Abstract
The antipsychotic drug pimozide has been found to exhibit anticancer effects. Previously, it was demonstrated that pimozide inhibits hepatocellular carcinoma (HCC) cell growth, but its pharmacodynamic characteristics remain unclear. The aim of the present study was to investigate the reversibility and mechanism of the ability of pimozide to inhibit cell proliferation in liver cancer. Cell viability was determined by Cell Counting Kit-8 and colony formation assay. The cell cycle distribution was analyzed by flow cytometry with Ki-67 and PI staining. ROS production of HCC cells was detected with DCFH-DA and inhibited with NAC treatment. Western blot assay was performed to detect the expression of related signaling molecules in HCC cells. Our results showed that pimozide promoted G0/G1 phase arrest in HCC cell lines without significant cell death. Its anti-proliferative effects on HCC cells were reversible, consistent with involvement of cell quiescence and reactive oxygen species (ROS) production. Pimozide enhanced inhibition of HCC cell proliferation by sorafenib. In conclusion, elucidation of pimozide's reversible proliferation inhibition in liver cancer and additive activity with a well-established anticancer drug warrants further exploration of the potential of pimozide as an adjuvant anticancer therapy.
Keywords: pimozide, hepatocellular carcinoma, quiescence, reversible proliferation inhibition, drug repurposing
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent and lethal malignancies in the world (1), with a 5-year survival rate of only 30–40% (2). HCC has a poor prognosis due to chemotherapy resistance and a high recurrence rate (3). Given the unsatisfactory state of current liver cancer treatment effectiveness, there is a great need for the development of new strategies for targeting HCC (4).
Drug repurposing is an important approach in cancer drug discovery and development (5,6). Notably, we previously found that pimozide, an FDA-approved compound used to treat chronic psychosis, exhibits anticancer effects on various types of carcinomas, including HCC (7), prostate cancer (8) and osteosarcoma (9). Pimozide has been reported to inhibit cell proliferation, colony formation, and sphere formation of these three cancer types (7–9). Pimozide also reduced migration of prostate and liver cancer cells (7). Additionally, pimozide increased the sensitivity of breast cancer cells to γ-irradiation treatment (10) and enhanced the anticancer effect of L-methyl-tryptophan (an indoleamine 2, 3-dioxygenase inhibitor) on melanoma cells (11). Furthermore, previously, we found that pimozide inhibited cancer cell growth through suppression of signal transducer and activator of transcription 3 (STAT3) activation (7–9) or Wnt/β-catenin signaling (12,13). In addition, pimozide has been shown to inhibit maintenance and tumorigenicity of HCC stem-like cells (14) and to induce reactive oxygen species (ROS) generation, which can impede cell proliferation (9).
In light of the aforementioned findings, the potential clinical anticancer efficacy of pimozide has piqued our interest. The toxicology profile of pimozide in human patients is well established owing to its long-term use in psychiatry. In addition, molecular targets involved in the suppression of cancer cell growth by pimozide have been identified. However, various factors affecting the drug's pharmacodynamic effects remain to be clarified, including whether the suppression of HCC cell proliferation by pimozide is reversible. Irreversible mechanisms include cell death, cell senescence and terminal differentiation (15–17), whereas reversible mechanisms include principally cell cycle arrest and cellular quiescence (18). The potential anticancer effect reversibility of pimozide would have direct effects on the dosage and administration cycle of the drug. Elucidation of these dynamics can accelerate the clinical translation of pimozide for oncological applications.
The aim of the present study was to investigate the type of proliferative inhibition produced by pimozide on liver cancer cells. We used cell proliferation assays with pan-caspase and RIP (receptor-interacting protein) kinase inhibitors to identify whether the effects of pimozide are associated with apoptosis or necrosis. In addition, we analyzed the effects of pimozide on the cell cycle. Importantly, we assessed the reversibility of the anti-proliferative effects of pimozide on liver cancer cells, the potential associations of the efficacy of pimozide with quiescence and ROS production, and the interaction of pimozide with sorafenib which is a tyrosine kinase inhibitor used to treat cancers affecting various organs.
Materials and methods
Cell lines and culture
The human liver cancer cell lines Sk-Hep1, Huh7 and HepG2 (provided by the Guangdong Provincial Key Laboratory of Liver Disease Research, Guangzhou, China) were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA), penicillin (100 U/ml) and streptomycin (100 µg/ml). The cell lines were maintained in 5% CO2 at 37°C. Huh7 and Sk-Hep1 cell lines were authenticated by short tandem repeat profiling analysis conducted by Procell Life Science & Technology Co., Ltd. (Wuhan, China).
