Abstract
Background
Pancreatic ductal adenocarcinoma remains a disease with a poor prognosis despite advances in surgery and systemic therapies. Neoadjuvant therapy strategies are a promising alternative to adjuvant chemotherapy. However, their role remains controversial. This meta-analysis aims to clarify the benefits of neoadjuvant therapy in resectable pancreatic ductal adenocarcinoma.
Methods
Eligible studies were identified from MEDLINE, Embase, Web of Science and the Cochrane Library. Studies comparing neoadjuvant therapy with a surgery first approach (with or without adjuvant therapy) in resectable pancreatic ductal adenocarcinoma were included. The primary outcome assessed was overall survival. A random-effects meta-analysis was performed, together with pooling of unadjusted Kaplan–Meier curve data.
Results
A total of 533 studies were identified that analysed the effect of neoadjuvant therapy in pancreatic ductal adenocarcinoma. Twenty-seven studies were included in the final data synthesis. Meta-analysis suggested beneficial effects of neoadjuvant therapy with prolonged survival compared with a surgery-first approach, (hazard ratio 0.72, 95% confidence interval 0.69–0.76). In addition, R0 resection rates were significantly higher in patients receiving neoadjuvant therapy (relative risk 0.51, 95% confidence interval 0.47–0.55). Individual patient data analysis suggested that overall survival was better for patients receiving neoadjuvant therapy (P = 0.008).
Conclusions
Current evidence suggests that neoadjuvant chemotherapy has a beneficial effect on overall survival in resectable pancreatic ductal adenocarcinoma in comparison with upfront surgery and adjuvant therapy. Further trials are needed to address the need for practice change.
Keywords: Pancreas, Cancer, Neoadjuvant, Surgery
Introduction
Pancreatic ductal adenocarcinoma (PDAC), the fourth largest cause of cancer-related mortality worldwide, is an aggressive malignancy with an unfavourable overall five-year survival rate of less than 4% over the past two decades despite recent advances in diagnostic imaging, surgical technique and perioperative care.1 Owing to the insidious nature of PDAC, the majority of patients present late, with advanced, metastatic disease, meaning that only a small cohort of patients are surgical candidates.
The current gold standard approach for management of localised PDAC with curative intent is up-front resection of the primary tumour and regional lymph nodes followed by adjuvant chemotherapy, established based on results of previous clinical trials such as the European Study Group for Pancreatic Cancer (ESPAC)-1 trial, which demonstrated a five-year survival rate of 21% in patients receiving adjuvant chemotherapy compared with 8% in patients who received no chemotherapy.2 More recent studies including the ESPAC-4 and the PRODIGY-24 trials shed further light on newer adjuvant strategies that improve disease free survival and overall survival in PDAC.3,4 The high morbidity rate and subsequent prolonged recovery time that accompany major resectional surgery may unfortunately have the effect of precluding patients from the timely receipt of adjuvant therapy.5 Even for those who successfully complete surgery and adjuvant therapy, a significant proportion of patients succumb to early locoregional or systemic metastatic disease recurrence, with median overall survival less than 20 months.6,7 Given this dismal prognosis, many experts consider PDAC a systemic condition from its onset, with micrometastases likely present in early stages of the disease.8
Owing to the need to improve long-term survival and resectability rates, and the failure of current multimodal approaches to achieve locoregional disease control, neoadjuvant chemotherapy strategies have been given increased attention and may be a promising alternative to a surgery-first approach. Numerous studies have reported beneficial effects on survival with neoadjuvant therapy, with or without concurrent radiotherapy,9–12 but outcome data from well-designed randomised studies are limited. The hypothesised benefits of neoadjuvant chemotherapy include early treatment of micrometastatic disease and improved resection rates by therapeutic tumour debulking.13,14
We performed a novel meta-analysis and systematic review with an enhanced secondary data analysis using an additional time-to-event data synthesis, based on the method described by Guyot et al in 2012 to achieve a close approximation to the original individual patient time-to-event data from which they were generated.15 In this study, we investigated the effect of neoadjuvant chemotherapy followed by resection compared with a surgery-first approach followed by adjuvant therapy. We also discuss the treatment benefits of neoadjuvant chemotherapy in terms of overall survival.
Methods
Search strategy
A systematic review was conducted in accordance with PRISMA guidelines for the reporting of meta-analyses.16 The literature search and data extraction were conducted independently by two authors (KR and PP) and final data including any discrepancies were resolved by consensus. A systematic search in MEDLINE (PubMed as the search engine), Embase, Web of Science and the Cochrane Library was conducted using a combination of the following keywords and MeSH headings: ‘pancreatic cancer’, ‘pancreatic ductal adenocarcinoma’, ‘pancreatic tumour’, ‘pancreatic neoplasm’, ‘neoadjuvant therapy’, ‘neoadjuvant’, ‘preoperative therapy’, ‘neoadjuvant chemotherapy’, ‘neoadjuvant chemoradiotherapy’, ‘resection’, ‘operative therapy’, ‘operation’ and ‘surgery’. All studies on patients with PDAC that provided a comparison analysis between patients treated with neoadjuvant chemotherapy followed by curative tumour resection and primarily resected patients (without neoadjuvant chemotherapy) were included.
