Abstract
BACKGROUND
In postoperative patients with breast cancer, the combination of an anthracycline and cyclophosphamide (AC) followed by a taxane is a standard regimen. In the current study, the authors examined whether AC could be safely omitted, and compared the effectiveness of paclitaxel versus docetaxel.
METHODS
Female postoperative patients with axillary lymph node‐positive breast cancer were eligible for enrollment in this phase 3, open‐label, randomized controlled trial at 84 centers in Japan. Patients were randomized to 4 cycles of doxorubicin at a dose of 60 mg/m2 and cyclophosphamide at a dose of 600 mg/m2 (AC) followed by 4 cycles of paclitaxel at a dose of 175 mg/m2 (ACpT) or AC followed by 4 cycles of docetaxel at a dose of 75 mg/m2 (ACdT), or 8 cycles of paclitaxel (PTx) or docetaxel (DTx) every 3 weeks. The primary endpoint was disease‐free survival (DFS). Secondary endpoints included overall survival adverse events. The authors adopted a 2 × 2 factorial design to examine the AC containing‐regimens (ACpT and ACdT) versus the AC free‐regimens (PTx and DTx), and the paclitaxel‐containing regimens (ACpT and PTx) versus the docetaxel‐containing regimens (ACdT and DTx).
RESULTS
Of 1060 patients, 1049 were treated and included in the intention‐to‐treat population. The DFS results did not demonstrate noninferiority between the AC‐containing and the AC‐free regimens (hazard ratio [HR], 1.19; 95% confidence interval [95% CI], 0.982‐1.448 [P noninferiority = .30]). Better outcomes were noted in patients treated with the docetaxel‐containing regimens compared with the paclitaxel‐containing regimens with respect to DFS (HR, 0.72; 95% CI, 0.589‐0.875 [P = .0008]) and overall survival (HR, 0.75; 95% CI, 0.574‐0.980 [P = .035]). Neutropenia, nausea, and vomiting were found to occur more often in the AC‐containing arms, whereas the incidence of edema was greater in the docetaxel‐containing treatment arms.
CONCLUSIONS
Noninferiority in DFS was not demonstrated between the AC‐containing and AC‐free regimens. Compared with a similar regimen of paclitaxel, docetaxel appeared to increase the DFS. Cancer 2017;123:759–68. © 2016 American Cancer Society.
Keywords: adjuvant therapy, docetaxel, doxorubicin and cyclophosphamide (AC)‐taxane, paclitaxel, randomized phase 3
Short abstract
In postoperative patients with breast cancer, the combination of an anthracycline and cyclophosphamide followed by a taxane is a standard regimen. The results of the current phase 3 study demonstrate that noninferiority in disease‐free survival is not shown between regimens containing the combination of doxorubicin plus cyclophosphamide and those that do not, and that compared with a similar regimen of paclitaxel, docetaxel increased disease‐free survival.
INTRODUCTION
The combination of an anthracycline and a taxane (4 cycles of doxorubicin plus cyclophosphamide [AC] followed by 4 cycles of paclitaxel) is the standard chemotherapeutic regimen for patients with lymph node‐positive breast cancer.1, 2, 3, 4 Unfortunately, rare but potentially fatal complications (cardiomyopathy and secondary leukemia) are associated with AC, for which no preventive regimens free of anthracyclines and cyclophosphamide offer safer clinical alternatives.5, 6, 7 To our knowledge, it also is not known whether paclitaxel or docetaxel is more effective for patients with breast cancer. In metastatic settings, docetaxel (at a dose of 100 mg/m2) has been compared with paclitaxel (at a dose of 175 mg/m2), in which both were administered every 21 days.8 The Eastern Cooperative Oncology Group 1199 trial compared the efficacy of paclitaxel and docetaxel in triweekly and weekly regimens in adjuvant settings.9
In the current study, we examined the clinical benefit of AC followed by a taxane regimen compared with an AC‐free regimen for patients with breast cancer in adjuvant settings. We also compared the clinical difference between the 2 taxanes given every 3 weeks.
