Abstract
Introduction
Standardised scoring systems for rheumatoid arthritis (RA) joint disease activity include Larsen score for radiographs, rheumatoid arthritis magnetic resonance imaging score (RAMRIS) for MRI and using the European League Against Rheumatisms-Outcome Measures in Rheumatology (EULAR-OMERACT) score for ultrasound (US) images. The aim of this prospective study was to investigate the relationship between histological synovitis and radiological synovitis, assessed by conventional X-ray, US and MRI of the wrist radiocarpal joint.
Methods
20 patients with treatment naive early RA (ERA) and 20 with long-standing RA (LRA) were enrolled in a 6-month prospective study. Patients with RA underwent US-guided synovial biopsy, X-ray and US of the wrist at enrolment and 6 months. MRI at baseline and also at 6 months for the ERA group, and scored with the RAMRIS system. X-ray was scored by Larsen score and US by the EULAR-OMERACT system. Synovial biopsy inflammation was determined by the Krenn score.
Results
In the ERA group at baseline, Krenn score was correlated strongly with both US combined score (r = 0.77 p < 0.001) and MRI synovitis score (r = 0.85 p < 0.001), while uncorrelated at 6 months. In the LRA group at baseline, these scores correlated strongly (r = 0.83, p < 0.001) to moderately (r = 0.61, p = 0.002), and persisted at 6 months for US score (r = 0.81 p < 0.001). For all patients with RA, change in Krenn score between baseline and 6 months was correlated with both change in US combined score (r = 0.65, p < 0.001) and change in MRI synovitis score (r = 0.50, p = 0.03).
Conclusion
The MRI RAMRIS synovitis score and EULAR-OMERACT US scoring system are sensitive measures of histological synovitis in LRA and ERA. After 6 months, this correlation persists in the established RA group, but not in the ERA group. Overall, decreases in MRI/US synovitis are associated with reductions in histological synovitis. The study validates the use of MRI RAMRIS and EULAR-OMERACT US scores as surrogate markers of histological synovitis in established RA and early untreated RA.
Keywords: rheumatoid arthritis, imaging, RAMRIS, EULAR-OMERACT ultrasound score, ultrasound-guided synovial biopsies, synovial pathotypes
Key messages.
What is already known about this subject?
Prospective studies incorporating EULAR-OMERACT ultrasound (US), MRI RAMRIS and radiograph Larsen standardised scores with repeat synovial histological analysis in rheumatoid arthritis (RA) have not been performed.
What does this study add?
This is the first prospective study evaluating in sequential synovial biopsies both the US EULAR-OMERACT and MRI RAMRIS scoring systems in treatment naive early RA and long-standing patients with RA and comparing with histological synovitis.
This study demonstrates that the MRI RAMRIS synovitis score and EULAR-OMERACT US scoring system are sensitive measures of histological synovitis in established RA and early untreated RA. Overall, change in histological synovitis over a 6-month period is correlated to both change in US EULAR-OMERACT score and the MRI RAMRIS scores.
How might this impact on clinical practice?
These findings validate the use of MRI RAMRIS and EULAR-OMERACT US scores as surrogate markers of histological synovitis in established RA and early untreated RA.
Introduction
The study of synovial biopsies from patients with rheumatoid arthritis (RA) has led to breakthroughs in disease understanding and the development of new targeted therapies. Current studies are investigating whether synovial tissue analysis can be used as an instrument for personalised medicine in RA for diagnosis, prognosis and treatment stratification.1 2 The development of ultrasound-guided synovial biopsy (USGSB) as a minimally invasive, safe and well-tolerated technique for retrieving synovial tissue has accelerated research in synovial histopathology.3–5 RA synovial pathology is heterogeneous, with some patients exhibiting high levels of inflammation with infiltrating lymphoid follicles while others have a more diffuse inflammatory alterations, and some almost without inflammation.6 This has led to recent studies proposing at least three different synovial subtypes (pathotypes) of RA, with different histology and gene expression.2 7 The association between baseline synovial pathotype and the joint imaging changes over time is currently unknown.
The development of standardised scoring systems for quantifying inflammatory and/or joint damage in RA by different imagining techniques has been rapidly evolving progressing from scoring systems for severity of erosive damage on conventional X-ray (eg, Larsen score) to more recent developments including the RAMRIS system to score MRI, and the EULAR-OMERACT system for ultrasound (US).8–11 US imaging has been found to have predictive value in relation to detecting subclinical disease, progression of structural damage and early detection of disease flare.12 13 The use of both grey scale (GS) and Doppler US modes have been standardised by the EULAR-OMERACT US group and a scoring system for RA disease activity for each joint has been developed.8
MRI has been shown to be able to predict disease progression in RA, and a system for scoring joint disease activity, RAMRIS, has been developed.9 A recent review on the association between MRI changes in arthritis and synovial pathobiology concluded that there was very limited data on this field and that prospective studies on well-characterised patients at defined stages of disease were needed.14
Importantly, prospective studies integrating all three imaging modalities and incorporating EULAR-OMERACT US, MRI RAMRIS and Larsen standardised scores with synovial histological analysis in RA have not been performed but are critical to not only elucidate the relationship between different imaging outcomes but also the relationship to histological synovitis. Therefore, the aim of this study is to investigate the relationship between histological synovitis and standardised US, MRI and radiographic measures of disease activity and damage.
Methods
Study design
The data presented in this manuscript originate from a prospective longitudinal study (three visits: baseline, 3-month visit and 6-month visit) that was conducted at Odense University Hospital (OUH), Denmark. (ClinicalTrials.gov: NCT02652299). All patients were recruited following written informed consent and the study was reviewed by the regional ethics review board (S-20140062) and the danish data protection agency (2008-58-0035).
Patients
Adult patients with RA were enrolled from Department of Rheumatology, OUH, and adult controls from Department of Orthopaedics, OUH. A total of 43 patients with RA were assessed for eligibility for the study of which three were excluded (two had calcium pyrophosphate deposition disease and one polymyalgia rheumatica). All patients with RA fulfilled ACR/EULAR 2010 classification criteria and had at least one swollen wrist. The group of patients with early RA (ERA group) (n=20) was newly diagnosed (<6 months) and treatment naive, and the long-standing RA (LRA group) had disease duration over 5 years. Treatment during the study period was according to Danish Rheumatological Association and EULAR RA treatment recommendations.15 Patients with high disease activity were offered intramuscular (IM) corticosteroid injection after the USGSB procedure (2 mL Depo-Medrol 40 mg/mL), no intra-articular (IA) injections were given as part of the biopsy procedures. In the LRA group, therapy was stable within 4 weeks prior to the 6 months of biopsy, with no IA/IM steroid, stable oral steroid dose and disease-modifying anti rheumatic drug (DMARD) was unchanged.
Patients in the control group were undergoing routine wrist arthroscopy at the Department of Orthopaedic Surgery, OUH, and had no history of inflammatory joint disease and no signs of inflammatory arthritis at clinical examination or on blood parameters. A total of 18 patients were assessed for eligibility for the study, and two were excluded as no synovial biopsy were retrieved during arthroscopy. The patients in the control group had minor orthopaedic diseases as ganglion cysts or mild osteoarthrosis.
Patient demographics including age, gender, diagnosis, disease duration, smoking status, joint biopsied, rheumatological medication (conventional synthetic DMARDs (csDMARD), biological DMARDs (bDMARDs) and corticosteroid), disease activity score in 28 joints with C-reactive protein (DAS28CRP) and use of IM or IA corticosteroid following biopsy were collected at baseline, 3 months and 6 months. Blood CRP was measured at all visits. Rheumatoid factor (RF) and anti-citrullinated proteins antibodies (ACPAs) were measured at baseline and at 6 months.
