Abstract
5-Fluorouracil (5-FU) dosing has traditionally been based on the body surface area (BSA) in colorectal cancer treatment. However, there is accumulating evidence that dosing based on BSA may be of limited use. The purpose of the present study was to evaluate the changes in 5-FU plasma levels and tumor response as well as the severity of adverse events in patients with cancer treated with 5-FU combined chemotherapy. The dosing amount of 5-FU was determined based on the BSA. Blood samples were collected, and 5-FU plasma levels in 15 patients with colorectal cancer were measured three times (0, 22 and 40 h before and after the start of infusion) during constant-infusion of 5-FU for 46 h by an immunoassay. 5-FU plasma levels were significantly higher at 22 and 40 h compared with at 0 h (P<0.001), when all 15 patients were analyzed. Notably, the tumor response of the partial response/stable disease group showed significant increases in 5-FU plasma levels at 40 h compared with at 22 h (P<0.01), while the progressive disease group showed no significant increase. In addition, the 5-FU plasma level in the adverse event level of grade ≥2 was higher than that of grade <2 at 40 h after the start of infusion. Collectively, these observations indicated that during continuous infusion of 5-FU, the 5-FU plasma level increased significantly, and the tumor response (such as partial response, stable or progressive disease) may be influenced by the increase of 5-FU plasma level from the start of infusion. Therefore, the 5-FU plasma level may be a predictive factor for maximizing the tumor response and minimizing the risk of severe adverse events.
Keywords: 5-fluorouracil, chemotherapy, colorectal cancer, plasma level, tumor response, adverse events
Introduction
Colorectal cancer is one of the leading causes of cancer mortality worldwide (1,2). Since the introduction of 5-fluorouracil (5-FU) in the 1960s, 5-FU has been widely used in colorectal cancer treatment, as well as for other malignancies, including gastric cancer, breast cancer, and head and neck cancer (3). Subsequently, 5-FU-based combination therapies, such as 5-FU plus folinic acid-oxaliplatin (FOLFOX) and 5-FU plus folinic acid-irinotecan (FOLFIRI) regimens, have been developed (4–6). Previously, the addition of anti-epidermal growth factor receptor monoclonal antibodies, such as cetuximab and panitumumab, and humanized anti-vascular growth factor receptor monoclonal antibodies, such as bevacizumab, have also proven to enhance the anti-tumor effect (7–12). Despite the development of combined therapy, 5-FU has continued to be the main drug for the treatment of colorectal cancer. However, the calculation of the dosing amount of 5-FU is still simply based only on the body surface area (BSA) (13).
Dosing based on BSA is considered convenient and easy to use, yet previous studies have indicated that many patients treated with 5-FU are not given the appropriate dose to achieve optimal plasma concentration (13,14). According to a previous study, only 20–30% of patients are treated with the appropriate therapeutic range, while 40–60% are under dosed and 10–20% are overdosed (15). Previous results have suggested the existence of interpatient and intrapatient pharmacokinetic (PK) variation in 5-FU clearance (13,16–19). Collectively, these previous results indicated that dosing based on BSA is of limited use (13,19,20). Therefore, the concept of direct monitoring of 5-FU plasma concentrations with appropriate dose adjustments has been introduced. A number of previous studies, including a large multicenter randomized trial, have shown that PK-guided dose adjustment of 5-FU in metastatic colorectal cancer has resulted in an improvement in therapeutic outcomes and reduced the severity of adverse events (14,20). The aim of the present study was to determine an optimal method of determining the 5-FU dosage for patients with colorectal cancer undergoing chemotherapy. In the present prospective study, the transition of 5-FU plasma levels in patients with colorectal cancer during treatment with 5-FU combined chemotherapy was monitored. Furthermore, the association between the tumor response and adverse events [classified by the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0] (21) based on the 5-FU plasma levels was evaluated.
