Abstract
Introduction
Redo parathyroidectomy for persistent/recurrent primary hyperparathyroidism is associated with a higher risk of complications and should be planned only with convincing localisation. We assessed whether 18fluorocholine positron emission tomography/computed tomography could identify parathyroid adenoma(s) in patients with persistent/recurrent primary hyperparathyroidism and negative conventional scans.
Materials and methods
A departmental database was used to identify patients with failed localisation attempts (sestamibi single photon emission computed tomography/computed tomography and/or computed tomography/magnetic resonance imaging and/or selective parathyroid hormone sampling) after previous unsuccessful surgery for primary hyperparathyroidism. 18Fluorocholine positron emission tomography was performed in all patients and redo surgery offered to those with positive findings.
Results
18Fluorocholine positron emission tomography incorporating arterial and portal phase enhanced computed tomography was performed in 12 patients with persistent/recurrent primary hyperparathyroidism (four men and eight women). Seven patients (58%) were cured after excision of adenomas located in ectopic positions (n = 3) or in anatomical position (n = 4). Five patients (42%) had persistent hypercalcaemia and repeat 18fluorocholine scan confirmed that the area highlighted on preoperative scans was excised. The arterial phase enhancement of the computed tomography was significantly different between cured and not-cured patients (P = 0.007). All seven cured patients had either a strong or weak enhancing pattern on computed tomography. Standardised uptake value at 60 minutes in patients with successful surgery (range 2.7–15.7, median 4.05) was higher than in patients with failed surgery (range 1.8–5.8, median 3.2) but was not statistically significant (P = 0.300).
Discussion
18fluorocholine scanning can identify elusive parathyroid adenomas, including those that are ectopic, and is useful in the management of patients with persistent/recurrent primary hyperparathyroidism when first-line scans are negative. The grading of the arterial phase of computed tomography can help to differentiate between true adenomas and false positive targets (lymph nodes).
Keywords: 18F-fluorocholine, PET/CT, Positron emission tomography/computed tomography, Oncology, Primary hyperparathyroidism, Parathyroidectomy
Introduction
Primary hyperparathyroidism (PHPT) affects up to 0.3% of the population and the only definitive cure is parathyroidectomy.1 Parathyroidectomy can be performed by a variety of approaches (bilateral neck exploration, minimally invasive approach, endoscopic, robotic) and the cure rates depend mostly on the surgeons’ experience.2,3 According to the Fourth National Audit Report from the British Association of Endocrine and Thyroid Surgeons, the rate of persistent/recurrent PHPT (p-PHPT) is 4.7%, and poses a significant problem regarding the future management of the patient.4 After failed neck exploration, the biochemical confirmation of the PHPT diagnosis is paramount. Once this is established, repeat diagnostic imaging is undertaken in order to locate the elusive parathyroid adenoma(s).5
The first-line localisation techniques used so far have been neck ultrasound scan, sestamibi scintigraphy (single photon emission computed tomography/computed tomography, SPECT/CT), magnetic resonance imaging (MRI) and arterial-enhanced multidetector computed tomography.2,5 Most surgeons usually reserve selective venous sampling as a second-line localisation technique due to its invasive nature and high cost.5 If the scans fail to localise the culprit gland, a blind reoperation is generally avoided due to the risk for a higher number of complications and failure to cure.
18Fluorocholine positron emission tomography/CT (FCH) scans are used for restaging in the setting of biochemical evidence of recurrence in patients with prostate cancer.6 In 2013, a study reported that FCH incidentally detected a hotspot in the neck of a patient which turned out to be a parathyroid adenoma.7 Since then, a number of small case series of patients and case reports have reported encouraging early results with the use of FCH scanning in patients with p-PHPT.7 A review from Kluijfhout et al in 2016 reported that the sensitivity of FCH scanning ranged from 80% to 100% and positive predictive value from 89% to 100% in several studies, with a total of 37 patients.8–16 A prospective study from Quak et al in patients with inconclusive ultrasound and MIBI single photon emission CT/CT showed a high sensitivity and positive predictive value of FCH for parathyroid adenoma detection.17
The aim of this study was to evaluate the usefulness of FCH scanning in patients with persistent/recurrent PHPT in the first cohort of patients in the United Kingdom.
