Abstract
The aim of this study was to assess the outcome of patients with aplastic anemia (AA), receiving rabbit anti‐thymocyte globulin (Thymoglobulin, SANOFI) and cyclosporin, as first line treatment. Eligible were 955 patients with AA, treated first line with Thymoglobulin, between 2001 and 2008 (n = 492), or between 2009 and 2012 (n = 463). The median age of the patients was 21 years (range 1‐84). Mortality within 90 days was 5.7% and 2.4%, respectively in the two time periods (P = .007).The actuarial 10‐year survival for the entire population was 70%; transplant free survival was 64%. Predictors of survival in multivariate analysis, were severity of the disease, patients age and the interval between diagnosis and treatment. Survival was 87% vs 61% for responders at 6 months versus nonresponders (P < .0001). The 10‐year survival of nonresponders at 6 months, undergoing a subsequent transplant (n = 110), was 64%, vs 60% for patient not transplantated (n = 266) (P = .1). The cumulative incidence of response was 37%, 52%, 65% respectively, at 90, 180, and 365 days. In multivariate analysis, negative predictors of response at 6 months, were older age, longer interval diagnosis treatment, and greater severity of the disease. In conclusion, early mortality is low after first line treatment of AA with Thymoglobulin, and has been further reduced after year 2008. Patients age, together with interval diagnosis—treament and severity of the disease, remain strong predictors of response and survival.
1. INTRODUCTION
Antithymocyte globulin (ATG) combined with cyclosporin a (CsA), is standard therapy for patients with acquired aplastic anemia (AA), lacking an HLA identical donor, or ineligible for transplantation because of age.1, 2 There are two animal sources of ATG, currently used in the clinic: horse and rabbit. First line treatment with rabbit ATG (Thymoglobulin, Sanofi, France) has been compared to horse ATG (ATGAM, Pfizer), in a prospective randomized trial at the National Insitute of Health (NIH, Bethesda),3 and has shown inferior results both in terms of response at 6 months (37% vs 68%) as well as 2‐year survival (76% vs 96%). Similar results were obtained in a matched pair analysis of the European Group for Blood and Marrow Transplantation (EBMT), with a 2‐year survival of 68% for rabbit ATG, compared to 86% for horse ATG.4 However, the number of patients involved in these two studies, and receiving Thymoglobulin, was small: 60 patients in the NIH study and 35 patients in the EBMT study. Conflicting results have come from retrospective studies, some confrming5, 6 and some disputing,7, 8, 9, 10 the superiority of horse versus rabbit ATG. Two recent meta‐analyzes have addressed the comparison of horse versus rabbit ATG for the treatment of AA, confirming superior response rate at 6 months with horse ATG, but with less clear results on mortality, or response beyond 6 months.11, 12 In addition, improved response rates with horse ATG, were driven mainly by studies with non‐Asian patients.11
The aim of the present study was to assess the outcome of a large number of patients, treated in Europe and in Asia with Thymoglobuline and CsA first line, in the past decade.
2. METHODS
2.1. Study design
This a single arm retrospective study aimed to assess the outcome of a large number of AA patients treated in Europe/Asia with first line rabbit ATG (Thymoglobuline) and CsA, between 2001 and 2012. The study was approved by the Ethical Committee of the Fondazione Policlinico Universitaro Gemelli, Roma, Italy. Data from existing databases were shared to create a newly constructed anonimised database, including information available from databases from the EBMT Society (European Group for Blood and Marrow Transplantation), Spain, Germany, a local Italian database and data from existing databases set up in Japan, China, Thailand, and Korea. All owners of the databases have accepted to share the information of their database. EBMT policy on datasharing was adopted for this project. Double entries have been excluded from this new database. The same applies to the data from the Asian centers. In addition each patient was double checked with unique patient number, date of birth and date of treatment, to make sure double entries are not included. Centers and Registries were contacted to sort inconsistencies and missing data. The database was locked on March 31st, 2016. The original data set contained 1212 patients: 168 were excluded because of unknown ATG brand; 67 because of a second treatment within 90 days; 22 because of lack of follow up data, and this left 955 patients evaluable for survival and 800 evaluable for response (Table 1): the 10‐year survival for patients with and without response data was comparable (P = .3). Severity of the disease was classified according to current criteria: very severe AA (neutrophil count <0.2 × 109/L), severe AA (neutrophil count 0.2–05 × 109/L), and non severe AA (neutrophil count >0.5 × 109/L). The inclusion criteria were as follows: patients with newly diagnosed acquired AA, aged 1–80 years, treated first line with rabbit ATG between 2001 and 2012, in the participating centers in Europe and Asia, and with updated follow up in 2015. Exclusion criteria were: constitutional AA, patients receiving first line treatment with horse ATG; patients treated before 2000 or after 2012, patients treated with other rabbit ATG brands, patients transplanted or getting an alternative treatment within 120 days after treatment initiation with Thymoglobuline.