Patients and methods
Patient treatment and eligibility
The present prospective study was performed using 15 patients with colorectal cancer treated with 5-FU combined chemotherapy at The Tobu Chiiki Hospital. All patients were subjected to standardized clinical practice, according to the Japanese Society for Cancer of the Colon and Rectum Guidelines 2016 for the Treatment of Colorectal Cancer (22). 5-FU combined chemotherapy included modified FOLFOX6 (mFOLFOX6) (n=6): Oxaliplatin 85 mg/m2 for 2 h, leucovorin (folinic acid) 200 mg/m2 for 2 h, 5-FU 400 mg/m2 bolus and 5-FU 2,400 mg/m2 continuous intravenous infusion for 46 h; or FOLFIRI (n=9): Irinotecan 150 mg/m2 for 2 h, leucovorin 150 mg/m2 for 2 h, 5-FU 400 mg/m2 bolus and 5-FU 2,400 mg/m2 continuous intravenous infusion for 46 h. All patients received the dose of 5-FU based on their BSA (5-FU 400 mg/m2 bolus and 5-FU 2,400 mg/m2 continuous intravenous infusion for both mFOLFOX6 and FOLFIRI regimens). The Eastern Cooperative Oncology Group Performance Status (ECOG-PS) classification (23) was used to evaluate the performance status (PS) of each patient. Patients were required to have a PS ≤2. The criteria for the classification of PS were as follows: i) PS 0, fully active, able to carry on pre-disease performances without restriction; ii) PS 1, restricted in physically strenuous activity but ambulatory and is able to carry out work of a light or sedentary nature; and iii) PS 2, ambulatory and capable of all selfcare but unable to carry out any work activities; up and about more than 50% of waking hours. All the patients included in the present study were treated under the guidance of professional medical staff members. Patients were required for 4 days in admission for treatment and were readmitted every 2 weeks for 3 months (a total of six admissions). The present study was approved by the Ethics Committee of Tobu Chiiki Hospital (approved December 21, 2015; IRB nos. 15 and 4), and performed between January 1, 2017 and March 31, 2017. The present study was conducted in accordance with the principles of the amended ‘Declaration of Helsinki and Ethical Guidelines for Epidemiological Research’ (established by the Japanese Government in 2008) (24). Written informed consent was obtained from all patients before they were enrolled in the present study. Patients with PS >2 or requiring radiation or dialysis, or those unable to provide informed consent were excluded.
Measurement of 5-FU plasma levels
Venous blood samples (10 ml) were collected three times during admission (prior to the start of infusion, and 22 and 40 h after the start of continuous 5-FU infusion). 5-FU plasma levels were measured using the My-5FU® assay, a competitive homogeneous nanoparticle agglutination immunoassay (FALCO Biosystems Ltd.). This assay is used for patients receiving 5-FU by continuous infusion to facilitate PK dose adjustment at the next cycle and drug monitoring to achieve an optimal plasma level of the drug. The assay uses two reagents and when they are mixed, the nanoparticles aggregate together. Therefore, the amount of aggregation of nanoparticles determines the 5-FU concentration in plasma samples. 5-FU plasma levels at 0 (prior to the start of infusion), 22 and 40 h after the start of continuous 5-FU infusion in each patient were calculated as median values of six admissions and statistically analyzed.
Analysis of tumor response and adverse events
Tumor response to treatment was classified according to Response Evaluation Criteria in Solid Tumors Group (RECIST 1.1) criteria (25) and was evaluated after the 3 month monitoring period. A complete response required the disappearance of every lesion. A partial response required a ≥30% reduction in the cross-sectional area of all lesions. Stable disease required a lesion size decrease <30%. Progressive disease categorization encompassed any situation in which any one lesion increased in cross-sectional size by >25% or a new lesion appeared.
Adverse events during chemotherapy were evaluated just after the infusion of 5-FU (for 46 h) on the 4th day of admission, according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 (21), based on physical examination and laboratory tests. The severity of adverse events was graded between 1 and 5. Grade 1 consists of the following criteria: i) Mild; ii) asymptomatic or mild symptoms; iii) clinical or diagnostic observations only; and iv) intervention not indicated. Grade 5 is associated with death. Adverse events were assessed on the last day before discharge for each patient.
