64.00) |
Abbreviations: IMRT Z intensity modulated radiation treatment; LSRT Z lens-sparing radiation treatment; VMAT Z volumetric modulated arc therapy.
Mean lens dose was calculated for each eye.
Table 2.
Treatment group characteristics
| Characteristic | Whole-eye radiation therapy (n = 60) | LSRT (n = 17) |
|---|---|---|
| Median no. of fractions | 18 | 18 |
| Median patient age at start of follow-up (months) | 22 | 21.1 |
| No. eyes (%) | ||
| International Intraocular | ||
| Retinoblastoma Classification | ||
| Group A | 0 | 1 (5.90) |
| Group B | 2 (3.30) | 6 (35.30) |
| Group C | 2 (3.30) | 3 (17.60) |
| Group D | 39 (65.00) | 3 (17.60) |
| Group E | 17 (28.33) | 3 (17.60) |
| Retinoblastoma of unknown stage | 0 | 1 (5.90) |
| Radiation therapy techniques for treated eyes | ||
| En face electrons | 6 (10.00) | 0 |
| IMRT | 52 (86.70) | 15 (88.20) |
| Lateral fields | 0 | 2 (11.8) |
| VMAT | 2 (3.30) | 0 |
| Sex of patient | ||
| Male | 36 (60.00) | 8 (47.06) |
| Female | 24 (40.00) | 9 (52.94) |
Abbreviations: IMRT Z intensity modulated radiation therapy; VMAT Z volumetric modulated arc therapy.
At the time of analysis with a median follow-up of51.8 months, cataracts developed in 71.7% of the eyes treated with WERT (43 eyes) compared with 35.3% for LSRT (6 eyes) and 5.9% for nontarget eyes (1 eye). Of the 50 eyes in which cataracts developed, 32 (64%) were implanted with IOLs.
Event-free survival
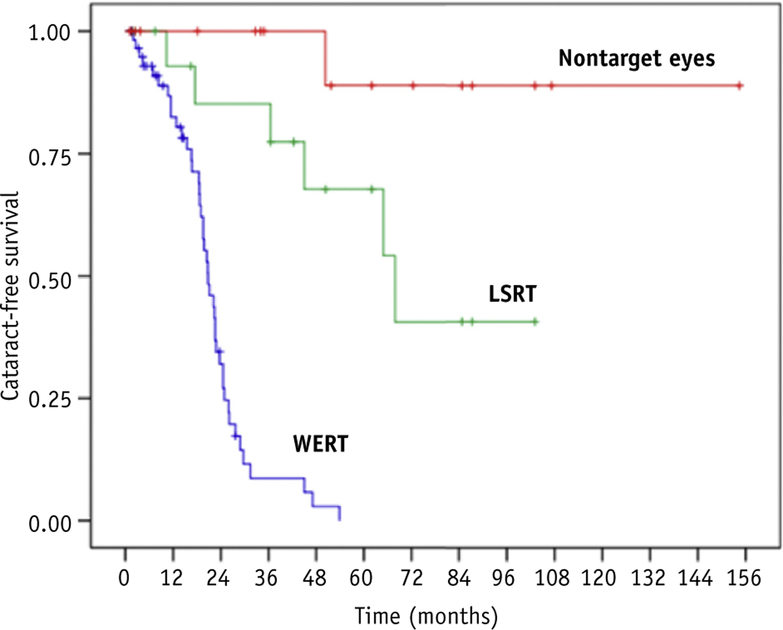
The estimated median EFS for the entire cohort was 24.9 months (95% confidence interval [CI], 19.0–30.7). The median EFS for WERT and LSRT was 20.8 months (95% CI, 17.8–23.8) and 67.9 months (95% CI 39.9–95.9), respectively, whereas the median EFS for nontarget fellow eyes was not reached (log-rank P value < .01). Figure 2 illustrates the Kaplan-Meier estimated EFS for each treatment group.
Fig. 2.
Kaplan-Meier estimated event-free survival curves by treatment group. Abbreviations: LSRT Z lens-sparing radiation treatment; WERT Z whole-eye radiation treatment.