Cell viability assay
Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8) assays (MedChem Express, Monmouth Junction, NJ, USA). Liver cancer cells were plated in 96-well plates (10,000 cells/well) and exposed to different concentrations of pimozide (5 and 10 µM) (MedChem Express) for 48 h. Kit-provided solution was added to each well and incubated at 37°C for 4 h. Absorbance at 450 nm was detected by a multi-well plate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). In addition, Sk-Hep1 and Huh7 cells were treated with 10 µM pimozide alone or with combination plus pan-caspase inhibitor Z-VAD-FMK (10 µM) or RIP kinase inhibitor necrostain 1 (10 µM) or 2 mM NAC (MedChem Express) for 48 h before being subjected to cell viability assay. In addition, Sk-Hep1 and Huh7 cells were treated with the 5 µM pimozide alone, sorafenib (MedChem Express) alone or combination for 48 h and then were subjected to cell viability assay.
Colony formation assay
The colony formation assay was performed as described previously (19). Liver cancer cells (500 cells/well) were plated in 10% FBS medium and treated with different concentrations of pimozide (5 and 10 µM). After incubation for 2 weeks, the cells were fixed with methanol and stained with crystal violet. Colony morphology was imaged under a stereomicroscope (magnification ×100). The colonies, defined as a cluster of at least 50 cells, were counted.
Cell cycle distribution assay
Cell cycle distribution was determined by propidium iodide (PI, Sigma-Aldrich; Merck KGaA) staining. Equal cell aliquots were seeded in 6-well plates and treated with pimozide for 48 h. The cells were harvested, washed with phosphate-buffered saline containing 0.1% bovine serum albumin, and vortexed with cold absolute ethanol. After addition of PI buffer (40 µg/ml, containing 100 µg/ml RNase), cells were analyzed by flow cytometry (CytoFLEX, Beckman Coulter, Inc.).
For quiescent cell detection, cells were washed, fixed with 0.5 ml of 4% paraformaldehyde in phosphate-buffered saline, and then incubated with an equal volume of 0.2% Triton-X. The fixed and permeabilized cells were washed, resuspended in staining buffer, and stained with Ki-67-FITC (BD Pharmigen). The stained cells were washed and resuspended in propidium iodide (PI) staining buffer. Cells in G0 phase were analyzed by flow cytometry (CytoFLEX, Beckman Coulter, Inc.) based on Ki-67 negativity and PI incorporation.
Annexin V/PI staining apoptosis assay
Apoptotic cells were detected with an Annexin V-FITC/PI Apoptosis Detection kit (Absin Bioscience, Shanghai, China) according to the manufacturer's instructions. Briefly, cells were treated with 10 µM pimozide for 48 h, submitted to Annexin V-FITC/PI staining, and then analyzed by flow cytometry (CytoFLEX, Beckman Coulter, Inc.).
ROS assay and ROS inhibition
ROS products were detected with 2,7-dichlorofluorescein diacetate (DCFH-DA), a fluorescent dye (Beyotime, Jiangsu, China). Briefly, after 10 µM pimozide treatment for 48 h, cells were washed and incubated with DCFH-DA (10 µM) for 30 min at 37°C in the dark. Then, cells were washed twice with DMEM and analyzed by flow cytometry at an excitation/emission wavelength of 488/525 nm. ROS production was inhibited with N-acetyl-l-cysteine (NAC).
Western blotting
Equal amounts of protein (20 µg/well) collected from cells with RIPA lysis buffer were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Merck Millipore, Billerica MA, USA). Blots were detected using primary antibodies against cell division protein kinase 6 (CDK6) (cat. no. ab124821; dilution 1:1,000), p-Rb (cat. no. ab173289; dilution 1:1,000) (Abcam, Cambridge, MA, USA), p27 (#3686; dilution 1:1,000), cyclin D1 (#2978; dilution 1:1,000), STAT3 (#12640; dilution 1:1,000), phosphorylated (p)-STAT3 (at tyrosine 705) (#9145; dilution 1:1,000), ERK1/2 (extracellular signal-regulated kinase 1 and 2) (#4695; dilution 1:1,000), phosphorylated (p)-ERK1/2 (#4376; dilution 1:1,000) (Cell Signaling Technology, Beverly, MA, USA) and β-actin (cat. no. sc-47778; dilution 1:1,000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Antibody binding was detected with an enhanced chemiluminescence kit (Immobilon Western Chemilum HRP Substrate, WBKLS0500, Merck Millipore).
Statistical analysis
The data were analyzed in GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA) and are presented as means with standard deviations (SDs). Student's t tests or analysis of one-way variance (ANOVA) followed by Dunnett's post hoc test for multiple comparisons were applied to assess the statistical differences between groups. P-values <0.05 were considered significant.