Materials and methods
The departmental database was used to identify patients with persistent/recurrent PHPT who had at least one previous failed parathyroid operation in our institution or elsewhere. The identified patients were only included in the study if all post-operative scans (ultrasound and sestamibi SPECT/CT) were both negative. Eligible patients were included in this prospective study and underwent a FCH scan in our institution. The case notes and electronic records were used to collect information regarding demographics, previous operations, laboratory tests and imaging scan results.
The technique involved intravenous injection of 300 MBq (0.008 Ci) 18F-ethylcholine. PET commenced 60 minutes later from skull base to upper thighs with second neck-to-carina acquisition at 90 minutes. Arterial-enhanced CT of the neck was performed with 80 ml iohexol (Omnipaque™ 300 (GE Healthcare) with 25 ml saline chase injected at 4 ml/second followed by portal venous CT body for attenuation correction. 120 kV reconstructed at 0.625 mm slices on either a GE-Discovery 690 or 710, 64-slice PET/CT scanner (GE, Milwaukee). PET/CT was reconstructed with QClear, Bayesian Penalised Likelihood reconstruction β400 (GE, Milwaukee).
If an area suspicious for a parathyroid adenoma was identified on the FCH scan (even small relatively equivocal choline foci), the patient underwent a focused exploration to remove the target suggested by the scan report. The operations were performed as day cases early in the morning and the patient was discharged home the same day. All patients returned for a postoperative follow-up appointment in the outpatient department at four weeks and again at six months. If the patient was not cured, they underwent a repeat FCH scan to prove that the initial PET choline avid target had been removed.
The histopathology reports were screened to confirm the excision of a parathyroid adenoma. Serum calcium and parathyroid hormone testing was used to confirm the biochemical cure from PHPT (or failure to cure).
At the end of the study, once all patients had undergone surgery based on the report of the FCH scans, all images were reviewed again by the reporting radiologist, unaware of the clinical outcomes. The confidence in reporting focal 18F-choline uptake was classified as definite, possible, equivocal or negative. The enhancing appearance on the CT arterial phase was described as strong arterial, weak arterial or non-enhancing.
The study was approved by the research and development department of our hospital. Consent was obtained from each participant after full explanation of the purpose and nature of all procedures used.
Statistical analysis was performed using GraphPad Prism version 6.00 for Windows. Data were tested for Gaussian distribution. Parametric data are presented as mean plus or minus standard deviation and compared using a T-test. Median and range are provided for data with non-Gaussian distribution and the Wilcoxon test was used for comparison. For all tests, statistical significance was considered for P < 0.05.
Results
Twelve patients with p-PHPT were included in the study. Their demographics, clinical and operative outcomes are presented in Table 1. Four patients were on cinacalcet preoperatively to control hypercalcemia.
Table 1.
Overview of the clinical characteristics of patients included in the study.