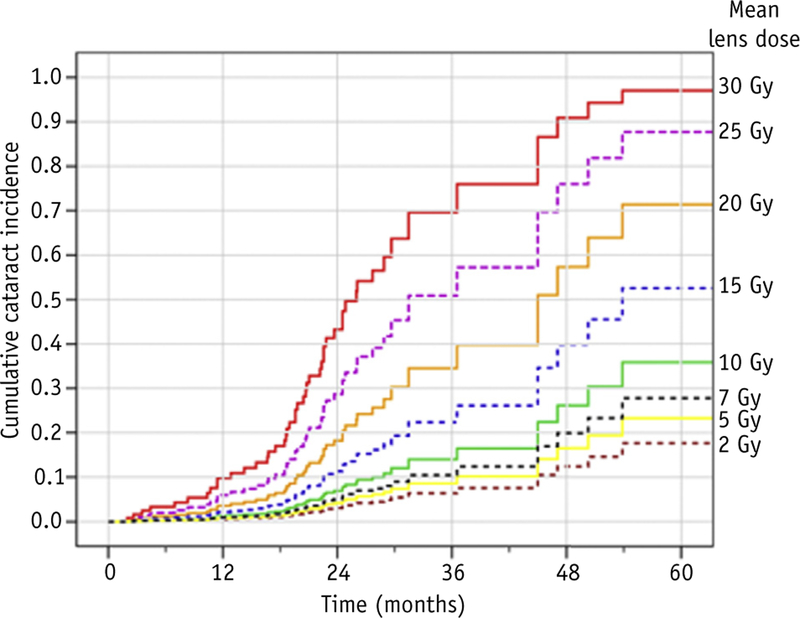
Univariate Cox proportional hazards analyses confirmed a significant difference in EFS among groups, with lower EFS hazard for LSRT versus WERT (hazard ratio, 0.108; 95% CI, 0.039–0.302; P < .001). Estimated cumulative cataract incidences for mean lens dose values of 2, 5, 7, 10, 15, 20, 25, and 30 Gy are illustrated in Figure 3. Increasing mean lens dose was associated with higher cataract hazard (P < .01).
Fig. 3.
Modeled cumulative cataract incidence over time by mean lens dose values.
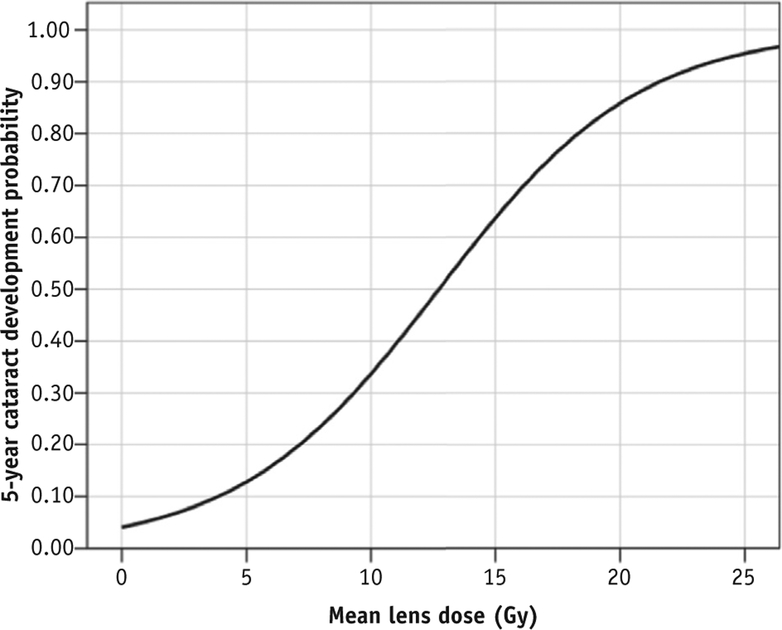
Lens dose response
The dose-response curve (Fig. 4) created from binary logistic regression demonstrates an estimated 5-year probability of cataract formation of approximately 35% in eyes that received a mean lens dose of approximately 10 Gy. Mean lens dose was significantly predictive of the 5-year cataract incidence (χ2 = 46.1; 1 degree of freedom; P < .01).
Fig. 4.
Mean lens dose-response curve with respect to 5-year cataract development probability.