Results
The anti-proliferative effect of pimozide on liver cancer cells is independent of drug-induced cell death
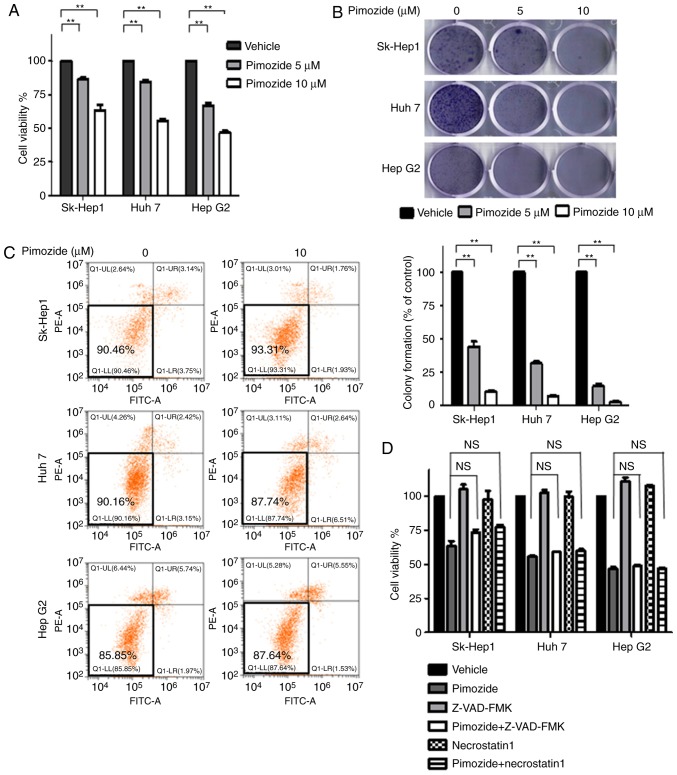
Proliferative assays showed that pimozide inhibited the growth of Sk-Hep1, Huh7 and HepG2 liver cancer cells in a dose-dependent manner (Fig. 1A). Colony formation assays showed similar inhibition of proliferation across the three cell lines (Fig. 1B). Annexin-V-FITC/PI double staining cell apoptosis assays showed that 10 µM pimozide did not induce obvious apoptosis in the liver cancer cells (Fig. 1C). Cell proliferation assays further showed that pan-caspase and RIP kinase inhibitors did not attenuate pimozide inhibition of cell growth in liver cancer cells (Fig. 1D), indicating that the suppression of proliferation was not associated with apoptosis or necrosis.
Figure 1.
The anti-proliferative effect of pimozide on liver cancer cells is not associated with cell death. Sk-Hep1, Huh7 and HepG2 cells were incubated with 5 and 10 µM pimozide for 48 h for cell viability (A) and colony formation (B) assays. (C) Apoptotic cells detected by Annexin V/PI staining. (D) Treatment of liver cancer cells with 10 µM pimozide alone or in combination with the pan-caspase inhibitor Z-VAD-FMK (10 µM) or the RIP kinase inhibitor necrostain 1 (10 µM) for 48 h before being subjected to cell viability assays. Representative images from one of three independent experiments are shown. Mean values are shown with SDs of three independent experiments; **P<0.01, compared to the control. NS, not significant.
Pimozide increases cell cycle arrest in HCC cells
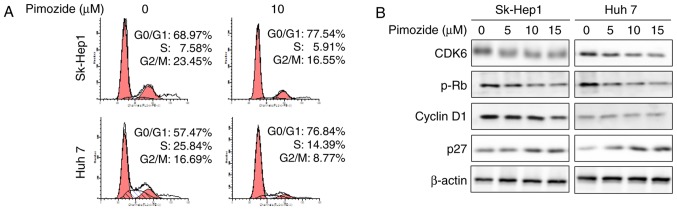
As shown in Fig. 2A, PI staining revealed that 14 h of 10 µΜ pimozide treatment significantly increased the number of cells in the G0/G1 phase in the Sk-Hep1 (68.97 to 77.54%; P<0.01) and Huh7 cells (57.47 to 76.84%). Western blots showed reduced expression of the cell cycle markers CDK6, p-Rb and cyclin D1 together with markedly increased p27 levels (Fig. 2B). These results indicated that the anti-proliferative effect of pimozide is associated with cell cycle arrest.
Figure 2.
Pimozide induces G0/G1 phase cell cycle arrest in HCC cells. (A) Cell cycle phase distribution of PI-stained Sk-Hep1 and Huh7 cells following treatment with 10 µM pimozide was determined by flow cytometry. Representative images from one of three independent experiments are shown. (B) Western blot analysis of cell cycle-related gene expression following 0, 5, 10 and 15 µM pimozide. Cell extracts were probed with antibodies against CDK6, p-Rb, cyclin D1 and p27 proteins. β-actin was used as a loading control. HCC, hepatocellular carcinoma; CDK6, cyclin dependent kinase 6.