| Patient | Sex | Preoperative calcium | Preoperative PTH | Age (years) | Cinacalcet use preoperatively | Preoperative disease status | Previous PHPT operations | Preoperative scans | DS last follow-upa |
| I | M | 2.7 | 14.5 | 47 | No | Persist. | 2 | MIBI, US | Cured |
| II | F | 2.7 | 24.8 | 52 | Yes | Persist. | 2 | MIBI, US, MRI, SVS | Persist. |
| III | F | 3.0 | 14.3 | 78 | No | Persist. | 1 | MIBI, US, SVS | Persist. |
| IV | F | 2.5 | H | 33 | Yes | Persist. | 1 | MIBI, US | Persist. |
| V | F | 2.7 | 21.2 | 81 | No | Persist. | 1 | MIBI, US | Cured |
| VI | F | 2.9 | 27.9 | 54 | No | Persist. | 1 | MIBI, US, MRI | Cured |
| VII | F | 2.8 | 15.6 | 56 | Yes | Persist. | 2 | MIBI, US, MRI, SVS | Cured |
| VIII | M | 3.0 | 13.6 | 26 | No | Recurr. | 2 | MIBI, US, SVS | Cured |
| IX | F | 56 | No | Persist. | 1 | MIBI, US, SVS | Cured | ||
| X | F | 3.1 | 21.8 | 53 | No | Persist. | 1 | MIBI, US | Persist. |
| XI | F | 59 | Yes | Persist. | 4 | MIBI, US | Cured | ||
| XII | F | 2.8 | 18.2 | 49 | No | Persist. | 1 | MIBI, US, SVS | Persist. |
a From date of choline operation till last follow-up; adjusted calcium normal values (2.2–2.6 mmol/l), PTH normal values (range 1.8–6.8 pmol/l).
MIBI, sestamibi single photon emission computed tomography/computed tomography; MRI, magnetic resonance imaging; Persist., persistent PHPT; PHPT, primary hyperparathyroidism; PTH, parathyroid hormone; PHPT, primary hyperparathyroidism; Recurr., recurrent PHPT; SVS, selective venous sampling; US, ultrasound.
All patients had negative sestamibi SPECT/CT and ultrasound scan after the initial failed neck exploration(s) for PHPT. In addition, three patients (II, VII, X) had a negative preoperative MRI scan and six patients (II, III, VII, VIII, IX and XII) also had a negative selective venous sampling (Table 1).
The result of the FCH scan and the histopathology details of the adenomas excised are presented in Table 2. All three cases with a choline-avid lesion in an ectopic position (one in prevertebral space and two medially to the common carotid artery) were cured. All five cases with p-PHPT after the reoperation had either non-enhancing targets (cases III, IV, X, XII) or a target with no CT correlation (case II) and histopathology in these cases only revealed the presence of lymph nodes. These five patients had repeat FCH scans after the operation and these showed that the ‘target’ identified on the preoperative FCH was removed (true false positive).
Table 2.
Correlation between the radiological and the histopathological findings of patients included in the study.
| Patient | FCH location | FCH scan result | SUVmaxa 60/90 | CT enhancing | CT size (mm) | Pathology | Weight (g)b | Size (mm)b | Postop. PTH |
| I | Anatomical | Behind left clavicle | 2.1/– | Weak arterial | 4 | Adenoma | 0.3 | 12 | 0.8 |
| II | Anatomical | Right inferior | 5.8/4.4 | No CT Correlation | - | Right thymus and LN | N/A | N/A | 8.8 |
| III | Anatomical | Right superior | 3.2/4.5 | Non-enhancing | 8 | LN | N/A | N/A | 11.0 |
| IV | Anatomical | Right inferior | 1.8/1.4 | Non-enhancing | – | LN | N/A | N/A | 14.3 |
| V | Ectopic | Prevertebral space | 3.6/2.2 | Strong | 10 | Adenoma | 0.5 | 15 | 7.3 |
| VI | Ectopic | Medial to right CCA bifurcation | 15.7/13.4 | Strong | 10 | Adenoma | 0.9 | 27 | 6.4 |
| VII | Anatomical | Right inferior | 2.7/2.7 | Weak arterial | 5 | Adenoma | 0.2 | 12 | 1.8 |
| VIII | Anatomical | Right inferior | 4.0/4.5 | Weak arterial | 6 | Adenoma | 15 | 3.4 | |
| IX | Ectopic | Medial to right CCA | 12.7/10.1 | Strong | 13 | Adenoma | 1.0 | 11 | 2.7 |
| X | Anatomical | Left inferior | 2.7/2.2 | Non-enhancing | – | LN | N/A | N/A | 13.8 |
| XI | Anatomical | Left inferior | 4.1/3.8 | Weak arterial | 5 | Adenoma | 1.0 | 15 | 3.6 |
| XII | Anatomical | Right inferior | 4.7/4.9 | Non-enhancing | – | LN | N/A | N/A | 21.0 |
a Of lesion removed during the operation at 60 and 90 minutes post administration.
b Of excised parathyroid adenoma on histopathology report; ‘anatomical’ refers to parathyroid adenomas identified in a typical position (e.g. close to the thyroid gland).