Statistical analysis
The plasma levels of 5-FU are presented as box-and-whisker plots (containing the minimum, first quartile, median, third quartile and maximum values), unless otherwise stated. The 5-FU plasma levels were measured three times (0 h prior to the start of infusion, 22 and 40 h after the start of continuous 5-FU infusion) in each patient, calculated as median values of six admissions, and statistically analyzed. Statistical significance was determined by one-way ANOVA with Bonferroni's post hoc test using GraphPad Prism 7 (GraphPad Software, Inc.). Statistical significance for the different tumor responses (complete response, partial response, stable disease and progressive disease) as well as the response rate and disease control rate between mFOLFOX6 and FOLFIRI was determined by a bivariate analysis using JMP 13 software (SAS Institute, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Patients
A total of 15 patients (5 female and 10 male) were enrolled in the present study. The patient characteristics are summarized in Table I. The mean age was 69.0±7.3 (mean ± SD, range of 52–79) years and the mean BSA was 1.57±0.18 m2. Based on the ECOG-PS classification (23), 1 patient was categorized as PS 0, 11 patients were categorized as PS 1, and 3 patients were categorized as PS 2. Only 1 patient had a tumor located in the descending colon, 10 patients had a tumor located in the sigmoid colon and 4 patients had a tumor located in the rectum. In total, 4 patients had a pathological diagnosis of well differentiated adenocarcinoma, 10 patients were diagnosed with moderately differentiated adenocarcinoma and 1 patient was diagnosed with papillary adenocarcinoma, according to the Japanese Classification of Colorectal Carcinoma (8th Edition) (26). A total of 9 patients enrolled were treated for a primary tumor, while 6 patients were treated for a recurrent tumor. Among the 9 patients, 8 patients were diagnosed as Stage IV and 1 patient was diagnosed as Stage IIIb, based on the Japanese Classification of Colorectal Carcinoma (8th Edition) (26) (data not shown). The 9 patients were diagnosed with resectable tumors, and thus they were not treated for a neoadjuvant setting. A total of 14 patients had metastasis. The most frequent metastatic site (6 patients) was the liver; other metastasis sites included the lung (1 patient) and peritoneum (2 patients). A total of 5 patients had multiple metastatic sites. A total of 11 out of 15 patients underwent operations.
Table I.
Patient characteristics.
| Characteristics | Value |
|---|---|
| Age (years) | 69.0±7.3a |
| Male/Female | 10/5 |
| BSA (m2) | 1.57±0.18a |
| ECOG-PS classification (0/1/2) | 1/11/3 |
| Tumor location (descending/sigmoid/rectum) | 1/10/4 |
| Pathological diagnosis (well/moderately/papillary) | 4/10/1 |
| Primary or recurrent tumor | 9/6 |
| Metastasis (+/−) | 14/1 |
| Location of metastasis (liver/lung/peritoneum/multiple) | 6/1/2/5 |
| Operation (+/−) | 11/4 |
Values are presented as the mean ± SD of 15 patients. BSA, body surface area; ECOG-PS, Eastern Cooperative Oncology Group-Performance Status.
Changes of the plasma level of 5-FU during infusion
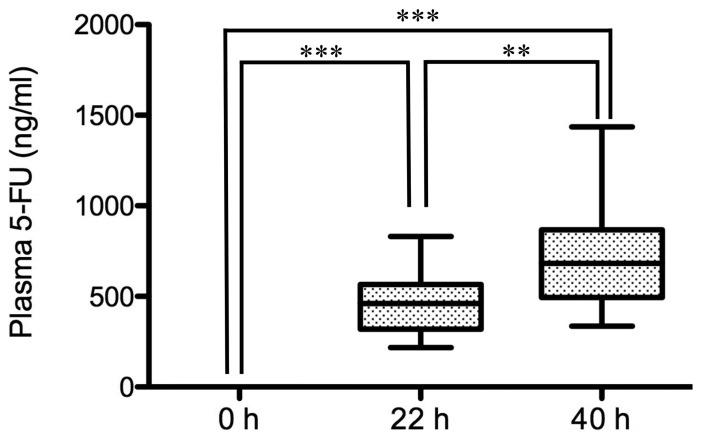
Before the start of infusion, the plasma level of 5-FU was 0 ng/ml in all patients (n=15). During infusion of 5-FU for 46 h, the level at 22 h was 460.5 ng/ml (median value), while the level at 40 h reached 682.0 ng/ml. The levels at 22 h and 40 h were significantly higher than that at 0 h (P<0.001; Fig. 1). Notably, the level at 40 h was significantly higher than that at 22 h (P<0.01).
Figure 1.
Plasma levels of 5-FU after the start of continuous infusion. Blood samples were collected prior to the start of infusion (0 h), 22 and 40 h after the start of continuous 5-FU infusion, and 5-FU plasma levels were measured. The plasma levels of 5-FU of 15 patients enrolled are presented as box-and-whisker plots. Values are compared among 0, 22 and 40 h. **P<0.01, ***P<0.001. 5-FU, 5-fluorouracil.
Tumor response and regimens
Among the 15 patients, 6 patients underwent chemotherapy of the mFOLFOX6 regimen and 9 patients underwent chemotherapy of the FOLFIRI regimen. The tumor response, according to RECIST 1.1 criteria showed no significant differences between mFOLFOX6 and FOLFIRI in the categories of complete response, partial response, stable disease and progressive disease (Table II). The response rate (proportion of patients who achieved complete or partial response) and the disease control rate (proportion of patients who achieved complete, partial response or stable disease) also showed no significant differences between mFOLFOX6 and FOLFIRI.