Synovial biopsies
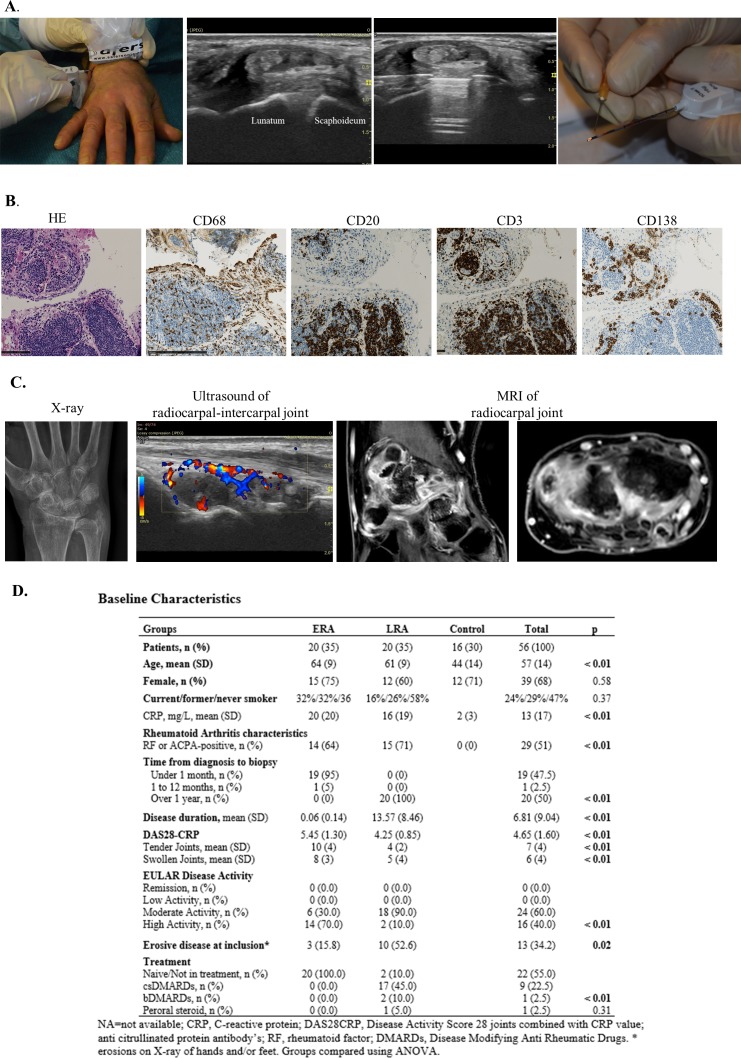
At baseline and 6-month visits, synovial biopsies were obtained by USGSB in a clean procedure room, as illustrated in figure 1A and previously described.3 All biopsies were taken from the same wrist at baseline and 6 months. Briefly, local anaesthetic was injected into the soft tissue up to the joint capsule and into the joint space and a Quick-Core biopsy needle (16-gauge; Cook Medical) was then guided by US and placed within the joint capsule to retrieve synovium. The biopsies were taken over the scaphoid and lunate junction in the radiocarpal joint (RCJ) of the wrist (figure 1A).
Figure 1.
Synovial biopsy procedure, histopathology, imaging and baseline characteristics. (A) The ultrasound-guided synovial biopsy procedure. (B) and (C) Visual comparison of imaging and synovial inflammation in a patient with RA where all imagning and biopsy procedure were done within 2 hours. Synovial biopsy staining showing typical lymphoid pathotype with lymphoid aggregates and positive staining for CD20, CD138 and CD3. Hand X-ray, Doppler US of the radiocarpal and intercarpal joints of the wrist and contrast-enhanced MRI of the wrist (one coronal and one sagittal view). (D) Baseline characteristics. ACPA, anti-citrullinated proteins antibody; ANOVA, analysis of variance; ERA, early rheumatoid arthritis; LRA, long-standing rheumatoid arthritis.
Control group synovial biopsies from the wrist were collected by traditional arthroscopy in an operating theatre, as previously described.16 Briefly, patients underwent general anaesthesia and up to 5 kg of traction across the wrist. Two standard arthroscopic portals were inserted into the RCJ, one for arthroscopic visualisation and the other for instrumentation. Synovial biopsies were obtained under direct visual inspection, using a Quick-Core biopsy needle (16-gauge; Cook Medical). A minimum of six synovial biopsies were retrieved per USGSB or arthroscopic procedure.
Half of the obtained biopsies were paraffin embedded and stained with haematoxylin and eosin, CD3 (T cells), CD20 (B cells), CD68 (macrophages) and CD138 (plasma cells) (figure 1B), as previously described.17 Only samples with intact lining layer were used for grading of tissue inflammation. Synovial tissue inflammation was quantified by application of the previously validated synovitis (Krenn) score (0–9), semiquantative scores (0–4) for CD3, CD20, CD68 and CD138 and by determining the dominant pathotype of the biopsies as either lymphoid (L), myeloid (M) or fibroid (F).6 18–20 The Krenn score was calculated as an average of three different biopsies in each patient, by scorer (SAJ) blinded for patient ID and patient group. Before and after scoring, agreement with a second observer (HDS) regarding score and sufficient quality of biopsies in selected samples was reached. Synovial pathotypes were described as fibroid defined as CD68 located in the sublining layer and CD3-CD20-CD138≤1; myeloid CD68 ≥2 and CD20 <2/CD138<2, and lymphoid CD20 ≥2 or CD138≥2.
Imaging
Synovial biopsies where obtained in the RCJ of the wrist over the scaphoid lunate junction (figure 1A). We therefore compared synovial tissue inflammation with US EULAR-OMERACT score of the RCJ intercarpal joint and the MRI RAMRIS RCJ score (figure 1C). For an overview of the Larsen, RAMRIS and EULAR-OMERACT scoring systems for the wrist, please see online supplementary figure S1.
rmdopen-2019-000951supp001.docx (36.2KB, docx)
Radiographic scoring
X-ray of hands and feet, measured at baseline and 6 months, were evaluated by the Larsen score system in time sequential order (wrist divided into four quadrants, with score from 0 to 5 in each) (online supplementary material figure S1).11 X-ray was evaluated in time sequential order by consultant in Radiology (TT) blinded for clinical data, US results and biopsy disease activity.
MRI
MRI of the hand and wrist on the side of the USGSB procedure was performed immediately prior (maximum 2 hours) to synovial biopsy, at inclusion and at 6 months for the ERA group and at inclusion for the LRA group and orthopaedic controls. Synovitis erosions and bone marrow oedema in the RCJ and distal radioulnar (RU) joint were scored in sequential order according to the RAMRIS system by a consultant in radiology (TT), blinded for clinical, US and histological data. MRI was performed with coronal short tau inversion recovery (STIR) and T1 water selective scan (WATS) and T1 WATS coronal and axial after gadolinium on a Philips Panorama 1T or Philips Ingenia 3T scanner using gadolinium contrast, in accordance with department guidelines for the patients with RA.
US imaging
Doppler US scanning of the hand and wrist was performed on the same side as the USGSB procedure by a rheumatologist (SAJ) experienced in musculoskeletal US, at baseline, 3 months and 6 months, immediately prior to USGSB. Using a General Electrics (GE) Logiq 9 US machine with a 4–15 MHz linear transducer (ML6-15, GE). Colour Doppler (CD), and not power Doppler (PD), was used as per EULAR-OMERACT scoring system guidelines when working with machines where CD is more sensitive than PD.12 21 The CD signal gain was set to a sensitivity just below the disappearance of colour noise. The evaluation for synovitis of the wrist was done following the EULAR-OMERACT US positions of the probe and scores for synovial hypertrophy (SH) (0–3) and blood flow in the synovium by CD (scoring from 0 to 3) was collected and finally a combined score (0–3) generated.8 The two scanning positions of the wrist were the longitudinal RU and the longitudinal RCJ intercarpal views.22 US scan images from 15 patients were also evaluated blindly by another US expert (HLI) reaching a kappa value of 0.83, when comparing to scores by SAJ.
Statistics
Cohen’s kappa statistic was used to assess the inter-rater agreement on evaluation of wrist US scans. Categorical variables were presented as numbers and percentages, and continuous variables as means with SD. To compare baseline characteristics, categorical variables between patient groups were analysed using χ2 test, and numerical variables between patient groups were analysed using two-sample t-tests, and analysis of variance, when appropriate. Tests for trends of values across the visits of the study (eg, CRP) were performed by linear regression with cluster robust standard errors. Comparisons over time of treatment, disease activity, wrist imaging data and synovial biopsy data among all patients with RA were performed using multinomial logistic regression with cluster robust standard errors for categorical variables, and linear regression with cluster robust standard errors for numerical variables.23 Pairwise correlations between synovial biopsy inflammation and disease activity registered using US or MRI scans were performed using unadjusted linear regression with cluster robust standard errors. Robust standard errors were used in the regression analyses to account for minor deviations from the model assumptions, and cluster robust standard errors were used to further account for repeated measurements in patients. Comparing numbers of IM and IA corticosteroid injections between the two RA groups during the study was done with Poisson regression with cluster robust standard errors. P values <0.05 were taken as statistically significant. Data were analysed on Stata V.15 (StataCorp, TX, USA).