CCA, Common carotid artery; CT, computed tomography; FCH, 18fluorocholine positron emission tomography/CT; LN, lymph nodes; N/A, Not applicable; PTH, parathyroid hormone (1.8–6.8 pmol/l); Postop, postoperative; SUV, standardised uptake values.
Table 3 presents a comparison of demographic, clinical and imaging-related variables between patients who were cured postoperatively and those with persistent hypercalcaemia. No significant differences were detected between the two groups in regards to the variables that were investigated. Two cases with maximum standardised uptake values (SUV) much higher than the others were confirmed to have adenomas.
Table 3.
Comparison of demographic, clinical and imaging related variables between patients that were cured postoperatively and the ones with persistent hypercalcemia.
| Cured (n = 7)mean (SD) | Persistence PHPT (n = 5)mean (SD) | P-valuea | Total (n = 12)mean (SD) | |
| Sex (male : female) | 2 : 5 | 0 : 5 | 0.470 | 2 : 10 |
| Age at date of operation (years)b | 54.1 (16.3) | 53.0 (16.1) | 0.907 | 53.7 (15.5) |
| Preoperative calcium | 2.8 (0.1) | 2.8 (0.2) | 0.945 | 2.8 (0.2) |
| Preoperative parathyroid hormonec | 18.6 (6.0) | 19.8 (4.5) | 0.748 | 19.1 (5.1) |
| SUVmax (60 minutes) | 6.4 (5.4) | 3.6 (1.6) | 0.300 | 5.3 (4.4) |
| SUVmax (90 minutes) | 6.1 (4.6) | 3.5 (1.6) | 0.252 | 4.9 (3.6) |
a ≤ 0.05 considered significant.
b Performed after choline scan.
c Adjusted calcium normal values (2.2–2.6 mmol/l), PTH normal values (1.8–6.8 pmol/l).
PHPT, primary hyperparathyroidism; SD, standard deviation; SUVmax, maximum standardised uptake values.
Illustrative examples of the results of the FCH scans and the intraoperative findings are presented in Figures 1 and 2 (corresponding to patients VII and XI, respectively).
Figure 1.
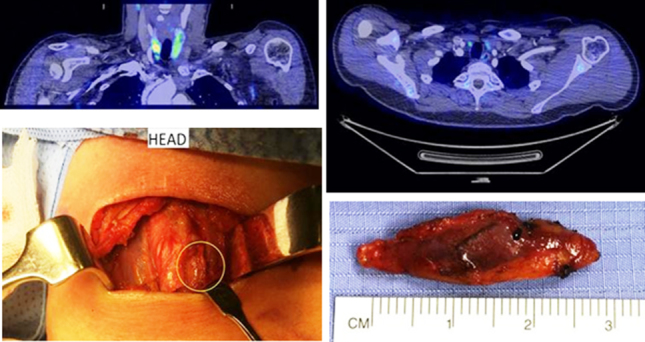
A 56-year-old woman with two previous failed neck explorations and negative selective venous sampling. The 18fluorocholine positron emission tomography/computed tomography (PET/CT) scan correctly identified a 5-mm faintly enhancing nodule immediately medial to the right common carotid artery and just separate from the right lobe of thyroid which was removed and the patient cured (axial and coronal fused PET/CT images on an standardised uptake values 0–6 scale using the arterial enhanced CT images, intraoperative picture and specimen size).
Figure 2.
