Abstract
The mechanism of the (CH3)2CCH2+H2O→(CH3)3COH reaction catalyzed by two strong acids (H2SO4 and HSbF6) was investigated theoretically using the ab initio MP2 and CCSD(T) methods and the aug-cc-pVDZ/LANL2DZ and aug-cc-pVTZ/LANL2DZ basis sets. The effects of surrounding solvent molecules were approximated by employing the polarized continuum solvation model. The most important findings include the observation that both acids are capable of catalyzing isobutene hydration but the reaction is predicted to proceed faster when the HSbF6 superacid plays the catalyst role.
Keywords: Organic chemistry, Physical chemistry, Catalysis, ab initio calculations, Reaction mechanism, Superacid
1. Introduction
Due to their wide range of applications, alcohols continue to arouse great interest in scientific community. Along with ethanol (commonly obtained in fermentation process) and methanol (synthesized primarily from synthesis gas), tert-butanol remains among the most popular alcohols used both in industry and laboratory. (CH3)3COH is utilized either directly as gasoline additive (where it acts as oxygenate, i.e., the substance facilitating complete combustion) or as a starting compound in synthesis of other oxygenates (such as methyl tert-butyl ether or ethyl tert-butyl ether) [1]. Apart from being used as a solvent [2], tert-butanol is also utilized to prepare potassium tert-butoxide which is a strong non-nucleophilic base used in Williamson ether synthesis [3] and has to be prepared in situ due to its high reactivity.
The hydration of alkenes is a prominent method for preparation of secondary and tertiary alcohols. Addition of water to alkenes can potentially lead to two products, one Markovnikov and one anti-Markovnikov. Primary laboratory method in which the main product is consistent with the Markovnikov's rule is the oxymercuration-reduction reaction (highly toxic mercury (II) acetate is used as a catalyst in this process) [4]. The most common laboratory way to obtain anti-Markovnikov product of alkene hydration is performing the Brown hydroboration [5]. This reaction, however, requires the use of extremely flammable and explosive borane as a catalyst. Alternative method of obtaining alcohols via hydration that is widely utilized in the industry (as it involves relatively safe catalyst) is the hydration of alkenes catalyzed by the acidic systems [6]. At present, H3PO4/SiO2 catalysts are used in this type of synthesis, however, major disadvantages of such processes include the relatively low alkene-to-alcohol conversion and the requirement of high purity of the alkene substrates [7]. Therefore, the use of sulfuric acid as a catalyst in alkene hydration reactions may still be considered reasonable. In 1965, Schubert and Lamm studied the mechanism of acid-catalyzed hydration of styrene and showed that the protonation of alkene is the rate determining step of this reaction [8]. This finding suggested that utilizing strong acids as catalyst should render the hydration of alkens more efficient. Indeed, several experiments conducted in the last few decades confirmed this assumption [9, 10].
The addition of water to isobutene leading to tert-butanol has been the subject of many considerations. In 1934, Lucas and Eberz found that the reaction rate of isobutene hydration performed at constant ionic strength depends linearly on the concentration of isobutene and the concentration of nitric acid [11]. Two decades later, Taft Jr demonstrated that the rate of isobutene hydration catalyzed by a mineral acid (HNO3 or H2SO4) is proportional to the Hammet acidity function (H0) of the catalyst employed [12] (Deckwer et al. arrived at similar conclusions while evaluating the rate constants of hydration of isobutene catalyzed by sulfuric acid) [13]. The Bielański group used H4SiW12O40 (dodecatungstosilicic acid) as a catalyst in the conversion of isobutene to tert-butanol performed in the gas phase [14], whereas Nakagawa, Tajima, and Hirao calculated heights of the energy barriers (spanning the 25–28 kcal/mol range) for the hydration of ethene catalyzed by H3PO4, H2SO4, and HClO4 acids [15].
As vaguely indicated above and demonstrated in many earlier reports, strong acids (and superacids in particular) can act as catalysts in many chemical reactions [16, 17, 18, 19]. Recently, our research group contributed to these studies by providing the mechanism of acid-catalyzed carbon monoxide hydrogenation yielding formaldehyde [20, 21] and predicting the ethanol-based (C2H5OH2)+(SbF6)− salt formation resulting from acetaldehyde hydrogenation in the presence of HSbF6 superacid [22]. In this contribution, we present our results and conclusions concerning the mechanism of isobutene hydration catalyzed either by a representative strong mineral acid (H2SO4) or by the fluoroantimonic superacid.