No year of publication limits were set, and only English text publications were included. The search was last updated on 1 January 2018. References of included publications were cross-checked for suitability for inclusion. Following the removal of duplicates, an initial review of titles and abstracts was conducted to identify articles of potential interest; these were then retrieved for full-text analysis and independent data extraction by the authors conducting the literature search. Reference lists of retrieved articles were hand searched for additional relevant references.
Selection criteria
All studies reporting data comparing outcomes for patients undergoing surgery (surgery-first approach) (with or without adjuvant therapy), with patients undergoing neoadjuvant chemotherapy followed by surgery for the treatment of PDAC with curative intent were included. Studies which included data on patients with borderline resectable PDAC were included in the final analysis, but studies focusing on ampullary and periampullary cancers, unresectable or metastatic pancreatic cancer were excluded. Complications recorded in our meta-analysis were as reported and defined by the respective study authors.
Assessment of methodological quality
Study quality was assessed using the Newcastle–Ottawa scale for cohort studies and the Cochrane Collaboration’s risk of bias tool for randomised controlled trials.17,18 The Newcastle–Ottawa scale assigns a score of 0–9, with points assigned on the basis of a sample’s representativeness of the exposed cohort, comparability of cohorts, control for confounding factors, and appropriateness of outcome selection and reporting. Based on the methodology in previous studies, a score of 7 or greater was defined as acceptable.19,20 The Cochrane tool considers several factors for each study to judge the risk of bias, including evidence of allocation concealment, extent of blinding, accounting of patients and outcome events, extent of outcome reporting, use of unvalidated outcomes measures (patient-reported outcomes).
Statistical analysis
Data were extracted and entered into an Excel spreadsheet. Meta-analysis was conducted using the logarithm of the hazard or odds ratio (hazard ratio, HR; odds ratio, OR), using a random-effects model, in Stata version 11.0. Data heterogeneity was assessed using the I2 test, and risk of publication bias was assessed with funnel plots and Egger’s test. Statistical significance was assumed at a level P < 0.05.
Where studies reported unadjusted data in the form of Kaplan–Meier curves, we conducted an additional time-to-event data synthesis. Using this method, Kaplan–Meier curve data were digitised with Engauge Digitizer, version 10.4 (Mark Mitchell), allowing individual patient-level data to be extracted using an algorithm that assumes constant censoring, using R (v 1.0.153, RStudio Inc). Reconstructed individual patient survival data were thus aggregated to create a combined survival curve, comparing the long-term outcomes of the two therapeutic strategies analysed using Stata.
Results
Literature research and characteristic of studies
In the initial search, 533 studies potentially relevant studies were identified, of which 493 were excluded as irrelevant after scanning titles or abstracts. A total of 40 studies were included for further full-text assessment, of which 27 studies fulfilled the inclusion criteria and were included in final data synthesis (Figure 1). Three studies were randomised controlled trials and 24 were retrospective cohort studies.
Figure 1.
PRISMA flowchart of search strategy and results.
Table 1 shows the study characteristics, including a quality assessment of each retrospective cohort study. The 27 studies included a total of 63,151 patients, 8,461 of which received neoadjuvant chemotherapy and 54,690 who were assigned to a surgery-first approach. The neoadjuvant and adjuvant therapy regimens varied greatly across each study, with specific regimen details not being available for some studies. However, the commonly used chemotherapy/radiotherapy or chemoradiotherapy regimens included (Table 2) gemcitabine/capecitabine (4 studies, n = 274), gemcitabine (10 studies, n = 2590), cisplatin (4 studies, n = 125), capecitabine (1 study, n = 40), 5-fluorouracil (9 studies, n = 626), folinic acid/fluorouracil/irinotecan/oxaliplatin (FOLFIRINOX; 2 studies, n = 188). The rate of patients receiving adjuvant therapy following surgical resection was reported by 15 studies and varied between 6.2% and 88%.
Table 1.
Demographics of studies.