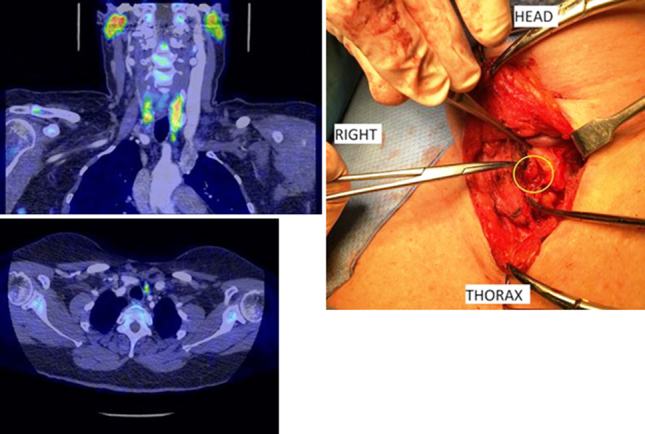
A 59-year-old woman with four previous failed neck explorations. The 18fluorocholine positron emission tomography/computed tomography (PET/CT) scan correctly identified a 5-mm mildly choline-avid focus (maximum standardised uptake value 4.1) immediately abutting the inferior pole of the left lobe of thyroid, intimately related to a small vessel, which was removed and the patient cured (axial and coronal fused PET/CT images on a standardised uptake values 0–6 scale using the arterial enhanced CT images and intraoperative picture).
The grading of the arterial-phase CT enhancement of the possible target in the FCH scan is presented in Table 4 and showed that there was a significant difference between the two groups; the cured group had either strong or weak arterial enhancement patterns while the p-PHPT group had non-enhancing or no CT correlation features (P = 0.007).
Table 4.
Grading of the computed tomography appearance (arterial phase) of the suspicious nodule of the 18fluorocholine positron emission tomography/computed tomography scan by the radiologist.
| Grading | Cured | Persistent PHPT | P-value | Total | |||
| (n) | (%) | (n) | (%) | (n) | (%) | ||
| Strong | 3 | 43 | 0 | 0.007 | 3 | 25 | |
| Weak | 4 | 57 | 0 | 4 | 33 | ||
| Non-enhancing | 0 | 4 | 80 | 4 | 33 | ||
| No CT correlation | 0 | 1 | 20 | 1 | 8 | ||
CT, computed tomography; FCH, 18fluorocholine positron emission tomography/CT; PHPT, primary hyperparathyroidism.
There was no significant difference between the cured and non-cured patients in regards to the confidence of the reporting radiologist that there is focal F-choline uptake in the suspicious nodule (P = 0.237; Table 5).
Table 5.
Grading of the confidence of the reporting radiologist in regards to whether there is focal F-choline uptake in the 18fluorocholine positron emission tomography/computed tomography scan.
| Grading | Cured (n) | Persistent PHPT (n) | P-value | Total | |
| (n) | (%) | ||||
| Definite | 4 | 1 | 0.237 | 5 | 42 |
| Possible | 2 | 1 | 3 | 25 | |
| Equivocal | 1 | 3 | 4 | 33 | |
PHPT, primary hyperparathyroidism.
All five patients with p-PHPT after the reoperation underwent a repeat FCH scan, which confirmed that the PET choline-avid target was indeed removed during the operation in our Institution.
An overview of the published literature (case series and case reports) is presented in Table 6.
Table 6.
Overview of the published literature on the use of 18Fluorocholine positron emission tomography in patients with hyperparathyroidism.