2. Methods
The structures of isobutene interacting with water molecule in the presence of either H2SO4 or HSbF6 catalyst were obtained by applying the second-order Møller-Plesset (MP2) perturbational method [23, 24, 25] with the aug-cc-pVDZ basis sets (for C, O, F, S, and H) [26] and with the Los Alamos National Laboratory (LANL) effective core potentials (ECP) with the appropriate valence basis set of double zeta quality (denoted LANL2DZ) for Sb [27, 28, 29]. The harmonic vibrational frequencies characterizing the stationary points were evaluated at the same theory level to assure that all the obtained structures correspond to true minima or first order saddle points on the potential energy surface. The intrinsic reaction coordinate (IRC) procedure [30, 31, 32, 33] (during which the reaction path is followed in both directions away from the transition state) was employed to confirm the corresponding minima for each transition structure.
The electronic energies of all stationary points were obtained by using a compound method, namely, by computing single point MP2 energies with the triple zeta aug-cc-pVTZ basis set for each structure previously optimized at the MP2/aug-pVDZ level, and then by adding the differences between the MP2/aug-pVDZ and CCSD(T)/aug-pVDZ energies (where CCSD(T) stands for the coupled-cluster method with single, double, and non-iterative triple excitations [34, 35, 36, 37]). Finally, the Gibbs free energies were obtained by combining such calculated electronic energies of the stationary points and the thermal and zero-point energy corrections as well as the entropy contributions (at T = 298.15K) computed at the same level of theory.
The reaction mechanisms were investigated in the liquid phase and the effects of surrounding solvent molecules (H2O or HF) were approximated by employing the polarized continuum solvation model (PCM) [38, 39, 40] within a self-consistent reaction field treatment, as implemented in the GAUSSIAN09 program (the default options for PCM and the dielectric constant of 78.36 and 84.20 were used for water and hydrogen fluoride, respectively) regarding to all structures investigated.
The partial atomic charges were evaluated by the Natural Bond Orbital (NBO) analysis scheme [41, 42, 43, 44, 45]. All calculations were performed using the GAUSSIAN09 (Rev. A.02) package [46].
Cartesian coordinates of all stationary point structures and their electronic energies and Gibbs free energies are gathered in the Supplementary Materials.
3. Results
3.1. The uncatalyzed hydration of isobutene
In order to verify the performance of acidic catalysts in the hydration of isobutene, the activation barrier for the uncatalyzed (CH3)2CCH2+H2O→(CH3)3COH reaction has to be determined for comparison. Hence, we investigated the direct H2O molecule attachment to isobutene (in the absence of any catalyst) in the liquid phase represented by two solvents, namely, water and hydrogen fluoride (as the H2SO4 and HSbF6 acids were selected to play the catalyst role in this process, see the forthcoming sections). Since our Gibbs free energy profiles for the uncatalyzed hydration of isobutene reveal that the relative energies of the stationary points (i.e., initial complex, transition state, and product) are nearly identical regardless the solvent considered (see Fig. 1), in this section we discuss our results jointly for these two solvents.
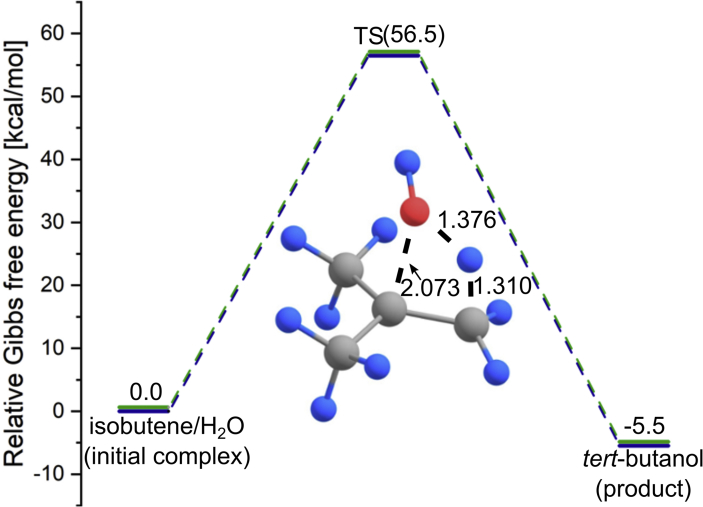
Fig. 1.