Reversibility of proliferative suppression by pimozide due to quiescence
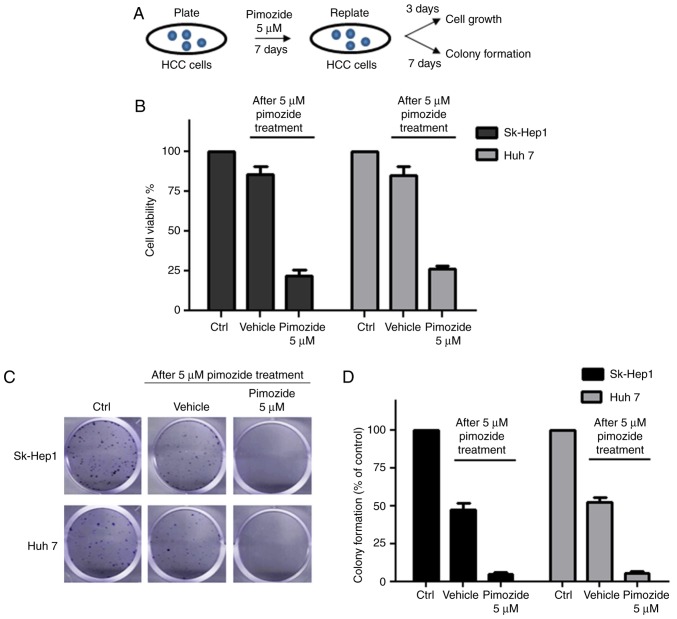
After treatment with 5 µM pimozide, HCC cells were re-plated in preparation for cell proliferative and colony formation assays, as illustrated in Fig. 3A. Increased cell counts and colony formation were observed in the pimozide-treated Sk-Hep1 and Huh7 cells following pimozide withdrawal, demonstrating restoration of proliferation and colony forming abilities (Fig. 3B and C). Following treatment with 5 µΜ pimozide for 1 week, Sk-Hep1 cells showed a decrease of 96.6±1.1% in colony number (Fig. 3D), while Sk-Hep1 cells without pimozide treatment displayed significant ability of colony formation (48.6±2.3%). Similar results were achieved in the Huh7 cells.
Figure 3.
Pimozide induces reversible proliferative suppression in HCC cells. (A) Experimental design diagram: 5 µM pimozide, Sk-Hep1 and Huh7 cells were re-plated for cell proliferative and colony formation assays. Analyses of cell proliferative (B) and colony formation (C) assays of Sk-Hep1 and Huh7 cells after pimozide treatment. (D) For the colony formation assay, the numbers of crystal violet-stained colonies were counted. The results were from 3 independent experiments. HCC, hepatocellular carcinoma.
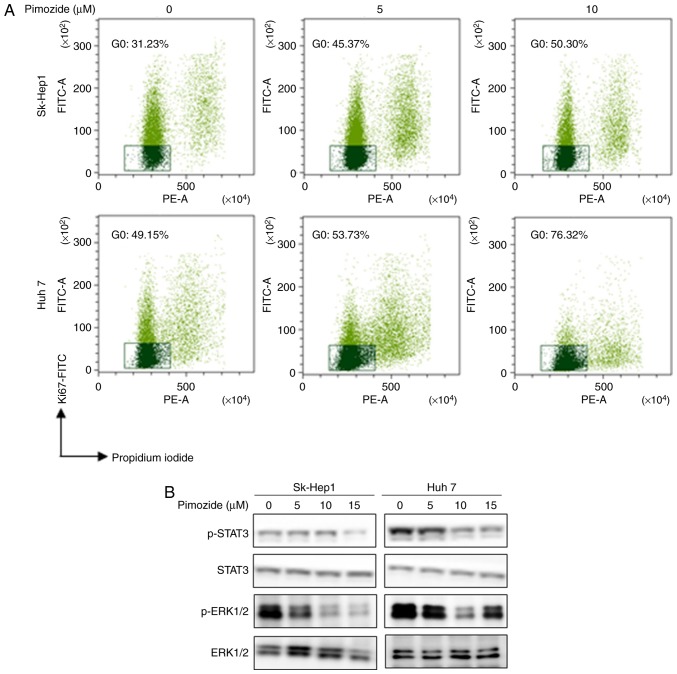
Ki-67 labelling, PI staining, and flow cytometry showed that pimozide increased the portion of cells in the quiescent G0 phase in a dose-dependent manner (Fig. 4A). After 10 µM pimozide treatment, Sk-Hep1 (31.23 to 50.30%) and Huh7 (49.15 to 76.32%) cells had significantly increased percentages of cells in the G0 phase. Western blot analysis showed that pimozide reduced p-STAT3 and p-ERK1/2 levels (Fig. 4B), indicating that pimozide induced HCC cell quiescence.
Figure 4.
Pimozide induces cellular quiescence in HCC cells. (A) Sk-Hep1 and Huh7 cells were incubated with 0, 5 and 10 µM pimozide for 48 h and subjected to Ki-67-FITC/PI staining to detect quiescent (G0 phase) cells. Representative flow cytometry data illustrating the Ki67−/PI− population gates for the G0 phase are shown with representative images from one of three independent experiments. (B) Western blot analysis of p-STAT3, STAT3, p-ERK1/2 and ERK1/2 in HCC cells after 0, 5, 10 and 15 µM pimozide treatment for 48 h. HCC, hepatocellular carcinoma.