Table 1.
Clinical characteristics of 955 AA patients
| Patients | 2001–2008 | 2009–2012 | P |
|---|---|---|---|
| N | 492 | 463 | |
| Median age | 21 (1–84) | 20 (1–81) | .5 |
| Gender M/F | 266/226 | 235/238 | .3 |
| Severity vSAA | 29% | 31% | <.01 |
| SAA | 32% | 46% | |
| nSAA | 27% | 19% | |
| unknown | 12% | 4% | |
| Int Dx‐ ATG (dd) | 15 (1–6941) | 21 (1–10175) | <.01 |
| Dose Thymo <2.5 | 85 | 167 | |
| 2.5‐3.5 | 192 | 87 | |
| >3.5 | 215 | 209 | <.01 |
| Treated EBMT/ASIA | 300/198 | 192/265 | <.01 |
| Response evaluabe | 413 | 387 | .8 |
| Median follow up | 1673 (6–5420) | 1192 (1–2658) | <.01 |
Abbreviations: AA, aplastic anemia; EBM, European Group for Blood and Marrow Tranplantation; Int Dx‐ATG, inerval diagnosis anti‐thymocyte globulin (ATG); nSAA, non severe AA; SAA, severe AA; Thymo, thymoglobulin; vSAA, very severe AA.
2.2. Primary end point
The primary objective was to assess overall survival for the use of Thymoglobuline and CsA, as first line immunosuppressive therapy for AA. Secondary end points were: 1 percentage of patients with response at day 90, 180, and 365, 2 early mortality (within 90 days after start of therapy) and late mortality (beyond 90 days after start of therapy) and corresponding causes of death, and 3 second line treatment for nonresponders or patients relapsing. An exploratory analysis was also planned to test for a link between total dose and survival or early mortality.
2.3. Response
Response was defined as complete (CR) with the following peripheral blood counts: Hb >120 gr/L, Platelets >100 × 109/L, Neutrophils >1.5 × 109/L. Partial response (PR) was defined as being transfusion independent, and not fulfilling the criteria for severe aplastic anemia. No response (NR) was defined as requiring transfusion support. Patients with response at fixed time points were considered responders at that time point. If data at specific time points were not available, the day of last transfusion after treatment, was used as cut off for response evaluation. These patients were classified as PR .
2.4. Comparison with retrospective data
To compare the outcome of patients in this study with patients reported in the prospective EBMT study comparing Thymoglobulin and lymphoglobulin,4 we selected only adults from the total number of patients: there were 558 adults aged 18–84 years, with a proportion of very severe, severe and non severe AA of 28%, 45%, 27%.
2.5. Statistical analysis
The Mann‐Whitney U test was used to compare continuous variables and Pearson χ2 test was used for categorical variables.
Univariate and multivariate survival analyzes were performed using the Cox proportional hazard model. All significant variables, alfa level two sided 5%, among those assessed in univariate analysis, were considered for the multivariate model. When calculating the cumulative incidence (CI) of response, the competing risk was death or a transplant. The logrank test was used for univariate comparison of survival curves, while the Gray test was used for univariate comparison of cumulative incidences. Transplant free survival was calculated with transplantation or death as an event.