| Reference | Year | Country | Data source | Patients (n) | Male (%) | NOS score |
| de Geus11 | 2017 | USA | RCS | 12857 | 35 | 8 |
| Townend38 | 2017 | Australia | RCS | 195 | 53 | 8 |
| Itchins39 | 2017 | Australia | RCS | 442 | 49 | 7 |
| Chen40 | 2017 | Chinese | RCS | 772 | 53.5 | 8 |
| Mokdad41 | 2016 | USA | RCS | 14941 | 51 | 7 |
| Mirkin42 | 2016 | USA | RCS | 18322 | 50.4 | 7 |
| Schubert43 | 2016 | Canada | RCS | 593 | 51.2 | 9 |
| Lufti44 | 2016 | USA | RCS | 7881 | 50.9 | 8 |
| Ferrone45 | 2015 | USA | RCS | 188 | 35.1 | 7 |
| Golcher46 | 2015 | Germany | RCT | 66 | 53 | n/a |
| Casadei47 | 2015 | Italy | RCT | 38 | 57.9 | n/a |
| Roland48 | 2015 | USA | RCS | 307 | 55 | 9 |
| Fujii49 | 2015 | Japan | RCS | 92 | n/a | 7 |
| Sho50 | 2014 | Japan | RCS | 184 | 53.8 | 8 |
| Papavasiliou51 | 2014 | USA | RCS | 309 | 47.6 | 7 |
| Tzeng52 | 2014 | USA | RCS | 167 | 54.5 | 7 |
| Cooper53 | 2014 | USA | RCS | 236 | 50.9 | 9 |
| Papalezova9 | 2012 | USA | RCS | 236 | 53.5 | 7 |
| Barugola54 | 2012 | Italy | RCS | 403 | 55 | 7 |
| Artinyan12 | 2011 | USA | RCS | 458 | 46.9 | 7 |
| Stokes55 | 2011 | USA | RCS | 170 | n/a | 8 |
| Satoi56 | 2009 | Japan | RCS | 68 | 48.5 | 7 |
| Stessin57 | 2008 | USA | RCS | 3885 | 48.3 | 9 |
| Vento58 | 2007 | Finland | RCS | 47 | 53.2 | 7 |
| Al-Sukhun59 | 2003 | USA | RCT | 41 | 43.9 | n/a |
| White60 | 2001 | USA | RCS | 111 | 54.1 | 8 |
| Spitz61 | 1996 | USA | RCS | 142 | n/a | 8 |
NOS, Newcastle–Ottawa Scale; RCS, retrospective cohort study; RCT, randomised controlled trial.
Table 2.
Details of studies.
| Reference | NAT (n) | No NAT (n) | NAT type | AT (n) | Resection rate (%) |
| de Geus11 | 1541 | 11316 | CR | n/a | n/a |
| Townend38 | 42 | 153 | GEM | 42 | 63 |
| Itchins39 | 87 | 355 | GEM,FOLFIRINOX | 376 | 79 |
| Chen40 | 102 | 670 | n/a | n/a | 100 |
| Mokdad41 | 2005 | 12936 | GEM | n/a | 100 |
| Mirkin42 | 1736 | 16586 | C, R, CR | n/a | 100 |
| Schubert43 | 377 | 216 | C, R, CR | 39 | 72.4 |
| Lufti44 | 1184 | 6697 | R | n/a | n/a |
| Ferrone45 | 101 | 87 | FOLFIRINOX | n/a | 45.9 |
| Golcher46 | 33 | 33 | GEMCAP | 7 | 19 |
| Casadei47 | 18 | 20 | GEM | 4 | 61.1 |
| Roland48 | 222 | 85 | GEM, 5-FU | 25 | 100 |
| Fujii49 | 21 | 71 | 5FU | 52 | 86 |
| Sho50 | 85 | 99 | GEM | 52 | 96 |
| Papavasiliou51 | 108 | 201 | 5-FU, GEMCAP | 136 | 100 |
| Tzeng52 | 115 | 52 | 5-FU, GEMCAP | 16 | 83 |
| Cooper53 | 153 | 83 | CR | 12 | 48 |
| Papalezova9 | 144 | 92 | GEM | 78 | 53 |
| Barugola54 | 41 | 362 | GEMCAP, CIS | 314 | 100 |
| Artinyan12 | 39 | 419 | R | n/a | n/a |
| Stokes55 | 40 | 130 | CAP | 150 | 40 |
| Satoi56 | 27 | 41 | 5-FU, CIS, GEM | 0 | n/a |
| Stessin57 | 70 | 3815 | 5-FU, GEM | n/a | n/a |
| Vento58 | 22 | 25 | GEM | n/a | 72 |
| Al-Sukhun59 | 20 | 21 | 5-FU, CIS | n/a | 15 |
| White60 | 37 | 74 | 5-FU, CIS | n/a | 84 |
| Spitz61 | 91 | 51 | 5-FU | n/a | 74 |
AT, adjuvant therapy; C, chemotherapy; CAP, capecitabine; CIS, cisplatin; CR, chemoradiotherapy; GEM, gemcitabine; GEMCAP, gemcitabine, capecitabine; FOLFIRINOX, folinic acid/fluorouracil irinotecan/oxaliplatin; NAT, neoadjuvant therapy; R, radiotherapy; 5-FU, fluorouracil.
Assessment of methodological quality
Study quality was moderate to high, with a median Newcastle–Ottawa score of 8 (range 7–9) for cohort studies. Across the 3 randomised controlled trials included, no more than one domain for any study showed evidence of limitations as defined by the Cochrane risk of bias tool, suggesting that the risk of bias for these studies was low.