Pimozide induces ROS generation in HCC cells
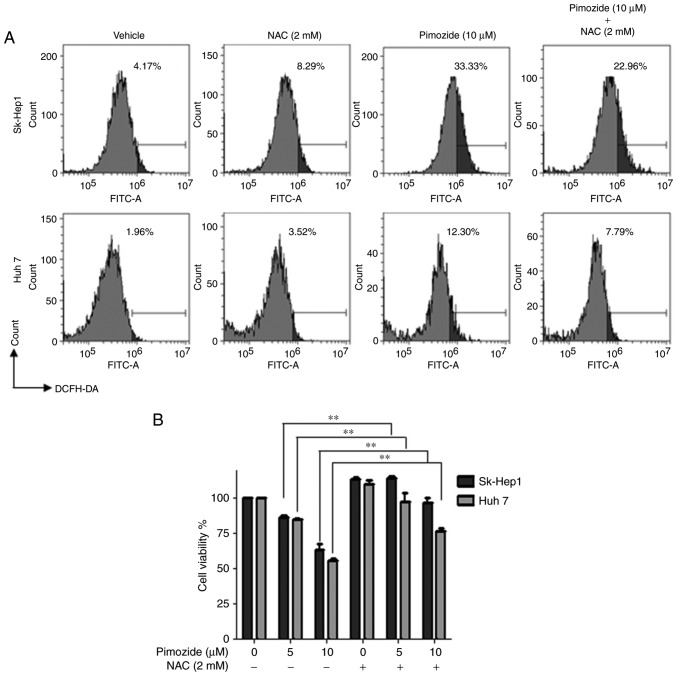
Flow cytometry after pimozide exposure (10 µΜ for 48 h) demonstrated increased ROS levels in the Sk-Hep1 and Huh7 cells and this effect was attenuated by the ROS scavenger NAC (Fig. 5A). NAC also reversed the pimozide-mediated inhibition of Sk-Hep1 and Huh7 cell proliferation (Fig. 5B).
Figure 5.
Pimozide treatment increases ROS generation in HCC cells. (A) Sk-Hep1 and Huh7 cells were treated with 10 µM pimozide, 2 mM NAC, or both for 48 h, and then stained with DCFH-DA. (A) Analysis of ROS production intensity among assayed cell populations. Representative images from one of three independent experiments are shown. (B) CCK-8 cell viability assay. Mean values from three independent experiments are shown with SDs, **P<0.01, compared to the control. ROS, reactive oxygen species; HCC, hepatocellular carcinoma; NAC, N-acetyl-l-cysteine.
Pimozide enhances the proliferative inhibition induced by tyrosine kinase inhibitor in HCC cells
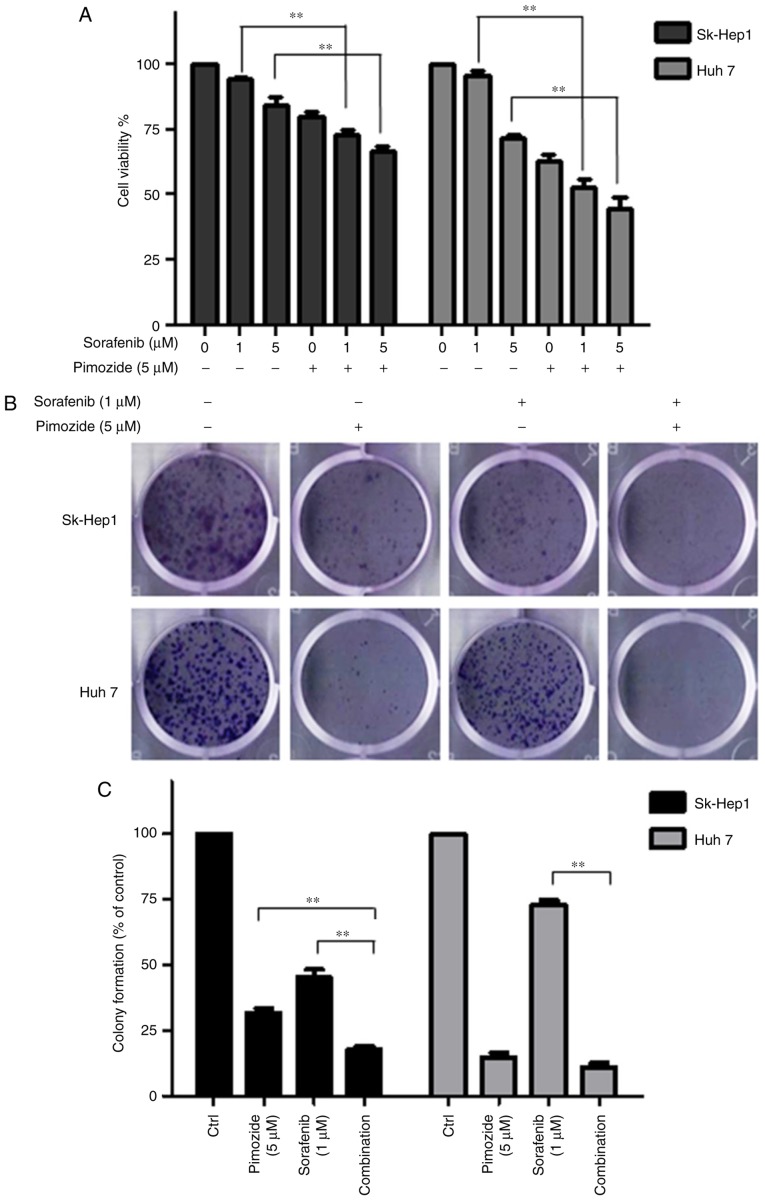
Cell viability (Fig. 6A) and colony formation assays (Fig. 6B and C) showed that 5 µΜ pimozide significantly enhanced the anticancer effect of 1 µΜ sorafenib in the Sk-Hep1 and Huh7 cell lines.