MATERIALS AND METHODS
Participants
Eligible participants were defined as female patients with postoperative breast cancer who were aged 18 to 70 years with histologically confirmed invasive disease and positive axillary lymph nodes diagnosed after sentinel lymph node biopsy or dissection; any estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) status; an Eastern Cooperative Oncology Group performance status of 0 to 1; and adequate hematologic, hepatic, and renal function. In terms of hormone status, patients who were positive for ER and/or PgR were ineligible initially. However, such eligibility was amended on June 14, 2003 to permit the enrollment of patients with any ER‐positive and PgR‐positive disease following data from the Cancer and Leukemia Group B (CALGB) 9344 trial1 showing that the survival benefits associated with taxanes are not only observed in patients with receptor‐negative disease.
Exclusion criteria included a history of myocardial infarction, congestive heart failure, or significant ischemic heart disease. Patients were required to initiate the protocol‐designated treatment within 84 days from the final surgical procedure.
The protocol was approved independently by the Institutional Review Board (IRB) at each institution/hospital before the initiation of patient enrollment. The first approval was given by the IRB of Aichi Prefectural Hospital (also known as Aichi Cancer Center Aichi Hospital) on September 4, 2001, and also by the IRB of the principal investigator's hospital (the National Cancer Center Hospital) on January 24, 2002. The protocol is available at http://www.csp.or.jp/cspor/en/n-sas_bc02.html. The study was registered to the Japanese University Hospital Medical Information Network (UMIN; clinical trial registration UMIN‐CTR C000000055). The UMIN Clinical Trial Registry satisfies the criteria of the International Committee of Medical Journal Editors. All patients provided written informed consent before participating in the current study.
Study Design and Procedures
The current trial examined 2 clinical hypotheses: 1) 8 cycles of a taxane are noninferior to 4 cycles of AC followed by 4 cycles of a taxane; and 2) one of the taxanes (paclitaxel or docetaxel) is superior or equivalent to the other.
We compared an AC‐containing versus an AC‐free regimen as well as 2 taxanes (paclitaxel vs docetaxel). Thus, we adopted a 2 × 2 factorial design with the following 4 treatment arms: 1) 4 cycles of doxorubicin at a dose of 60 mg/m2 and cyclophosphamide at a dose of 600 mg/m2 (AC) followed by 4 cycles of paclitaxel at a dose of 175 mg/m2 (ACpT; control arm); 2) AC followed by 4 cycles of docetaxel at a dose of 75 mg/m2 (ACdT); 3) 8 cycles of paclitaxel at a dose of 175 mg/m2 (PTx); and 4) 8 cycles of docetaxel at a dose of 75 mg/m2 (DTx). Treatments in all arms were provided every 3 weeks (Fig. 1). Prophylactic use of granulocyte colony‐stimulating factor was prohibited during the study period.
Figure 1.
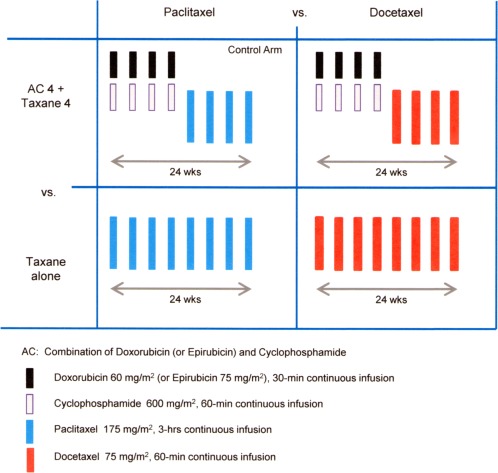
2 × 2 factorial design. Two factors were compared: (1) the combination of doxorubicin plus cyclophosphamide (AC) followed by taxanes versus a taxane alone; and (2) paclitaxel versus docetaxel. There were 4 treatment arms: 1) 4 cycles of intravenous (iv) doxorubicin (at a dose of 60 mg/m2) plus iv cyclophosphamide (at a dose of 600 mg/m2); 2) AC followed by 4 cycles of iv paclitaxel (at a dose of 175 mg/m2) in the control arm and AC followed by 4 cycles of iv docetaxel (at a dose of 75 mg/m2); 3) 8 cycles of iv paclitaxel (at a dose of 175 mg/m2); and 4) 8 cycles of iv docetaxel (at a dose of 175 mg/m2). All treatment arms were comprised of 8 cycles. Each treatment was administered every 3 weeks.
