Abstract
It is essential to know and understand the anatomy of the tarsometatarsal (TMT) joint (Lisfranc joint) to achieve a correct diagnosis and proper treatment of the injuries that occur at that level.
Up to 20% of Lisfranc fracture-dislocations go unnoticed or are diagnosed late, especially low-energy injuries or purely ligamentous injuries. Severe sequelae such as post-traumatic osteoarthritis and foot deformities can create serious disability.
We must be attentive to the clinical and radiological signs of an injury to the Lisfranc joint and expand the study with weight-bearing radiographs or computed tomography (CT) scans.
Only in stable lesions and in those without displacement is conservative treatment indicated, along with immobilisation and initial avoidance of weight-bearing.
Through surgical treatment we seek to achieve two objectives: optimal anatomical reduction, a factor that directly influences the results; and the stability of the first, second and third cuneiform-metatarsal joints.
There are three main controversies regarding the surgical treatment of Lisfranc injuries: osteosynthesis versus primary arthrodesis; transarticular screws versus dorsal plates; and the most appropriate surgical approach.
The surgical treatment we prefer is open reduction and internal fixation (ORIF) with transarticular screws or with dorsal plates in cases of comminution of metatarsals or cuneiform bones.
Cite this article: EFORT Open Rev 2019;4:430-444. DOI: 10.1302/2058-5241.4.180076
Keywords: Lisfranc joint, Lisfranc fracture-dislocation, tarsometatarsal joint, diagnosis, treatment, results
Introduction
The term ‘Lisfranc injury’ refers strictly to an injury in which one or more of the metatarsals are displaced with respect to the tarsus. This name is attributed to a French surgeon and gynaecologist of the Napoleonic era who was the first to describe the injury in 1815 and to describe an amputation at that level.1 The use of this term is very broad and can refer to a low-energy sports injury or a high-energy lesion, as well as lesions that are purely ligamentous or those that are associated with fractures of the metatarsals, cuneiform bones, scaphoid bone or cuboid bone.
Lisfranc injuries are infrequent, at approximately 0.2% of all fractures, although in 20% of cases they are not diagnosed or are diagnosed late.2,3 However, early and accurate diagnosis of these injuries are fundamental requirements for their appropriate treatment and to prevent long-term sequelae.
Men are two to four times more likely to suffer a Lisfranc joint injury, possibly because they participate more frequently in high-speed activities. These injuries are common in the third decade of life.
The majority (87.5%) are closed injuries and are becoming more frequent in athletes, in whom it is common to see subtle Lisfranc injuries.4,5 These are injuries that have occurred in sports such as soccer, gymnastics and running (Fig. 1).6
Fig. 1.
Mechanism of indirect injury in fracture-dislocations of the Lisfranc joint [tarsometatarsal (TMT)] joint: longitudinal force with the foot in plantar flexion.
In this article, we will review the main concepts related to the diagnosis and treatment of Lisfranc joint injuries.
Relevant anatomy of the Lisfranc joint (tarsometatarsal joint)
The Lisfranc joint or tarsometatarsal joint (TMT) is formed by the five metatarsals that articulate with the three cuneiform bones and the cuboid bone. The first, second and third cuneiform-metatarsal joints are characterised by high stability with little or no mobility; however, joints between the fourth and fifth metatarsals and cuboid bone have greater mobility, which is necessary for adapting the foot to the ground.
Bone stability is determined by the trapezoidal shape of the base of the first three metatarsals, with their respective cuneiform bones forming a stable arch known as the ‘transverse arch or Roman arch’ with the second TMT joint as the keystone. It is a joint in which the second metatarsal is embedded in a shroud between the first and third cuneiform bones. In fact, a shallow second TMT joint mortise is considered a risk factor for suffering a Lisfranc injury.7,8
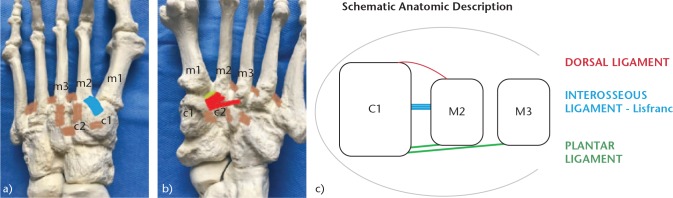
It is also important to understand the ligamentous structures that stabilise the Lisfranc joint (Fig. 2). These structures include the following: 1) TMT dorsal and plantar ligaments that cross each TMT joint – the dorsal ligaments are weaker, which explains why the displacement is often dorsal; 2) inter-metatarsal ligaments that join the second to the fifth metatarsals – there is no inter-metatarsal ligament between the first and the second metatarsals. However, there is a plantar interosseous ligament from the lateral zone of the first cuneiform bone to the medial zone of the second metatarsal, commonly known as the Lisfranc ligament; and (3) the Lisfranc ligament complex, formed by the TMT ligaments of the first and second radius and by the Lisfranc ligament. The latter is the strongest and thickest, which is why it is essential to maintain the second metatarsal and consequently maintain the midfoot arch.
Fig. 2.
Anatomy of the TMT joint: (a) Dorsal view. In blue, dorsal TMT ligament first cuneiform to second metatarsal (c1-m2). In brown, inter-metatarsal ligaments, which do not exist between the first and second metatarsals (m1-m2). (b) Plantar view. In red, plantar TMT ligament; in green, interosseous ligament (ligament of Lisfranc), exclusive between the first cuneiform and the second metatarsal (c1-m2). (c) Schematic anatomic description.
Clinical presentation, radiological study and classification
We should suspect a Lisfranc joint injury in patients who report pain in the midfoot or in trauma patients who present other lesions that could mask the diagnosis. An early diagnosis is important to preventing sequelae and functional disability or acute complications such as compartment syndrome, which require urgent treatment with fasciotomy.
High-energy injuries are easier to diagnose (Fig. 3). They are characterised by foot deformities associated with pain, swelling and an inability to walk. They are produced by a direct trauma, as occurs in traffic accidents, and are often associated with severe involvement of the soft tissues and neurovascular damage.7 In these cases, we must request a radiographic non-weight-bearing study based on three views: 1) anteroposterior (AP) (Fig. 4a): the alignment between the medial edge of the second metatarsal and the medial edge of the second cuneiform bone should be checked. The distance between the bases of the first and second metatarsals should not exceed 2 mm. The ‘fleck sign’ (Fig. 4b), a small bone fragment in the first inter-metatarsal space, indicates the avulsion of the Lisfranc ligament; 2) internal oblique (Fig. 4c): the alignment between the medial border of the cuboid bone and the medial border of the fourth metatarsal should be checked; and 3) lateral (Fig. 4d): the dorsal/plantar displacement of the metatarsals should be assessed.
Fig. 3.
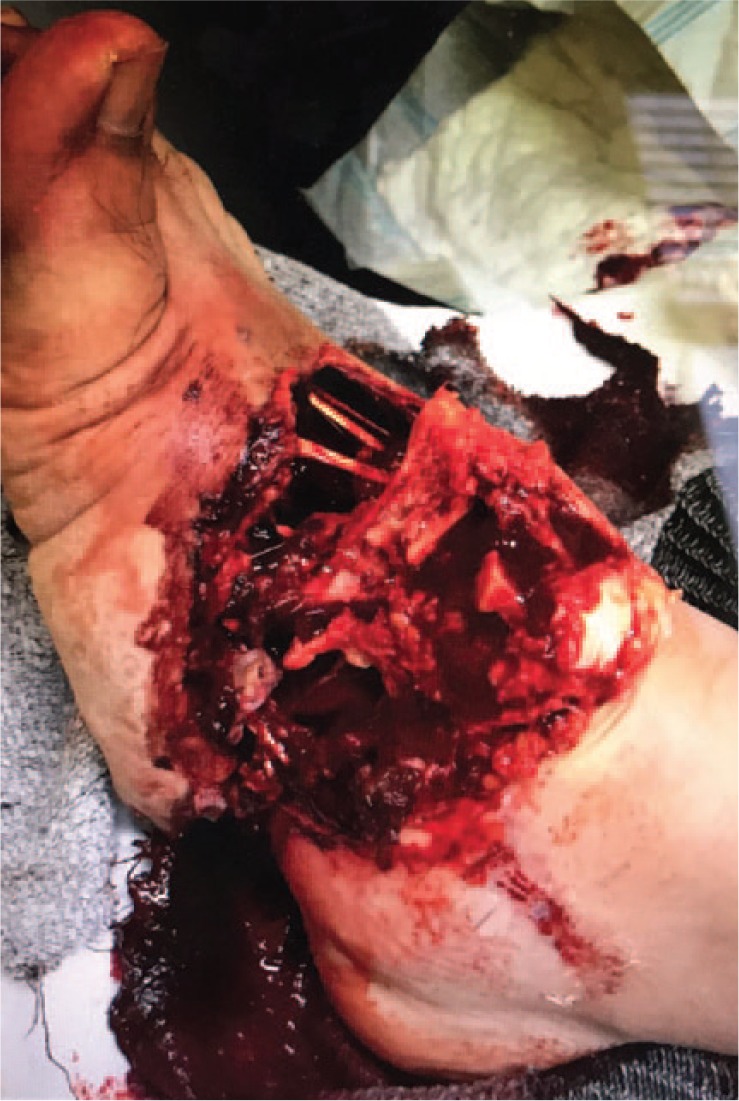
Open fracture of the Lisfranc and Chopart joints produced in a traffic accident (high-energy mechanism).
Fig. 4.