Figure 6.
Pimozide enhances the proliferative inhibition of sorafenib in HCC cells. Sk-hep1 and Huh7 cells were treated with 5 µM pimozide, sorafenib, or both, and then subjected to cell viability (A) and colony formation (B) assays. Representative images from one of three independent experiments are shown. (C) Mean values from three independent experiments are shown with SDs. The data are summarized from 3 independent experiments, **P<0.01, compared to the control. HCC, hepatocellular carcinoma.
Discussion
Drug effect reversibility is an important factor in determining drug administration mode for optimizing efficacy and persistence while avoiding adverse secondary effects (20). The same drug may produce different effects on different types of tumor cells, mainly due to the particular cell type's genetic background (14). Although pimozide was found to promote the apoptosis of breast cancer (10,21,22), prostate cancer and melanoma cells (23), our data showed that pimozide did not promote apoptosis of liver cancer cells. Moreover, the inhibition of liver cancer cell proliferation by primozide was not affected by pan-caspase or RIP kinase inhibition. These results indicate that the mechanism of the anti-proliferative effects of pimozide on liver cancer cells may not involve drug-induced cell death.
The present observation of the reversible suppression of liver cancer cell proliferation was associated with pimozide-induced cellular quiescence (the resting, non-proliferative G0 phase of the cell cycle) (24,25). Quiescence has been posited to be a drug effect-escape mechanism (26). However, while in this dormant state, cancer cells are not causing harm (27). Because proliferative suppression due to cellular quiescence is transient and reversible, HCC cells will return to a proliferating state if they re-enter the cell cycle (28).
With respect to the mechanism, it was found that HCC cellular quiescence was associated with reduced phosphorylation of STAT3 and ERK1/2. The time-sensitive antitumor activity of pimozide observed suggests that long-term maintenance of pimozide would be needed to maintain its anticancer efficacy. Because pimozide is a well-known antagonist of serotonin 5-hydroxytryptamine receptor 7 (5HT7), we examined 5HT7 expression in liver cancer cell lines and found that they indeed had elevated 5HT7 expression (data not shown). Further research is needed to probe the association of 5HT7 expression with cellular quiescence in HCC cells.
Pimozide is an FDA approved drug shown to have no adverse effects on hepatic or hematopoietic cells. Pimozide is well tolerated in mice without significant effects on body weight (7,29). To date, a lethal dose for pimozide in humans has not been established. The median lethal doses for pimozide in mice and rats are 228 and 5,120 mg/kg, respectively [DrugBank no. DB01100 (30)]. The dose of pimozide used in this study is substantially lower than commonly used clinical doses. Therefore, there is good reason to be optimistic that pimozide may be a safe drug for treating cancer.
Drug repurposing requires a new drug application clinical entry point (31). For example, metformin, a biguanide-class antidiabetic medication, was repurposed as an adjuvant therapy for breast (32) and bladder cancer (33). Similarly, we have been exploring the potential of pimozide to be an adjuvant anticancer therapy. Given the mediocre clinical efficacy of sorafenib alone in patients with HCC, it is hoped that its efficacy may be enhanced by combining it with another intervention (34). Previously, carfilzomib has been shown to work synergistically with sorafenib in inhibiting the proliferation, survival and metastasis of HCC cells in vitro (35). Here, we showed that pimozide can enhance the proliferative inhibition of sorafenib in HCC cells, suggesting that it has the potential to improve HCC outcomes as an adjuvant therapy.