The Gibbs free energy profile of the uncatalyzed (CH3)2CCH2+H2O→(CH3)3COH reaction in the liquid phase and the structure of the corresponding transition state. The relative Gibbs free energies of the stationary points obtained for the H2O solvent (green) and HF solvent (brown) differ by less than 0.05 kcal/mol whereas the geometrical parameters of the corresponding transition states (TS) differ by less than 0.001 Å (for bond lengths) and 0.01° (for angles). Relative Gibbs free energies (obtained at T = 298.15K) are given in kcal/mol, selected bond lengths in Å.
The initial complex (consisting of the (CH3)2CCH2 and H2O reactants) and the (CH3)3COH product are connected through a single transition state (TS) whose structure is depicted in Fig. 1. The imaginary vibration (1870 i cm−1) found for this TS corresponds to the simultaneous H–OH stretching (the H–O bond elongates to 1.376 Å) and formation of the C–O and C–H bonds. The partial atomic charges resulting from the NBO population analysis performed for this transition state structure confirm that the mechanism of this process can be considered an electrophilic addition as the partial charges on the OH fragment (i.e., nucleophilic agent) sum up to -0.558e, the partial charge on the H atom (i.e., electrophilic agent) that is being attached to isobutene was calculated to be +0.419e whereas the charges on two carbon atoms involved were found to be +0.260e (for the tertiary carbon which is the target of the electrophilic attack) and -0.757e (for the carbon atom which is the target of the nucleophilic attack).
The height of the kinetic barrier for the uncatalyzed (CH3)2CCH2+H2O→(CH3)3COH reaction is rather large (56.5 kcal/mol, see Fig. 1) which indicates that such a process should not be considered plausible. Since other evaluations of this barrier are not available in the literature, we compare our estimation to the barrier predicted for the H2O attachment to ethylene and reported by Nakagawa et al. [15] who obtained (at the BLYP/6-31G(d) theory level) a similar value of 46.1 kcal/mol.
Taking into account a significant height of the activation barrier for the uncatalyzed reaction, one may conclude that the formation of tert-butanol by combining isobutene and H2O, although thermodynamically favorable by 5.5 kcal/mol (as evaluated at the theory level employed), should not proceed at any noticeable rate in the absence of catalysts.
3.2. The hydration of isobutene catalyzed by the H2SO4 acid
The mechanism of the isobutene hydration might be changed by the introduction of a catalyst. Since the sulfuric acid is commonly used to catalyze hydration reactions of various alkenes, we chose the H2SO4 molecule to play the catalyst role while investigating the mechanism of the (CH3)2CCH2+H2O→(CH3)3COH process.
The Gibbs free energy profile corresponding to the H2SO4-catalyzed hydration of isobutene yielding tert-butanol is presented in Fig. 2, whereas the equilibrium structures of all stationary points are depicted in Fig. 3 (initial complex, intermediate products, and final product) and Fig. 4 (transition states). The reaction begins with formation of the initial complex consisting of mutually interacting (yet structurally intact) isobutene, H2SO4, and H2O molecules.
Fig. 2.
The Gibbs free energy profile of the isobutene hydration catalyzed by H2SO4. The relative Gibbs free energies (at T = 298.15) of the stationary points are obtained for the H2O solvent. Relative Gibbs free energies are given in kcal/mol.