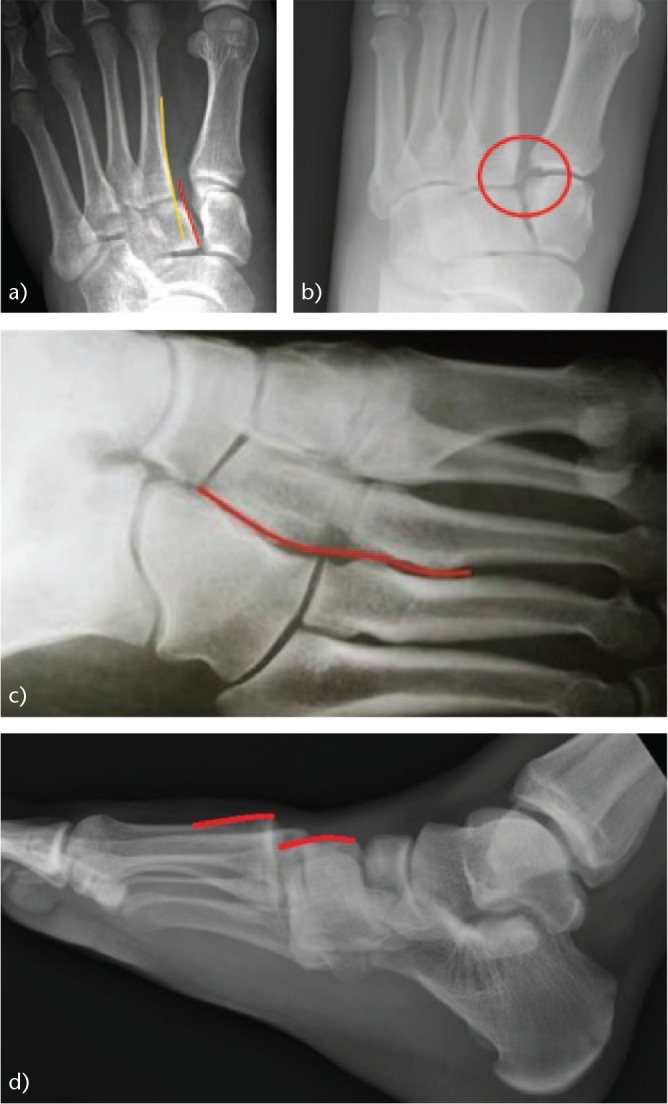
Radiological study of a lesion of the Lisfranc joint: (a) Anteroposterior (AP) radiograph. Note the discontinuity of the medial cortex of the second metatarsal (m2) with the medial cortical of the second cuneiform (c2) (yellow and red lines). (b) ‘Fleck sign’, fracture-avulsion of the Lisfranc ligament (circle). (c) Internal oblique radiograph, showing continuity of the medial cortex of the cuboid and the medial cortex of the fourth metatarsal (m4) (red line). (d) Lateral radiograph showing dorsal dislocation of the metatarsals (red lines).
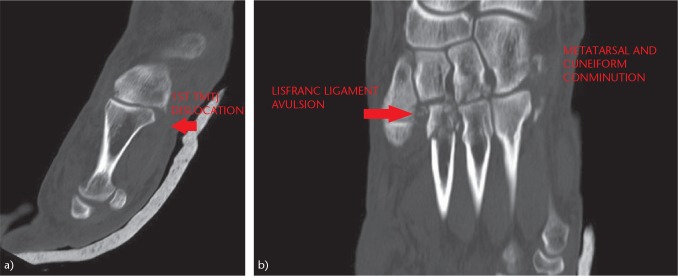
A computed tomography (CT) scan (Fig. 5) allows a more accurate assessment of the Lisfranc joint. It allows the diagnosis of more subtle fractures and subluxations that are not observed in simple radiographs. The CT scan is generally used for surgical planning. Magnetic resonance imaging (MRI) is very useful for detecting soft-tissue injuries and ligamentous injuries. It presents a sensitivity and predictive value of up to 94% in determining instability of the Lisfranc joint and can therefore be useful for the diagnosis of the subtle Lisfranc injury.9,10
Fig. 5.
Study of the Lisfranc joint by means of CT scan: (a) CT scan allows an accurate description of subtle lesions of the TMT joint. (b) Comminution of the cuneiforms and bases of the metatarsals. Increased space between the first and second metatarsals, and fracture-avulsion of the Lisfranc ligament (‘fleck sign’).
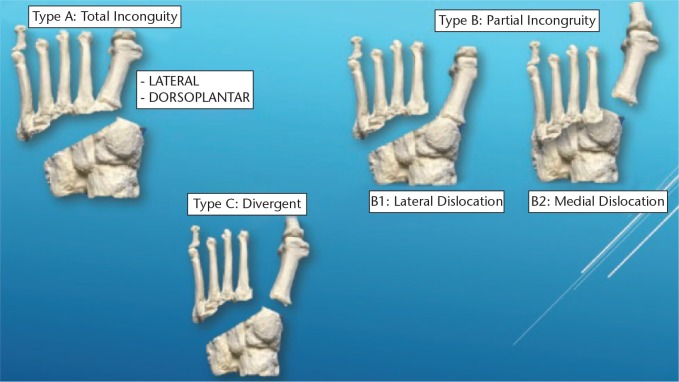
The classification of Lisfranc lesions was originally described by Quenu and Kuss in 1909 based on the concept of the three columns. The lesions were classified as homolateral, isolated and divergent.4 The classification was modified by Hardcastle et al in 1982: in type A, all metatarsals are displaced in one direction and there is a total incongruence; in type B, there is partial incongruence with one or more displaced metatarsals; and type C has a divergent pattern of incongruence. Myerson proposed a modification of the classification that showed greater complexity of the lesion (Fig. 6).9
Fig. 6.
The 1986 Myerson classification for Lisfranc fracture-dislocations.9
Subtle injuries of the Lisfranc joint
Diagnosing low-energy injuries can be challenging and requires a high level of suspicion on the part of the orthopaedic surgeon. Patients usually present with pain and swelling without deformity and they can often walk. Historically, patients with subtle Lisfranc subluxation feel or hear a ‘pop’ in their midfoot, followed by initial pain with the weight-bearing, then decreasing pain. Midfoot sprains are the second most frequent injury to athletes' feet after the metatarsophalangeal (MTF) joint injury. The following cases are highly suggestive of a Lisfranc lesion: 1) plantar ecchymosis at the level of the midfoot (Fig. 7); 2) pain on palpation or manipulation of the TMT joints.11 The manoeuvres of provocation of passive abduction and pronation of the forefoot can be useful while keeping the fixed hindfoot with the other hand; 3) altered sensitivity in the back of the first inter-metatarsal space can help to achieve an early diagnosis. Lisfranc lesions are associated with a post-traumatic neuropathy of the medial terminal branch of the deep peroneal nerve and a decrease in two-point discrimination; 4) The ‘piano key test’, which consists of moving the head of the affected metatarsal while firmly holding the midfoot and hindfoot, is useful in identifying and isolating the affected TMT joint; and 5) an increase in the distance between the hallux and the second finger, known as a ‘positive gap’, which correlates with inter-cuneiform instability.
Fig. 7.
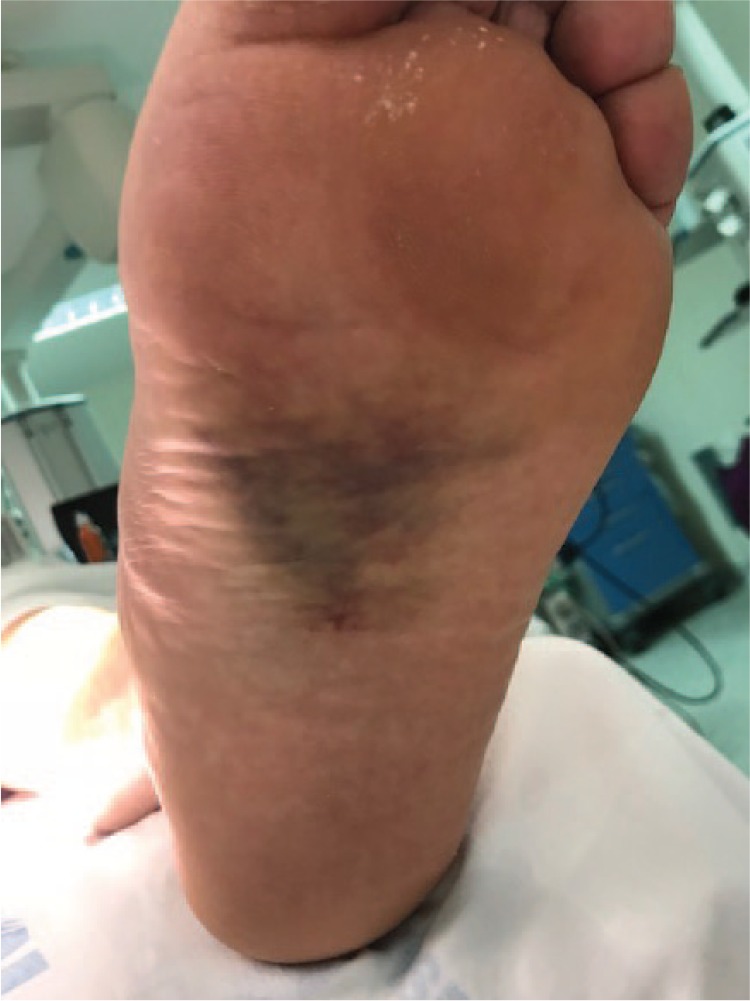
Plantar ecchymosis is a pathognomonic sign of Lisfranc injury.
In these cases, radiographs should be performed on both feet. If pain does not allow them to be performed, the radiographs can be performed under regional anaesthesia of the ankle.10 Weight-bearing radiographs are superior to the radiographs performed under stress manoeuvres, in which the applied force is limited compared with that of the entire weight of the body. It has recently been shown that an angle of 28.9° in the AP radiograph of the foot improves visualization of the Lisfranc joint in most patients.12,13 It has been observed that the cavus midfoot is more prevalent in patients with Lisfranc lesions. This finding is important because it provides a radiological parameter that can provide a high index of suspicion.14
In 2002, Nunley and Vertullo developed a new classification for subtle lesions of the Lisfranc joint, produced by a low-energy mechanism, typical of athletes who suffered an axial load on standing in plantar flexion and slightly rotated.10 According to these authors, three stages could be observed: stage I, patients who could not practice sports, without displacement between the first and second metatarsals on weight-bearing radiographs but with a positive bone scintigraphy; stage II, diastasis between the first and second metatarsals of 2 mm to 5 mm on the AP radiographs on weight-bearing but without loss of the medial longitudinal arch on lateral radiographs on weight-bearing; stage III, diastasis > 5 mm and loss of the medial longitudinal arch, described as a decrease in the distance between the plantar border of the fifth metatarsal and the plantar border of the first cuneiform bone.