In conclusion, suppression of liver cancer cell proliferation by pimozide was found to involve promotion of cellular quiescence and to be reversible. Furthermore, pimozide was shown to act synergistically with sorafenib, suggesting that it should be further explored as an adjuvant therapy for use with anticancer drugs such as sorafenib in liver cancer treatment with its dosage respecting the reversibility of its effects.
Acknowledgements
We thank the other members of the laboratory of Ji Kunmei for their critical comments.
Funding
This work was supported by the National Natural Science Foundation of China (81602595 to JJC), the Natural Science Foundation of Shenzhen City (JCY20170818142053544 to JJC), the Medical Scientific Research Foundation of Guangdong Province (A2019522 to JJC) and 2016 Discipline Construction of Shenzhen City.
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
JJC and KJ conceived and designed the study. JJC and LNZ collected and analyzed the data. JJC, LNZ NC and ZZ performed the experiments. JJC and KJ were responsible for the reagents/materials/analysis tools. JJC and KJ wrote the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y) 2014;10:153–161. [PMC free article] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the united states from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoshida Y, Fuchs BC, Tanabe KK. Prevention of hepatocellular carcinoma: Potential targets, experimental models, and clinical challenges. Curr Can Drug Targets. 2012;12:1129–1159. doi: 10.2174/15680096112091129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cervello M, McCubrey JA, Cusimano A, Lampiasi N, Azzolina A, Montalto G. Targeted therapy for hepatocellular carcinoma: Novel agents on the horizon. Oncotarget. 2012;3:236–260. doi: 10.18632/oncotarget.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boguski MS, Mandl KD, Sukhatme VP. Drug discovery. Repurposing with a difference. Science. 2009;324:1394–1395. doi: 10.1126/science.1169920. [DOI] [PubMed] [Google Scholar]
- 6.Knapp S. New opportunities for kinase drug repurposing and target discovery. Br J Cancer. 2018;118:936–937. doi: 10.1038/s41416-018-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JJ, Cai N, Chen GZ, Jia CC, Qiu DB, Du C, Liu W, Yang Y, Long ZJ, Zhang Q. The neuroleptic drug pimozide inhibits stem-like cell maintenance and tumorigenicity in hepatocellular carcinoma. Oncotarget. 2017;8:17593–17609. doi: 10.18632/oncotarget.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou W, Chen MK, Yu HT, Zhong ZH, Cai N, Chen GZ, Zhang P, Chen JJ. The antipsychotic drug pimozide inhibits cell growth in prostate cancer through suppression of STAT3 activation. Int J Oncol. 2016;48:322–328. doi: 10.3892/ijo.2015.3229. [DOI] [PubMed] [Google Scholar]
- 9.Cai N, Zhou W, Ye LL, Chen J, Liang QN, Chang G, Chen JJ. The STAT3 inhibitor pimozide impedes cell proliferation and induces ROS generation in human osteosarcoma by suppressing catalase expression. Am J Transl Res. 2017;9:3853–3866. [PMC free article] [PubMed] [Google Scholar]
- 10.Strobl JS, Melkoumian Z, Peterson VA, Hylton H. The cell death response to gamma-radiation in MCF-7 cells is enhanced by a neuroleptic drug, pimozide. Breast Cancer Res Treat. 1998;51:83–95. doi: 10.1023/A:1006046604062. [DOI] [PubMed] [Google Scholar]
- 11.Jia H, Ren W, Feng Y, Wei T, Guo M, Guo J, Zhao J, Song X, Wang M, Zhao T, et al. The enhanced antitumour response of pimozide combined with the IDO inhibitor L-MT in melanoma. Int J Oncol. 2018;53:949–960. doi: 10.3892/ijo.2018.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fako V, Yu Z, Henrich CJ, Ransom T, Budhu AS, Wang XW. Inhibition of wnt/β-catenin signaling in hepatocellular carcinoma by an antipsychotic drug pimozide. Int J Biol Sci. 2016;12:768–775. doi: 10.7150/ijbs.14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren Y, Tao J, Jiang Z, Guo D, Tang J. Pimozide suppresses colorectal cancer via inhibition of Wnt/β-catenin signaling pathway. Life Sci. 2018;209:267–273. doi: 10.1016/j.lfs.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Gonçalves JM, Silva CAB, Rivero ERC, Cordeiro MMR. Inhibition of cancer stem cells promoted by Pimozide. Clin Exp Pharmacol Physiol. 2019;46:116–125. doi: 10.1111/1440-1681.13049. [DOI] [PubMed] [Google Scholar]
- 15.Yan D, Parker RE, Wang X, Frye SV, Earp HS, III, DeRyckere D, Graham DK. MERTK promotes resistance to irreversible EGFR tyrosine kinase inhibitors in non-small cell lung cancers expressing wild-type EGFR family members. Clin Can Res. 2018;24:6523–6535. doi: 10.1158/1078-0432.CCR-18-0040. [DOI] [PubMed] [Google Scholar]