Meta-analysis
Meta-analysis of studies that reported hazard ratios demonstrated better survival outcomes with neoadjuvant chemotherapy compared with a surgery-first approach (Figure 2; HR 0.72, 95% confidence interval, CI, 0.69–0.76). Data heterogeneity was high (I2 = 77.9%, P < 0.001).
Figure 2.

Forest plot of survival rates following neoadjuvant chemotherapy compared with surgery first (top) and adjuvant compared with no adjuvant therapy (bottom).
Considering postoperative treatment regimens alone (independent of whether or not patients underwent neoadjuvant chemotherapy), patients receiving adjuvant therapy demonstrated better survival outcomes (Figure 2; HR 0.62, 95% CI 0.60–0.65), with low data heterogeneity (I2 = 10.7%, P = 0.347).
The rate of histopathologically negative (R0) resection margins was significantly improved with neoadjuvant chemotherapy compared with the surgery-first approach (RR 0.51, 95% CI 0.47–0.55), with high data heterogeneity (I2 = 94.3%, P < 0.001; Figure 3).
Figure 3.

Forest plot of negative (R0) resection margins for patients receiving neoadjuvant chemotherapy compared with surgery first (CI, confidence interval; RR, relative risk).
Morbidity rates were significantly reduced for patients receiving neoadjuvant chemotherapy compared with the surgery-first approach (RR 0.81, 95% CI 0.73–0.90), with high data heterogeneity (I2 = 66.8%, P = 0.001; Figure 4).
Figure 4.
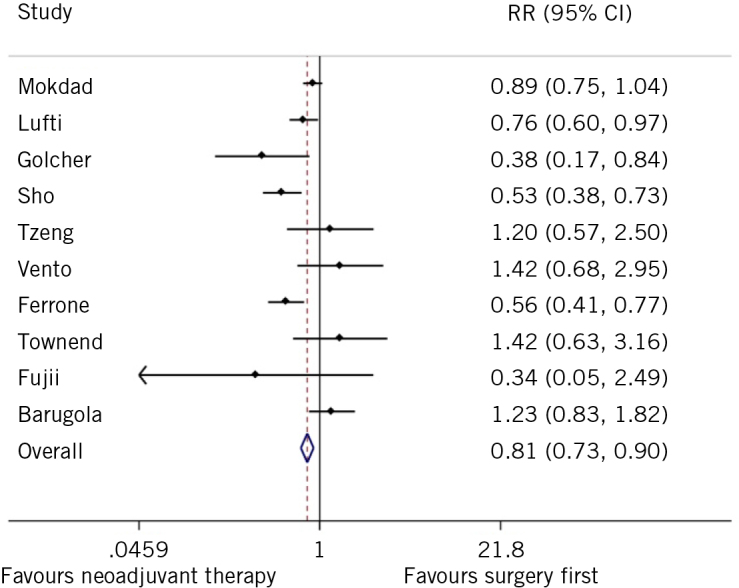
Forest plot of morbidity rates for neoadjuvant chemotherapy compared with surgery first.
The risk of publication bias was visually assessed as low (Figure 5), with no significant bias risk as measured by Egger’s test p = 0.859. Data synthesis of studies that did not report hazard ratios but did report Kaplan–Meier curves demonstrated improved median survival of 30.9 (95% CI 24.3–38.3) months with neoadjuvant chemotherapy compared with 23.8 (95% CI 21.9–25.2) months with surgery first (HR 1.15, 95% CI 1.00–1.33; log rank P = 0.0085 (Figure 6).
Figure 5.
Funnel plot of hazard ratios for overall survival comparing neoadjuvant chemotherapy with surgery first (HR, hazard ration; SE, standard error).
Figure 6.
Kaplan–Meier survival curve for overall survival for neoadjuvant chemotherapy and surgery first for patients with pancreatic ductal adenocarcinoma with number at risk values (P = 0.008).
Discussion
This systematic review and meta-analysis suggests a significant survival advantage for patients receiving neoadjuvant chemotherapy followed by resection with curative intent compared with patients who went straight to surgery. Furthermore, there was a significant difference in survival, with poorer outcomes for patients who did not receive postoperative postoperative chemotherapy compared with those who received adjuvant chemotherapy. Similarly, histopathological results were also improved, with improved rates of R0 resection with neoadjuvant chemotherapy followed by surgery.