Table II.
Tumor response and 5-fluorouracil regimens.
| Tumor response | mFOLFOX6 (n=6) | FOLFIRI (n=9) | P-value |
|---|---|---|---|
| Complete response | 0 | 0 | N/A |
| Partial response | 2 | 3 | >0.99 |
| Stable disease | 1 | 3 | 0.60 |
| Progressive disease | 3 | 3 | 0.52 |
| Response rate | 33% | 33% | >0.99 |
| Disease control rate | 50% | 67% | 0.52 |
In total, 6 patients were treated with the mFOLFOX6 regimen and 9 patients were treated with the FOLFIRI regimen. The number of patients with different tumor responses (complete response, partial response, stable disease and progressive disease) in each regimen are listed. The response rate is defined as the proportion of patients with complete or partial response among each regimen. The disease control rate is defined as the proportion of patients with complete, partial response or stable disease among each regimen. Statistical significance was determined by a bivariate analysis. There was no significant difference in each tumor response, response rate or disease control rate between the two regimens. mFOLFOX, modified 5-fluorouracil plus folinic acid-oxaliplatin; FOLFIRI, 5-fluorouracil plus folinic acid-irinotecan; N/A, not applicable.
Changes of the plasma level of 5-FU in different tumor response groups
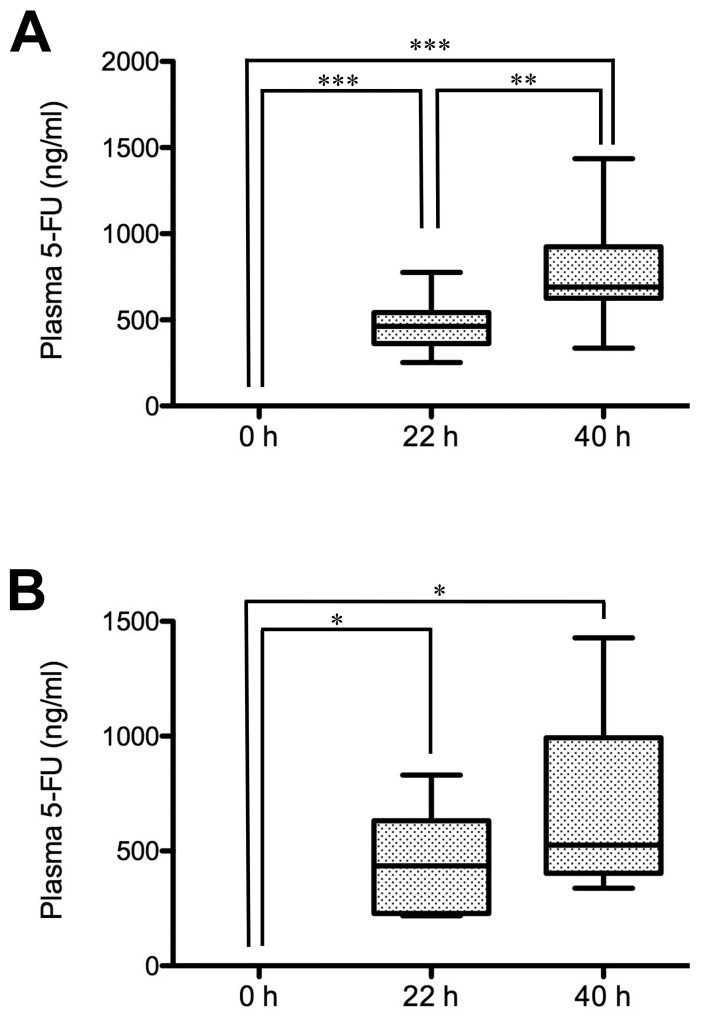
The plasma levels of 5-FU in the patients with different tumor responses were examined. In the partial response/stable disease group, the 5-FU plasma level at 22 h after the start of infusion was 460.5 ng/ml (median value), while the level at 40 h reached 690.9 ng/ml (Fig. 2A). The levels at 22 and 40 h were significantly higher than at 0 h (P<0.001). Notably, the level at 40 h was significantly higher than at 22 h (P<0.01). In the progressive disease group, the 5-FU plasma level at 22 h after the start of infusion was 435.1 ng/ml, while the level at 40 h reached 525.9 ng/ml (Fig. 2B). The levels at 22 h and 40 h were significantly higher than at 0 h (P<0.05). However, the level at 40 h was not significantly higher than at 22 h.
Figure 2.