Discussion
Our study describes a large single-institution cohort of patients over an 18-year period with a single disease followed closely with detailed eye evaluations by ophthalmologists; thus, our cataract rate should closely approximate the population cataract rate in patients with Rb who undergo irradiation. Of patients in whom cataracts were diagnosed after RT, 64% had IOL implantation, confirming an opacity affecting vision, although additional patients with documented posterior subcapsular cataracts were not offered an IOL because of the poor prospect of useful vision.
In our study, most patients were treated with 36 Gy but had a wide range of mean lens doses, allowing for 2 predictive models. Cataract risk with Cox regression allowed us to select discrete doses and estimate cumulative incidences over time, whereas logistic regression estimated risk over a continuous range of doses at a chosen follow-up interval. For example, time-dependent cataract incidence at a lens dose of 10 Gy is an estimated 10% at 24 months and increases to 15% at the 36 months (Fig. 3). By excluding eyes with less than 5 years of follow-up, the logistic model estimates a 35% risk by 5 years with a dose of 10 Gy (see Fig. 4). We can combine both model predictions to crudely estimate dose-dependent cataract risk. For example, logistic analysis estimates a 5-year cataract risk of 20% with a 7-Gy mean lens dose, whereas the Cox regression model predicts that cataracts will develop in approximately 25% of eyes with 7 Gy 5 years after RT. We may interpret that a mean lens dose of 7 Gy is associated with a 20% to 25% risk for cataract development by 5 years.
Although the use of RT for Rb has declined, its use is now generally reserved for the most critical clinical situations, often in monocular patients. Thus, lens-sparing therapy in these situations is of utmost importance. Our results refine lens dose constraints, particularly in children who have radiation dose exposure to the orbit. Our analysis on cataract incidence in patients with Rb who undergo radiation benefits from a significantly long follow-up period for a large number of patients irradiated uniformly. We can, therefore, confidently quantify and model our experience with LSRT and compare with WERT. The profoundly superior EFS for LSRT validates the assumed risk reduction with lower lens doses.16,17 We believe our lens dose data would apply to other highly conformal RT techniques such as proton therapy (either passively scattered or intensity modulated).
Cataract outcomes in Rb have also been reported in prior studies involving photon-based WERT and LSRT, as shown in Table 3.1,18–28 Most of these studies do not go beyond reporting crude incidences, whereas we provide comprehensive time-to-event analyses and dose-response modeling. For LSRT, cataract incidence rates range widely from 2% to 83%.1,18,20,21,25,26 Many of these reports (Table 3) consist of small sample sizes,21 have short follow-up,1,18,20,21, or fail to describe whether ophthalmologic examinations were performed for cataract assessment.20
Table 3.
Review of retinoblastoma cataract outcomes and lens dose recommendations
| Series | Year | Cohort age (mo) | N (eyes) | RT technique | RT fields | Rx Dose (Gy) | Median follow-up (mo) | Cataract (%) |
|---|---|---|---|---|---|---|---|---|
| Toma et al18 | 1995 | Children | 67 | LSRT | Ant-Lat | 40 | 35 | 2 |
| Hungerford et al19 | 1995 | Children | 175 | WERT | Ant or Ant-Lat | 35–44 | 108 | 100* |
| McCormick et al20 | 1988 | 1–60 | 97 | LSRT | Ant-Lat, Lat | 38.5–50 | 33 | 9 (Ant-Lat), 0 (Lat) |
| Foote et al21 | 1989 | 0.5–39 | 25 | LSRT, WERT | Lat LSRT, Ant WERT | 39–51 | 33 | 29 (LSRT), 9 (WERT)* |
| Schipper et al22 | 1985 | 0.5–91 | 54 | LSRT | Lat | 45 | 72 | 33†,‡ |
| Amendola et al23 | 1990 | Birth-60 | 11 | WERT | Oblique wedged pair | 35–45 | 40 | 46 |
| Choi et al24 | 2010 | 2–65 | 32 | WERT | Ant or Ant-Lat | 35–55 | 150 | 28§ |
| Phillips et al25 | 2003 | 1–58 | 47 | LSRT | Ant or Ant-Lat | 20–40 | 128 | 23 |
| Blach et al26 | 1996 | Children | 180 | LSRT | Ant-Lat, Lat | 38–50 | 101 versus 52 | 22 (overall) |
| Egbert et al27 | 1978 | 3–84 | 38 | WERT | Lat | 35–60 | 30–252 | 66 |
| Hernandez et al28 | 1996 | 0.5–16 | 34 | WERT | Ant-Lat | 34.5–49.5 | 35 | 41 |
| Scott et al1 | 1999 | 0.2–60 | 58 | LSRT | Lat | 36–54 | 37 | 17 (LSRT), 37 (Lat) |
| Chodick et al30 | 2009 | Children | 828 | WERT | Ant, Lat, Ant-Lat, Brachy | 15–115 | 384 | NAǁ |
| Mouw et al34 | 2014 | 0.2–30 | 60 | Proton | Proton | RBE: 40–46.8 | 96 | 6 |
Abbreviations: Ant Z anterior; Brachy Z brachytherapy; Lat Z lateral; LSRT Z lens-sparing radiation therapy; RBE Z relative biologic effectiveness; Rx Z prescription; WERT Z whole-eye radiation therapy.