3. RESULTS
3.1. Early mortality
The number of patients who died within 90 days from treatment was 39 (4%), whereas 167 died beyond day +90 (17%). Causes of death were hemorrhage (n = 7), unspecified infections (n = 28) and fungal infections (n = 4). Early mortality <90 days, was age dependent: 1,9%, 2,5%, 4,8% and 16,3% for age groups 0–20, 21–40, 41–60, >60 years (P < .0001). When stratified for treatment era, early mortality was 6% in 2001–2008, compared to 2% in 2009–2012 (P < .001). Early mortality was also reduced in each age group, 0–20, 21–40, 41–60, >60 years as follows: 3.07% to 0.8%, 3.85% to 0.9%, 5.0% to 4.7%, 22% to 9%, respectively. Early mortality correlated with severity of the disease, and was higher in patients with very severe AA (7%) as compared to patients with severe or non severe AA (3%) (P = .006).
3.2. Late mortality
Fiftyeight patients died between 91 ‐ 365 days, and 109 patients died beyond one year. In the first year mortality rates were similar between nontransplant and transplanted patients (6,9% vs 4,4%, P = .1). Between 1 and 2 years mortality was 3.4% vs 10.8%, respectively for nontransplanted and transplanted patients (P < .01). Between 2 and 5 years, mortality was 3,9% vs 9,1% (P = .004); beyond 5 years from ATG, mortality was 1,7% for not transplanted vs 2.8% for transplanted patients (P = .1).
3.3. Response to treatment
Eight hundred patients were evaluable for response . The cumulative incidence of response (CI), which included complete and partial responses, at 90 days was 37% (95% CI 33–40%); at 180 days it was 52% (95% CI 48–55%) and at 365 days it was 65% (95% CI 61–68%).The CI of the competing event (death) in the 3 time periods was respectively 5% (95% CI 3–6%), 11% (95% CI 9–13%) and 17% (95% CI 14–20%). The probability of response at 6 months was 52% in the 2 time periods (≤2008>). Of 464 nonresponders on day +90, 27% were classified as responders on day +180, and 46% at day +365.
The strongest predictor of response at 6 months was the interval diagnosis‐treatment, 0–30 or >30 days from diagnosis: the response was respectively 62% and 54% (Greys test 0.05). The second prognostic factor was the age group (0–20, 21–40, 41–60, >60 years) with response at 6 months of 55%, 52%, 47% and 38% (P = .06 Greys test). Severity of the disease had an impact on response but not significant (P = .1). The dose of ATG was stratified in 3 groups <=2.5 mg/kgx5 (n = 253), 2.5‐3.5 mg/kg × 5 (n = 278) and =>3.75 mg/kgx5 (n = 424), 3.75 mg/kg being the canonical Thymoglobulin dose: the response rate at 6 months in patients <20 years of age, was 56%, 60%, 67% (P = .1) for the 3 doses; in adults over 20 years of age the response at 6 months was 58%, 42%, 69% respectively (P < .001).
3.4. Relapse
Relapse was reported in 16% of responding patients: their survival at 10 years is 57%; it was 59% for patients receiving a second course of ATG and 69% for patients receiving a transplant (P = .5)
3.5. Second line treatment
Three hundred and sixtythree patients received second line treatment, including patients not responding and patients relapsing after first line therapy. ATG was given in 133 and a transplant in 230 patients: the median age was significantly higher in patients given a second course of ATG (36 years; range 0.6–81) compared to transplant patients (21 years; range 1–68) (P < .0001). The severity of the disease was also different in the second ATG group, compared to the transplant patients, as follows: very severe AA,18% vs 36%, severe AA 41% vs 44% and non severe AA, 40% vs 19% respectively (P = .0001). The 10 year actuarial survival was 64% for ATG and 70% for hemopoietic stem cell transplatation (HSCT) (P = .3). In a multivariate analysis, only for patients receiving a second treatment, results were as follows: (a) survival of severe (P = .7) and non severe AA (P = .7) was comparable to very severe patients; (b) the year of treatment (<=/>2008) was not predictive (P = .9); (c) type of second line treatment (ATG or HSCT) was not predictive (RR = 1.16, P = .6), and (d) only patients over the age of 60 had an increased risk of death (RR 2.8, P = .002).