Results
Patients
Data from a total of 96 synovial biopsy procedures were included from 56 patients (20 ERA, 20 LRA and 16 controls). Baseline characteristics are presented in figure 1D. When comparing the ERA, LRA and control group significant differences in age, CRP level and RF and/or ACPA positivity (all p<0.01, all estimates lowest in the control group) were demonstrated. The ERA had significantly higher disease activity and CRP level (both p<0.01) compared with the LRA group while there was higher prevalence of erosive disease in the LRA group (p=0.02). All patients in the ERA group were treatment naive and two in the LRA group had declined active therapy.
Treatments with csDMARD, bDMARDs and corticosteroid during the study period are presented in detail in online supplementary material table S2. Of the patients with ERA, 70% were taking methotrexate monotherapy at 6 months and 15% a combination of or other csDMARDS, 10% had stopped treatment due to personal preference and 5% declined any treatment during the study. Of the patients with LRA, five patients were treated with a bDMARD by 6 months and three commenced treatment during the course of the study. At 6 months, both groups had one patient taking oral corticosteroid treatment.
Immediately after the first synovial biopsy, 85% (17/20) versus 45% (9/20) in the ERA and LRA groups, respectively, accepted IM corticosteroid. There was no difference in the number of patients receiving oral or parenteral (IM or IA) steroid during the study between the ERA and LRA groups (online supplementary table S1).
Change in disease activity, imaging scores and histopathological assessments between baseline and 6 months
We next evaluated changes in disease activity, X-ray, MRI and US scores and histopathological assessments during the course of the study in the ERA and LRA groups. We demonstrated a significant reduction in DAS28CRP in both groups (p<0.001) although CRP was only significantly reduced in the ERA group (p=0.01) (table 1). We found no significant progression in Larsen score of the wrist on X-ray during the study period in neither group (table 1).
Table 1.
Disease activity, imaging and histopathology baseline and 6 months
| ERA group | Control group* | |||||
| Visit Baseline |
Visit 3months |
Visit 6months |
P value† | Baseline | P value‡ | |
| N | 20 | 19 | 20 | 16 | ||
| DAS28CRP, mean (SD) | 5.2 (1.0) | 2.9 (1.2) | 2.7 (1.1) | < 0.001 | ||
| CRP, mean (SD) | 20.4 (19.8) | 12.0 (13.7) | 8.7 (12.8) | 0.01 | 1.8 (2.3) | < 0.001 |
| Wrist Larsen score, mean (SD) | 0.3 (0.7) | 0.3 (0.8) | 0.91 | 0.0 (0.0) | 0.09 | |
| US EULAR-OMERACT score | ||||||
| Radiocarpal intercarpal joints combined score (0–3) | 2.1 (0.8) | 1.9 (0.7) | 1.6 (0.6) | 0.07 | ||
| SH score (0–3) | 1.9 (0.8) | 1.9 (0.7) | 1.6 (0.6) | 0.17 | ||
| CD score (0–3) | 1.7 (1.0) | 1.2 (0.9) | 1.0 (0.7) | 0.001 | ||
| MRI RAMRIS score | ||||||
| Radiocarpal joint | ||||||
| Synovitis (0–3)§ | 1.1 (0.9) | 0.8 (0.5) | 0.02 | 0.3 (0.5) | 0.003 | |
| Bone erosions (0–10) | 0.2 (0.4) | 0.1 (0.4) | 1.00 | 0.0 (0.0) | 0.08 | |
| Bone oedema (0–3) | 0.1 (0.3) | 0.1 (0.2) | 0.31 | 0.00 (0.00) | 0.16 | |
| Synovial biopsy Krenn score |
||||||
| Lining cell layer, mean (SD) | 2.1 (0.8) | 1.8 (0.7) | 0.19 | 1.6 (0.9) | 0.09 | |
| Stromal activation, mean (SD) | 2.1 (0.6) | 1.6 (0.6) | 0.02 | 1.4 (0.6) | 0.002 | |
| Infiltrates, mean (SD) | 1.9 (0.7) | 1.6 (0.8) | 0.27 | 1.3 (0.6) | 0.02 | |
| Total score, mean (SD) | 6.0 (1.5) | 5.0 (1.5) | 0.01 | 4.2 (1.7) | 0.002 | |
| Specific markers | ||||||
| CD20, mean (SD) | 1.2 (1.1) | 0.8 (09) | 0.06 | 0.3 (0.5) | 0.001 | |
| CD3, mean (SD) | 2.0 (1.2) | 1.9 (0.9) | 0.70 | 0.7 (0.7) | < 0.001 | |
| CD68, mean (SD) | 2.8 (0.9) | 2.4 (0.8) | 0.19 | 1.6 (0.8) | < 0.001 | |
| CD138, mean (SD) | 1.3 (1.2) | 1.1 (0.9) | 0.45 | 0.3 (0.6) | 0.002 | |
| LRA group | ||||||
| DAS28CRP, mean (SD) | 4.3 (0.6) | 3.2 (1.0) | 2.6 (1.1) | < 0.001 | ||
| CRP, mean (SD) | 15.6 (18.9) | 10.3 (11.4) | 10.3 (16.3) | 0.08 | 1.8 (2.2) | 0.002 |
| Wrist Larsen score, mean (SD) | 3.7 (5.5) | 3.7 (5.6) | 0.49 | 0.0 (0.0) | 0.006 | |
| US EULAR-OMERACT score | ||||||
| Radiocarpal intercarpal joints combined score (0–3) | 2.3 (0.8) | 2.1 (0.9) | 1.9 (0.8) | 0.17 | ||
| SH (0–3) | 2.1 (0.8) | 2.0 (0.9) | 1.9 (0.8) | 0.24 | ||
| CD (0–3) | 2.0 (1.0) | 1.6 (1.0) | 1.3 (1.1) | 0.02 | ||
| MRI RAMRIS score | ||||||
| Radiocarpal joint | ||||||
| Synovitis (0–3) | 2.0 (1.2) | 0.3 (0.5) | < 0.001 | |||
| Bone erosions (0–10) | 1.2 (1.3) | 0.0 (0.0) | < 0.001 | |||
| Bone oedema (0–3) | 0.3 (0.7) | 0.0 (0.0) | 0.08 | |||
| Synovial biopsy Krenn score |
||||||
| Lining cell layer, mean (SD) | 2.3 (0.8) | 2.1 (0.8) | 0.24 | 1.6 (0.9) | 0.01 | |
| Stromal activation, mean (SD) | 2.2 (0.8) | 1.9 (0.7) | 0.27 | 1.4 (0.6) | 0.003 | |
| Infiltrates, mean (SD) | 2.2 (0.7) | 2.2 (0.5) | 0.77 | 1.3 (0.6) | < 0.001 | |
| Total score, mean (SD) | 6.8 (1.9) | 6.1 (1.6) | 0.17 | 4.2 (1.7) | < 0.001 | |
| Specific markers | ||||||
| CD20, mean (SD) | 1.8 (1.5) | 1.5 (1.2) | 0.40 | 0.3 (0.5) | < 0.001 | |
| CD3, mean (SD) | 2.5 (1.2) | 2.2 (1.0) | 0.48 | 0.8 (0.7) | < 0.001 | |
| CD68, mean (SD) | 3.0 (0.7) | 2.8 (0.8) | 0.39 | 1.6 (0.8) | < 0.001 | |
| CD138, mean (SD) | 2.1 (1.2) | 2.0 (1.3) | 0.74 | 0.3 (0.6) | < 0.001 | |
Significant p values in bold.
*Six patients in control group had MRI without contrast.
†Test for trend.
‡P value. The same control group values versus, respectively, ERA and LRA baseline.
§One in ERA group, no MRI due to claustrophobia.
CD, colour Doppler; CRP, C-reactive protein; DAS28CRP, disease activity score in 28 joints with CRP level included; ERA, early rheumatoid arthritis; LRA, long-standing rheumatoid arthritis; SH, synovial hypertrophy.
In both the ERA and LRA groups, there was a significant reduction in the US Doppler score (both with a mean reduction of 0.7, respectively (p=0.001 and p=0.02)) during the study period, while the decrease in the total score was not significant (p=0.07 and p=0.17).