While surgical resection is the only known curative treatment for pancreatic cancer, even in the event of R0 surgical resection there remains a local recurrence rate of 50–80% and a 25–50% chance of developing distant metastases.21–23 To date, the reported effectiveness of adjuvant therapy has been mixed. Various phase three trials investigating the role of adjuvant therapy in pancreatic adenocarcinoma have shown minimal improvements in disease-free and overall survival.22–25 The ESPAC-3 trial investigating the optimal duration and timing of adjuvant therapy in post resection pancreatic cancer demonstrated shortcomings in adjuvant therapy by showing that only two-thirds of patients completed the full course of chemotherapy, and that failure to complete was associated with significantly poorer overall survival (median survival of 28.0 months vs 14.6 months).3 However, the ESPAC-4 trial showed not only an improved overall survival with gemcitabine and capecitabine (28.0 months, 95% CI 23.5–31.5) compared with gemcitabine alone (25.5 months, 95% CI 22.7–27.9; HR 0.82, 95% CI 0.68–0.98, p = 0.032) but also a reduction in adverse events. Additionally, the PRODIGY-24 trial demonstrated significant improvements in disease-free and overall survival with FOLFIRINOX, a promising agent in neoadjuvant strategies compared with gemcitabine alone.4
While adjuvant therapy has been proven to play a beneficial role in the postoperative treatment of pancreatic cancer, the significant morbidity associated with pancreatic resection means that a significant proportion of patients may fail to recover sufficiently from surgery to receive adjuvant chemotherapy within an appropriate treatment window.26 In contrast, neoadjuvant treatment is independent of surgical morbidity and a phase II trial by Heinrich et al demonstrated the safety profile of this approach in 28 patients with resectable cancer receiving gemcitabine and cisplatin for two months before resection.27 This approach showed adequate toleration to systemic therapy with minimal grade III toxicities and a low morbidity rate.27 Histological and cytological responses showed a median survival of 26.5 months.27
Neoadjuvant therapy, an evolving treatment paradigm in pancreatic cancer and already well-established concepts for rectal and gastric cancer,28,29 has numerous potential benefits to address the shortcomings of adjuvant therapies. First, while postoperative morbidity may subsequently preclude adjuvant therapy and reduce survival,30 this limitation is not present when administering preoperative (neoadjuvant) therapy. Second, preoperative therapy is hypothesised to generate free radical agents, which display optimum benefit in a pre-resection, well-oxygenated environment compared with a relatively hypoxic post-surgical environment.31,32 Finally, restaging evaluation following neoadjuvant therapy and before planned surgery to detect any rapidly progressive systemic disease can serve as a means to prognosticate and risk stratify patients to determine those that may or may not benefit from radical surgical approaches. Evans has suggested that approximately 25% of patients who embark on preoperative treatment do not proceed to resection of their primary tumour due to disease progression, thus sparing these patients from the morbidity and mortality associated with surgical intervention.33 There may also be advantages with neoadjuvant therapy with respect to postoperative complications; at least one study reported lower rates of pancreatic leak and leak-associated morbidity and mortality.34
It must be noted that the results of our meta-analysis should be interpreted with the following limitations in mind. Owing to the lack of high-powered randomised controlled trials, the majority of our data have been analysed from retrospective cohort studies. As with most such studies, there is an inherited selection bias risk, despite relatively high study quality, as measured by the Newcastle–Ottawa scale. In this case, one must be particularly cognisant of the risk of selection bias wherein patients who failed to complete or deteriorated during neoadjuvant therapy were not clearly reported in the majority of studies. Excluding patients in the neoadjuvant therapy cohort with toxicity or tumour progression (thereby failing to progress to surgery) in addition to including all patients who had surgery first inappropriately favours neoadjuvant therapy. The included studies are not analysed by the ‘intention-to-treat’ principle and further data from prospective randomised controlled trials are needed to counter these biases.
Further to this, some of the included cohort studies are analyses of large nationwide databases with more than 10,000 patients.11 These studies are given greater weight over smaller studies in our meta-analysis even when using a random-effects model. The results of the meta-analysis therefore reflect the results of these large-scale studies.
Similarly, patients who had neoadjuvant therapy have not been randomised to the intervention in most included studies, so there is probably a high selection bias in this group compared with the group of patients undergoing direct surgery. Additionally, multiple neoadjuvant regimens are included in the meta-analyses. Unfortunately, subgroup analyses for the different regimens was not possible because of the limited availability of specific treatment strategies provided by registries.
In recent years, advancements in pancreatic cancer management has seen the introduction of newer chemotherapeutic regimens such as FOLFIRNOX and gemcitabine-nab-paclitaxel and their subsequent integration into the neoadjuvant treatment approach. In this study we included only two studies using FOLFIRINOX chemotherapy. As more studies become available on this topic, this will help to further delineate the neoadjuvant therapeutic paradigm.
Our meta-analysis is limited by high data heterogeneity and an inability to differentiate between subgroups of patients who did or did not receive adjuvant therapy (independent of neoadjuvant therapy/surgery first), for purposes of analysis. We attempted to limit data heterogeneity by separately assessing adjusted hazard ratio data (via meta-analysis) and non-adjusted data (pooled Kaplan–Meier curves), with both pooled datasets producing similar results. Additional shortcomings include specific limitations of the data sources of some of the studies, such as the SEER registry and Cancer Surveillance Program database, which do not capture information such as chemotherapy regimen used or resectional margin status. However, it should be noted that analysis of published data in the literature examining the benefit of neoadjuvant chemoradiotherapy for resectable pancreatic cancer, no widespread consensus exists regarding the use of radiation and chemoradiotherapy regimens.