Recently, the introduction of a fourth category to the Myerson-modified Hardcastle classification has been proposed, which corresponds to the so-called subtle injury of the Lisfranc joint or lesion type D.15 It is subdivided into D1, distance from the first cuneiform bone (c1) to the second metatarsal (m2) [(c1-m2)] ⩽ 2 mm, does not require surgical fixation; and D2, distance between c1 and m2 > 2 mm, which requires surgical fixation. Type D2 can be purely ligamentous (D2L) or with bone avulsion (D2B).
Sivakumar et al consider that this modification offers two advantages:15
1) greater simplicity, given the entire spectrum of Lisfranc joint injuries is incorporated into a single classification; and 2) it uses as a reference the distance between c1 and m2, which has proven to be more precise and sensitive in terms of determining a Lisfranc injury.16
Treatment of Lisfranc injuries
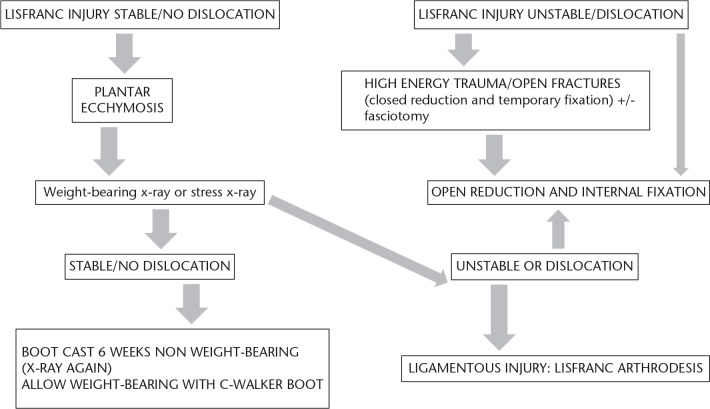
Lisfranc lesions that go unnoticed or are treated inappropriately can result in progressive deformity, instability and post-traumatic osteoarthritis (OA), which can occur in up to half of the cases of Lisfranc fracture dislocations (Fig. 8).4
Fig. 8.
Treatment protocol recommended by us for fracture-dislocations of the Lisfranc joint.
Non-surgical treatment
There is only one circumstance in which non-surgical treatment is indicated and in which the results of this treatment are good and patients can return to their sports activity levels before the injury. They are Lisfranc ligament sprains, which are stable and non-displaced lesions that correspond to stage I of the Nunley and Vertullo classification. They can be treated non-surgically with a plaster boot without weight-bearing for six weeks.10 If, after that period, pain has disappeared in the midfoot, a gradual return to specific sports activity is allowed using an orthopaedic insole to discharge the medial longitudinal arch. If at six weeks the pain persists, an orthopaedic boot with weight-bearing is used for four more weeks.
Surgical treatment
Patients with displaced or unstable Lisfranc joint injuries require surgical treatment in order to achieve anatomic reduction.1,2 The risk of post-traumatic OA will depend on the quality of the reduction.17
In high-energy injuries, such as traffic accidents, it is important to consider a possible compartment syndrome and, if the suspicion is high, perform a fasciotomy.5 If there is a large displacement of the metatarsals, it is necessary to perform an axial alignment and stabilisation with Kirschner wires (K-wires) or an external fixator as a first measure in the emergency department.18 This approach will allow better healing of the soft tissues and will reduce the risk of a compartment syndrome.
The definitive surgical intervention must be deferred ten to 15 days until the healing of the soft tissues and the appearance of wrinkles on the skin (wrinkle sign).
Our surgical technique in Lisfranc injuries (high or low energy)
We place the patient in the supine position with a cushion under the ipsilateral thigh and we use a support to maintain the knee in 90º flexion, which helps us perform reduction and bone fixation (Fig. 9).
Fig. 9.
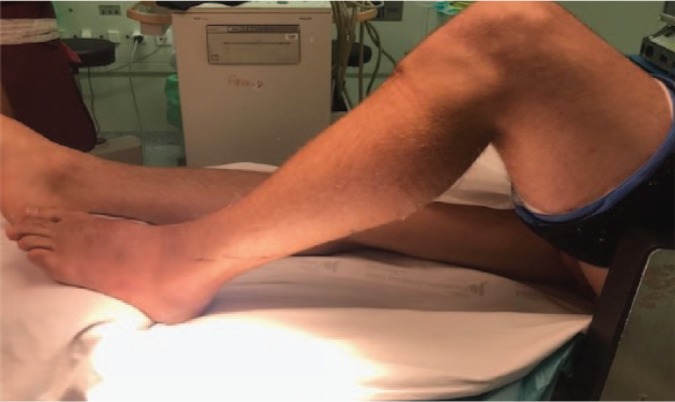
Position that we usually use on the surgical table to facilitate the placement of the osteosynthesis material.
We usually perform open surgery. If there is involvement of the third TMT or instability of the fourth or fifth metatarsals, we use a double approach, making a longitudinal incision in the first inter-metatarsal space and another longitudinal incision in line with the fourth metatarsal. We perform a reduction from the medial to the lateral direction. We start by reducing the first cuneiform-metatarsal joint with K-wires and bone fixation with two non-cannulated 3.5-mm screws, from the first metatarsal to the first cuneiform. Then, we use a second screw from the first cuneiform to the first metatarsal.
The next step is to reduce the space between the first and second metatarsals. To this end, we use a reduction clamp between the medial zone of the first cuneiform bone and the lateral zone of the second metatarsal, and we perform bone fixation with a non-cannulated screw of 4 or 4.5 mm from the first cuneiform to the base of the second metatarsal (Fig. 10). The next screw goes from the second metatarsal to the second cuneiform once the joint has been reduced. Through the incision on the fourth metatarsal, the third tarsal-metatarsal joint is evaluated and reduced if necessary. Bone fixation is performed using two crossed 3.5-mm non-cannulated screws or a 3.5-mm non-cannulated screw and a K-wire. Each joint must be stabilised with two screws or a screw and a K-wire. If there is an injury to the fourth and fifth metatarsals, they will be stabilised with K-wires between the metatarsal and the cuboid bone, which are removed at six weeks.
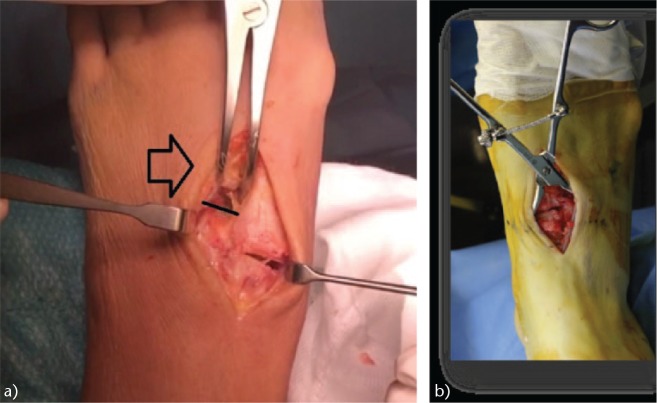
Fig. 10.
Intra-operative images: (a) note the separation between the first and second metatarsals (black arrow) that causes instability due to rupture of the Lisfranc ligament complex (black line). (b) Reduction and closure of the first intermetatarsal space.
If there is a fracture or comminution of the bases of the metatarsals or of the cuneiform bones (Figs 11 and 12), we use dorsal plates for the stabilisation of the corresponding joint; however, we always use the one known as the ‘Lisfranc screw’ between the first cuneiform bone and the base of the second metatarsal. In Lisfranc lesions that are purely ligamentous, we also perform this same surgical technique.
Fig. 11.
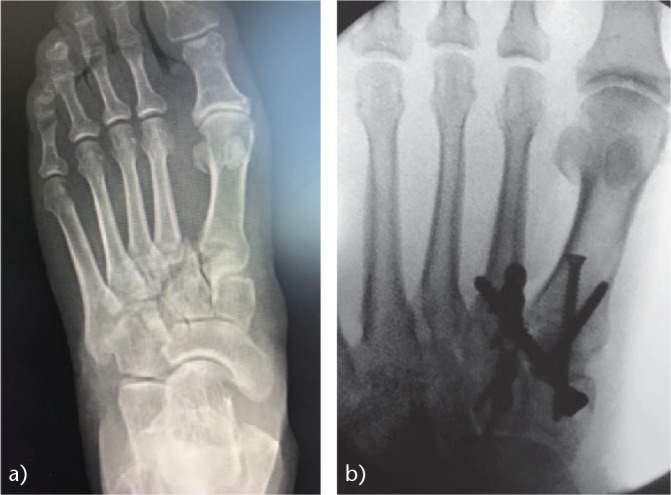
Osteosynthesis of a Lisfranc lesion: (a) comminuted fracture of the base of the second metatarsal; (b) the first inter-metatarsal space was reduced with a Lisfranc screw and fixed with a dorsal plate on the second cuneiform-metatarsal joint.
Fig. 12.
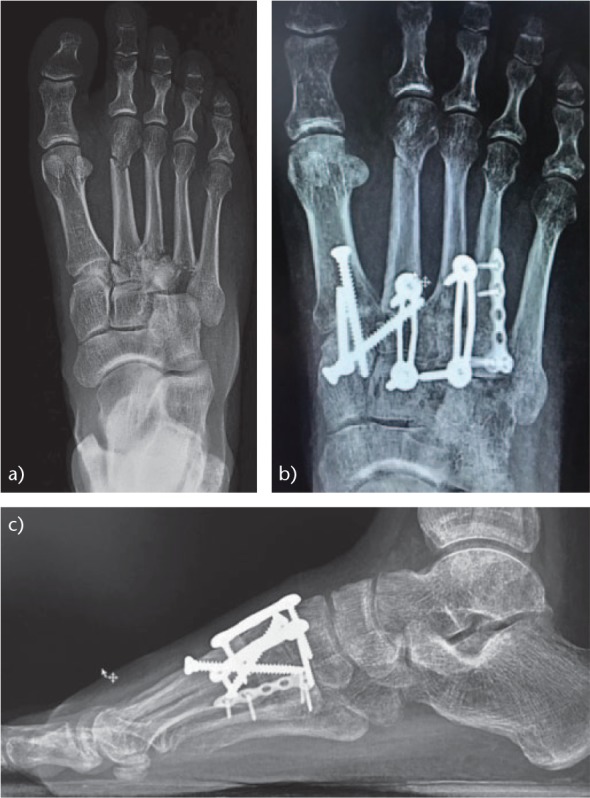
Surgical treatment of Lisfranc lesion: (a) comminuted fracture of the second, third and fourth metatarsal bases. Although there was no clear increase in inter-metatarsal space, there was ligamentous instability. (b) Post-operative anteroposterior (AP) projection. ORIF of the first column was performed and stabilisation of the second and third rays with a Lisfranc screw and dorsal plates. Osteosynthesis of the base of the fourth metatarsal was also performed. The joint between the fourth and fifth metatarsals and the cuboid were not fixed, given that they are articulations of adaptation to the ground and must have mobility. (c) Post-operative lateral projection.