When evaluating wrist RCJ RAMRIS scores at baseline, we demonstrated a significantly higher level of synovitis and bone erosions, but not bone oedema (p=0.03, p<0.01 and p=0.3) in the LRA compared with the ERA group at baseline (table 1). In the ERA group, in which paired baseline and 6 months MRI scans were performed, we found a significant decline in synovitis (p=0.02), but not in bone erosions or bone oedema (table 1).
Next, we evaluated the baseline histopathological scores of the synovial biopsies in the ERA, LRA and control groups. We demonstrated a significantly lower level of synovitis (mean Krenn total score ERA: 6.00, LRA: 6.75 vs control 4.19) and infiltration by CD20, CD3, CD68 and CD138-positive cells in the control versus ERA and LRA groups (p<0.001, table 1) but no difference between ERA and LRA groups (data not shown).
In the ERA and LRA groups, in which sequential synovial biopsies were available, we demonstrated a significant reduction in the synovitis score in the ERA (p=0.01), but not the LRA group (p=0.17) at 6 months (table 1). There was no reduction in specific marker scores in neither the ERA nor LRA group over the study period (table 1).
Pairwise correlations between disease activity, synovial inflammation, US EULAR-OMERACT and MRI RAMRIS scores: baseline and 6-month visits
To explore the relationship between disease activity, histological synovitis and radiological scores further, we calculated pairwise correlations between these scores for both the ERA and LRA groups at baseline and 6-month visits (table 2). In the baseline ERA and LRA groups, the Krenn score correlated with both the EULAR-OMERACT US combined score (r=0.77 p<0.001 and r=0.83 p<0.001) and with the RAMRIS MRI synovitis score (r=0.85 p<0.001 and r=0.61, p=0.002) (table 3). At 6 months in the ERA group, significant correlations were not seen while in the LRA group, in which only US data were available, significant correlations persisted (r=0.81 p<0.001) (table 2). Due to the non-significant correlations between imagining measures and Krenn score in the ERA group at 6-month data, detailed analyses of the correlations between the Krenn score components (inflammatory infiltrates, lining cell layer and synovial stroma) and imagining data were performed, see Supplementary material table S5. The results show that in the ERA 6-month data, synovial stroma score is correlated to EULAR-OMERACT US combined score (r=0.54, p<0.01). Further in the ERA 6-month group inflammatory infiltrates is correlated to MRI bone oedema (r=0.46, p<0.01).
Table 2.
Pairwise correlation between DAS28CRP, Krenn score, EULAR-OMERACT US scores and RAMRIS MRI scores of the RCJ of the wrist. Baseline correlations (below the diagonal) and 6-month data (above the diagonal)
| ERA | DAS28-CRP | Krenn score | Larsen score* | EO US combined | EO US Doppler | EO US GS | MRI synovitis | MRI erosion | MRI bone oedema |
| DAS28CRP | – | r=0.34 p=0.09 | r=0.04 p=0.82 | r=−0.14 p=0.57 |
r=−0.22 p=0.31 |
r=−0.15 p=0.51 |
r=0.10 p=0.72 |
r=−0.21 p=0.34 |
r=−0.24 p=0.33 |
| Krenn score | r=0.32 p=0.09 | – | r=0.00 p=1.00 |
r=0.18 p=0.38 | r=0.10 p=0.72 |
r=0.18 p=0.44 |
r=0.14 p=0.65 | r=−0.10 p=0.80 |
r=−0.48 p=0.24 |
| Larsen X-ray score* | r=0.09 p=0.68 | r=0.13 p=0.19 | – | r=0.03 p=0.90 |
r=−0.40 p= 0.03 |
r=0.03 p=0.90 |
r=0.27 p=0.14 | r=−0.19 p=0.09 |
r=−0.10 p=0.34 |
| EO US combined score | r=0.12 p=0.34 |
r=0.77 p< 0.001 | r=0.23 p=0.21 |
– |
r=
0.62
p= 0.001 |
r=1† p< 0.001 | r=0.17 p=0.11 |
r=
0.26
p= 0.01 |
r=
0.14
p= 0.02 |
| EO US Doppler score | r=0.08 p=0.66 |
r=
0.72
p< 0.001 |
r=0.14 p=0.17 | r=0.88 p< 0.001 | – |
r=
0.62
p< 0.001 |
r=
0.41
p= 0.02 |
r=
0.40
p< 0.001 |
r=
0.32
p< 0.001 |
| EO US GS score | r=0.19 p=0.24 |
r=
0.86
p< 0.001 |
r=0.29 p=0.08 |
r=0.92 p< 0.001 |
r=
0.77
p< 0.001 |
– | r=0.27 p=0.14 |
r=
0.26
p= 0.01 |
r=
0.14
p= 0.02 |
| MRI synovitis score | r=0.23 p=0.25 | r=0.85 p< 0.001 |
r=
0.37
p= 0.002 |
r=
0.73
p< 0.001 |
r=
0.68
p< 0.001 |
r=
0.83
p< 0.001 |
– |
r=
0.44
p= 0.03 |
r=0.09 p=0.11 |
| MRI erosion score | r=0.10 p=0.36 | r=0.00 p=1.00 | r=−0.18 p=0.09 | r=−0.03 p=0.91 | r=0.11 p=0.60 | r=0.03 p=0.91 |
r=0.08 p=0.60 |
– | r=0.55 p=0.26 |
| MRI bone oedema score | r=0.09 p=0.36 | r=0.13 p=0.43 | r=−0.15 p=0.17 | r=0.20 p=0.25 | r=0.27 p=0.07 | r=0.25 p=0.06 | r=0.12 p=0.49 |
r=
0.79
p= 0.03 |
– |
| LRA | |||||||||
| DAS28CRP | – | r=0.45 p= 0.04 | r=0.32 p=0.16 |
r=
0.57
p= 0.01 |
r=
0.62
p= 0.002 |
r=
0.50
p= 0.003 |
NA | NA | NA |
| Krenn score | r=−0.03 p=0.87 | – | r=0.20 p=0.53 |
r=
0.81
p< 0.001 |
r=
0.63
p= 0.002 |
r=
0.77
p< 0.001 |
NA | NA | NA |
| Larsen X-ray score* | r=0.06 p=0.75 | r=0.42 p=0.08 | – | r=0.37 p=0.17 |
r=
0.56
p= 0.01 |
r=0.24 p=0.35 | NA | NA | NA |
| EO US combined score | r=0.36 p=0.14 | r=0.83 p< 0.001 |
r=
0.44
p= 0.02 |
– |
r=
0.72
p< 0.001 |
r=
0.96
p< 0.001 |
NA | NA | NA |
| EO US Doppler score | r=0.35 p=0.09 |
r=
0.75
p< 0.001 |
r=0.38 p=0.06 |
r=
0.82
p< 0.001 |
– |
r=
0.65
p< 0.001 |
NA | NA | NA |
| EO US GS score | r=0.35 p=0.13 |
r=
0.79
p< 0.001 |
r=0.16 p=0.51 |
r=
0.93
p< 0.001 |
r=
0.73
p< 0.001 |
– | NA | NA | NA |
| RAMRRIS synovitis score | r=0.42 p= 0.04 | r=0.61 p= 0.002 |
r=
0.48
p= 0.003 |
r=
0.72
p= 0.002 |
r=
0.74
p< 0.001 |
r=
0.58
p= 0.004 |
– | NA | NA |
| MRI erosion score | r=0.42 p=0.10 |
r=0.27 p=0.13 |
r=
0.59
p= 0.004 |
r=
0.51
p= 0.008 |
r=
0.53
p= 0.008 |
r=0.31 p=0.13 |
r=
0.55
p= 0.004 |
– | NA |
| MRI bone oedema score | r=0.27 p=0.37 |
r=0.13 p=0.32 |
r=
0.37
p= 0.03 |
r=
0.32
p= 0.01 |
r=
0.29
p= 0.01 |
r=0.27 p=0.06 |
r=0.37 p=0.07 |
r=
0.75
p= 0.01 |
– |
Comparisonby unadjusted linear regression with robust cluster estimation. NA: Not available, as the LRA did not have 6-month MRI. Significant correlations in bold.
*Total Larsen wrist score (all quadrants).
†The combined US scores are the same as the GS scores in the ERA 6-month visit group.
DAS28CRP, disease activity score in 28 joints with CRP level included; EO, EULAR-OMERACT; ERA, early rheumatoid arthritis; GS, grey scale; LRA, long-standing rheumatoid arthritis; RCJ, radiocarpal joint; US, ultrasound.