All cataracts occurred within 2 years.
Cataract rate was 100% when lens dose was 25 Gy or higher.
Also reported a minimum cataractogenic dose of 8 Gy.
Also reported a median event-free survival of 69 months.
Reported a cataract odds ratio of 6 (5 Gy vs. 2.5 Gy).
Eyes treated with LSRT in our cohort had a median follow-up of more than 60 months and a 35.3% incidence of cataracts. Several studies have reported lower cataract incidence rates using D-shaped lateral photon beams.18,20,21,26 Although lateral beams may provide greater lens sparing compared with IMRT, the orbital bone dose would be higher. In a dosimetry report, Krasin et al demonstrated that IMRT has superior orbital bone sparing.29
Schipper et al reported that 8 Gy was the lowest dose associated with cataract in their LSRT cohort.22 The applicability of their data is limited because their results are from the 2-dimensional treatment planning era, when robust lens dose calculations were not available. The largest Rb cataract study to date was reported by Chodick et al, who assessed more than 800 eyes.30 They reported an average interval of 51 years between RT and cataract extraction for eyes treated with a single course of WERT. However, their report included patients treated before 1984 with outdated RT techniques. Furthermore, data regarding cataract surgery were self-reported by patients. The authors also conceded that their results likely underestimated the true cataract rate because their study did not account for cataracts that may have been diagnosed and observed. Foote et al provided the only known series to compare cataract outcomes between WERT and LSRT.21 Unexpectedly, they reported a cataract incidence of 9% for their WERT patients and 29% for their LSRT patients, which involved D-shaped lateral beams. However, their sample size was limited to 25 eyes with a median follow-up of only 33 months.
Hall et al analyzed the 5-year cataract risk in patients receiving total body irradiation (TBI).31 They produced a dose-response model in their metaregression, which predicts that at 10 Gy (equivalent single dose), the 5-year cataract risk is more than 90%, in contrast to approximately 35% as predicted by our models. One notable difference between our studies is that the Hall et al model used single-dose TBI fractionation which is known to be very different biologically than the same dose delivered over several fractions.32 Only a third of the studies included in their report had fractionated TBI regimens. Pooling the fractionated TBI data and using weighted means, we calculated a 5-year cataract risk of 34% at a dose of 13 Gy, which is comparable to our model predication of 40% at the same dose.
Limitations of this study pertain to the retrospective nature of our data collection. Although our outcome was carefully documented by ophthalmologists, it is possible that eye examinations were primarily focused on assessing tumor response, so a cataract could have been missed. However, given the frequency of examinations, it would be unlikely that a child would have had a significant delay in diagnosis after the onset of a lens opacity. In addition, this is not a randomized study, and patients were assigned to treatment groups based on disease extent factors such as the presence of vitreous seeding. It is possible, although unlikely, that other biologic factors associated with higher stage or vitreous seeding are associated with increased cataract risk independent of lens radiation dose. Cataracts can also be associated with intravitreal melphalan33; however, these data were not collected because intravitreal melphalan would have been offered only to the most recent patients over the 18-year study period.