Despite the promising findings of our meta-analysis, neoadjuvant therapy does not represent a panacea in treatment of PDAC. A 2010 meta-analysis by Gillen et al found response rates of 29.1% for neoadjuvant therapy in resectable PDAC.35 The same study also reported no significant differences in overall survival between neoadjuvant therapy and no-neoadjuvant therapy patient groups, although over 20 further studies on this topic have since been published (and are included in this review).
Further research is required before neoadjuvant treatment in resectable PDAC can be confidently recommended as the new gold standard. Further randomised trials are desirable to determine both the efficacy of neoadjuvant therapy, but also the most effective neoadjuvant chemotherapy regimen. A recent German randomised controlled trial comparing neoadjuvant therapy with straight-to-surgery for PDAC was terminated early due to poor accrual of patients.36 Going forward, multicentre trials will be required to achieve sufficient sample sizes and successful patient recruitment. To this end, we await the results of the randomised, controlled, multicentre randomised phase III PREOPANC trial by the Dutch Pancreatic Cancer Group,37 and a further multicentre feasibility trial (ESPAC 5F) currently recruiting in the UK.
Conclusion
This review represents a contemporaneous and comprehensive review regarding the role of neoadjuvant therapies in resectable pancreatic using the best available evidence for resection rates and survival estimates. On the basis of current evidence, it would appear that resection and survival rates after neoadjuvant therapy and surgery are improved compared to treatment with surgery alone. The overall five-year survival rate for pancreatic cancer has remained virtually static at approximately 5% since the 1970s, despite surgical, oncological and technological advances over the years. This is in stark contrast to the prognosis of several other solid cancers, which have seen significant improvements in the same time period. There remains a strong need for high-quality randomised prospective studies investigating the role of neoadjuvant therapies in pancreatic cancer.
References
- 1.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993; (1): 68–733. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Stocken DD, Friess H et al. . A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; (12): 1,200–1,210. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Palmer DH, Ghaneh P et al. . Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017; (10073): 1,011–1,024. [DOI] [PubMed] [Google Scholar]
- 4.Edeline J, Bonnetain F, Phelip JM et al. . Gemox versus surveillance following surgery of localized biliary tract cancer: Results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. J Clin Oncol 2017; (4 Suppl): 225.29148892 [Google Scholar]
- 5.Aloia TA, Aloia TE, Lee JE et al. . Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg 2007; (3): 347–355. [DOI] [PubMed] [Google Scholar]
- 6.Iacobuzio-Donahue CA, Fu B, Yachida S et al. . DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009; (11): 1,806–1,813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnerlich JL, Luka SR, Deshpande AD et al. . icroscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg 2012; (8): 753–760. [DOI] [PubMed] [Google Scholar]
- 8.Sohal DPS, Walsh RM, Ramanathan RK, Khorana AA. Pancreatic adenocarcinoma: treating a systemic disease with systemic therapy. J Natl Cancer Inst 2014; (3): dju011. [DOI] [PubMed] [Google Scholar]
- 9.Papalezova KT, Tyler DS, Blazer DG et al. . Does preoperative therapy optimize outcomes in patients with resectable pancreatic cancer? J Surg Oncol 2012; (1): 111–118. [DOI] [PubMed] [Google Scholar]
- 10.Lutfi W, Talamonti MS, Kantor O et al. . Perioperative chemotherapy is associated with a survival advantage in early stage adenocarcinoma of the pancreatic head. Surgery 2016; (3): 714–724. [DOI] [PubMed] [Google Scholar]
- 11.de Geus SWL, Eskander MF, Bliss LA et al. . Neoadjuvant therapy versus upfront surgery for resected pancreatic adenocarcinoma: A nationwide propensity score matched analysis. Surgery 2017; (3): 592–601. [DOI] [PubMed] [Google Scholar]
- 12.Artinyan A, Anaya DA McKenzie S et al. . Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer 2011; (10): 2,044–2,049. [DOI] [PubMed] [Google Scholar]
- 13.Embuscado EE, Laheru D, Ricci F et al. . Immortalizing the complexity of cancer metastasis: genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol Ther 2005; (5): 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dholakia AS, Kumar R, Raman SP et al. . Mapping patterns of local recurrence after pancreaticoduodenectomy for pancreatic adenocarcinoma: a new approach to adjuvant radiation field design. Int J Radiat Oncol Biol Phys 2013; (5): 1,007–1,015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyot P, Ades AE, Ouwens MJNM, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012; : 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J et al. . The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med 2009; (7): e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells G, Shea B, O ’Connell D et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta- analyses. The Ottawa Hospital, 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (cited April 2019). [Google Scholar]