Plasma levels of 5-FU in the patients with different tumor responses after the start of continuous infusion. Blood samples of patients in (A) the partial response/stable disease group and (B) the progressive disease group were collected prior to the start of infusion (0 h), 22 and 40 h after the start of continuous 5-FU infusion, and 5-FU plasma levels were measured. The plasma levels of 5-FU of the partial response/stable disease group (n=9) and the progressive disease group (n=6) are presented as box-and-whisker plots. Values are compared among 0, 22 and 40 h. *P<0.05, **P<0.01, ***P<0.001. 5-FU, 5-fluorouracil.
Furthermore, the mean 5-FU plasma level at 40 h after the start of infusion between the partial response/stable disease group and the progressive disease group was compared (Fig. 2). The 5-FU plasma level of the partial response/stable disease group (776.2±286.9 ng/ml; mean ± SD) was higher than that of the progressive disease group (681.9±369.4 ng/ml; data not shown), although there was no significant difference between the two groups, as assessed by one-way ANOVA analysis with Bonferroni's post hoc test.
Adverse events in different tumor response groups
Table III shows the adverse events of grade ≥2 in the different tumor response groups; grade 2, according to CTCAE v5.0, which is defined according to the following criteria: i) Moderate; ii) minimal, local or noninvasive intervention indicated; and iii) limiting age-appropriate instrumental activities of daily living. In the partial response group, numbness was the most common adverse event (75%). In the stable disease group, numbness and diarrhea were observed (both 50%). In the progressive disease group, numbness (80%), appetite loss (60%) and hand foot syndrome (60%) were observed. Adverse events were evaluated after the infusion of 5-FU for 46 h on the 4th day of admission. Therefore, this suggests that the 5-FU plasma level at 40 h after the start of infusion may reflect the results of the adverse events.
Table III.
Tumor response and severity of adverse events.
| Adverse events | Partial response, % | Stable disease, % | Progressive disease, % |
|---|---|---|---|
| Hand foot syndrome | 50 | 0 | 60 |
| Fatigue | 0 | 25 | 40 |
| Edema | 0 | 25 | 40 |
| Stomatitis | 50 | 25 | 40 |
| Appetite loss | 25 | 25 | 60 |
| Numbness | 75 | 50 | 80 |
| Neutropenia | 0 | 0 | 20 |
| Nausea/Vomit | 25 | 0 | 0 |
| Facial flushing | 0 | 0 | 20 |
| Diarrhea | 0 | 50 | 20 |
The proportion of adverse events among patients of Grade ≥2 (n=13) treated with modified 5-fluorouracil plus folinic acid-oxaliplatin or 5-fluorouracil plus folinic acid-irinotecan is shown for each tumor response (partial response, n=4; stable disease, n=4; progressive disease, n=5). Grade 2 is defined according to the following criteria of the Common Terminology Criteria for Adverse Events v5.0: i) Moderate; ii) minimal, local or noninvasive intervention indicated; and iii) limiting age-appropriate instrumental activities of daily living.
Plasma levels of 5-FU in the patients with different severities of adverse events
Among the 15 patients, 13 patients were included in the adverse event level of grade ≥2. In the partial response/stable disease group, 8 out of 9 patients were included in the adverse event level of grade ≥2, whereas in the progressive disease group, 5 out of the 6 patients were included to have an adverse event level of grade ≥2. 5-FU plasma levels at 40 h after the start of infusion in the adverse event level of grade ≥2 (13 patients in the partial response/stable disease and progressive disease groups) reached 690.9 ng/ml (median value), while the 5-FU plasma level for the adverse event level of grade <2 (2 patients in the partial response/stable disease and progressive disease groups) was 591.6 ng/ml (Fig. 3). Although there was no statistical significance between the groups of grades ≥2 and <2, the 5-FU plasma level was ~100 ng/ml higher in the group of grades ≥2 than that in the group of grade <2.
Figure 3.
Plasma levels of 5-FU in the patients with different severities of adverse events at 40 h after the start of infusion. Plasma levels of 5-FU in patients with the severity of adverse events (grade <2 and ≥2) were compared at 40 h after the start of infusion. The plasma levels of 5-FU of grade <2 (n=2) and ≥2 (n=13) are expressed in box-and-whisker plots. 5-FU, 5-fluorouracil.