Table 3.
Pairwise correlation between DAS28CRP, Krenn score, EULAR-OMERACT US combined score and RAMRIS MRI synovitis score of the RCJ of the wrist
| All patients and all visits | Krenn score | Larsen X-ray score* | EULAR-OMERACT US combined score | RAMRIS MRI synovitis score |
| DAS28CRP | r=0.31 p= 0.002 | r=−0.02 p=0.77 |
r=
0.27
p= 0.01 |
r=
0.26
p= 0.01 |
| Krenn score | – |
r=
0.35
p= 0.03 |
r=
0.72
p< 0.001 |
r=
0.66
p< 0.001 |
| Larsen X-ray score* | – |
r=
0.35
p= 0.02 |
r=
0.53
p< 0.001 |
|
| EULAR-OMERACT US combined score | – |
r=
0.61
p< 0.001 |
Significant correlations in bold. Comparison by unadjusted linear regression with clusterrobust standard errors.
*Total Larsen wrist score (all quadrants)
DAS28CRP, disease activity score in 28 joints with CRP level included; RCJ, radiocarpel joint; US, ultrasound.
Larsen score was not correlated with Krenn score at any point in any group
In the ERA and LRA groups, at baseline, the RAMRIS MRI synovitis score was correlated to EULAR-OMERACT US combined score (table 2, r=0.73, p<0.001 and r=0.72, p=0.002). In the ERA group, in which paired US and MRI data were available at 6 months, a weak correlation between combined US score and MRI erosions and bone marrow oedema (table 2, r=0.40 and r=0.32, both p<0.05) was observed while US Doppler also correlated to MRI synovitis (table 2, r=0.41 p=0.02).
Next, we evaluated whether the relationship between histological synovitis and MRI and US scores persisted if all patients at all visits were combined. Therefore, we first combined data for all visits and all patients (table 3), and second segregated patients into ERA and LRA groups combining baseline and 6-month visits (figure 2).
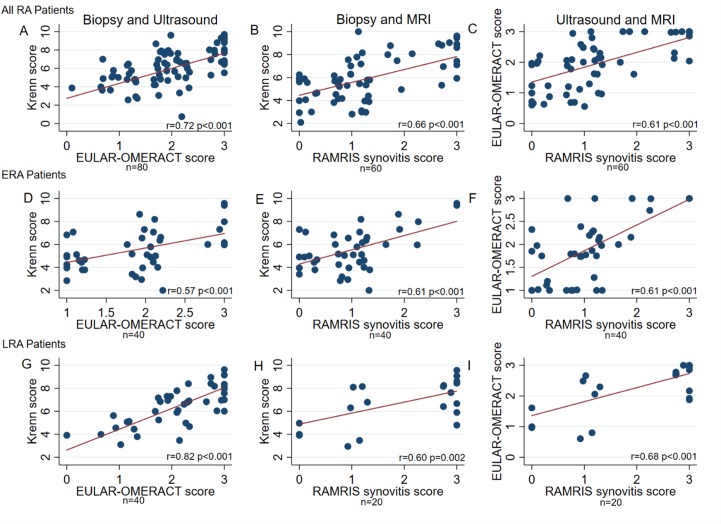
Figure 2.
RCJ of the wrist pairwise comparisons of synovial biopsy inflammation, US EULAR-OMERACT combined score and MRI RAMRIS synovitis score in all patients with RA, and in patients with ERA and LRA separately. Legend: Comparison between inflammation in synovial biopsies using the Krenn score, combined US EULAR-OMERACT score and the MRI RAMRIS synovitis score in the RCJ of the wrist of all patients with RA (A,B,C), and hereafter divided into ERA (D,E,F) and LRA (G,H,I) patient groups. Comparison by unadjusted linear regression with robust cluster estimation. ERA, early RA, LRA, long-standing RA; RA, rheumatoid arthritis; RCJ, radiocarpal joint.
We initially compared imaging scores and histological synovitis scores. We found a significant and strong correlation between histological synovitis scores and RCJ EULAR-OMERACT US combined score (r=0.72, table 3), a relationship that persisted if the ERA and LRA groups were considered separately (ERA group, r=0.57 (figure 2D) and LRA, r=0.82 (figure 2G)); all p<0.001). In addition, when comparing the EULAR-OMERACT US CD and SH scores in all patients, we demonstrated significant correlations with the synovitis score (CD r=0.63 and GS r=0.71; p<0.01), a relationship that again persisted if the ERA and LRA groups were considered separately (ERA: CD r=0.53 and SH r=0.6; LRA: CD r=0.69 and SH r=0.78; all p<0.01).
When comparing the MRI RCJ RAMRIS synovitis score for all patients, we found a moderate correlation with Krenn score (r=0.66, figure 2B), which was also seen if the ERA and LRA groups were considered separately (r=0.61 and LRA r=0.60 (figure 2E, H), p<0.01). We found no correlation between RCJ MRI RAMRIS bone marrow oedema score and histological synovitis (overall r=0.18, p=0.26, ERA r=0.08, p=0.41, LRA r=0.13, p=0.39). RCJ RAMRIS erosion score was weakly correlated to histological synovitis score (r=0.30, p=0.03). In the orthopaedic control group, Krenn score was not correlated to MRI RAMRIS synovitis score (r=0.40, p=0.20), and neither was MRI oedema or erosion scores (data not shown).
Finally, we demonstrated a significant correlation between EULAR-OMERACT US score and MRI RAMRIS synovitis score (r=0.61, p<0.001; figure 2C) which was also seen if the ERA and LRA groups were considered separately (r=0.61 and r=0.68; both p<0.001, figure 2F, I). Synovitis on MRI correlated moderately to strongly to US score components SH and CD (overall: CD r=0.70 and SH r=0.64, ERA: CD r=0.63 and SH r=0.67, LRA: CD: r=0.74 and SH: r=0.58; all p<0.01).
Overall, our results suggest that both US and MRI synovitis are robust measures of histologic synovitis and that both MRI and US are strongly correlated with Krenn score.
Pairwise correlations between differences in clinical, imaging and histological scores between baseline and 6 months
To determine whether significant correlations existed between changes in clinical, imaging and histological scores between baseline and 6 months, we calculated pairwise correlations between the delta changes (6-month baseline) for each score (table 4).
Table 4.
Pairwise correlation between change in DAS28CRP, Krenn score, EULAR-OMERACT US combined score and RAMRIS MRI synovitis score of the RCJ of the wrist during the study
| All patients with RA All visits |
ΔKrenn score | ΔLarsen score* | ΔEO US combined | ΔEO US Doppler | ΔEO US GS | ΔMRI synovitis† | ΔMRI erosions | ΔMRI BM oedema |
| ΔDAS28CRP | r=0.25 p=0.12 |
r=0.18 p=0.29 |
r=
0.38
p= 0.02 |
r=
0.45
p= 0.004 |
r=
0.35
p= 0.02 |
r=
0.52
p= 0.02 |
r=−0.12 p=0.61 |
r=0.34 p=0.16 |
| ΔKrenn score | – | r=0.01 p=0.95 |
r=
0.65
p< 0.001 |
r=
0.51
p< 0.001 |
r=
0.63
p< 0.001 |
r=
0.50
p= 0.03 |
r=−0.20 p=0.39 |
r=0.14 p=0.55 |
| ΔLarsen score* | – | r=0.06 p=0.71 |
r=−0.04 p=0.81 |
r=0.05 p=0.77 |
r=0.14 p=0.58 |
r=0.06 p=0.82 |
r=0.06 p=0.81 |
|
| ΔEO US combined | – |
r=
0.66
p< 0.001 |
r=
0.96
p< 0.001 |
r=
0.59
p< 0.001 |
r=−0.52 p= 0.02 |
r=−0.11 p=0.67 |
||
| ΔEO US Doppler | – |
r=
0.62
p< 0.001 |
r=
0.61
p= 0.005 |
r=−0.38 p=0.09 |
r=0.07 p=0.76 |
|||
| ΔEO US GS | – |
r=
0.67
p= 0.001 |
r=−0.53 p= 0.02 |
r=−0.08 p=0.74 |
||||
| ΔMRI synovitis† | – | r=−0.27 p=0.24 |
r=−0.15 p=0.54 |
|||||
| ΔMRI erosions | – | r=0.00 p=1.00 |
ΔChange between 6-month visit and baseline visit data.