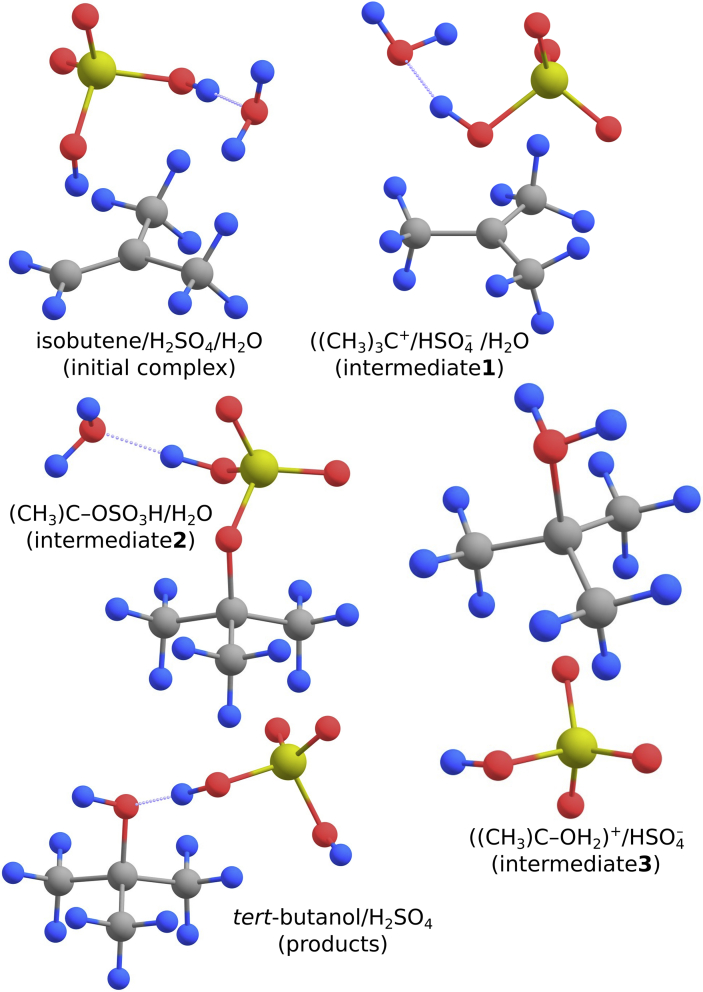
Fig. 3.
The MP2/aug-cc-pVDZ equilibrium structures of the initial complex, intermediate products and final products predicted for the isobutene hydration catalyzed by H2SO4.
Fig. 4.
The MP2/aug-cc-pVDZ structures of the transition states predicted for the isobutene hydration catalyzed by H2SO4. Selected bond lengths are given in Å.
Next, the reaction path bifurcates as the initial complex might evolve either to the ((CH3)3C)+/HSO4–/H2O intermediate product (labeled intermediate1 in Figs. 2 and 3) or to the ((CH3)3C–OH2)+/HSO4– intermediate product (labeled intermediate3 in Figs. 2 and 3). The former path involves the protonation of isobutene by H2SO4 and proceeds through the transition state labeled TS1 in Figs. 2 and 4 whereas the latter path consists of simultaneous isobutene protonation and H2O molecule attachment to the central carbon atom and proceeds via the transition state labeled TS1’ in Figs. 2 and 4. The kinetic barrier that has to be surmounted to form the ((CH3)3C–OH2)+/HSO4– intermediate product was found to be 19.6 kcal/mol while the barrier for the formation of ((CH3)3C)+/HSO4–/H2O (intermediate1) was predicted to be smaller (16.9 kcal/mol), see Fig. 2. Once the ((CH3)3C–OH2)+/HSO4– (intermediate3) is formed, it should spontaneously (i.e., through a negligibly small kinetic barrier) evolve to the final products (tert-butanol and regenerated H2SO4 catalyst, see the equilibrium structure of the (CH3)3COH/H2SO4 complex in Fig. 3).
Alternatively, when the intermediate1 is formed after passing the TS1 saddle point, the reaction path bifurcates once again as the ((CH3)3C)+/HSO4–/H2O complex might evolve either to the (CH3)3C–OSO3H/H2O complex (consisting of the tert-butyl carbocation neutralized by the HSO4– anion attached to it, labeled intermediate2 in Figs. 2 and 3) or to the ((CH3)3C–OH2)+/HSO4– (intermediate3) described above. The kinetic barriers for the transformation of intermediate1 to intermediate2 (through the TS2’) or to intermediate3 (via the TS2) are very similar (3.3 kcal/mol and 2.6 kcal/mol, respectively) despite the structural differences in the corresponding saddle points. As explained above, the formation of the intermediate3 is followed by its immediate (due to the negligibly small kinetic barrier) evolution to the final products consisting of tert-butanol and regenerated H2SO4. By contrast, the intermediate2 represents a very stable complex whose relative energy approaches that of the final reaction products, hence its transformation to (CH3)3COH/H2SO4 might be problematic. In addition, the evolution from (CH3)3C–OSO3H/H2O to ((CH3)3C–OH2)+/HSO4– requires passing relatively large kinetic barrier (via TS3, see Figs. 2 and 4) of 24.9 kcal/mol. Once this barrier is overcome, the ((CH3)3C–OH2)+/HSO4– (intermediate3) is generated and then the formation of the (CH3)3COH/H2SO4 final product follows.