| Author (date) | Patients (n) | PHPT type | Sex | Age, years (range) | Preop. calcium | Preop. PTH | Dose used | Timing | Success |
| Quak (2013)7 | 1 | Primary | 1M | 71 | 2.84 | 91.8 pg/l (< 80) | – | – | 1/1 |
| Orevi (2014)16 | 40 | Primary/tertiary | – | 55 (21–72) | 2.8 | 15.9 pmol/l | 370 MBq | 11 minutes | 24/27 |
| Lezaic (2014)13 | 24 | Primary | 4M, 20F | (42–77) | Med : 2.7 | Med:83.5 pg/mL | 100 MBq | 60 minutes | 36/40 |
| Van Raalte (2015)14 | 2 | Primary | – | 69, 66 | 2.73, 2.96 | 32.3 pmol/l, 19.0 pmol/l | 175 MBq | 40 minutes | 2/2 |
| Michaud (2015)15 | 17 | Primary/secondary/lithium | 5M, 12F | 52 (25–75) | X | 280 pg/ml (range 61–1946 pg/ml) | 3 MBq/kg | 10 minutes | 16/17 |
| Rep (2015)18 | 43 | Primary | 8M, 35F | 59.6 (11) | 2.8 | 311.5 ng/l | 100 MBq | 5 minutes, 1 hour, 2 hours | 40/43 |
| Kluijfhout (2015)11 | 5 | Primary | – | – | – | – | 2 MBq/kg | – | 4/5 |
| Kluijfhout (2016)10 | 44 | Primary/tertiary | 11M, 33F | 58.9 (31–80) | 1.08 | 2.11 | 2 MBq/kg | – | 33/35 |
| Behera (2016)9 | 2 | Primary | 2M | 38, 58 | – | – | – | – | 2/2 |
| Hocevar (2017)19 | 151 | Primary | 30M, 121F | 61.1 (13–82) | – | – | 100 MBq | 5 minutes, 1 hour | 122/126 |
| Quak (2018)17 | 25 | Primary | 10M, 15F | 58.9 (14.2) | 2.76 | 94.8 ng/l | 1,5 MBq/kg | 60 minutes | 19/25 |
PHPT, Primary hyperparathyroidism; PTH,: Parathyroid hormone, Adjusted calcium normal values (2.2-2.6 mmol/L), PTH normal values (1.8-6.8 pmol/l, 10-65 ng/l).
Discussion
This study reports the outcomes of redo parathyroidectomy in the first cohort of patients in the UK who underwent an FCH scan for persistent/recurrent PHPT. The results demonstrate that FCH scanning can identity elusive parathyroid glands and lead to successful parathyroidectomies and definite cure in more than 50% of the patients in this highly selected group, with one or more previous unsuccessful operations and numerous negative ‘conventional’ scans.
The FCH scan was performed only in patients with p-PHPT with at least one failed neck exploration and several negative localisation scans. The use of the FCH was based on the sparse data published in the last six years (summarised in Table 6). The cohort of patients included in this study represents a very challenging subgroup of patients with PHPT as they all had previous negative neck exploration for PHPT and negative scans. In contrast, these highly selected patients appear rarely in the other studies published to date. For example, only three such patients were included in the group of 33 patients reported by Kluijfhout et al.11
The weight and size of the parathyroid lesions removed in this case series resembled the experience of Michaud et al and Lezaic et al,11,15 who reported that most of the abnormal parathyroid glands weighted close to 0.1 g or more and measured around 10 mm. As such, it is evident that it is not the small size of the gland that results in failed localisation by traditional scans, to identify it but possibly a combination of factors such as distorted anatomy due to fibrosis and scarring and an altered pattern of feeding blood vessels. FCH scan overcomes these limitations by exploiting a functional aspect of the parathyroid glands with excellent anatomical resolution.
Furthermore, the majority of cured patients in our study had a missed gland in an anatomic position that highlights the importance of the first operation as a golden opportunity to cure patients with PHPT. Unfortunately, there are no other published studies on the location of the elusive gland in FCH scans for persistent/recurrent PHPT in patients with negative ultrasound and sestamibi SPECT/CT. The ability of FCH scans to identify parathyroid adenomas in ectopic positions has been confirmed in our study and has been previously reported by others.11,14,16
In our study, it was clear that all the proven parathyroid adenomas were either strongly or weakly enhancing on arterial phase CT and that non-enhancing, Choline-avid nodules were typically lymph nodes. In this very difficult patient population, the combination with arterial enhanced CT appears to be a good test, as the choline study helps to guide the identification of small potential adenomas. There is a great deal of enhancement and contrast visible on these arterial enhanced CT scans.