After the surgery, we immobilise the limb with a splint that is maintained for two to three weeks. At that time, the stitches are removed and the patient uses an orthopaedic non-weight-bearing walker boot for four to six more weeks. During this period, the boot can be removed to perform ankle dorsiflexion exercises. After that period, we allow weight-bearing with a walker boot and an interior insole with a medial discharge arch for four more weeks. Sports activity is allowed for eight to 12 months, depending on the clinical and radiographic evolution. We do not routinely perform hardware removal.
Controversies
Osteosynthesis versus primary arthrodesis
The traditional treatment for Lisfranc lesions is open reduction and internal fixation (ORIF). However, some authors believe that primary partial arthrodesis offers better results and a lower rate of re-operations. It has even been shown that Lisfranc lesions of purely ligamentous low energy have better functional results with a primary arthrodesis.1–4
In a meta-analysis, Smith et al found no significant differences in terms of functional results or in terms of non-anatomical reduction when comparing the two techniques.19 There were only significant differences in the re-operation rate for hardware removal when ORIF was used.
The rate of re-operations can influence decision-making. However, Buda et al in a recent article concluded that if we exclude hardware removal, patients with Lisfranc lesions treated with ORIF do not have a higher rate of re-operation (29.5%) compared with those who are treated with a primary partial arthrodesis (29.6%).20 The most common causes of re-operation are post-traumatic OA in patients treated with ORIF and non-union in those treated with primary arthrodesis.
In a prospective and randomised trial conducted in patients with purely ligamentous Lisfranc lesions, in those for whom a Lisfranc arthrodesis was performed, better functional results were observed in the short and medium term, with patients reaching up to 92% of their previous level of activity in the post-operative period. In the case of ORIF, they only reached 65% of their previous level of activity.19 In clinical practice, orthopaedic surgeons are often reluctant to perform an arthrodesis on young and active patients with subtle lesions of the Lisfranc joint (Figs 13 and 14).
Fig. 13.
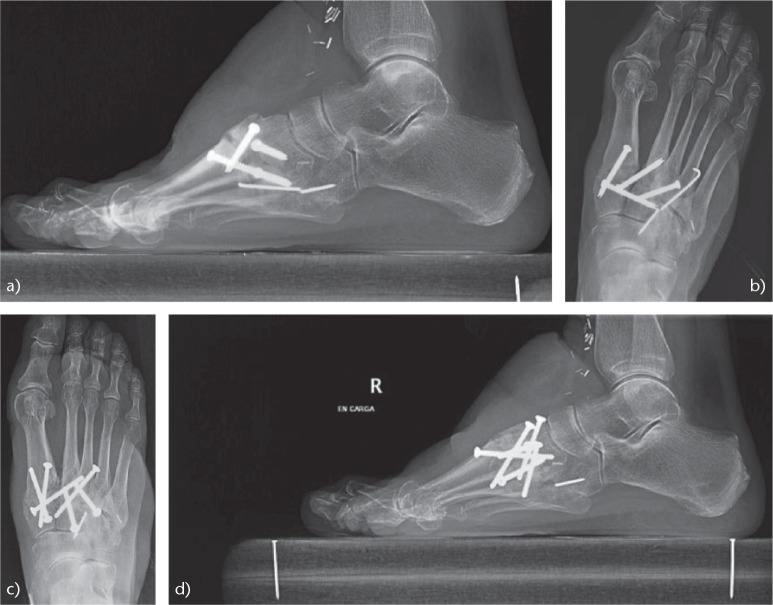
Painful post-traumatic OA after a non-anatomical reduction of a Lisfranc injury. Arthrodesis of the Lisfranc joint was performed with complete relief of symptoms: (a) Lateral view before the arthrodesis; (b) AP radiograph before the arthrodesis; (c) AP view after the arthrodesis; (d) lateral radiograph after the arthrodesis.
Fig. 14.
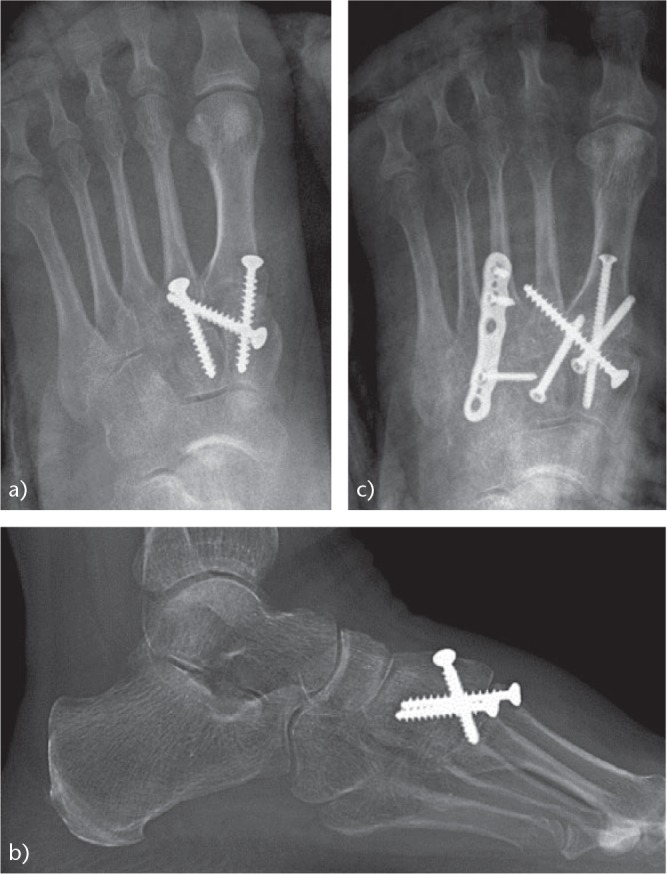
Another case of post-traumatic OA of the Lisfranc joint due to a non-anatomical reduction associated with instability of Lisfranc joint: (a) AP view before the arthrodesis; (b) lateral radiograph before the arthrodesis; (c) radiograph after the arthrodesis. The result was satisfactory.
ORIF: transarticular screws versus dorsal bridge plates
Fixation with screws offers a rapid recovery of activities and a low incidence of secondary displacement compared with the technique of bone fixation with K-wires.21 K-wires are easy to insert and remove, but have shown up to 32% secondary displacement or suboptimal reduction.22 Thus, they are reserved only for injuries of the lateral column (fourth and fifth metatarsals).
A biomechanical study in cadavers showed that cortical screws and cannulated screws with distal thread have a similar clamping force and equal resistance to deformation with partial weight-bearing.23 Therefore, cannulated screws with partial thread can simplify the surgical procedure without sacrificing the strength of the fixation.
The use of transarticular screws has been questioned because they produce joint damage (2% to 6%). For this reason, dorsal plates with screws that do not cross the joint have been used in recent years. These plates provide stability without compromising the cartilage. In a biomechanical study comparing osteosynthesis with transarticular screws and dorsal plates, it was confirmed that the two methods had a similar efficacy in reducing and resisting the TMT joint to displacement on weight-bearing.24
In a retrospective study recently published by Kirzner et al, it has been observed that patients treated with a dorsal bridge plate had significantly better functional and radiological results than those treated with transarticular screws or a combination of plate and screws.25 In addition, complete Lisfranc lesions, homolateral or divergent, had poorer results regardless of the modality of fixation. Plates do not cause joint damage, but they do require a wider surgical exposure and can increase soft-tissue irritation.
In our experience, we reserve the use of dorsal bridge plates combined with transarticular screws for cases in which there is comminution of the cuneiform bones or the base of the metatarsals, given that in these cases the screws do not provide the necessary stability.
In the case of an arthrodesis of the Lisfranc joint, a plantar plate plus a compression screw appear to be superior and is more in line with the biomechanical principles of the Association for the Study of Internal Fixation (ASIF) because the plate is located on the side of greater tension.26 Plantar plates have demonstrated that they adapt better to the contour of the bone, diminishing irritation to soft tissues and providing them better coverage. In a biomechanical study in cadavers, Klos et al have demonstrated that plantar plates give greater stability and rigidity to the arthrodesis than dorsomedial plates.27
Which surgical approach is more appropriate?
The classic approach used for open Lisfranc fracture-dislocation surgery is the one described above. An incision in the first intermetatarsal space allows us to access the first and second TMT joints; if necessary, a second longitudinal incision is made in line with the fourth metatarsal.
When we make two incisions, soft-tissue complications can arise due to the narrow skin bridges we create. Of the complications reported in the literature, 0% to 9% of patients develop a superficial infection and 0% to 13% experience a delay in the healing of the surgical wounds.16,17
Philpott et al have described a modification of the classic dorsal approach.28 They make a single longitudinal incision on the second metatarsal, beginning at the TMT joint and extending to the MTF joint. From this single incision, they develop intervals or windows to access each of the TMT joints. These authors stated that the approach was a viable alternative with a rate of wound complications comparable to previously reported approaches. Up to 25% of alterations in sensitivity and 8% of cutaneous necroses were observed after a transverse incision,29 which is a superficial incision to the neurovascular bundle that allows us to access each of the TMT joints through small windows.