Summing up, our calculations confirm that sulfuric acid effectively reduces the kinetic barrier that has to be surmounted to transform isobutene into tert-butanol. As we demonstrated, this conversion might be accomplished by following a few different reaction paths each of which involves the isobutene protonation as the initial step (that may or may not be associated with the simultaneous H2O attachment). Also, our results indicate that if the (CH3)3C–OSO3H/H2O complex is formed along the reaction path, the overall process should become slower as the conversion of this intermediate product into tert-butanol becomes the rate limiting step.
3.3. The hydration of isobutene catalyzed by the HSbF6 superacid
The mechanism of isobutene hydration catalyzed by HSbF6 superacid is much simpler than the mechanism involving H2SO4 as a catalyst and described in the preceding section. As demonstrated in the Gibbs free energy profile shown in Fig. 5, the reactants form the initial complex (consisting of nearly non-deformed isobutene, HSbF6, and H2O systems stabilized mainly by the intermolecular hydrogen bonds).
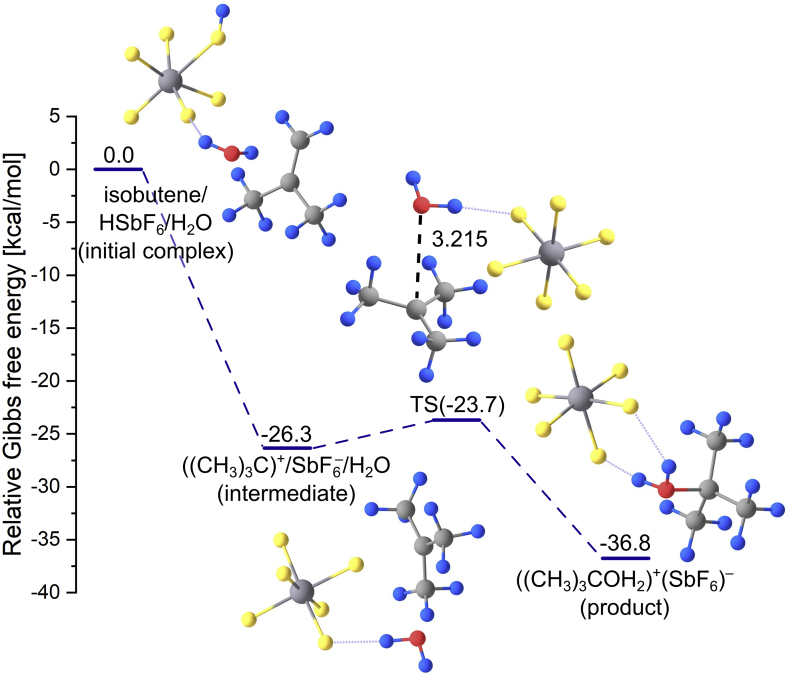
Fig. 5.
The Gibbs free energy profile of the isobutene hydration catalyzed by HSbF6. The relative Gibbs free energies (at T = 298.15) of the stationary points are obtained for the HF solvent. Relative Gibbs free energies are given in kcal/mol.
Once the isobutene/HSbF6/H2O initial complex is formed, it spontaneously converts to the intermediate product due to negligibly small (<0.5 kcal/mol) kinetic barrier separating (CH3)2CCH2/HSbF6/H2O from ((CH3)3C)+/SbF6–/H2O. This first reaction step involves the protonation of isobutene by the HSbF6 superacid and the energy of such generated intermediate product (consisting of tertiary ((CH3)3C)+ carbocation interacting with the SbF6– anion and water molecule) is lower by 26.3 kcal/mol than that of the initial complex. Next, a small kinetic barrier of 2.6 kcal/mol has to be surmounted (see Fig. 5 for the corresponding transition state structure labeled TS) to form the reaction product. During this step, H2O molecule is being attached via its oxygen atom to the tertiary carbon atom of ((CH3)3C)+ carbocation. Hence, the final product of the isobutene hydration catalyzed by HSbF6 represents the ((CH3)3COH2)+(SbF6)– ionic compound rather than the complex of tert-butanol and regenerated acid catalyst, see Fig. 5. However, one should recall that superacid-catalyzed reactions very often lead to ionic salts instead of desired covalent products and their further transformation into preferred products requires proper quenching (usually performed by utilizing Na2CO3 or NaHCO3/H2O) [47].