- 16.Abdallah SM, Hirsh V. Irreversible tyrosine kinase inhibition of epidermal growth factor receptor with afatinib in EGFR activating mutation-positive advanced non-small-cell lung cancer. Curr Oncol. 2018;25(Suppl 1):S9–S17. doi: 10.3747/co.25.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sagot I, Laporte D. Quiescence, an individual journey. Curr Genet. 2019;65:695–699. doi: 10.1007/s00294-018-00928-w. [DOI] [PubMed] [Google Scholar]
- 18.Heldt FS, Barr AR, Cooper S, Bakal C, Novák B. A comprehensive model for the proliferation-quiescence decision in response to endogenous DNA damage in human cells. Proc Natl Acad Sci USA. 2018;115:2532–2537. doi: 10.1073/pnas.1715345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai N, Xie SJ, Qiu DB, Jia CC, Du C, Liu W, Chen JJ, Zhang Q. Potential effects of α-mangostin in the prevention and treatment of hepatocellular carcinoma. J Funct Foods. 2016;26:309–318. doi: 10.1016/j.jff.2016.08.014. [DOI] [Google Scholar]
- 20.Agarwal SM, Pal D, Gupta M, Saini R. Insight into discovery of next generation reversible TMLR inhibitors targeting EGFR activating and drug resistant T790M mutants. Curr Can Drug Targets. 2017;17:617–636. doi: 10.2174/1568009617666170330112842. [DOI] [PubMed] [Google Scholar]
- 21.Rybczynska M, Spitaler M, Knebel NG, Boeck G, Grunicke H, Hofmann J. Effects of miltefosine on various biochemical parameters in a panel of tumor cell lines with different sensitivities. Biochem Pharmacol. 2001;62:765–772. doi: 10.1016/S0006-2952(01)00715-8. [DOI] [PubMed] [Google Scholar]
- 22.Dakir EH, Pickard A, Srivastava K, McCrudden CM, Gross SR, Lloyd S, Zhang SD, Margariti A, Morgan R, Rudl PS, El-Tanani M. The anti-psychotic drug pimozide is a novel chemotherapeutic for breast cancer. Oncotarget. 2018;9:34889–34910. doi: 10.18632/oncotarget.26175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strobl JS, Kirkwood KL, Lantz TK, Lewine MA, Peterson VA, Worley JF., III Inhibition of human breast cancer cell proliferation in tissue culture by the neuroleptic agents pimozide and thioridazine. Cancer Res. 1990;50:5399–5405. [PubMed] [Google Scholar]
- 24.Coller HA, Sang L, Roberts JM. A new description of cellular quiescence. PLoS Biol. 2006;4:e83. doi: 10.1371/journal.pbio.0040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alarcón T, Jensen HJ. Quiescence: A mechanism for escaping the effects of drug on cell populations. J R Soc Interface. 2011;8:99–106. doi: 10.1098/rsif.2010.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiore APZP, Ribeiro PF, Bruni-Cardoso A. Sleeping beauty and the microenvironment enchantment: Microenvironmental regulation of the proliferation-quiescence decision in normal tissues and in cancer development. Front Cell Dev Biol. 2018;6:59. doi: 10.3389/fcell.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recasens A, Munoz L. Targeting cancer cell dormancy. Trends Pharmacol Sci. 2019;40:30229–30233. doi: 10.1016/j.tips.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Nelson EA, Walker SR, Xiang M, Weisberg E, Bar-Natan M, Barrett R, Liu S, Kharbanda S, Christie AL, Nicolais M, et al. The STAT5 inhibitor pimozide displays efficacy in models of acute myelogenous leukemia driven by FLT3 mutations. Genes Cancer. 2012;3:503–511. doi: 10.1177/1947601912466555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhattarai D, Singh S, Jang Y, Hyeon Han S, Lee K, Choi Y. An insight into drug repositioning for the development of novel anti-cancer drugs. Curr Top Med Chem. 2016;16:2156–2168. doi: 10.2174/1568026616666160216153618. [DOI] [PubMed] [Google Scholar]
- 32.Samuel SM, Varghese E, Varghese S, Büsselberg D. Challenges and perspectives in the treatment of diabetes associated breast cancer. Cancer Treat Rev. 2018;70:98–111. doi: 10.1016/j.ctrv.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 33.El-Arabey AA. Correction to: New insight for metformin against bladder cancer. Genes Environ. 2018;40:16. doi: 10.1186/s41021-018-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 35.Jiang C, Xu R, Li XX, Zhou YF, Xu XY, Yang Y, Wang HY, Zheng XFS. Sorafenib and carfilzomib synergistically inhibit the proliferation, survival, and metastasis of hepatocellular carcinoma. Mol Cancer Ther. 2018;17:2610–2621. doi: 10.1158/1535-7163.MCT-17-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.