Our study has not been able to identify significant differences between the groups of cured and non-cured patients that could be used as prognostic markers for the diagnostic accuracy of the FCH scan. This is in alignment with the results from Kluijfhout et al,10 who showed no significant differences in age, sex, ratio of preoperative calcium, use of cinacalcet, history of neck surgery, and concomitant multinodular goitre between these two groups.
A number of aspects of the FCH scan remain to be explored further, including the optimal time and dose for scanning and the cost effectiveness of the scan. Rep et al have showed that one hour is enough to provide differentiation between the SUV values of thyroid and parathyroid adenomas while scanning after that time point offers no added information.18 Our study has also shown that there was no added value in 90-minute PET imaging compared with 60-minute imaging. The cost effectiveness relationship of the FCH scan depends mostly on whether it can replace any of the traditional imaging such as ultrasound and sestamibi SPECT/CT. Currently, its main benefit is derived in the small group of patients with negative ultrasound and sestamibi SPECT/CT and failed neck exploration, who should represent approximately 5% of a large cohort of patients with PHPT. In this highly selected group, a successful FCH used as a second-line imaging modality may provide significant cost savings if it leads to a successful parathyroidectomy by abolishing the need for further follow-ups, blood tests and repeated imaging.
The strength of this study is the use of the FCH scan in a very challenging group of patients – those with double negative localisation and failed operation(s). This particular group of patients would have received no surgical treatment if it was not for the FCH results and, for them, the clinical benefit is readily easily apparent. Furthermore, the patients with p-HPT after the operation in our institution had a repeat FCH scan that confirmed that we had indeed removed the PET choline avid target and as such the initial FCH scan was indeed false positive.
The authors acknowledge the limitations of this study including the small number of patients recruited and its single-centre nature. Future multi-institutional studies and collaborations could overcome these limitations.
Conclusion
In summary, this is the first study that focuses on the usefulness of FCH scanning in patients with previously failed parathyroid exploration(s) and subsequent negative imaging with ultrasound and sestamibi SPECT/CT. Imaging with FCH was found to be able to identify elusive parathyroid adenomas, even in ectopic positions, and can prove useful in the management of patients with p-PHPT when first-line scans are negative. The grading of the CT arterial phase can help differentiate between true adenomas and false positive targets (lymph nodes) and should be taken into account when interpreting the FCH scan.
Acknowledgements
Data presented as poster at the 2017 meeting of the British Association of Endocrine and Thyroid Surgeons in Belfast, October 2017.
References
- 1.Kebebew E, Clark OH. Parathyroid adenoma, hyperplasia, and carcinoma: localization, technical details of primary neck exploration, and treatment of hypercalcemic crisis. Surg Oncol Clin N Am 1998; (4): 721–748. [PubMed] [Google Scholar]
- 2.Palazzo FF, Delbridge LW. Minimal-access/minimally invasive parathyroidectomy for primary hyperparathyroidism. Surg Clin North Am 2004; (3): 717–734. [DOI] [PubMed] [Google Scholar]
- 3.Christakis I, Palazzo F. Minimal invasive parathyroidectomy : Watkinson JC, Scott-Coombes DM (). Tips and Tricks in Endocrine Surgery. London: Springer; 2014, 259–266. [Google Scholar]