It seems important to notice that choosing the HSbF6 superacid to play the catalyst role in the hydration of isobutene yielding tert-butanol is predicted to proceed very fast because the only kinetic barrier on the reaction path is very small (2.6 kcal/mol).
4. Conclusions
On the basis of our ab initio calculations carried out using the CCSD(T) and MP2 methods with the aug-cc-pVDZ/LANL2DZ and aug-cc-pVTZ/LANL2DZ basis sets (including the effects of surrounding solvent molecules approximated by employing the polarized continuum solvation model) performed to study the mechanism of the (CH3)2CCH2+H2O→(CH3)3COH reaction catalyzed by either the H2SO4 or HSbF6 acid, we arrive at the following conclusions:
-
(i)
The uncatalyzed isobutene hydration yielding tert-butanol might proceed according to the electrophilic addition mechanism but the activation barrier predicted for this reaction is very large (56.5 kcal/mol in the liquid phase) which renders this process highly unlikely.
-
(ii)
The use of the sulfuric acid effectively reduces the kinetic barrier that has to be surmounted to transform isobutene into tert-butanol. H2SO4-catalyzed (CH3)2CCH2+H2O→(CH3)3COH reaction might proceed along a few different paths involving the isobutene protonation as the initial step. Possible formation of the (CH3)3C–OSO3H/H2O complex as the intermediate product is predicted to decrease the rate of the overall process as its further conversion to tert-butanol requires overcoming a large kinetic barrier of ca. 25 kcal/mol.
-
(iii)
The isobutene hydration is predicted to proceed very fast when catalyzed by the HSbF6 superacid as only one small kinetic barrier of ca. 3 kcal/mol has to be surmounted along the reaction path.
Declarations
Author contribution statement
Jakub Brzeski: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Piotr Skurski: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Polish Ministry of Science and Higher Education grant No. DS 530-8375-D499-19 (to P. S.) and Wroclaw Centre for Networking and Supercomputing (http://wcss.pl) grant No. 455.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Meusinger R. Qualitative and quantitative determination of oxygenates in gasolines using 1H nuclear magnetic resonance spectroscopy. Anal. Chim. Acta. 1999;391:277–288. [Google Scholar]
- 2.Kim J.Y., Park J., Hwang H., Kim J.K., Song I.K., Choi J.W. Catalytic depolymerization of lignin macromolecule to alkylated phenols over various metal catalysts in supercritical tert-butanol. J. Anal. Appl. Pyrolysis. 2015;113:99–106. [Google Scholar]
- 3.Wang Y. Potassium tert-butoxide. Synlett. 2011;19:2901–2902. [Google Scholar]
- 4.Pasto D.J., Gontarz J.A. Mechanism of the oxymercuration of substituted cyclohexenes. J. Am. Chem. Soc. 1971;93:6902–6908. [Google Scholar]
- 5.Brown H.C. Hydroboration—a powerful synthetic tool. Tetrahedron. 1961;12:117–138. [Google Scholar]
- 6.Kresge J., Chiang Y., Fitzgerald P.H., McDonald R.S., Schmid G.H. General acid catalysis in the hydration of simple olefins. The mechanism of olefin hydration. J. Am. Chem. Soc. 1971;93:4907–4908. [Google Scholar]
- 7.Weissermel H., Arpe H.-J. In: Industrial Organic Chemistry. third ed. Sora K., editor. Wiley-VCH Verlag GmbH; Weinheim: 1997. pp. 191–196. ch. 8. [Google Scholar]
- 8.Schubert W.M., Lamm B. The acid-catalyzed hydration of styrene. J. Am. Chem. Soc. 1966;88:120–124. [Google Scholar]
- 9.Delmas M., Gaset A. Supported acid catalysis with ion-exchange resins IV. 1,3-dioxacyclohexanes obtained from polymerized formaldehyde and aromatic alkenes of plant origin in organic media: discussion of the reaction mechanism. J. Mol. Catal. 1982;17:51–63. [Google Scholar]