Discussion
5-FU dosing has traditionally been based on BSA in colorectal cancer treatment. However, there is accumulating evidence that the dosing based on BSA may be of limited use (13). The purpose of the present study was to evaluate the changes in 5-FU plasma levels and the tumor response as well as the severity of adverse events in patients with cancer treated with 5-FU combined chemotherapy (mFOLFOX6 and FOLFIRI). The dosing amount of 5-FU was determined based on the BSA, and the transition of 5-FU plasma levels in 15 patients with colorectal cancer was monitored three times (0,22 and 40 h) before and after the start of infusion during constant-infusion of 5-FU for 46 h. The present study demonstrated that 5-FU plasma levels were significantly higher at 22 and 40 h compared with at 0 h, when all 15 patients were analyzed. Notably, the partial response/stable disease group showed significant increases in 5-FU plasma levels at 40 h compared with at 22 h, although the progressive disease group showed no significant increase. In addition, the 5-FU plasma level in the adverse event level of grade ≥2 was higher than that of grade <2 at 40 h after the start of infusion.
A significant increase in the 5-FU plasma level at 40 h compared with 22 h after the start of infusion was observed. In order to explain the wide range of increases in 5-FU plasma levels, a number of previous studies suggested that dihydropyrimidine dehydrogenase (DPD), an initial rate-limiting enzyme related to 5-FU metabolism, plays a role in determining the plasma levels of 5-FU (27–30). Therefore, the significant increase in 5-FU plasma levels after continuous intravenous infusion (particularly between 22 and 40 h) observed in the partial response/stable disease group may be due to the decreased 5-FU-metabolizing activity of DPD. However, the non-significant increase of 5-FU plasma level in the progressive disease group (between 22 and 40 h) may suggest that the 5-FU-metabolizing activity of DPD is higher than that of the partial response/stable disease group. Furthermore, the present results suggested that the tumor response may be influenced by the increase of 5-FU plasma level (possibly based on the low DPD activity) after the start of continuous infusion, as observed in the higher plasma 5-FU level in the partial response/stable disease group, compared with the progressive disease group, suggesting that a high DPD activity may be associated with a poor prognosis in the tumor response. However, DPD activity was not evaluated in the present study. Therefore, DPD activity should be evaluated in future studies.
In contrast to a previous study (18), in which the 5-FU plasma level reached a plateau at 22 h after the continuous infusion with an electronical pump, the present study showed that the 5-FU plasma level continued to significantly increase between 22 and 40 h after infusion. In the previous study (18), 5-FU continuous infusion relied on an elastomeric infusion pump that is suitable for ambulatory patients treated in an out-patient clinic. In the present study, professional staff members constantly monitored the electronical infusion pump to prevent the discontinuity of 5-FU during admission. Therefore, the infusion methods used in the present study may explain a continuous increase of 5-FU plasma levels during 40 h from the start of admission. Furthermore, the increase in the 5-FU plasma level may also explain the adverse events occurring in the partial response/stable disease group. However, it is not possible to conclude that an elastomeric pump is inferior to an electronical infusion pump. Previous studies have suggested that patients prefer elastomeric balloon infusion pump rather than an electronical infusion pump, as the weight and size of elastomeric infusion pump is low (31,32). However, it has been reported that the accuracy in delivery rate of an elastomeric infusion pump is lower compared with an electronical infusion pump (33,34). Therefore, the accuracy of the delivery rate in an electronical infusion pump may contribute to the significant increase of the plasma level of 5-FU at 40 h in the present study. Furthermore, it has also been suggested in a previous study utilizing an electronical infusion pump that acute toxicity was correlated with a high 5-FU plasma level (35). Taking these observations into consideration, it was hypothesized that the high plasma level of 5-FU observed in the partial response/stable disease group may reflect the severity in adverse events shown in the present study.
However, the present study has several limitations. The number of patients enrolled in the present study was limited and the tumor response was evaluated for only a short term of 3 months. Therefore, future studies should monitor patients for a longer period to evaluate the prognosis and overall survival. However, despite these limitations, the present study supports the accumulating evidence that measuring 5-FU plasma levels may contribute to the improvement of a positive tumor response as well as to minimize the risk of severe adverse events (14,20). Furthermore, it is difficult to discuss the effect of 5-FU for tumor response and adverse event in the protocol of FOLFOX or FOLFIRI using oxaliplatin or irinotecan, respectively. However, to the best of our knowledge, a standardized method has not yet been developed to evaluate the plasma levels of oxaliplatin or irinotecan. Notably, a previous study has revealed that a higher plasma level of 5-FU undergoing a regimen of continuous 5-FU plus leucovorin with an electronical infusion pump was correlated with more severe toxicities (35). The present study also indicated that a higher 5-FU plasma level results in a positive tumor response. Although the plasma levels of oxaliplatin and irinotecan were not measured in the present study, these previous studies (15,17,36) likely support the hypothesis that a significant increase of 5-FU plasma at 40 h is associated with the severity of toxicity as well as a positive tumor response.