Results
Van Hoeve et al have shown that the walking speed of patients treated for a Lisfranc lesion is slower than in healthy patients. Significant differences were found between the two groups in terms of joint balance in the midfoot during the take-off phase (push-off) in the sagittal plane (flexion/extension).30
Table 1 shows the main publications and results regarding ORIF in Lisfranc lesions.22,25,31–54 Table 2 summarises the main complications that can occur in the treatment of Lisfranc fracture-dislocations.2,3,17 Table 3 summarises the literature and results of primary arthrodesis in TMT joint injuries.55–57 Table 4 summarises the comparative studies of ORIF versus primary arthrodesis in lesions of the Lisfranc joint.16,19,20,58,60
Table 1.
Main studies on the treatment of Lisfranc fracture-dislocations with open reduction and internal fixation (ORIF)
| Authors | Year | Comments |
|---|---|---|
| Hesp et al31 | 1984 | This study analysed 24 cases. In the long run, functional and radiological results depended on the accuracy of reduction. For good anatomical results, immediate closed or, if needed, ORIF by percutaneous K-wires was paramount. |
| Perez-Blanco et al32 | 1988 | Open treatment was recommended if minor displacement persisted. Routine K-wire fixation was advised for all cases. Results were evaluated in 28 patients with a mean age of 34 years and a mean follow-up of 6.3 years. Treatment included closed reduction, occasionally followed by K-wire fixation. If closed reduction was not achieved, ORIF was performed. Results were evaluated according to Hardcastle's scoring system. On that basis, 20 good, 5 fair, and 3 poor results were obtained and there was 1 early amputation. Good results were associated with an accurate reduction. |
| Bandac and Botez33 | 2012 | This study analysed 31 patients. The average follow-up period was 44 months. The results were assessed using the Baltimore PFS and AOFAS mid-foot scoring scale. Ten patients had an excellent outcome on the PFS scale, 8 were classified as good, and 13 as fair and poor. Of all techniques of surgical treatment used, the highest scores were achieved by internal fixation with screws. Eight patients (25.8%) developed post-traumatic OA of the TMT joints. |
| Marin-Peña et al34 | These authors studied 32 patients. Initial reduction and secondary displacement were measured by the Myerson scale. Long-run radiographical data were classified as good, fair or poor results. Mean follow-up was 14 years. Seventeen patients with anatomic close reduction but instability were treated with closed reduction and K-wire fixation followed by cast immobilization. Eight patients with stable anatomic close reduction were treated with closed reduction and cast. Seven patients with unacceptable closed reduction were treated with ORIF and K-wire stabilization. The analysis of radiological long-term data showed 15 patients with good results, 8 with fair results and 9 with poor results. There was no statistically significant difference between overall PFS scores and different types of treatment, Hardcastle long-term radiological scores or Hardcastle type of fracture. | |
| Schepers et al22 | 2013 | In this study, 28 patients were analysed. The outcomes demonstrated that ORIF with screws or plate resulted in better reduction and better maintenance of reduction in both low- and high-energy Lisfranc injuries. Six patients were treated with closed reduction and percutaneous fixation and 22 with ORIF. Sixteen patients were treated with K-wires only (6 closed, 10 open), 7 with screws with or without K-wires, and 5 with medial plating with or without K-wires. In the closed reduction group, 2/6 (33%) reductions were considered acceptable vs 19 of 22 (86%) in the open group. All 6 secondary displacements occurred in the K-wire fixation group (37.5%) vs none in the rigid fixation group. |
| Demirkale et al35 | 2013 | In this report, 32 patients were studied. Mean follow-up was 55.5 months. The comparison of treatment results showed that those patients with high-grade soft-tissue injuries had lower AOFAS and FADI scores compared with Tscherne Grade 1 injuries. The overall negative impact of the severity of soft-tissue injury on functional results had similar significance with regard to post-traumatic OA and fracture type. There was also a statistically significant difference between outcome measures and post-reduction quality. Patient age and delayed surgery had no statistically significant effect on the final result. |
| Hu et al36 | 2014 | In this prospective comparative study (level of evidence II), 60 patients with primarily isolated Lisfranc joint injury were treated with ORIF and dorsal plate fixation (n=32) or ORIF and screw fixation (n=28). The patients were followed on average for 31 months. Open reduction and dorsal plate fixation for a dislocated Lisfranc injury had better short- and medium-term results and a lower re-operation rate than standard screw ORIF. Of the dorsal plate fixation group, radiographic analysis showed anatomic reduction in 29 patients (90.6%, 29/32) and non-anatomic reduction in three patients. In the screw fixation group, radiographic analysis showed anatomic reduction in 23 patients and non-anatomic reduction in five patients (82.1%, 23/28). |
| Crates et al37 | 2015 | Subtle Lisfranc injuries failing non-operative treatment were successfully stabilised using either a dual screw or suture button technique. Of 36 patients analysed, 16 (44.44%) were successfully treated non-operatively and 20 (55.56%) required surgery after non-operative treatment had failed. Of those treated operatively, 9 (45%) were stabilized with dual screws and 11 (55%) with dual suture buttons. The mean follow-up period was 36 months. The AOFAS scores significantly improved from the pre- to final post-treatment values. Of the 9 feet stabilized with dual screws, 7 (77.78%) required screw removal during the observation period. |
| Li et al38 | 2015 | This study showed that anatomical reduction of Lisfranc injury can be achieved by ORIF with the miniplate and hollow screw. Normal structure of the Lisfranc joint was regained to a great extent; injured ligaments were also repaired. This method offered an excellent curative effect, avoided post-operative complications and improved the patients' quality of life. All injuries were closed. The time interval between injury and operation was 6.6 days on average. All patients were followed up for 18 to 24 months (average 20 months). Anatomic reduction was achieved in all patients according to images. According to the AOFAS score, 5 cases were defined as excellent, 3 as good and 2 cases fair. During follow-up, there was no wound infection or complications except for OA in 2 cases. Healing time was in the range of 3 to 6 months (average 3.6 months). |
| Dubois-Ferrière et al39 | 2016 | This study analysed 61 patients treated surgically for an injury to the TMT joint complex. Patients underwent either ORIF with transarticular screws or primary arthrodesis when joint comminution at the TMT level was such that ORIF was not possible. Radiographic evidence of OA was noted in 44 (72.1̵) of the patients and symptomatic OA in 54.1%, the latter having poorer results. Risk factors for OA were non-anatomic reduction, fracture classification of Myerson type C and a history of smoking. Two to 24 years following surgical treatment to restore and maintain joint anatomy for Lisfranc injuries, these authors found satisfactory clinical outcome scores and a large number of patients who had returned to their previous level of functioning and employment, with little need for secondary procedures. |
| McHale et al40 | 2016 | The study analysed 28 NFL athletes who sustained Lisfranc injuries. More than 90% of NFL athletes returned to play in the NFL at a median of 11.1 months from time of injury. |
| van Koperen et al41 | 2016 | This study described the results of 34 patients treated with bridge plating after TMT fracture dislocations compared with transarticular screw fixation. Bridge plating was used in 21 patients. In 13 patients, K-wires or transarticular screws or a combination were used. The median follow-up period was 49 months. The implants were removed in 10/13 patients in the transarticular group and in 17/21 patients in the bridge plating group. The incidence of wound complications was comparable in both groups. The median AOFAS score was lower in the transarticular group (77 vs 66). The FFI score was 18 in both groups. Patient satisfaction was 90% in the bridge plating group and 80% in the transarticular group. Bridge plating for Lisfranc injuries led to at least similar results compared with transarticular fixation in terms of functional outcomes and patient satisfaction. |
| Ahmad and Jones42 | 2016 | In this grade I of evidence study, 40 patients with acute Lisfranc injuries were studied. Through randomisation, 20 and 20 patients received bio-absorbable vs steel screws, respectively. Bio-absorbable screws provided short-term results that were comparable and not significantly different from steel screws for treating unstable Lisfranc injuries. Both methods were predictable in improving function and pain, but using absorbable screws eliminated the need for hardware removal after such trauma. |
| Del Vecchio et al43 | 2016 | In this study, 5 patients with a diagnosis of Lisfranc low-energy lesion were treated with a novel surgical technique characterized by minimal osteosynthesis performed through a minimally invasive approach. The authors performed a closed reduction and minimally invasive stabilisation with a bridge plate and a screw after achieving a closed anatomical reduction. According to the radiological criteria established, the joint reduction was anatomical in 4 patients, almost anatomical in 1 patient, and non-anatomical in none of the patients. The mean score according to the VAS at the end of the follow-up period was 1.4 points over 10. |
| Lien et al44 | 2016 | These authors described a combined innovative portal arthroscopy and fluoroscopy-assisted reduction and fixation in 10 cases of subtle injury of the Lisfranc joint. Of the 10 patients, 3 had excellent outcomes, 6 had good outcomes and 1 had a fair outcome. The Lisfranc distance, foot arch height and function of the foot were restored clinically; all measurements showed statistically significant differences. |
| Cassinelli et al45 | 2016 | A delayed ORIF of 8 patients with missed, low-energy Lisfranc injury was performed and resulted in decreased pain. In this series, a fair to good functional outcome was observed; the ability to return to work or previous sports was possible for all patients studied. Average age at surgery was 39.8 years and the mean time to surgery from injury was 15.1 weeks. There were no radiographic signs of a late diastasis at the Lisfranc joint. All patients, including 2 workers compensation cases, returned to work and all were able to return to their prior sporting activity. |
| Qu et al46 | 2016 | This study analysed 20 cases of severe open Lisfranc fracture and dislocation. Treatment was performed through one-stage internal fixation with K-wires in association with vacuum sealing drainage. This technique led to fast anatomical reduction, stabilised bony structure, fast-soft tissue recovery and good short-term follow-up results. The average time of internal fixation surgery was 47 minutes. There were 3 cases of wound-edge necrosis; however, there were no cases of skin necrosis around the incision, or deep infection. The mean time of the first hospital stay was 16.1 days. |