Comparison by unadjusted linear regression.
*Total Larsen wrist score (all quadrants).
†Only the ERA group underwent two MRI scans. Significant correlations in bold.
BM, bone marrow; EO, EULAR-OMERACT;ERA, early rheumatoid arthritis; GS, grey scale; RCJ, radiocarpal joint; US, ultrasound.
Change in DAS28CRP was weakly correlated with change in RCJ combined EULAR-OMERACT US (r=0.38, p=0.02) and moderately with change in RAMRIS MRI synovitis score (r=0.52, p=0.02), but not with change in Krenn score (r=0.25, p=0.12) nor Larsen score (r=0.18, p=0.29) (table 4).
Change in Krenn score was moderately correlated with change in combined EULAR-OMERACT US score (r=0.65, p<0.001) and with change in RAMRIS MRI synovitis score (r=0.50, p=0.03) (table 4). No significant correlation was found between delta RAMRIS bone marrow oedema or erosion scores and histological synovitis (table 4). Change in wrist Larsen score was not correlated to any of the disease activity measures.
The wrists radioulnar joint
As all analyses so far have been performed on the RCJ (location of synovial tissue sampling) to determine whether a similar relationship existed in another location within the wrist joint, we also evaluated the relationship between US and MRI scores in the radioulnar (RU) joint of the wrist. Between baseline and 6 months, we demonstrated a significant reduction in EULAR-OMERACT US combined score and MRI RAMRIS synovitis score in the ERA group (p=0.006 and p=0.02, respectively, Supplementary Table S2). In the LRA group, in which only US data were available at 6 months, we found no significant reduction in the EULAR-OMERACT US combined score (Supplementary Table S2). For all patients and all visits, we found a moderate correlation between the combined EULAR-OMERACT US score and distal ulna MRI RAMRIS synovitis score of overall r=0.58 (p<0.001), ERA r=0.26 (p=0.09) and LRA r=0.85 (p<0.001).
Synovial pathotypes, disease activity and imaging scores
Finally, we evaluated the relationship between synovial pathotypes and clinical disease activity and radiological scores, respectively. We found a significantly higher number of fibroid (F) (9 vs 3 and 2) and lower number of lymphoid (L) pathotype (1 vs 10 and 13) within the control versus ERA and LRA groups. No significant difference in pathotype distribution between ERA and LRA groups were found at baseline (p=0.15). In total, 42.5% (17/40) patients changed pathotype during the study period (online supplementary table S3).
When evaluating clinical disease activity measures, we found a significant difference in level of CRP at baseline and 6 months, with the lowest level in the fibroid group, which was not found in controls (table 5, Baseline: p=0.02, 6 months: p=0.01, and control group: p=0.39). We found no differences in CRP between lymphoid and myeloid (M) pathotypes at baseline or 6 months (p=0.58 and p=0.52, respectively). At baseline, there was significant differences in Larsen score between the fibroid and lymphoid, but not between the myeloid and lymphoid pathotypes (table 5, overall: p=0.02, L vs M p=0.42 and L vs F p=0.01). No differences in CRP were found between the lymphoid and myeloid pathotypes at baseline or at 6 months (p=0.43 and p=0.52, respectively).
Table 5.
Pathotype, disease activity and radiocarpal wrist joint imagining and biopsy data
| Pathotype | All patients with RA | Control group | ||||||||||
| Baseline biopsy (n=40) | 6-month biopsy (n=40) | Baseline (n=16) | ||||||||||
| Lymphoid | Myeloid | Fibroid | P value | Lymphoid | Myeloid | Fibroid | P value | Lymphoid | Myeloid | Fibroid | P value | |
| n (%) | 23 (57.5) | 12 (30.0) | 5 (12.5) | 16 (40.0) | 22 (55.0) | 2 (5.0) | 1 (6.3) | 6 (37.5) | 9 (56.3) | |||
| C-reactive protein, mean (SD) | 20.9 (22.3) | 17.5 (14.0) | 6.4 (7.0) | 0.02 | 11.6 (18.5) | 8.3 (11.4) | 2.3 (0.4) | 0.01 | 1.0 (.) | 2.8 (3.5) | 1.1 (0.6) | 0.39 |
| DAS28CRP, mean (SD) | 4.9 (0.9) | 4.6 (1.0) | 4.5 (1.2) | 0.63 | 2.9 (1.1) | 2.5 (1.1) | 1.6 (0.0) | < 0.01 | ||||
| ACPA and/or RF positive, n (%) | 17 (74) | 7 (58) | 4 (80) | 0.57 | 12 (75) | 16 (73) | 0 (0) | < 0.01 | 0 (0) | 0 (0) | 0 (0) | |
| Wrist Imaging Scores | ||||||||||||
| X-ray Larsen, mean (SD) | 2.8 (4.6) | 1.5 (4.3) | 0.0 (0.0) | 0.02 | 4.8 (6.3) | 0.4 (0.7) | 1.0 (1.4) | 0.04 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | |
| US EULAR-OMERACT score | ||||||||||||
| Combined score, mean (SD) | 2.5 (0.7) | 1.9 (0.7) | 1.2 (0.4) | < 0.01 | 2.0 (0.7) | 1.6 (0.7) | 2.0 (0.0) | 0.08 | ||||
| Grey scale, mean (SD) | 2.4 (0.7) | 1.8 (0.6) | 1.0 (0.0) | < 0.01 | 1.9 (0.7) | 1.6 (0.7) | 2.0 (0.0) | 0.08 | ||||
| Colour Doppler, mean (SD) | 2.3 (0.8) | 1.4 (0.9) | 1.0 (0.7) | < 0.01 | 1.7 (1.0) | 0.9 (0.8) | 1.0 (1.4) | 0.07 | ||||
| RAMRIS score in wrist radiocarpal joint* | ||||||||||||
| Synovitis (0–3), mean (SD) | 2.1 (1.0) | 1.1 (1.0) | 0.2 (0.4) | < 0.01 | 1.2 (0.4) | 0.7 (0.5) | 0.03 | 1.0 (.) | 0.0 (0.0) | 0.3 (0.5) | < 0.01 | |
| Bone erosion (0–10), mean (SD) | 0.9 (1.2) | 0.5 (1.0) | 0.2 (0.4) | 0.12 | 0.2 (0.4) | 0.1 (0.3) | 0.75 | 0.0 (.) | 0.0 (0.0) | 0.0 (0.0) | ||
| Bone oedema (0–3), mean (SD) | 0.3 (0.7) | 0.0 (0.0) | 0.0 (0.0) | 0.03 | 0.0 (0.0) | 0.1 (0.3) | 0.34 | 0.0 (.) | 0.0 (0.0) | 0.0 (0.0) | ||
| Synovial tissue inflammation | ||||||||||||
| Krenn score, mean (SD) | 7.1 (1.6) | 5.9 (1.5) | 4.2 (0.4) | < 0.01 | 6.2 (1.7) | 5.0 (1.4) | 6.0 (0.0) | < 0.01 | 7.0 (.) | 4.8 (0.7) | 3.4 (1.7) | < 0.01 |
| Specific markers (0–4) | ||||||||||||
| CD20, mean (SD) | 2.3 (1.2) | 0.7 (0.5) | 0.0 (0.0) | < 0.01 | 2.1 (1.1) | 0.5 (0.5) | 0.0 (0.0) | < 0.01 | 1.0 (.) | 0.5 (0.5) | 0.0 (0.0) | 0.05 |
| CD3, mean (SD) | 2.8 (1.0) | 1.7 (0.6) | 0.4 (0.5) | < 0.01 | 2.5 (1.0) | 1.8 (0.7) | 1.0 (0.0) | < 0.01 | 2.0 (.) | 1.0 (0.6) | 0.4 (0.5) | < 0.01 |
| CD68, mean (SD) | 3.3 (0.8) | 2.5 (0.5) | 2.0 (0.0) | < 0.01 | 3.0 (0.8) | 2.4 (0.7) | 2.0 (1.4) | 0.08 | 3.0 (.) | 2.0 (0.6) | 1.1 (0.6) | < 0.01 |
| CD138, mean (SD) | 2.5 (1.1) | 0.6 (0.5) | 0.0 (0.0) | < 0.01 | 2.6 (0.6) | 0.9 (0.6) | 0.0 (0.0) | < 0.01 | 2.0 (.) | 0.3 (0.5) | 0.1 (0.3) | < 0.01 |
*LRA not MRI at 6 months, one patient with ERA did not undergo MRI due to claustrophobia, six control MRI without use of contrast agent. Seven controls without wrist X-ray before biopsy. Significant p values in bold. Comparison by unadjusted linear regression with cluster robust standard errors.