Finally, clinical experience and judgment must be used before establishing constraints based on these data because careful interpretation is necessary with any predictive model. In this retrospective study, we demonstrate the long-term EFS advantage for LSRT compared with WERT. We caution that cataract risk in irradiated patients with Rb may not apply to patients in other age groups with other conditions. However, our dose-response models are comparable to other reported series and may guide future RT planning decisions when one must balance the potential risk of local tumor control with LSRT against the benefit of avoiding a cataract, which is often surgically treatable.
Conclusions
We report the first clinical data demonstrating significantly improved EFS in patients with Rb treated with LSRT in the modern era. Through lens dose-response modeling, we validate a mean lens dose threshold of 7 Gy to keep the cataract risk below 25%. Although RT is used significantly less often for Rb owing to advances in chemotherapy delivery options, these findings are relevant for refining lens dose constraints, particularly in children with monocular vision who may receive radiation dose near the orbit.
Supplementary Material
Footnotes
Conflicts of interest: none.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2018.12.004.
References
- 1.Scott IU, Murray TG, Feuer WJ, et al. External beam radiotherapy in retinoblastoma: Tumor control and comparison of 2 techniques. Arch Ophthalmol 1999;117:766–770. [DOI] [PubMed] [Google Scholar]
- 2.Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975–2004. Br J Ophthalmol 2009;93:21–23. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Galindo C, Orbach DB, VanderVeen D. Retinoblastoma. Pediatr Clin North Am 2015;62:201–223. [DOI] [PubMed] [Google Scholar]
- 4.Abramson DH. Retinoblastoma in the 20th century: Past success and future challenges the Weisenfeld lecture. Invest Ophthalmol Vis Sci 2005;46:2683–2691. [DOI] [PubMed] [Google Scholar]
- 5.Krengli M, Hug EB, Adams JA, et al. Proton radiation therapy for retinoblastoma: Comparison of various intraocular tumor locations and beam arrangements. Int J Radiat Oncol Biol Phys 2005;61:583–593. [DOI] [PubMed] [Google Scholar]
- 6.Ainsbury EA, Bouffler SD, Dörr W, et al. Radiation cataractogenesis: A review of recent studies. Radiat Res 2009;172:1–9. [DOI] [PubMed] [Google Scholar]
- 7.Blakely EA, Kleiman NJ, Neriishi K, et al. Radiation cataractogenesis: Epidemiology and biology. Radiat Res 2010;173:709–717. [DOI] [PubMed] [Google Scholar]
- 8.Imhof SM, Mourits MP, Hofman P, et al. Quantification of orbital and mid-facial growth retardation after megavoltage external beam irradiation in children with retinoblastoma. Ophthalmology 1996;103: 263–268. [DOI] [PubMed] [Google Scholar]
- 9.Gallie BL, Budning A, DeBoer G, et al. Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiotherapy. Arch Ophthalmol 1996;114:1321–1328. [DOI] [PubMed] [Google Scholar]
- 10.Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: An extended follow-up. J Clin Oncol 2005;23:2272–2279. [DOI] [PubMed] [Google Scholar]
- 11.Abramson DH, Shields CL, Munier FL, et al. Treatment of retinoblastoma in 2015: Agreement and disagreement. JAMA Ophthalmol 2015;133:1341–1347. [DOI] [PubMed] [Google Scholar]
- 12.Munier FL, Gaillard M-C, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: From prohibition to conditional indications. Br J Ophthalmol 2012;96:1078–1083. [DOI] [PubMed] [Google Scholar]
- 13.Munier FL, Verwey J, Pica A, et al. New developments in external beam radiotherapy for retinoblastoma: From lens to normal tissue-sparing techniques. Clin Experiment Ophthalmol 2008;36:78–89. [DOI] [PubMed] [Google Scholar]
- 14.Linn Murphree A Intraocular retinoblastoma: The case for a new group classification. Ophthalmol Clin North Am 2005;18:41–53, viii. [DOI] [PubMed] [Google Scholar]