3.6. Univariate analysis on survival
With a median follow up of 1416 days (range 1–5420), the overall survival at 10 years, for all 955 patients, is 70% (Figure 1), and 76% when patients were censored as surviving at the time of transplant. The following variables were strong pretreatment predictors of survival in univariate analysis: 1 patients age, with a 10‐year survival of 80%, 70%, 49%, 38% of patients in the age group 0–20, 21–40, 41–60, and over 60 years (P < .0001) (Figure 2A); 2 the interval diagnosis treatment, with survival ranging from 75% for patients treated within 1 month from diagnosis, 62% for interval 1–4 months, to 51% for treatment beyond 120 days from diagnosis (P = .001) (Figure 2B); 3 the severity of the disease was also predictive, with a 10‐year survival of 66%, 73%, 70% for neutrophil counts of <0.2, 0.2‐0.5 and >0.5 × 109/L (P = .006). Response to treatment was the fourth predictor in univariate analysis: 10‐year survival was 96% for patients classified as complete remissions (CR) at 6 months, 82% for partial remissions (PR) and 61% for nonresponders at 6 months (P < .0001) (Figure 2C). Because of the high survival of 376 nonresponders at 6 months, we further investigated the role of allogeneic HSCT: survival was 64% for 110 patients allografted and 60% for patients receiving a second course of ATG or supportive care untill response (n = 266) (P = .1) (Figure 2D).
Figure 1.
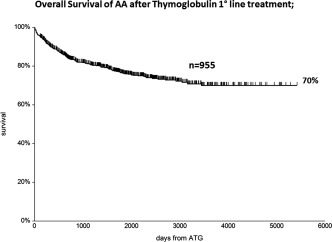
Ten year overall survival of 955 patients with aplastic anemia (AA) treated with Thymoglobulin and cyclosporine first line
Figure 2.
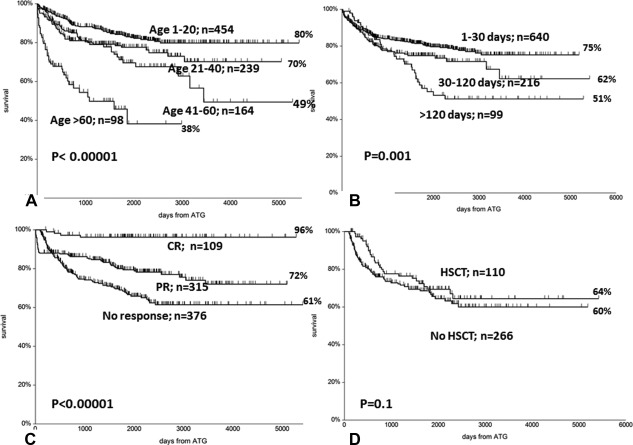
(A) Survival of patients stratified according to age: a significant negative effect is seen for patients above the age of 40 and 60 years. (B) Survival of patients stratified according to the interval between diagnosis and treatment with ATG (in days): a significant advantage for patients treated early. (C) Survival of patients stratified according to response to treatment at 6 months: CR = complete remisson; PR= partial remission; and no response. (D) No difference in survival for patients identified as nonresponders at 6 months, according to whether they were transplanted (HSCT) or not (no HSCT). Transplantation occurred at a median interval of 245 days (range 120‐ 2034) from Thymoglobulin treatment
3.7. Multivariate analysis on survival
In multivariate analysis, factors predicting overall survival were as follows (Table 2): severity of the disease, with a death risk ratio (RR) of 0.48, for patients with a neutrophil count over 0.5 × 109/L, compared to patients with a neutrophil count of <0.2 × 109/L; older age, with a death RR of 5.08 for patients over the age of 60, compared to patients <20 years old; an interval between diagnosis and treatment, with a death RR of 1.62, for patients treated beyond 120 days from diagnosis, as compared to patients treated within one month. There was a trend for better survival in females, than males (P = .07). The dose of ATG used, treatment of patients in Europe orAsia, and the year of treatment, were not significant predictors of survival, although the death risk ratio was 0.77 for the most recent period of treatment (Table 2).
Table 2.