- 10.Kresge A.J., Yin Y. Kinetics and mechanism of the acid-catalyzed hydration of dihydro-1,4-dioxin. J. Phys. Org. Chem. 1989;2:43–50. [Google Scholar]
- 11.Lucas H.J., Liu Y.-P. The hydration of unsaturated compounds. III. The hydration rate of trimethylethylene in aqueous solutions of acids 1. J. Am. Chem. Soc. 1934;56:2138–2140. [Google Scholar]
- 12.Taft R.W. The dependence of the rate of hydration of isobutene on the acidity function, H0, and the mechanism for olefin hydration in aqueous acids. J. Am. Chem. Soc. 1952;74:5372–5376. [Google Scholar]
- 13.Deckwer W.D., Popovic M., Allenbach U. Rate constants of the sulfuric acid catalyzed hydration of isobutene from bubble column studies. React. Kinet. Catal. L. 1975;3:449–454. [Google Scholar]
- 14.Poźniczek J., Małecka-Lubańska A., Micek-Ilnicka A., Bielański A. Gas phase hydration of isobutene to tert-butyl alcohol on H4SiW12O40 as the catalyst. Appl. Catal., A. 1999;176:101–109. [Google Scholar]
- 15.Nakagawa Y., Tajima N., Hirao K. A theoretical study of catalytic hydration reactions of ethylene. J. Comput. Chem. 2000;21:1292–1304. [Google Scholar]
- 16.Olah G.A., Laali K., Farooq O. Aromatic substitution. 52. Superacid-catalyzed carbonylation of aromatics with carbon monoxide. J. Org. Chem. 1985;50:1483–1486. [Google Scholar]
- 17.Olah G.A., Wang Q., Surya Prakash G.K. Trifluoromethanesulfonic acid catalyzed electrophilic sulfuration of alkanes (cycloalkanes) with elemental sulfur to dialkyl (dicycloalkyl) sulfidesls. J. Am. Chem. Soc. 1990;112:3697–3698. [Google Scholar]
- 18.Olah G.A., Surya Prakash G.K., Török B., Török M. Effect of acid/hydrocarbon ratio, temperature and contact time on the isobutane-isobutylene alkylation with trifluoromethanesulfonic acid. Catal. Lett. 1996;40:137–142. [Google Scholar]
- 19.Surya Prakash G.K., Paknia F., Kulkarni A., Narayanan A., Wang F., Rasul G., Mathew T., Olah G.A. Taming of superacids: PVP-triflic acid as an effective solid triflic acid equivalent for Friedel–Crafts hydroxyalkylation and acylation. J. Fluorine Chem. 2015;171:102–112. [Google Scholar]
- 20.Rybacka O., Czapla M., Skurski P. The formation of formaldehyde via the carbon monoxide hydrogenation catalyzed by the HSbF6 superacid. Theor. Chem. Acc. 2017;136:140. doi: 10.1039/c7cp03362a. [DOI] [PubMed] [Google Scholar]
- 21.Rybacka O., Czapla M., Skurski P. Mechanisms of carbon monoxide hydrogenation yielding formaldehyde catalyzed by the representative strong mineral acid, H2SO4, and Lewis–Brønsted superacid, HF/AlF3. Phys. Chem. Chem. Phys. 2017;19:18047–18054. doi: 10.1039/c7cp03362a. [DOI] [PubMed] [Google Scholar]
- 22.Rybacka O., Skurski P. Mechanism of the ethanol-based (C2H5OH2)+(SbF6)− salt formation by the superacid-catalyzed acetaldehyde hydrogenation. Theor. Chem. Acc. 2018;137:121. [Google Scholar]
- 23.Møller C., Plesset M.S. Note on an approximation treatment for many-electron systems. Phys. Rev. 1934;46:618–622. [Google Scholar]
- 24.Head-Gordon M., Pople J.A., Frisch M.J. MP2 energy evaluation by direct methods. Chem. Phys. Lett. 1988;153:503–506. [Google Scholar]
- 25.Frisch M.J., Head-Gordon M., Pople J.A. A direct MP2 gradient method. Chem. Phys. Lett. 1990;166:275–280. [Google Scholar]
- 26.Kendall R.A., Dunning T.H., Jr., Harrison R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992;96:6796–6806. [Google Scholar]
- 27.Hay P.J., Wadt W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985;82:270–283. [Google Scholar]
- 28.Wadt W.R., Hay P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985;82:284–298. [Google Scholar]
- 29.Hay P.J., Wadt W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985;82:299–310. [Google Scholar]