In conclusion, during continuous infusion of 5-FU, the 5-FU plasma level increased significantly. The tumor response may be influenced by the increase of 5-FU plasma level from the start of infusion. The 5-FU plasma level may be a predictive factor for maximizing the tumor response and minimizing the risk of severe adverse events.
Acknowledgements
The authors would like to thank Mr Hiroshi Yamane and Mr Makoto Miyazaki (FALCO Biosystems Ltd.) for measuring the plasma levels of 5-FU in the present study.
Funding
Funding was provided from Takeda Pharmaceutical Company Ltd. (grant. no. RS2016A001043) and Chugai Pharmaceutical Co., Ltd. (grant. no. AC-1-20170518213426-569401).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YA and TO designed the research. YA, NS, TS, KK, KNa, AN, MK, TW, KNi and TO performed the clinical study. YA, TO and IN analyzed the data. YA and IN prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Tobu Chiiki Hospital (approved December 21, 2015; IRB nos. 15 and 4), and performed between January 1, 2017 and March 31, 2017. The present study was conducted in accordance with the principles of the amended ‘Declaration of Helsinki and Ethical Guidelines for Epidemiological Research’ (established by the Japanese Government in 2008). Written informed consent was obtained from all patients before they were enrolled in the present study.
Patient consent for publication
All the participants enrolled in the present study provided written informed consent for the publication of any associated data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Benson AB, III, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:370–398. doi: 10.6004/jnccn.2017.0059. [DOI] [PubMed] [Google Scholar]
- 3.Mayer RJ. Moving beyond fluorouracil for colorectal cancer. N Engl J Med. 2000;343:963–964. doi: 10.1056/NEJM200009283431309. [DOI] [PubMed] [Google Scholar]
- 4.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 6.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 9.Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 10.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB, III, Eastern Cooperative Oncology Group Study E3200 Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 11.Wu M, Rivkin A, Pham T. Panitumumab: Human monoclonal antibody against epidermal growth factor receptors for the treatment of metastatic colorectal cancer. Clin Ther. 2008;30:14–30. doi: 10.1016/j.clinthera.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, Andre T, Chan E, Lordick F, Punt CJ, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–4713. doi: 10.1200/jco.2010.28.15_suppl.3565. [DOI] [PubMed] [Google Scholar]
- 13.Saam J, Critchfield GC, Hamilton SA, Roa BB, Wenstrup RJ, Kaldate RR. Body surface area-based dosing of 5-fluoruracil results in extensive interindividual variability in 5-fluorouracil exposure in colorectal cancer patients on FOLFOX regimens. Clin Colorectal Cancer. 2011;10:203–206. doi: 10.1016/j.clcc.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Patel JN, O'Neil BH, Deal AM, Ibrahim JG, Sherrill GB, Olajide OA, Atluri PM, Inzerillo JJ, Chay CH, McLeod HL, Walko CM. A community-based multicenter trial of pharmacokinetically guided 5-fluorouracil dosing for personalized colorectal cancer therapy. Oncologist. 2014;19:959–965. doi: 10.1634/theoncologist.2014-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saif MW, Choma A, Salamone SJ, Chu E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: A rational approach to improving therapeutic outcomes. J Natl Cancer Inst. 2009;101:1543–1552. doi: 10.1093/jnci/djp328. [DOI] [PubMed] [Google Scholar]
- 16.Gamelin E, Boisdron-Celle M, Delva R, Regimbeau C, Cailleux PE, Alleaume C, Maillet ML, Goudier MJ, Sire M, Person-Joly MC, et al. Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: Results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J Clin Oncol. 1998;16:1470–1478. doi: 10.1200/JCO.1998.16.4.1470. [DOI] [PubMed] [Google Scholar]
- 17.Ychou M, Duffour J, Kramar A, Debrigode C, Gourgou S, Bressolle F, Pinguet F. Individual 5-FU dose adaptation in metastatic colorectal cancer: Results of a phase II study using a bimonthly pharmacokinetically intensified LV5FU2 regimen. Cancer Chemother Pharmacol. 2003;52:282–290. doi: 10.1007/s00280-003-0658-0. [DOI] [PubMed] [Google Scholar]