ACPA, anti-citrullinated proteins antibody; DAS28CRP, disease activity score in 28 joints with CRP level included; ERA, early RA; LRA, long-standing RA; RA, rheumatoid arthrits; RF, rheumatoid factor.
At baseline, the lymphoid pathotype had significantly higher EULAR-OMERACT combined score and MRI RAMRIS synovitis score, compared with other pathotypes (table 5, overall; both p<0.01, US: L vs M p=0.02 and L vs F p<0.001, MRI: L vs M p=0.007 and L vs F p<0.001). This pattern persisted at 6 months for MRI synovitis (L vs M, p=0.03), but not for US score (table 5, overall: p=0.03 and p=0.08, respectively, L vs M p=0.14). Overall, no differences were found, in MRI erosions or bone oedema of the RCJ between pathotypes, at baseline or 6 months. In subgroup analyses comparing the lymphoid to the fibroid pathotype at baseline, the lymphoid pathotype had more MRI erosions and MRI bone marrow oedema than the fibroid pathotype (p=0.04 and p=0.03).
The Krenn score was significantly higher in the lymphoid group at baseline (L vs M and L vs F both p<0.05), while at 6 months it was significantly higher in the myeloid but not fibroid pathotype (L vs M p=0.02 L vs F p=0.57).
Finally, we analysed whether baseline pathotype was associated with significant differences in change in clinical disease activity, histological synovitis and US and MRI scores between baseline and 6 months (online supplementary table S3). We found no significant differences in CRP, DAS28CRP, Larsen, MRI, US or Krenn score change during the study period between the three pathotypes.
Overall, our results suggest that a lymphoid pathotype is associated with higher levels of synovial inflammation, US and MRI synovitis.
Discussion
We herein present a prospective study evaluating the relationship between histopathology and MRI, US and radiographic scores in two cohorts of well-characterised patients with early therapy naive and established RA both at baseline and at 6-month follow-up. Our results demonstrate a number of important findings. First, histological synovitis is strongly correlated with both US EULAR-OMERACT score and the MRI RAMRIS score at baseline. Second, there is a strong correlation between EULAR-OMERACT US score and MRI RAMRIS score. Third, change in histological synovitis over a 6-month period is correlated to both change in US EULAR-OMERACT score and the MRI RAMRIS scores. Finally, we demonstrate that a lymphoid synovial pathotypes is significantly associated with high levels of synovial inflammation, MRI and US synovitis.
To our knowledge, this is the first prospective study evaluating, in sequential synovial biopsies, both the US EULAR-OMERACT and MRI RAMRIS scoring systems in patients with ERA and LRA and comparing with histological synovitis. The safety and tolerability of the minimal invasive USGSB procedures has made it possible to study this relationship.5
There is an increasing use of US in the routine care of patients with RA and US and MRI as research tools in assessing disease activity. Therefore, the validation of scores such as the EULAR-OMERACT US synovitis score and MRI RAMRIS score against histological synovitis (an objective measure) is essential to reduce inter-reader and intrareader variability, thereby improving early disease detection and disease monitoring.24 25 Previous studies on US and synovial histological changes support our findings, although these studies are limited by the lack of incorporation of the standardised US EULAR-OMERACT system and/or incorporation of repeat synovial biopsies.26–29 Similar observations have also been reported in previous cohorts evaluating MRI and histological synovitis although limitations in terms of prospective study design, MRI standardisation, including application of MRI RAMRIS score, direct comparison with biopsied joint, wide interval between biopsy and MRI and variations in patient disease activity, duration and concomitant therapy have made interpretation of data challenging.14 30 Importantly, we demonstrate that not only do the OMERACT and RAMRIS scores correlate strongly with histological synovitis but in addition show that the two scoring systems correlate with each other. However, it is remarkable to note that in the ERA group at 6 months the correlation between histological synovitis, MRI and US synovitis was lost. This observation could be a putative result of variations in response of histological and radiological measures of synovitis in early versus established RA. According to this, exploiting the ‘window of opportunity’ through intensive treatment initiation in some patients with ERA could result in a faster decrease in synovial vascularisation and hypertrophy found on MRI and US imagining scores than normalisation of histopathological changes measured in the Krenn score. In our analysis of the subcomponents of the Krenn score (online supplementry table S5, online supplementary material), we show that US Doppler data are not correlated to any of the Krenn components in ERA visit 6-month data, which is not seen in any other group. The Krenn score does not include immunohistochemistry markers (eg, CD31), which could be used for a more precise assessment of synovial neovascularisation which could be of a central role in the ERA groups synovitis score.20 Further studies of this relationship in patients with ERA with longer observation periods are needed to evaluate why some patients with marked disease activity reduction on imaging still have ongoing histological synovitis progress.
Finally, our results demonstrate that the lymphoid pathotype is significantly associated with synovial inflammation and higher US EULAR-OMERACT combined score and MRI RAMRIS synovitis score at baseline compared with other pathotypes. The lymphoid pathotype could therefore be a potential future prognostic marker. Although we did not find significant differences between the pathotypes in change of imaging scores or Krenn scores this is likely due to the relatively small number of patients in this study. Furthermore, more data on pathotypes in RA are needed as it could be affected by disease duration, treatment and others confounding factors. Importantly, two biopsy-driven randomised multicentre clinical trials (Response—Resistance to Tituximab vs Tocilizumab in RA31 and Stratification of Biologic Therapies for RA by Pathobiology32) are due to report in the near future on how baseline pathotype affect treatment response.2
We believe the presented results are robust for a number of reasons. First, validated scoring systems for radiographs, US and MRI and histological synovitis were applied. Second, two well-characterised patient groups with early and established RA were included along with a control group. Including ERA and LRA ensures that the results are generalisable. Patients within the control group had significantly lower MRI, US and histological synovitis than patients with RA and there was no correlation between RAMRIS score and histological synovitis in the control group. Furthermore, the controls had a different synovial pathotype composition with majority of the fibroid pathotype. Previous studies comparing synovial osteoarthrosis and RA synovial biopsies inflammation confirm these histological findings.33 34 A further strength of our study is the rigid timing for clinical examination, imaging and synovial sampling which were all performed within 1–2 hours eliminating the fluctuations in disease activity that have been previously clearly documented to exist. The study has limitations including that the LRA group did not undergo MRI at 6 months, there was no erosive progression in the wrist in the RA group by neither Larsen score nor RAMRIS score, only few of the patients with RA had fibroid pathotype, and corticosteroids injections were allowed in all patients which could affect synovial inflammation. Due to the large number of analyses, multiple testing could be a problem and the borderline-significant results should be interpreted with this in mind. Furthermore, treatment was as per routine clinical care not per protocol.
In summary, this study demonstrates that the MRI RAMRIS synovitis score and EULAR-OMERACT US scoring system are sensitive measures of histological synovitis in established RA and early untreated RA. This relationship persists during the study period in the established RA group despite effective treatment, but not in the ERA after 6 months. This suggest that ERA and LRA have different responses to treatment intensification, possibly due to an immunological ‘window of opportunity’ in the ERA group. Overall, significant decreases in MRI/US synovitis are associated with significant reductions in histological synovitis. These findings validate the use of MRI RAMRIS and EULAR-OMERACT US scores as surrogate markers of histological synovitis in established RA and early untreated RA. Synovial pathotypes have differences at baseline in degree of synovial inflammation and US and MRI imagining scores.
Acknowledgments
We are indebted to all the study subjects and personnel contributing data to this study.
Footnotes
Contributors: All authors have contributed substantially in the process of completing this study, specified as follows: Conception of the study: SAJ. Designing the study: SAJ and HL. Aggregation of data: SAJ and HL. Statistics: SAJ and PVL. Interpretation of data: All authors. Drafting and revising, final approval and agreement to be accountable: All authors.