Multivariate Cox analysis on survival
| Baseline | Compared value | RR | 95%CI | P |
|---|---|---|---|---|
| Severity <200 PMN | 200–500 PMN | 0.58 | 0.41‐0.82 | .001 |
| >500 PMN | 0.47 | 0.32‐0.71 | .0003 | |
| Age group <20 years | 20–40 years | 1.43 | 0.97‐2.12 | .06 |
| 40–60 years | 2.01 | 1.31‐3.08 | .001 | |
| >60 years | 5.08 | 3.22‐8.02 | <.00001 | |
| Dose <2.5 mg/kg | 2.5‐3.5 | 1.33 | 0.87‐2.03 | .17 |
| >3.5 | 1.04 | 0.65‐1.67 | .85 | |
| Int Dx‐ ATG <30 days | 30–120 days | 1.57 | 1.10‐2.25 | .01 |
| >120 days | 1.66 | 1.08‐2.55 | .02 | |
| EBMT/ASIA EBMT | ASIA | 0.98 | 0.64‐1.50 | .9 |
| Year of ATG 2001–2008 | >2008 | 0.77 | 0.56‐1.07 | .12 |
| Gender Male | female | 0.76 | 0.57‐1.03 | .07 |
Abreviations: As in Table 1; PMN = neutrophils; Dose: indicates mg/kg/day and should be multiplied ×5, for the total dose in mg/kg.
3.8. Causes of death
The major cause of death was infections, overall 12%, followed by hemorrhages (2.6%). Transplant related causes of death included GvHD and graft rejection, accounting for 10% of transplant patients. In 12 cases the cause of death was acute myeloid leukemia (1.3%).
4. DISCUSSION
This is a large retrospective study on patients with acquired AA treated first line with Thymoglobuine and CsA, addressing 4 major issues: early mortality, response rates, second line treatment, and survival.
Early mortality was studied because of previous reports, suggesting a higher mortality in patients receiving Thymoglobulin, as compard to patients given horse ATG,3, 4, 13 possibly becaue of greater immunosuppressive activity of Thymoglobulin, leading to increased risk of infectious complications.3
In the present study, the overall early mortality was 6% for patients treated 2001–2008, and 2% in the period 2009–2012. This is true for all age groups, with current mortality within 90 days, ranging from 1.6%, for patients aged 1–59 years, to 9% for patients over 60: the reduction in the latter group is particularly significant (22% to 9%,) . In other words the current risk of early death, within the first 3 months, for patients treated first line with Thymoglobulin and CsA, is less than 2%, for younger patients (≤60 years), and 9% above 60 years of age. The use of a lower dose of Thymoglobulin, was not associated with reduced toxicity. There were only 30 patients above the age of 70: their overall early mortality is 33%, suggesting caution in the use of Thymoglobulin and CsA, in this elderly population. Causes of early death were mainly infectious: we did not collect data on the use of G‐CSF, which has been reported to reduce infectious complications after treatment with horse ATG,14 and we can not therefore comment on its use after first line rabbit ATG. However, it should be pointed out that mortality was not reduced in the EBMT randomized study.14
Response was recorded in 800 patients: response at 6 months was 52% which compares favorably with the 40% response in the EBMT study on 35 patients4 and 37% response in the NIH study on 60 patients.3 The major predictor of response was the interval between diagnosis and treatment, best response being achieved in patients treated within 1 month from diagnosis. The dose of Thymoglobulin seems to have an effect, with best responses seen in both children and adults, receiving the classic dose of 3.75 mg/kg/day × 5 days, but a randomized study comparing 2.5 mg/kg/day vs 3.75 mg/kg/day is ongoing in Asia, and will answer this question. Response at 6 months was, as expected, a strong predictor of survival, with 10‐year survival of 96% for complete responders, 72% for partial responders and 61% for nonresponders. We were particularly surprised with the 10‐year survival of 61% for patients classified as nonresponders at 6 months, and we hypothesized that transplantation may have been the main salvage therapy. We thus compared 10‐year survival of nonresponders at 6 months, receiving an allogeneic HSCT (n = 110), with survival of patients not allografted (n = 266), and found it was comparable: 64% for HSCT and 60% for patients receiving a second immunosuppressive treatment, or supportive care untill