- 18.Kaldate RR, Haregewoin A, Grier CE, Hamilton SA, McLeod HL. Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6. Oncologist. 2012;17:296–302. doi: 10.1634/theoncologist.2011-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capitain O, Asevoaia A, Boisdron-Celle M, Poirier AL, Morel A, Gamelin E. Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: A phase II, proof-of-concept study. Clin Colorectal Cancer. 2012;11:263–267. doi: 10.1016/j.clcc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Gamelin E, Delva R, Jacob J, Merrouche Y, Raoul JL, Pezet D, Dorval E, Piot G, Morel A, Boisdron-Celle M. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: Results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2099–2105. doi: 10.1200/JCO.2007.13.3934. [DOI] [PubMed] [Google Scholar]
- 21.Cho J, Yoon J, Kim Y, Oh D, Kim SJ, Ahn J, Suh GY, Nam SJ, Mitchell SA. Linguistic validation of the US national cancer institute's patient-reported outcomes version of the common terminology criteria for adverse events in Korean. J Glob Oncol. 2019;5:1–10. doi: 10.1200/JGO.18.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34. doi: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correa GT, Bandeira GA, Cavalcanti BG, Santos FB, Rodrigues Neto JF, Guimaraes AL, Haikal DS, De Paula AM. Analysis of ECOG performance status in head and neck squamous cell carcinoma patients: Association with sociodemographical and clinical factors, and overall survival. Support Care Cancer. 2012;20:2679–2685. doi: 10.1007/s00520-012-1386-y. [DOI] [PubMed] [Google Scholar]
- 24.Skierka AS, Michels KB. Ethical principles and placebo-controlled trials-interpretation and implementation of the Declaration of Helsinki's placebo paragraph in medical research. BMC Med Ethics. 2018;19:24. doi: 10.1186/s12910-018-0262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Ishiguro S. Pathological diagnosis of colorectal cancer according to Japanese classification of colorectal carcinoma. Nihon Rinsho. 2011;69(Suppl 3):S325–S329. (In Japanese) [PubMed] [Google Scholar]
- 27.Pinedo HM, Peters GF. Fluorouracil: Biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–1664. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- 28.Lu ZH, Zhang R, Diasio RB. Purification and characterization of dihydropyrimidine dehydrogenase from human liver. J Biol Chem. 1992;267:17102–17109. [PubMed] [Google Scholar]
- 29.Diasio RB, Lu Z. Dihydropyrimidine dehydrogenase activity and fluorouracil chemotherapy. J Clin Oncol. 1994;12:2239–2242. doi: 10.1200/JCO.1994.12.11.2239. [DOI] [PubMed] [Google Scholar]
- 30.Diasio RB, Johnson MR. Dihydropyrimidine dehydrogenase: Its role in 5-fluorouracil clinical toxicity and tumor resistance. Clin Cancer Res. 1999;5:2672–2673. [PubMed] [Google Scholar]
- 31.Zahnd D, Aebi S, Rusterholz S, Fey MF, Borner MM. A randomized crossover trial assessing patient preference for two different types of portable infusion-pump devices. Ann Oncol. 1999;10:727–729. doi: 10.1023/A:1008334313918. [DOI] [PubMed] [Google Scholar]
- 32.Capdevila X, Macaire P, Aknin P, Dadure C, Bernard N, Lopez S. Patient-controlled perineural analgesia after ambulatory orthopedic surgery: A comparison of electronic versus elastomeric pumps. Anesth Analg. 2003;96:414–417. doi: 10.1097/00000539-200302000-00022. table of contents. [DOI] [PubMed] [Google Scholar]
- 33.Ganapathy S, Amendola A, Lichfield R, Fowler PJ, Ling E. Elastomeric pumps for ambulatory patient controlled regional analgesia. Can J Anaesth. 2000;47:897–902. doi: 10.1007/BF03019672. [DOI] [PubMed] [Google Scholar]
- 34.Kaye T. Prolonged infusion times with disposable elastomeric infusion devices. Am J Hosp Pharm. 1994;51:533–534. [PubMed] [Google Scholar]
- 35.Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, Maillart PJ, Goudier MJ, Burtin PC, Delva RG, Lortholary AH, Gesta PH, Larra FG. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer. 1996;77:441–451. doi: 10.1002/(SICI)1097-0142(19960201)77:3<441::AID-CNCR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Blaschke M, Blumberg J, Wegner U, Nischwitz M, Ramadori G, Cameron S. Measurements of 5-FU plasma concentrations in patients with gastrointestinal cancer: 5-FU levels reflect the 5-FU dose applied. J Cancer Ther. 2012;3:28–36. doi: 10.4236/jct.2012.31004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.