- 30.Fukui K. The path of chemical reactions - the IRC approach. Acc. Chem. Res. 1981;14:363–368. [Google Scholar]
- 31.Hratchian H.P., Schlegel H.B. Accurate reaction paths using a Hessian based predictor–corrector integrator. J. Chem. Phys. 2004;120:9918–9924. doi: 10.1063/1.1724823. [DOI] [PubMed] [Google Scholar]
- 32.Hratchian H.P., Schlegel H.B. Using Hessian updating yo increase the efficiency of a Hessian based predictor-corrector reaction path following method. J. Chem. Theory Comput. 2005;1:61–69. doi: 10.1021/ct0499783. [DOI] [PubMed] [Google Scholar]
- 33.Hratchian H.P., Schlegel H.B. Chapter 10 - Finding minima, transition states, and following reaction pathways on ab initio potential energy surfaces. Theory Appl. Comput. Chem. 2005:195–249. [Google Scholar]
- 34.Cížek J. On the Use of the cluster expansion and the technique of diagrams in calculations of correlation effects in atoms and molecules. Adv. Chem. Phys. 1969;14:35–89. [Google Scholar]
- 35.Bartlett R.J., Purvis G.D., III Many-body perturbation theory, coupled-pair many-electron theory, and the importance of quadruple excitations for the correlation problem. Int. J. Quantum Chem. 1978;14:561–581. [Google Scholar]
- 36.Purvis G.D., III, Bartlett R.J. A full coupled-cluster singles and doubles model: the inclusion of disconnected triples. J. Chem. Phys. 1982;76:1910–1918. [Google Scholar]
- 37.Scuseria G.E., Janssen C.L., Schaefer H.F., III An efficient reformulation of the closed-shell coupled cluster single and double excitation (CCSD) equations. J. Chem. Phys. 1988;89:7382–7387. [Google Scholar]
- 38.Miertuš S., Scrocco E., Tomasi J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981;55:117–129. [Google Scholar]
- 39.Miertuš S., Tomasi J. Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem. Phys. 1982;65:239–245. [Google Scholar]
- 40.Cossi M., Barone V., Cammi R., Tomasi J. Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996;255:327–335. [Google Scholar]
- 41.Foster J.P., Weinhold F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980;102:7211–7218. [Google Scholar]
- 42.Reed E., Weinhold F. Natural bond orbital analysis of near-Hartree-Fock water dimer. J. Chem. Phys. 1983;78:4066–4073. [Google Scholar]
- 43.Reed E., Weinstock R.B., Weinhold F. Natural population analysis. J. Chem. Phys. 1985;83:735–746. [Google Scholar]
- 44.Carpenter J.E., Weinhold F. Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J. Mol. Struct. 1988;46:41–62. [Google Scholar]
- 45.Reed E., Curtiss L.A., Weinhold F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988;88:899–926. [Google Scholar]
- 46.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., Li X., Caricato M., Marenich A.V., Bloino J., Janesko B.G., Gomperts R., Mennucci B., Hratchian H.P., Ortiz J.V., Izmaylov A.F., Sonnenberg J.L., Williams-Young D., Ding F., Lipparini F., Egidi F., Goings J., Peng B., Petrone A., Henderson T., Ranasinghe D., Zakrzewski V.G., Gao J., Rega N., Zheng G., Liang W., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Throssell K., Montgomery J.A., Jr., Peralta J.E., Ogliaro F., Bearpark M.J., Heyd J.J., Brothers E.N., Kudin K.N., Staroverov V.N., Keith T.A., Kobayashi R., Normand J., Raghavachari K., Rendell A.P., Burant J.C., Iyengar S.S., Tomasi J., Cossi M., Millam J.M., Klene M., Adamo C., Cammi R., Ochterski J.W., Martin R.L., Morokuma K., Farkas O., Foresman J.B., Fox D.J. Gaussian, Inc.; Wallingford CT: 2016. Gaussian 16, Revision B.01. [Google Scholar]
- 47.Olah G.A., Surya Prakash G.K., Molnár Á., Sommer J. John Wiley and Sons; New York: 2009. Superacid Chemistry. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.