response. Because response was available in only 800 patients, and we had studied the effect of transplantation in patients classified as nonresponders at 6 months, we also compared HSCT (n = 207) and a second course of ATG (n = 122), in the entire patient population: patients allocated to HSCT were significantly younger and had significantly more cases with a neutrophil count 0.2 × 109/L. It would have been inappropriate to compare ATG and HSCT in such different patient populations. We therefore ran a multivariate Cox analysis, including patients age, severity of the disease, type of treatment, and year of treatment: the only significant predictor was the patients age above 60 years, whereas the risk ratio of mortality for allografted patients was 1.16 as compared to ATG (P = .6); also severity of the disease, as identified by neutrophils counts, was not predictive of survival. This study suggests that responses can occur late, possibly beyond 365 days, which was the last time point of assessment, in agreement with data from the Spanish study.7
Survival was the primary end point of this study, since we were concerned with results of two prospective studies showing rather poor two year survival of patients receiving Thymoglobulin, in the order of 60%, significantly less when compared to horse ATG, in the order of 80%.3, 4 However, some retrospective studies had shown more promising results in first and second line treatment with rabbit ATG.7, 8, 9, 10, 15 In the present study on 955 patients, the overall survival at 10 years was 70%, 76% after censoring patients as surviving at the time of transplant, and transplant free survival was 64%. When looking at predictors, we found 3 major significant variables: patients age, interval between diagnosis and treatment, and severity of the disease. These variable have already been shown to predict outcome in patients receiving ATG.16, 17 We could not show an effect on survival of different schedules of Thymoglobulin, ranging from 2.5 mg/kg/day ×5 to 3.75 mg/kg/day ×5, although the latter regimen had a greater proportion of responders both in patients under the age of 20 years, as well as in older patients.
Finally, we compared the adult population from this data base (n = 558), with 35 patients of the prospective EBMT study, receiving Thymoglobulin first line: the two groups were comparable for age (median 37) and severity of the disease, with an excess of very severe AA in the current larger study. The cumulative incidence of response at 6 months, was 40% in the EBMT study4 and 58% in the adults of our study. Survival at 2 years was 68% in the EBMT study and 81% in the current study. It is difficult to explain why results should be different: the number of patients could be one issue, since the EBMT study was based only on 35 patients receiving rabbit ATG. Also a recent meta‐analysis on 1636 patients, shows no difference in survival between patients receiving horse or rabbit ATG.11
In conclusion, this study suggests that first line treatment of AA with Thymoglobulin is currently associated with a low early mortality within 90 days: for patients above 60 years, and especially above 70 years of age, early infectious complications suggest caution. Response rates at 6 months seem higher, compared to what as been reported in two prospective trials, but still somewhat lower than responses at 6 months with horse ATG: it may be that responses after rabbit ATG are delayed. This is confirmed by the fact that survival of nonresponders at 6 months, is comparable in patients receiving or not an allogeneic transplant. In consideration of the exciting results reported with the triple combination of horse ATG, CSA and eltrombopag,18 it will be interesting to test the same combination also with rabbit ATG.
AUTHOR CONTRIBUTIONS
AB, CV designed the study, analyzed the data, and wrote the manuscript. RO created the data base and analyzed the data. AB, HS, BH, CD, SK, XZ, XC, SI, SC, DCJ, SG, MTVL, YZ, and CV contributed patients and revised the manuscript.
AKNOWLEDGMENTS
This study was supported by an unrestricted grant of SANOFI, France.