- 18.Higgins JPT, Altman DG, Gøtzsche PC et al. . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; : d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Meijer VE, Kalish BT, Puder M, IJzermans JNM. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg 2010; (9): 1,331–1,339. [DOI] [PubMed] [Google Scholar]
- 20.Tilney HS, Sains PS, Lovegrove RE et al. . Comparison of outcomes following ileostomy versus colostomy for defunctioning colorectal anastomoses. World J Surg 2007; (5): 1,143–1,152. [DOI] [PubMed] [Google Scholar]
- 21.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg 1997; (2): 195–200. [DOI] [PubMed] [Google Scholar]
- 22.Aoyama T, Murakawa M, Katayama Y et al. . Impact of postoperative complications on survival and recurrence in pancreatic cancer. Anticancer Res 2015; (4): 2,401–2,409. [PubMed] [Google Scholar]
- 23.Fischer R, Breidert M, Keck T et al. . Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol 2012; (2): 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oettle H, Post S, Neuhaus P et al. . Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer. JAMA 2007; (3): 267. [DOI] [PubMed] [Google Scholar]
- 25.Hsu CC, Herman JM, Corsini MM et al. . Adjuvant chemoradiation for pancreatic adenocarcinoma: the johns hopkins hospital—mayo clinic collaborative study. Ann Surg Oncol 2010; (4): 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russ AJ, Weber SM, Rettammel RJ et al. . Impact of selection bias on the utilization of adjuvant therapy for pancreas adenocarcinoma. Ann Surg Oncol 2010; (2): 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinrich S, Schäfer M, Weber A et al. . Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity. Ann Surg 2008; (6): 1,014–1,022. [DOI] [PubMed] [Google Scholar]
- 28.Sauer R, Liersch T, Merkel S et al. . Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase iii trial after a median follow-up of 11 years. J Clin Oncol 2012; (16): 1,926–1,933. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham D, Allum WH, Stenning SP et al. . Perioperative Chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; (1): 11–20. [DOI] [PubMed] [Google Scholar]
- 30.Erridge S, Pucher PH, Markar SR et al. . Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg 2017; (11): 1,433–1,442. [DOI] [PubMed] [Google Scholar]
- 31.Evans DB, Rich TA, Byrd DR et al. . Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 1992; (11): 1,335–1,339. [DOI] [PubMed] [Google Scholar]
- 32.Pilepich MV, Miller HH. Preoperative irradiation in carcinoma of the pancreas. Cancer 1980; (9): 1,945–1,949. [DOI] [PubMed] [Google Scholar]
- 33.Douglas B. Resectable pancreatic cancer: the role for neoadjuvant/preoperative therapy. HPB (Oxford) 2006; (5): 365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng T-Y, Sheth K, White RR et al. . Effect of neoadjuvant chemoradiation on operative mortality and morbidity for pancreaticoduodenectomy. Ann Surg Oncol 2006; (1): 66–74. [DOI] [PubMed] [Google Scholar]
- 35.Gillen S, Schuster T, Meyer Zum Büschenfelde C et al. . Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. Seiler C, editor. PLoS Med 2010; (4): e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golcher H, Brunner TB, Witzigmann H et al. . Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer. Strahlenther Onkol 2015; (1): 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Versteijne E, van Eijck CHJ, Punt CJA et al. . Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials 2016; (1): 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Townend P, de Reuver PR, Chua TC et al. . Histopathological tumour viability after neoadjuvant chemotherapy influences survival in resected pancreatic cancer: Analysis of early outcome data. ANZ J Surg 2017; [DOI] [PubMed] [Google Scholar]
- 39.Itchins M, Arena J, Nahm CB et al. . Retrospective cohort analysis of neoadjuvant treatment and survival in resectable and borderline resectable pancreatic ductal adenocarcinoma in a high volume referral centre. Eur J Surg Oncol [Internet]. 2017;1–7. Available from: http://dx.doi.org/10.1016/j.ejso.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Liu G, Wang K et al. . Neoadjuvant radiation followed by resection versus upfront resection for locally advanced pancreatic cancer patients: a propensity score matched analysis. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mokdad AA, Minter RM, Zhu H et al. . Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: A propensity score matched analysis. J Clin Oncol 2017; (5): 515–22. [DOI] [PubMed] [Google Scholar]
- 42.Mirkin KA, Hollenbeak CS, Wong J. Survival impact of neoadjuvant therapy in resected pancreatic cancer: A Prospective Cohort Study involving 18,332 patients from the National Cancer Data Base. Int J Surg [Internet]. 2016; : 96–102. Available from: http://dx.doi.org/10.1016/j.ijsu.2016.08.523 [DOI] [PubMed] [Google Scholar]