| Hill et al47 | 2017 | In this analysis of paediatric Lisfranc injuries, 56 children treated for bony or ligamentous Lisfranc injuries were analysed. Overall, 51% of fractures and 82% of sprains were sports-related. A total of 34% of the cohort underwent ORIF, which was more common among patients with closed physes (67%). Full weight-bearing was allowed in ORIF patients at a mean of 14.5 weeks, compared with 6.5 weeks in the non-operative group. Complications were rare (4%) and included physeal arrest in 1 patient and a broken, retained implant in 1 patient. |
| Balazs et al48 | 2017 | Twenty-one military personnel sustaining Lisfranc injuries had high rates of persistent pain and disability, even after optimal initial surgical treatment. Median patient age was 23 years and mean follow-up was 43 months. Within this cohort, 14 patients were able to return to military service, while 7 required a disability separation from the armed forces. Of the 18 patients who underwent initial fixation, 8 were subsequently revised to midfoot arthrodesis for persistent pain. |
| McHale et al49 | 2017 | Lisfranc injuries identified at the NFL Combine had an adverse effect on an NFL athlete's draft status, draft position and overall play during initial NFL seasons. In particular, residual displacement of the Lisfranc joint had a detrimental effect on the first 2 seasons of NFL play that could lead to long-lasting negative effects on the athlete's career. A total of 41/2162 (1.8%) Combine participants were identified with Lisfranc injuries, of whom 26/41 (63.4%) were managed operatively. Players who underwent surgery were more likely to go undrafted compared with players managed non-operatively (38.5% vs 13.3%, operative vs non-operative management, respectively) and featured a poorer NFL draft pick position (155.6 vs 109). Lisfranc-injured players when compared with controls were noted to have poorer outcomes in terms of NFL draft position (142 vs 111.3, Lisfranc-injured players vs controls, respectively), NFL career length 2 years or longer (62.5% vs 69.6%) and number of games played (16.9 vs 23.3) and started (6.8 vs 10.5) within the first 2 years of their NFL career. Radiographs demonstrated that 17/41 (41.5%) athletes had residual Lisfranc joint displacement > 2 mm compared with the contralateral foot. Lisfranc-injured athletes with > 2 mm residual displacement, when compared with matched controls, had a poorer draft position (156.9 vs 111.2 for Lisfranc-injured players vs controls, respectively) and fewer games played (14.4 vs 23.3) and started (3.1 vs 10.5). Athletes with > 2 mm residual displacement featured poorer results across all assessed NFL variables vs athletes with residual displacement of ⩽ 2 mm. |
| Vosbikian et al50 | 2017 | Thirty-eight patients were analysed. Minimum follow-up of 3 years. The average age at the time of surgery was 34.2 years. At final follow-up, 31 patients were available for assessment (81.6%). Minimally invasive methods of treating low-energy Lisfranc injuries with less soft-tissue stripping and disruption were described in this series. They were a valuable tool to optimise outcomes while minimizing the potential morbidity of more traditional, open techniques. Percentage recovery compared to their preinjury functional level averaged 91.4%. There were no complications in this series. Twenty-two patients underwent screw removal electively at an average of 6.9 months following the index procedure. No patients had undergone any additional operative procedures, nor had any objective evidence of midfoot collapse or OA at the time of the final follow-up. |
| Lau et al51 | 2017 | Functional results after Lisfranc fractures were most dependent on the quality of anatomical reduction and not the choice of fixation implant used. Fifty patients who underwent surgical fixation of Lisfranc injuries over a 6-year period in 1 of 3 treatment arms were reviewed: trans-articular screw fixation alone, dorsal bridge plating alone or a combination of dorsal bridge and trans-articular screw fixation. A high-quality anatomical reduction was the best predictor of functional outcomes. Injury type by Myerson classification systems or open vs closed status was also not significantly associated with any fixation group. Open exposures were more likely to result in soft-tissue complications, but there were no significant differences in metalware failure or need for removal. |
| Hawkinson et al52 | 2017 | This study not only reinforced the importance of initial anatomic reduction and the poor outcomes of post-traumatic OA but also suggested that salvage arthrodesis portends poor outcomes in a highly active population. Most notably, it found no significant difference in return to duty rates between ORIF and primary arthrodesis despite the inclusion of more ‘missed’ and chronic injuries in the primary arthrodesis group. This suggested that primary arthrodesis could be a viable option in a young and active population regardless of treatment timing. This study reviewed 171 low-energy closed TMT dislocations and fracture dislocations. Outcomes were defined as return to active duty and separation from service. The data demonstrated no significant differences between ORIF and primary arthrodesis as well as significantly lower return to duty rates among those who underwent salvage arthrodesis. There was no association between increased time from injury to treatment and the observed outcomes. |
| Mora et al53 | 2018 | Most patients who sustained a Lisfranc injury could return to sport and physical activity after ORIF. Patients should be counselled pre-operatively that approximately 1 in 3 might experience continued pain at the injury site. Thirty-three adult patients aged ⩽ 55 years who presented with a Lisfranc injury and underwent ORIF using a Lisfranc screw combined with bridge plating were analysed. Mean age and follow-up were 31.2 years and 2.9 years, respectively. Post-operatively, 31 patients (94%) were able to return to some form of sport. Twenty-two patients (66%) returned to playing sport at or above their pre-injury level. Of the 11 patients who played less sport, 6 had ongoing pain and the remaining 5 were asymptomatic but were participating less frequently because of other lifestyle reasons. In addition, of the 33 patients, 11 (33%) had some degree of ongoing pain that might limit their ability to return to sports and physical activities. |
| Kirzner et al25 | 2018 | A total of 108 patients were treated for a Lisfranc fracture-dislocation. Of these, 38 underwent trans-articular screw fixation, 45 dorsal bridge plating and 25 a combination technique. Injuries were assessed pre-operatively according to the Myerson classification system. Patients treated with dorsal bridge plating had better functional and radiological results than those treated with trans-articular screws or a combination technique. Significantly better functional outcomes were seen in the bridge plate group. Functional outcomes were dependent on the quality of the reduction. A trend was noted indicating that plate fixation is associated with a better anatomical reduction. Myerson types A and C2 significantly predicted a poorer functional outcome, suggesting that total incongruity in either a homolateral or divergent pattern leads to poorer outcomes. The greater the number of columns fixed, the poorer the outcome. |
| Singh et al54 | 2018 | A total of 47 athletes with Lisfranc injuries were identified, having 23 ligamentous injuries and 24 fractures. Thirty-five (75%) were treated operatively. Among NFL players, 29 (83%) returned to play, taking 10 months to do so. Overall, NFL players started fewer games 2 and 3 seasons following surgery and showed a significant decline in performance 1 season after return compared with preinjury levels (21%). Offensive players had a significantly greater decline in statistical performance compared with their defensive counterparts. Although professional NFL athletes returned to play at a high rate (83%) following Lisfranc injury, their league participation and performance were significantly decreased on return. Ligamentous and bony injuries had similar prognoses; however, offensive players showed greater declines in performance compared with defensive players. |
ORIF, open reduction and internal fixation; K-wire, Kirschner wire; PFS, Painful Foot Score; AOFAS, American Orthopaedic Foot and Ankle Society; OA, osteoarthritis FADI, Foot and Ankle Disability Index; TMT, tarsometatarsal; NFL, National Football League; VAS, visual analogue scale.
Table 2.
Main complications after open reduction and internal fixation (ORIF) reported in the literature
| Compartment syndrome | 2.6% |
| Skin problems | 3.6% |
| Infection | 1.5% |
| Deep vein thrombosis | 0.5% |
| Reflex sympathetic dystrophy | 1% |
| Screw problems | 16% |
| Lisfranc post-traumatic OA | 49% |
| Arthrodesis | 7.8% |
OA, osteoarthritis.
Table 3.
Main studies on the treatment of Lisfranc fracture-dislocations with primary arthrodesis
| Authors | Year | Comments |
|---|---|---|
| Reinhardt et al55 | 2012 | Treatment of both primarily ligamentous and combined osseous and ligamentous Lisfranc injuries with partial partial arthrodesis produced good clinical and patient-based results. Twenty-five patients (12 ligamentous, 13 combined), median age 46 years, were followed for an average of 42 months. At the last follow-up, patients reported an average return to 85% of their pre-injury activity level. Twenty-one patients (84%) expressed satisfaction with their result; at the latest follow-up, the mean VAS score was 1.8 out of 10. Three patients showed radiographic signs of post-traumatic OA of adjacent joints. |
| MacMahon et al56 | 2016 | Thirty-eight patients were analysed. Mean age at surgery was 31.8 years and mean follow-up was 5.2 years. Pre-operatively, 47.1% were high-impact, and post-operatively, 44.8% were high-impact. Most patients were able to return to their previous physical activities following partial primary arthrodesis for a Lisfranc injury, many of which were high-impact. However, the decreased participation or increase in difficulty of some activities suggested that some patients experienced post-operative limitations in exercise. Compared to pre-operatively, perceived difficulty was the same in 66% and increased in 34% of physical activities. Participation levels were improved in 11%, the same in 64% and impaired in 25% of physical activities. In regard to return to physical activity, 97% of respondents were satisfied with their operative outcome. |
| Lui et al57 | 2016 | This article describes a minimally invasive approach of arthroscopic arthrodesis of the involved TMT joints. The arthroscopic procedure was performed through the junction portals of the involved articulation. It had the advantages of better cosmesis, less wound complication, less bone resection and more thorough joint debridement. However, it was contra-indicated if there was an associated significant foot deformity or shortening of the involved foot rays. |
VAS, visual analogue scale; OA, osteoarthritis; TMT, tarsometatarsal.
Table 4.