Funding: SAJ is supported by grants from The Danish Rheumatism Association and Odense University Hospital PhD Fund and Fund for clinical research.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Denmark, Odense: The SynRA study is approved by the regional ethics review board (S-20140062) and the Danish Data Protection Agency (2008-58-0035).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Orr C, Vieira-Sousa E, Boyle DL, et al. . Synovial tissue research: a state-of-the-art review. Nat Rev Rheumatol 2017;13:463–75. 10.1038/nrrheum.2017.115 [DOI] [PubMed] [Google Scholar]
- 2. Humby FC, Al Balushi F, Lliso G, et al. . Can Synovial Pathobiology Integrate with Current Clinical and Imaging Prediction Models to Achieve Personalized Health Care in Rheumatoid Arthritis? Front Med 2017;4 10.3389/fmed.2017.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly S, Humby F, Filer A, et al. . Ultrasound-guided synovial biopsy: a safe, well-tolerated and reliable technique for obtaining high-quality synovial tissue from both large and small joints in early arthritis patients. Ann Rheum Dis 2015;74:611–7. 10.1136/annrheumdis-2013-204603 [DOI] [PubMed] [Google Scholar]
- 4. Humby F, Kelly S, Hands R, et al. . Use of ultrasound-guided small joint biopsy to evaluate the histopathologic response to rheumatoid arthritis therapy: recommendations for application to clinical trials. Arthritis & Rheumatology 2015;67:2601–10. 10.1002/art.39235 [DOI] [PubMed] [Google Scholar]
- 5. Just SA, Humby F, Lindegaard H, et al. . Patient-reported outcomes and safety in patients undergoing synovial biopsy: comparison of ultrasound-guided needle biopsy, ultrasound-guided portal and forceps and arthroscopic-guided synovial biopsy techniques in five centres across Europe. RMD Open 2018;4:e000799 10.1136/rmdopen-2018-000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pitzalis C, Kelly S, Humby F. New learnings on the pathophysiology of RA from synovial biopsies. Current Opinion in Rheumatology 2013;25:334–44. 10.1097/BOR.0b013e32835fd8eb [DOI] [PubMed] [Google Scholar]
- 7. Dennis G, Holweg CTJ, Kummerfeld SK, et al. . Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther 2014;16 10.1186/ar4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Möller I, Janta I, Backhaus M, et al. . The 2017 EULAR standardised procedures for ultrasound imaging in rheumatology. Ann Rheum Dis 2017;76:1974–9. 10.1136/annrheumdis-2017-211585 [DOI] [PubMed] [Google Scholar]
- 9. Ejbjerg B, et al. The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas: the wrist joint. Annals of the Rheumatic Diseases 2005;64(suppl_1):i23–47. 10.1136/ard.2004.031823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Heijde D, Landewé R. Are conventional radiographs still of value? Current Opinion in Rheumatology 2016;28:310–5. 10.1097/BOR.0000000000000279 [DOI] [PubMed] [Google Scholar]
- 11. Larsen A. How to apply Larsen score in evaluating radiographs of rheumatoid arthritis in long-term studies. J Rheumatol 1995;22:1974–5. [PubMed] [Google Scholar]
- 12. Terslev L, Naredo E, Aegerter P, et al. . Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce-Part 2: reliability and application to multiple joints of a standardised consensus-based scoring system. RMD Open 2017;3:e000427 10.1136/rmdopen-2016-000427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paulshus Sundlisæter N, Aga A-B, Olsen IC, et al. . Clinical and ultrasound remission after 6 months of treat-to-target therapy in early rheumatoid arthritis: associations to future good radiographic and physical outcomes. Ann Rheum Dis 2018;77 10.1136/annrheumdis-2017-212830 [DOI] [PubMed] [Google Scholar]
- 14. Humby F, Mahto A, Ahmed M, et al. . The Relationship Between Synovial Pathobiology and Magnetic Resonance Imaging Abnormalities in Rheumatoid Arthritis: A Systematic Review. J Rheumatol 2017;44:1311–24. 10.3899/jrheum.161314 [DOI] [PubMed] [Google Scholar]
- 15. Smolen JS, Landewé R, Bijlsma J, et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 16. Wolf JM, Dukas A, Pensak M. Advances in wrist arthroscopy. J Am Acad Orthop Surg 2012;20:725–34. 10.5435/JAAOS-20-11-725 [DOI] [PubMed] [Google Scholar]
- 17. Tak PP, Smeets TJM, Daha MR, et al. . Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis & Rheumatism 1997;40:217–25. 10.1002/art.1780400206 [DOI] [PubMed] [Google Scholar]
- 18. Krenn V, Perino G, Rüther W, et al. . 15 years of the histopathological synovitis score, further development and review: A diagnostic score for rheumatology and orthopaedics. Pathology - Research and Practice 2017;213:874–81. 10.1016/j.prp.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 19. Tak PP, Breedveld FC. Analysis of serial synovial biopsies as a screening method for predicting the effects of therapeutic interventions. JCR: Journal of Clinical Rheumatology 1997;3 10.1097/00124743-199708000-00002 [DOI] [PubMed] [Google Scholar]
- 20. Najm A, le Goff B, Venet G, et al. . IMSYC immunologic synovitis score: A new score for synovial membrane characterization in inflammatory and non-inflammatory arthritis. JointBone Spine 2019;86 10.1016/j.jbspin.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 21. Torp‐Pedersen S, Christensen R, Szkudlarek M, et al. . Power and Color Doppler Ultrasound Settings for Inflammatory Flow: Impact on Scoring of Disease Activity in Patients With Rheumatoid Arthritis. Arthritis & Rheumatology 2015;67:386–95. 10.1002/art.38940 [DOI] [PubMed] [Google Scholar]
- 22. Hammer HB, Bolton-King P, Bakkeheim V, et al. . Examination of intra and interrater reliability with a new ultrasonographic reference atlas for scoring of synovitis in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 2011;70:1995–8. 10.1136/ard.2011.152926 [DOI] [PubMed] [Google Scholar]
- 23. MA Computing Robust Standard Errors for Within‐groups Estimators. 49 Oxford bulletin of Economics and Statistics, 1987. [Google Scholar]
- 24. D’Agostino M-A, Terslev L, Aegerter P, et al. . Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce; Part 1: definition and development of a standardised, consensus-based scoring system. RMD Open 2017;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Østergaard M, Peterfy CG, Bird P, et al. . The OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging (MRI) Scoring System: Updated Recommendations by the OMERACT MRI in Arthritis Working Group. J Rheumatol 2017;44:1706–12. 10.3899/jrheum.161433 [DOI] [PubMed] [Google Scholar]
- 26. Purkayastha N, Humby F, Rocher-Ros V, et al. . Wrist ultrasound - the model method for grey-scale and power Doppler scoring. Ann Rheum Dis 2019;78 10.1136/annrheumdis-2018-213977 [DOI] [PubMed] [Google Scholar]
- 27. Andersen M, Ellegaard K, Hebsgaard JB, et al. . Ultrasound colour Doppler is associated with synovial pathology in biopsies from hand joints in rheumatoid arthritis patients: a cross-sectional study. Ann Rheum Dis 2013. [DOI] [PubMed] [Google Scholar]
- 28. Najm A, Orr C, Gallagher L, et al. . Knee joint synovitis: study of correlations and diagnostic performances of ultrasonography compared with histopathology. RMD Open 2018;4 10.1136/rmdopen-2017-000616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vordenbäumen S, Sewerin P, Lögters T, et al. . Inflammation and vascularisation markers of arthroscopically-guided finger joint synovial biospies reflect global disease activity in rheumatoid arthritis. Clin Exp Rheumatol 2014;32:117–20. [PubMed] [Google Scholar]
- 30. Vordenbäumen S, Schleich C, Lögters T, et al. . Dynamic contrast-enhanced magnetic resonance imaging of metacarpophalangeal joints reflects histological signs of synovitis in rheumatoid arthritis. Arthritis Res Ther 2014;16 10.1186/s13075-014-0452-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Response - Resistance to Rituximab versus Tocilizumab in RA. Available: http://www.r4ra-nihr.whri.qmul.ac.uk
- 32. Stratification of Biologic Therapies for RA by Pathobiology. Available: http://www.matura-mrc.whri.qmul.ac.uk
- 33. Mucke J, Hoyer A, Brinks R, et al. . Inhomogeneity of immune cell composition in the synovial sublining: linear mixed modelling indicates differences in distribution and spatial decline of CD68+ macrophages in osteoarthritis and rheumatoid arthritis. Arthritis Res Ther 2016;18 10.1186/s13075-016-1057-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krenn V, Morawietz L, Burmester G-R, et al. . Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology 2006;49:358–64. 10.1111/j.1365-2559.2006.02508.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2019-000951supp001.docx (36.2KB, docx)