- 43.Shubert CR, Bergquist JR, Groeschl RT et al. . Overall survival is increased among stage III pancreatic adenocarcinoma patients receiving neoadjuvant chemotherapy compared to surgery first and adjuvant chemotherapy: An intention to treat analysis of the National Cancer Database. Surgery [Internet]. 2016. October [cited 2017 Oct 7]; (4): 1,080–96. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0039606016302379 [DOI] [PubMed] [Google Scholar]
- 44.Lutfi W, Talamonti MS, Kantor O et al. . Perioperative chemotherapy is associated with a survival advantage in early stage adenocarcinoma of the pancreatic head. Surgery [Internet]. 2016; (3): 714–24. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0039606016302045 [DOI] [PubMed] [Google Scholar]
- 45.Ferrone CR, Marchegiani G, Hong TS et al. . Radiological and Surgical Implications of Neoadjuvant Treatment With FOLFIRINOX for Locally Advanced and Borderline Resectable Pancreatic Cancer. Ann Surg [Internet]. 2015. January [cited 2017 Nov 9]; (1): 12–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25599322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golcher H, Brunner TB, Witzigmann H et al. . Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer. Strahlentherapie und Onkol [Internet]. 2015; (1): 7–16. Available from: http://link.springer.com/10.1007/s00066-014-0737-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casadei R, Di Marco M, Ricci C et al. . Neoadjuvant Chemoradiotherapy and Surgery Versus Surgery Alone in Resectable Pancreatic Cancer: A Single-Center Prospective, Randomized, Controlled Trial Which Failed to Achieve Accrual Targets. J Gastrointest Surg 2015; (10): 1802–12. [DOI] [PubMed] [Google Scholar]
- 48.Roland CL, Yang AD, Katz MHG et al. . Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol 2015; (4): 1,168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujii T, Yamada S, Murotani K. Inverse Probability of Treatment Weighting Analysis of Upfront Surgery Versus Neoadjuvant Chemoradiotherapy Followed by Surgery for Pancreatic Adenocarcinoma with Arterial Abutment. 2015; (39): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sho M, Akahori T, Tanaka T et al. . Optimal indication of neoadjuvant chemoradiotherapy for pancreatic cancer. Langenbeck’s Arch Surg 2015; (4): 477–85. [DOI] [PubMed] [Google Scholar]
- 51.Papavasiliou P, Hoffman JP, Cohen SJ et al. . Impact of preoperative therapy on patterns of recurrence in pancreatic cancer. 2014; 34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tzeng CWD, Cao HST, Lee JE et al. . Treatment Sequencing for Resectable Pancreatic Cancer: Influence of Early Metastases and Surgical Complications on Multimodality Therapy Completion and Survival. J Gastrointest Surg 2014; (1): 16–25. [DOI] [PubMed] [Google Scholar]
- 53.Cooper AB, Holmes HM, Ka J et al. . Role of Neoadjuvant Therapy in the Multimodality Treatment of Older Patients with Pancreatic Cancer. 2014; 111–20. [DOI] [PubMed] [Google Scholar]
- 54.Barugola G,DM, Partelli S, D M, Crippa S, D M et al. . Outcomes after resection of locally advanced or borderline resectable pancreatic cancer after neoadjuvant therapy. 2012; 132–9. [DOI] [PubMed] [Google Scholar]
- 55.Stokes JB, Nolan NJ, Stelow EB et al. . Preoperative Capecitabine and Concurrent Radiation for Borderline Resectable Pancreatic Cancer. Ann Surg Oncol [Internet]. 2011; (3): 619–27. Available from: http://link.springer.com/10.1245/s10434-010-1456-7 [DOI] [PubMed] [Google Scholar]
- 56.Satoi S, Yanagimoto H, Toyokawa H, Takahashi K. Surgical Results After Preoperative Chemoradiation Therapy for Patients With Pancreatic Cancer. 2009; (3): 2–3. [DOI] [PubMed] [Google Scholar]
- 57.Stessin AM, Meyer JE, Sherr DL. Neoadjuvant Radiation Is Associated With Improved Survival in Patients With Resectable Pancreatic Cancer: An Analysis of Data From the Surveillance, Epidemiology, and End Results (SEER) Registry. Int J Radiat Oncol Biol Phys 2008; (4): 1,128–33. [DOI] [PubMed] [Google Scholar]
- 58.Vento P, Mustonen H, Joensuu T et al. . Impact of preoperative chemoradiotherapy on survival in patients with resectable pancreatic cancer. World J Gastroenterol [Internet]. 2007. June 7 [cited 2017 Oct 7]; (21): 2,945–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17589944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Sukhun S, Zalupski MM, Ben-Josef E et al. . Chemoradiotherapy in the treatment of regional pancreatic carcinoma: a phase II study. Am J Clin Oncol. 2003; (6): 543–9. [DOI] [PubMed] [Google Scholar]
- 60.White RR, Hurwitz HI, Morse MA et al. . Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas. Ann Surg Oncol [Internet]. 2001. December [cited 2017 Oct 7]; (10): 758–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11776488 [DOI] [PubMed] [Google Scholar]
- 61.Spitz FR, Abbruzzese JL, Lee JE et al. . Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol [Internet]. 1997. March [cited 2017 Oct 7]; (3): 928–37. Available from: http://ascopubs.org/doi/10.1200/JCO.1997.15.3.928 [DOI] [PubMed] [Google Scholar]