- 4.British Association of Endocrine and Thyroid Surgeons Fourth National Audit Report 2012. Henley: Dendrite Clinical Systems; 2012. [Google Scholar]
- 5.Christakis E, Perrier N. Evaluation and management of persistent or recurrent hyperparathyroidism In: Cameron J, Cameron A (eds). Current Surgical Therapy, 12th ed Amsterdam: Elsevier; 2016, 792–797. [Google Scholar]
- 6.Hodolic M. Imaging of prostate cancer using 18F-choline PET/computed tomography. PET Clin 2017; (2): 173–184. [DOI] [PubMed] [Google Scholar]
- 7.Quak E, Lheureux S, Reznik Y et al. F18-choline, a novel PET tracer for parathyroid adenoma? J Clin Endocrinol Metab 2013; (8): 3,111–3,112. [DOI] [PubMed] [Google Scholar]
- 8.Kluijfhout WP, Pasternak JD, Drake FT et al. Use of PET tracers for parathyroid localization: a systematic review and meta-analysis. Langenbecks Arch Surg 2016; (7): 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behera A, Damle NA. Incremental role of 18F-fluorocholine PET/CT over technetium-99m-labeled MIBI scan in hyperparathyroidism. Indian J Endocrinol Metab 2016; (6): 888–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluijfhout WP, Vorselaars WM, van den Berk SA et al. Fluorine-18 fluorocholine PET-CT localizes hyperparathyroidism in patients with inconclusive conventional imaging: a multicenter study from the Netherlands. Nucl Med Comm 2016; (12): 1,246–1,252. [DOI] [PubMed] [Google Scholar]
- 11.Kluijfhout WP, Vorselaars WM, Vriens MR B et al. Enabling minimal invasive parathyroidectomy for patients with primary hyperparathyroidism using Tc-99m-sestamibi SPECT-CT, ultrasound and first results of (18)F-fluorocholine PET-CT. Eur J Radiol 2015; (9): 1,745–1,751. [DOI] [PubMed] [Google Scholar]
- 12.Michaud L, Burgess A, Huchet V et al. Is 18F-fluorocholine-positron emission tomography/computerized tomography a new imaging tool for detecting hyperfunctioning parathyroid glands in primary or secondary hyperparathyroidism? J Clin Endocrinol Metab 2014; (12): 4,531–4,536. [DOI] [PubMed] [Google Scholar]
- 13.Lezaic L, Rep S, Sever MJ et al. (1)(8)F-Fluorocholine PET/CT for localization of hyperfunctioning parathyroid tissue in primary hyperparathyroidism: a pilot study. Eur J Nucl Med Mol Imaging 2014; (11): 2,083–2,089. [DOI] [PubMed] [Google Scholar]
- 14.van Raalte DH, Vlot MC, Zwijnenburg A, ten Kate RW. F18-Choline PET/CT: a novel tool to localize parathyroid adenoma? Clin Endocrinol (Oxf) 2015; (6): 910–912. [DOI] [PubMed] [Google Scholar]
- 15.Michaud L, Balogova S, Burgess A et al. A pilot comparison of 18F-fluorocholine PET/CT, ultrasonography and 123I/99mTc-sestaMIBI dual-phase dual-isotope scintigraphy in the preoperative localization of hyperfunctioning parathyroid glands in primary or secondary hyperparathyroidism: influence of thyroid anomalies. Medicine (Baltimore) 2015; (41): e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orevi M, Freedman N, Mishani E et al. Localization of parathyroid adenoma by (1)(1)C-choline PET/CT: preliminary results. Clin Nucl Med 2014; (12): 1,033–1,038. [DOI] [PubMed] [Google Scholar]
- 17.Quak E, Blanchard D, Houdu B et al. F18-choline PET/CT guided surgery in primary hyperparathyroidism when ultrasound and MIBI SPECT/CT are negative or inconclusive: the APACH1 study. Eur J Nucl Med Mol Imaging 2018; (4): 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rep S, Lezaic L, Kocjan T et al. Optimal scan time for evaluation of parathyroid adenoma with [(18)F]-fluorocholine PET/CT. Radiol Oncol 2015; (4): 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hocevar M, Lezaic L, Rep S et al. Focused parathyroidectomy without intraoperative parathormone testing is safe after pre-operative localization with (18)F-Fluorocholine PET/CT. Eur J Surg Oncol 2017; (1): 133–137. [DOI] [PubMed] [Google Scholar]