Bacigalupo A, Oneto R, Schrezenmeier H, et al. First line treatment of aplastic anemia with thymoglobuline in Europe and Asia: Outcome of 955 patients treated 2001‐2012. Am J Hematol. 2018;93:643–648. 10.1002/ajh.25081
REFERENCES
- 1. Aljurf M, Al‐Zahrani H, Van Lint MT, et al. Standard treatment of acquired SAA in adult patients 18–40 years old with an HLA‐identical sibling donor. Bone Marrow Transplant. 2013;48(2):178–179. [DOI] [PubMed] [Google Scholar]
- 2. Killick SB, Bown N, Cavenagh J, et al. British Society for Standards in Haematology. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187–207. [DOI] [PubMed] [Google Scholar]
- 3. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit ATG in acquired aplastic anmia. N Engl J Med. 2011;365(5):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marsh JC, Bacigalupo A, Schrezenmeier H, et al. European blood and marrow transplant group severe aplastic anaemia Working Party. Prospective study of rabbit antithymocyte globulin and cyclosporine for aplastic anemia from the EBMT Severe Aplastic Anaemia Working Party. Blood. 2012;119(23):5391–5396. [DOI] [PubMed] [Google Scholar]
- 5. Jeong DC, Chung NG, Cho B, et al. Long‐term outcome after immunosuppressive therapy with horse or rabbit antithymocyte globulin and cyclosporine for severe aplastic anemia in children. Haematologica. 2014;99(4):664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deyell RJ, Shereck EB, Milner RA, Schultz KR. Immunosuppressive therapy without hematopoietic growth factor exposure in pediatric acquired aplastic anemia. Pediatr Hematol Oncol. 2011;28(6):469–478. [DOI] [PubMed] [Google Scholar]
- 7. Vallejo C, Montesinos P, Polo M, et al. Bone marrow failure Spanish study group (Pethema‐GETH). Rabbit antithymocyte globulin versus horse antithymocyte globulin for treatment of acquired aplastic anemia: a retrospective analysis. Ann Hematol. 2015;94(6):947–954. [DOI] [PubMed] [Google Scholar]
- 8. Chen C, Xue HM, Xu HG, et al. Rabbit‐antithymocyte globulin combined with cyclosporine A as a first‐line therapy: improved, effective, and safe for children with acquired severe aplastic anemia. J Cancer Res Clin Oncol. 2012;138(7):1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki T, Kobayashi H, Kawasaki Y, et al. Efficacy of combination therapy with anti‐thymocyte globulin and cyclosporine A as a first‐line treatment in adult patients with aplastic anemia: a comparison of rabbit and horse formulations. Int J Hematol. 2016;104(4):446–453. [DOI] [PubMed] [Google Scholar]
- 10. Chuncharunee S, Wong R, Rojnuckarin P, et al. Efficacy of rabbit antithymocyte globulin as first‐line treatment of severe aplastic anemia: an Asian multicenter retrospective study. Int J Hematol. 2016;104(4):454–461. [DOI] [PubMed] [Google Scholar]
- 11. Yang N, Chen J, Zhang H, et al. Horse versus rabbit antithymocyte globulin in immunosuppressive therapy of treatment‐naïve aplastic anemia: a systematic review and meta‐analysis. Ann Hematol. 2017;96(12):2031–2043. [DOI] [PubMed] [Google Scholar]
- 12. Hayakawa J, Kanda J, Akahoshi Y, et al. Meta‐analysis of treatment with rabbit and horse antithymocyte globulin for aplastic anemia. Int J Hematol. 2017;105(5):578–586. [DOI] [PubMed] [Google Scholar]
- 13. Atta EH, Lima CBL, Dias DSP, et al. Predictors of early mortality after rabbit antithymocyte globulin as first‐line treatment in severe aplastic anemia. Ann Hematol. 2017;96(11):1907–1914. [DOI] [PubMed] [Google Scholar]
- 14. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in newly‐diagnosed severe aplastic anemia patients receiving antithymocyte globulin (ATG), cyclosporine, with or without G‐CSF: a study of the SAA Working Party of the EBMT. Blood. 2011;117(17):4434–4441. [DOI] [PubMed] [Google Scholar]
- 15. Di Bona E, Rodeghiero F, Bruno B, et al. Rabbit antithymocyte globulin (r‐ATG) plus cyclosporine and granulocyte colony stimulating factor is an effective treatment for aplastic anaemia patients unresponsive to a first course of intensive immunosuppressive therapy. Br J Haematol. 1999;107(2):330–334. [DOI] [PubMed] [Google Scholar]
- 16. Scheinberg P, Young NS. How I treat aplastic anemia. Blood. 2012;120:1185–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Locasciulli A, Oneto R, Bacigalupo A. A, et al. on the behalf of the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (SAA‐WP, BMT). Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2007;92(1):11–8. [DOI] [PubMed] [Google Scholar]
- 18. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376(16):1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]