- 15.Reisner ML, Viégas CMP, Grazziotin RZ, et al. Retinoblastoma—comparative analysis of external radiotherapy techniques, including an IMRT technique. Int J Radiat Oncol Biol Phys 2007;67:933–941. [DOI] [PubMed] [Google Scholar]
- 16.Henk JM, Whitelocke RA, Warrington AP, et al. Radiation dose to the lens and cataract formation. Int J Radiat Oncol Biol Phys 1993;25: 815–820. [DOI] [PubMed] [Google Scholar]
- 17.Eldebawy E, Parker W, Abdel Rahman W, et al. Dosimetric study of current treatment options for radiotherapy in retinoblastoma. Int J Radiat Oncol 2012;82:e501–e505. [DOI] [PubMed] [Google Scholar]
- 18.Toma NM, Hungerford JL, Plowman PN, et al. External beam radiotherapy for retinoblastoma: II. Lens sparing technique. Br J Ophthalmol 1995;79:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hungerford JL, Toma NM, Plowman PN, et al. External beam radiotherapy for retinoblastoma: I. Whole eye technique. Br J Ophthalmol 1995;79:109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormick B, Ellsworth R, Abramson D, et al. Radiation therapy for retinoblastoma: Comparison of results with lens-sparing versus lateral beam techniques. Int J Radiat Oncol Biol Phys 1988;15: 567–574. [DOI] [PubMed] [Google Scholar]
- 21.Foote RL, Garretson BR, Schomberg PJ, et al. External beam irradiation for retinoblastoma: Patterns of failure and dose-response analysis. Int J Radiat Oncol Biol Phys 1989;16:823–830. [DOI] [PubMed] [Google Scholar]
- 22.Schipper J, Tan KEWP, van Peperzeel HA. Treatment of retinoblastoma by precision megavoltage radiation therapy. Radiother Oncol 1985;3:117–132. [DOI] [PubMed] [Google Scholar]
- 23.Amendola BE, Lamm FR, Markoe AM, et al. Radiotherapy of retinoblastoma: A review of 63 children treated with different irradiation techniques. Cancer 1990;66:21–26. [DOI] [PubMed] [Google Scholar]
- 24.Choi SY, Kim M-S, Yoo S, et al. Long term follow-up results of external beam radiotherapy as primary treatment for retinoblastoma. J Korean Med Sci 2010;25:546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips C, Sexton M, Wheeler G, et al. Retinoblastoma: Review of 30 years’ experience with external beam radiotherapy. Australas Radiol 2003;47:226–230. [DOI] [PubMed] [Google Scholar]
- 26.Blach LE, McCormick B, Abramson DH. External beam radiation therapy and retinoblastoma: Long-term results in the comparison of two techniques. Int J Radiat Oncol Biol Phys 1996;35:45–51. [DOI] [PubMed] [Google Scholar]
- 27.Egbert PR, Donaldson SS, Moazed K, et al. Visual results and ocular complications following radiotherapy for retinoblastoma. Arch Ophthalmol 1978;96:1826–1830. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez JC, Brady LW, Shields JA, et al. External beam radiation for retinoblastoma: Results, patterns of failure, and a proposal for treatment guidelines. Int J Radiat Oncol Biol Phys 1996;35:125–132. [DOI] [PubMed] [Google Scholar]
- 29.Krasin MJ, Crawford BT, Zhu Y, et al. Intensity-modulated radiation therapy for children with intraocular retinoblastoma: Potential sparing of the bony orbit. Clin Oncol (R Coll Radiol.) 2004;16:215–222. [DOI] [PubMed] [Google Scholar]
- 30.Chodick G, Kleinerman RA, Stovall M, et al. Risk of cataract extraction among adult retinoblastoma survivors. Arch Ophthalmol 2009;127:1500–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall MD, Schultheiss TE, Smith DD, et al. Dose response for radiation cataractogenesis: A meta-regression of hematopoietic stem cell transplantation regimens. Int J Radiat Oncol Biol Phys 2015;91:22–29. [DOI] [PubMed] [Google Scholar]
- 32.Olch AJ. Tumors of the central nervous system In: Pediatric Radio-therapy: Planning and Treatment. Boca Raton, FL: CRC Press; 2013. p. 97–169. [Google Scholar]
- 33.Suzuki S, Aihara Y, Fujiwara M, et al. Intravitreal injection of melphalan for intraocular retinoblastoma. Jpn J Ophthalmol 2015;59: 164–172. [DOI] [PubMed] [Google Scholar]
- 34.Mouw KW, Sethi RV. Yeap BY, et al. Proton radiotherapy for the treatment of retinoblastoma. Int J Radiat Oncol Biol Phys 2014;90: 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.