Main studies comparing primary arthrodesis versus open reduction and internal fixation (ORIF) in Lisfranc fracture-dislocations
| Authors | Year | Comments |
|---|---|---|
| Sheibani-Rad et al16 | 2012 | This is a systematic review of the literature comparing the 2 most common procedures for Lisfranc fractures: primary arthrodesis and ORIF. This study highlighted that both procedures yield satisfactory and equivalent results. It was concluded that there might be a slight advantage to performing a primary arthrodesis for Lisfranc joint injuries in terms of clinical results. |
| Smith et al19 | 2016 | These authors performed a systematic review of the literature. They examined whether ORIF or primary arthrodesis led to: 1) fewer re-operations for hardware removal; 2) less frequent revision surgery; 3) higher patient outcome scores; and 4) more frequent anatomic reduction. The risk ratio for hardware removal was 0.23, indicating more hardware removal for ORIF than primary arthrodesis. For other revision surgery, the risk ratio for ORIF was 0.36 favouring neither. Similarly, neither was favoured using patient-reported outcomes. When considering the risk of non-anatomic alignment, neither was favoured. The surgeon should consider the increased risk of hardware removal along with its associated morbidity and discuss this with the patient pre-operatively when considering ORIF for Lisfranc injuries. |
| Qiao et al58 | 2017 | A comparative retrospective study of 25 patients with acute or subacute Lisfranc complex injuries was conducted. All patients were classified by Myerson classification. Eight patients were treated with primary arthrodesis, whereas 17 patients received non-fusion operations. Primary arthrodesis had advantages compared with primary ORIF: reduced foot deformity rates, sustained biomechanical morphology of the feet, fewer complications, higher level of function recovery, shorter time of surgical procedures and higher AOFAS score. According to this research, primary arthrodesis might be a better choice for treating Lisfranc injury. All fractures healed for both the arthrodesis group and the non-fusion group. Complications occurred in 8 patients (8/17, 47%) in the non-primary arthrodesis group, including the second and third phalanx abduction (1), talipes cavus (2), eversion deformity of front foot (3), eversion deformity of calcaneus (1), as well as post-operative infection (1). Only 2 patients (2/8, 25%) in the arthrodesis group experienced complications. One was a limitation of motion of the front foot and pain during walking; the other was an eversion deformity of the front foot. |
| Cochran et al59 | 2017 | Thirty-two active duty military personnel were studied. All were operatively managed for low-energy Lisfranc injuries. Primary arthrodesis was performed on 14 patients with ORIF in 18. The average age was 28 years. Low-energy Lisfranc injuries treated with primary arthrodesis had a lower implant removal rate, an earlier return to full military activity, and better fitness test scores after 1 year, but there was no difference in FAAM scores after 3 years. The primary arthrodesis group returned to full duty at an average of 4.5 months, whereas the ORIF group returned at an average of 6.7 months. There were no differences between the 2 groups in the FAAM scores at an average of 35 months. Implant removal was performed on 15 (83%) in the ORIF group and 2 (14%) in the primary arthrodesis group. |
| Albright et al60 | 2018 | From a healthcare system's standpoint, primary arthrodesis would clearly be the preferred treatment strategy for predominantly ligamentous Lisfranc injuries and dislocations. These authors conducted a formal cost-effectiveness analysis using a Markov model and decision tree to explore the healthcare costs and health outcomes associated with a scenario of ORIF vs primary arthrodesis for 45 years post-operatively. The outcomes assessed included long-term costs, quality-adjusted life-years (QALYs) and incremental cost per QALY gained. The costs were evaluated from the healthcare system perspective and are expressed in U.S. dollars at a 2017 price base. ORIF was always associated with higher costs compared with primary arthrodesis and was less effective in the long term. When calculating the cost required to gain 1 additional QALY, the primary arthrodesis group cost $1429/QALY and the ORIF group cost $3958/QALY. The group undergoing primary arthrodesis overall spent, on average, $43 192 less than the ORIF group, and primary arthrodesis was overall a more effective technique. |
| Buda et al20 | 2018 | When excluding planned removal of hardware, patients with Lisfranc injuries treated with ORIF did not demonstrate a higher rate of re-operation compared with those undergoing primary arthrodesis. Adult patients who sustained closed, isolated Lisfranc injuries with or without fractures and who underwent ORIF or primary arthrodesis with a minimum follow-up of 12 months were analysed. Some 217 patients were analysed (mean follow-up, 62.5 months), of which 163 (75.1%) underwent ORIF and 54 (24.9%) underwent primary arthrodesis. Overall and including planned procedures, patients treated with ORIF had a significantly higher rate of return to the operation room (75.5%) compared with those in the primary arthrodesis group (31.5%). When excluding planned hardware removal, however, there was no difference in reoperation rates between the 2 groups (29.5% in the ORIF group and 29.6% in the primary arthrodesis group). Risk factors correlating with unplanned return to the operation room included deep infection, delayed wound healing and high-energy trauma. |
AOFAS, American Orthopedic Foot and Ankle Society; FAAM, Foot and Ankle Ability Measure.
Conclusions
High-energy Lisfranc fracture-dislocations are easy to diagnose; however, we should pay special attention to those patients with midfoot pain and plantar haematoma, given they almost certainly present a lesion of the TMT joint. If not diagnosed, sequelae such as OA, instability and foot deformity are possible. The current trend is the surgical treatment of all lesions that show instability with weight-bearing or an initial displacement that indicates a rupture or detachment of the Lisfranc ligament complex.
All the authors agree that the most important factor to obtaining satisfactory functional and radiographic results is achieving optimal anatomical reduction, even independently of the technique used or the osteosynthesis material used. On the other hand, no statistically significant differences were observed, either in functional results or in the degree of reduction, between osteosynthesis and primary partial arthrodesis. Only primary partial arthrodesis has shown better results in purely ligamentous Lisfranc lesions.
According to biomechanical studies, there are no differences between the use of transarticular screws and dorsal plates in terms of reduction and displacement with weight-bearing. Only one retrospective clinical study has shown better results with the use of dorsal plates. However, the severity of the injury and the degree of reduction were, according to aforementioned study, the most determining factors. Prospective studies should be conducted comparing screws and plates in fractures with similar characteristics. For now, we advocate the use of transarticular screws, except for in cases of excessive comminution of the joint facets, for which we instead use dorsal plates or primary arthrodesis.
Footnotes
ICMJE Conflict of interest statement: The author declares no conflict of interest relevant to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Cassebaum WH. Lisfranc fracture-dislocations. Clin Orthop Relat Res 1963;30(30):116–129. [PubMed] [Google Scholar]
- 2. Desmond EA, Chou LB. Current concepts review: Lisfranc injuries. Foot Ankle Int 2006;27(8):653–660. [DOI] [PubMed] [Google Scholar]
- 3. Myerson MS, Cerrato R. Current management of tarsometatarsal injuries in the athlete. Instr Course Lect 2009;58:583–594. [PubMed] [Google Scholar]
- 4. Stavlas P, Roberts CS, Xypnitos FN, Giannoudis PV. The role of reduction and internal fixation of Lisfranc fracture-dislocations: a systematic review of the literature. Int Orthop 2010;34(8):1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benirschke SK, Meinberg E, Anderson SA, Jones CB, Cole PA. Fractures and dislocations of the midfoot: Lisfranc and Chopart injuries. J Bone Joint Surg [Am] 2012;94(14):1325–1337. [DOI] [PubMed] [Google Scholar]
- 6. DeOrio M, Erickson M, Usuelli FG, Easley M. Lisfranc injuries in sport. Foot Ankle Clin 2009;14(2):169–186. [DOI] [PubMed] [Google Scholar]
- 7. Peicha G, Labovitz J, Seibert FJ, et al. The anatomy of the joint as a risk factor for Lisfranc dislocation and fracture-dislocation. An anatomical and radiological case control study. J Bone Joint Surg [Br] 2002;84(7):981–985. [DOI] [PubMed] [Google Scholar]
- 8. Raikin SM, Elias I, Dheer S, et al. Prediction of midfoot instability in the subtle Lisfranc injury. Comparison of magnetic resonance imaging with intraoperative findings. J Bone Joint Surg [Am] 2009;91(4):892–899. [DOI] [PubMed] [Google Scholar]
- 9. Myerson MS, Fisher RT, Burgess AR, Kenzora JE. Fracture dislocations of the tarsometatarsal joints: end results correlated with pathology and treatment. Foot Ankle 1986;6(5):225–242. [DOI] [PubMed] [Google Scholar]
- 10. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: lisfranc injuries in the athlete. Am J Sports Med 2002;30(6):871–878. [DOI] [PubMed] [Google Scholar]
- 11. Aronow MS. Treatment of the missed Lisfranc injury. Foot Ankle Clin 2006;11(1):127–142, ix. [DOI] [PubMed] [Google Scholar]
- 12. Pourcho AM, Liu YH, Milshteyn MA. Electrodiagnostically confirmed posttraumatic neuropathy and associated clinical exam findings with Lisfranc injury. Foot Ankle Int 2013;34(8):1068–1073. [DOI] [PubMed] [Google Scholar]
- 13. Rankine JJ, Nicholas CM, Wells G, Barron DA. The diagnostic accuracy of radiographs in Lisfranc injury and the potential value of a craniocaudal projection. AJR Am J Roentgenol 2012;198(4):W365–369. [DOI] [PubMed] [Google Scholar]
- 14. Podolnick JD, Donovan DS, DeBellis N, Pino A. Is pes cavus alignment associated with Lisfranc injuries of the foot? Clin Orthop Relat Res 2017;475(5):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sivakumar BS, An VVG, Oitment C, Myerson M. Subtle Lisfranc injuries: A topical review and modification of the classification system. Orthopedics 2018;41(2):e168–e175. [DOI] [PubMed] [Google Scholar]
- 16. Sheibani-Rad S, Coetzee JC, Giveans MR, DiGiovanni C. Arthrodesis versus ORIF for Lisfranc fractures. Orthopedics 2012;35(6):e868–e873. [DOI] [PubMed] [Google Scholar]
- 17. Lau S, Howells N, Millar M, et al. Plates, screws, or combination? Radiologic outcomes after Lisfranc fracture dislocation. J Foot Ankle Surg 2016;55(4):799–802. [DOI] [PubMed] [Google Scholar]
- 18. Herscovici D, Jr, Scaduto JM. Acute management of high-energy lisfranc injuries: A simple approach. Injury 2018;49(2):420–424. [DOI] [PubMed] [Google Scholar]
- 19. Smith N, Stone C, Furey A. Does open reduction and internal fixation versus primary arthrodesis improve patient outcomes for Lisfranc trauma? A systematic review. Clin Orthop Relat Res 2016;474(6):1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buda M, Kink S, Stavenuiter R, et al. Reoperation rate differences between open reduction internal fixation and primary arthrodesis of Lisfranc injuries. Foot Ankle Int 2018;39(9):1089–1096. [DOI] [PubMed] [Google Scholar]
- 21. Watson TS, Shurnas PS, Denker J. Treatment of Lisfranc joint injury: current concepts. J Am Acad Orthop Surg 2010;18(12):718–728. [DOI] [PubMed] [Google Scholar]
- 22. Schepers T, Oprel PP, Van Lieshout EM. Influence of approach and implant on reduction accuracy and stability in Lisfranc fracture-dislocation at the tarsometatarsal joint. Foot Ankle Int 2013;34(5):705–710. [DOI] [PubMed] [Google Scholar]
- 23. Rozell JC, Chin M, Donegan DJ, Hast MW. Biomechanical comparison of fully threaded solid cortical versus partially threaded cannulated cancellous screw fixation for Lisfranc injuries. Orthopedics 2018;41(2):e222–e227. [DOI] [PubMed] [Google Scholar]
- 24. Alberta FG, Aronow MS, Barrero M, et al. Ligamentous Lisfranc joint injuries: a biomechanical comparison of dorsal plate and transarticular screw fixation. Foot Ankle Int 2005;26(6):462–473. [DOI] [PubMed] [Google Scholar]
- 25. Kirzner N, Zotov P, Goldbloom D, Curry H, Bedi H. Dorsal bridge plating or transarticular screws for Lisfranc fracture dislocations: a retrospective study comparing functional and radiological outcomes. Bone Joint J 2018;100-B(4):468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klos K, Gueorguiev B, Mückley T, et al. Stability of medial locking plate and compression screw versus two crossed screws for lapidus arthrodesis. Foot Ankle Int 2010;31(2):158–163. [DOI] [PubMed] [Google Scholar]
- 27. Klos K, Simons P, Hajduk AS, et al. Plantar versus dorsomedial locked plating for Lapidus arthrodesis: a biomechanical comparison. Foot Ankle Int 2011;32(11):1081–1085. [DOI] [PubMed] [Google Scholar]
- 28. Philpott A, Lawford C, Lau SC, et al. Modified dorsal approach in the management of Lisfranc injuries. Foot Ankle Int 2018;39(5):573–584. [DOI] [PubMed] [Google Scholar]
- 29. Vertullo CJ, Easley ME, Nunley JA. The transverse dorsal approach to the Lisfranc joint. Foot Ankle Int 2002;23(5):420–426. [DOI] [PubMed] [Google Scholar]
- 30. van Hoeve S, Stollenwerck G, Willems WP, et al. Gait analysis and functional outcome in patients after Lisfranc injury treatment. Foot Ankle Surg 2018;24(6):535–541. [DOI] [PubMed] [Google Scholar]
- 31. Hesp WL, van der Werken C, Goris RJ. Lisfranc dislocations: fractures and/or dislocations through the tarso-metatarsal joints. Injury 1984;15(4):261–266. [DOI] [PubMed] [Google Scholar]
- 32. Pérez Blanco R, Rodríguez Merchán C, Canosa Sevillano R, Munuera Martínez L. Tarsometatarsal fractures and dislocations. J Orthop Trauma 1988;2(3):188–194. [DOI] [PubMed] [Google Scholar]
- 33. Bandac RC, Botez P. Lisfranc midfoot dislocations: correlations between surgical treatment and functional outcomes. Rev Med Chir Soc Med Nat Iasi 2012;116(3):834–839. [PubMed] [Google Scholar]
- 34. Marín-Peña OR, Viloria Recio F, Sanz Gómez T, Larrainzar Garijo R. Fourteen years follow up after Lisfranc fracture-dislocation: functional and radiological results. Injury 2012;43(suppl 2):S79–S82. [DOI] [PubMed] [Google Scholar]
- 35. Demirkale I, Tecimel O, Celik I, et al. The effect of the Tscherne injury pattern on the outcome of operatively treated Lisfranc fracture dislocations. Foot Ankle Surg 2013;19(3):188–193. [DOI] [PubMed] [Google Scholar]
- 36. Hu SJ, Chang SM, Li XH, Yu GR. Outcome comparison of Lisfranc injuries treated through dorsal plate fixation versus screw fixation. Acta Ortop Bras 2014;22(6):315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crates JM, Barber FA, Sanders EJ. Subtle Lisfranc subluxation: results of operative and nonoperative treatment. J Foot Ankle Surg 2015;54(3):350–355. [DOI] [PubMed] [Google Scholar]
- 38. Li BL, Zhao WB, Liu L, et al. Efficacy of open reduction and internal fixation with a miniplate and hollow screw in the treatment of Lisfranc injury. Chin J Traumatol 2015;18(1):18–20. [DOI] [PubMed] [Google Scholar]
- 39. Dubois-Ferrière V, Lübbeke A, Chowdhary A, et al. Clinical outcomes and development of symptomatic osteoarthritis 2 to 24 years after surgical treatment of tarsometatarsal joint complex injuries. J Bone Joint Surg [Am] 2016;98-A(9):713–720. [DOI] [PubMed] [Google Scholar]
- 40. McHale KJ, Rozell JC, Milby AH, Carey JL, Sennett BJ. Outcomes of Lisfranc Injuries in the National Football League. Am J Sports Med 2016;44(7):1810–1817. [DOI] [PubMed] [Google Scholar]
- 41. van Koperen PJ, de Jong VM, Luitse JS, Schepers T. Functional outcomes after temporary bridging with locking plates in Lisfranc injuries. J Foot Ankle Surg 2016;55(5):922–926. [DOI] [PubMed] [Google Scholar]
- 42. Ahmad J, Jones K. Randomized, prospective comparison of bioabsorbable and steel screw fixation of Lisfranc injuries. J Orthop Trauma 2016;30(12):676–681. [DOI] [PubMed] [Google Scholar]
- 43. DelVecchio JJ Ghioldi M Raimondi N De Elias M. Minimally invasive medial plating of low-energy Lisfranc injuries: preliminary experience with five cases. Adv Orthop 2016;2016:4861260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lien SB, Shen HC, Lin LC. Combined innovative portal arthroscopy and fluoroscopy-assisted reduction and fixation in subtle injury of the Lisfranc joint complex: analysis of 10 cases. J Foot Ankle Surg 2017;56(1):142–147. [DOI] [PubMed] [Google Scholar]
- 45. Cassinelli SJ, Moss LK, Lee DC, Phillips J, Harris TG. Delayed open reduction internal fixation of missed, low-energy Lisfranc injuries. Foot Ankle Int 2016;37(10):1084–1090. [DOI] [PubMed] [Google Scholar]
- 46. Qu W, Ni S, Wang Z, et al. Severe open Lisfranc injuries: one-stage operation through internal fixation associated with vacuum sealing drainage. J Orthop Surg Res 2016;11(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hill JF, Heyworth BE, Lierhaus A, Kocher MS, Mahan ST. Lisfranc injuries in children and adolescents. J Pediatr Orthop B 2017;26(2):159–163. [DOI] [PubMed] [Google Scholar]
- 48. Balazs GC, Hanley MG, Pavey GJ, Rue JP. Military personnel sustaining Lisfranc injuries have high rates of disability separation. J R Army Med Corps 2017;163(3):215–219. [DOI] [PubMed] [Google Scholar]
- 49. McHale KJ, Vopat BG, Beaulieu-Jones BR, et al. Epidemiology and outcomes of Lisfranc injuries identified at the National Football League scouting combine. Am J Sports Med 2017;45(8):1901–1908. [DOI] [PubMed] [Google Scholar]
- 50. Vosbikian M, O’Neil JT, Piper C, Huang R, Raikin SM. Outcomes after percutaneous reduction and fixation of low-energy Lisfranc injuries. Foot Ankle Int 2017;38(7):710–715. [DOI] [PubMed] [Google Scholar]
- 51. Lau S, Guest C, Hall M, et al. Functional outcomes post Lisfranc injury-transarticular screws, dorsal bridge plating or combination treatment? J Orthop Trauma 2017;31(8):447–452. [DOI] [PubMed] [Google Scholar]
- 52. Hawkinson MP, Tennent DJ, Belisle J, Osborn P. Outcomes of Lisfranc injuries in an active duty military population. Foot Ankle Int 2017;38(10):1115–1119. [DOI] [PubMed] [Google Scholar]
- 53. Mora AD, Kao M, Alfred T, et al. Return to sports and physical activities after open reduction and internal fixation of Lisfranc injuries in recreational athletes. Foot Ankle Int 2018;39(7):801–807. [DOI] [PubMed] [Google Scholar]
- 54. Singh SK, George A, Kadakia AR, Hsu WK. Performance-based outcomes following Lisfranc injury among professional American football and rugby athletes. Orthopedics 2018;41(4):e479–e482. [DOI] [PubMed] [Google Scholar]
- 55. Reinhardt KR, Oh LS, Schottel P, Roberts MM, Levine D. Treatment of Lisfranc fracture-dislocations with primary partial arthrodesis. Foot Ankle Int 2012;33(1):50–56. [DOI] [PubMed] [Google Scholar]
- 56. MacMahon A, Kim P, Levine DS, et al. Return to sports and physical activities after primary partial arthrodesis for Lisfranc injuries in young patients. Foot Ankle Int 2016;37(4):355–362. [DOI] [PubMed] [Google Scholar]
- 57. Lui TH. Arthroscopic tarsometatarsal arthrodesis. Arthrosc Tech 2016;5(6):e1311–e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qiao YS, Li JK, Shen H, et al. Comparison of arthrodesis and non-fusion to treat Lisfranc injuries. Orthop Surg 2017;9(1):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cochran G, Renninger C, Tompane T, Bellamy J, Kuhn K. Primary arthrodesis versus open reduction and internal fixation for low-energy Lisfranc injuries in a young athletic population. Foot Ankle Int 2017;38(9):957–963. [DOI] [PubMed] [Google Scholar]
- 60. Albright RH, Haller S, Klein E, et al. Cost-effectiveness analysis of primary arthrodesis versus open reduction internal fixation for primarily ligamentous Lisfranc injuries. J Foot Ankle Surg 2018;57(2):325–331. [DOI] [PubMed] [Google Scholar]