Patients were randomly assigned at the Comprehensive Support Project for Oncological Research (CSPOR) Data Center to 1 of 4 treatment arms (Fig. 2) with equal probability using the method of minimization over 6 stratification factors. These factors included tumor size (<3.0 cm vs ≥ 3.0 cm), hormone receptor status (both ER negative and PgR negative or others), HER2 status (2+, 3+, or others), surgical procedure (breast‐conserving surgery or mastectomy), number of positive axillary lymph nodes (1‐3, 4‐9, or ≥ 10), and institution. Data from HER2 fluorescence in situ hybridization assays were not available. Because the current study was an open‐label trial, no masking protocols were required. The randomization algorithm was designed and executed by a biostatistician (YO).
Figure 2.
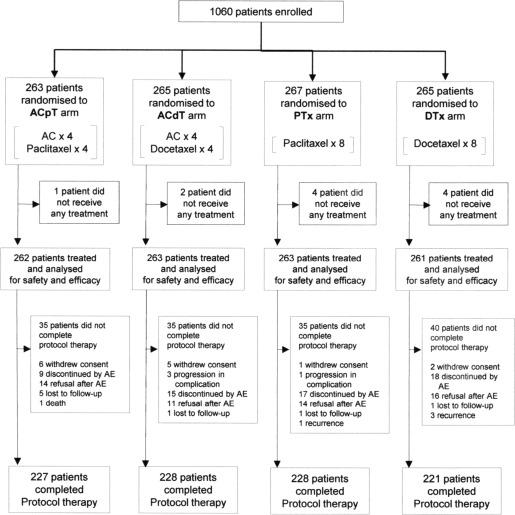
Consolidated Standards Of Reporting Trials (CONSORT) diagram. Treatment arms were the control arm, which was 4 cycles of the combination of doxorubicin plus cyclophosphamide (AC) followed by 4 cycles of paclitaxel (ACpT); 4 cycles of AC followed by 4 cycles of docetaxel (ACdT); 8 cycles of paclitaxel without AC (PTx); and 8 cycles of docetaxel without AC (DTx). AE indicates adverse event.
In all treatment arms, patients whose tumors were positive for ER, PgR, or both received tamoxifen or an aromatase inhibitor for 5 years after the completion of chemotherapy. Patients with HER2‐positive breast cancer were not treated with trastuzumab unless distant metastases were detected because adjuvant therapy with trastuzumab was not approved in Japan until February 2008, which was 22 months after enrollment was closed for the current trial.
Endpoints
The planned study period included 3 years of patient enrollment and at least 5 years with a maximum of 10 years of follow‐up for each patient until the occurrence of events. The primary endpoint was disease‐free survival (DFS). Secondary endpoints included overall survival (OS), adverse events, and health‐related quality of life. DFS was defined as the period from randomization until the date of the first event, including local/regional disease recurrence, distant metastasis, invasive contralateral breast cancer, and death from any cause. OS was defined as the period from randomization until the date of death, regardless of cause. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria (version 2.0). All eligible patients, patients with disease recurrence, and records of patients who died were reviewed by the central committee for safety and efficacy.
Statistical Methods
The current trial was designed to confirm the noninferiority between AC‐containing (ACpT and ACdT) and AC‐free (PTx and DTx) regimens with regard to DFS and to guarantee sufficient statistical power. The secondary hypothesis, the comparison of 2 taxanes (ACpT and PTx vs ACdT and DTx), was the exploratory issue in this trial and requires confirmation via subsequent meta‐analyses of other studies. We used the estimated hazard ratio (HR) and/or its confidence interval (CI) for the DFS analysis due to the respective survival data.
The HRs of the regimen of cyclophosphamide, methotrexate, and 5‐fluorouracil (CMF) compared with nontreatment, AC compared with CMF, and AC plus a taxane compared with AC from the Early Breast Cancer Trialists' Collaborative Group and Cancer and Leukemia Group B (CALGB) 9344 trials were 0.76, 0.88, and 0.78 to 0.86, respectively. The threshold for noninferiority hypothesis verification (Δ) sets use of a taxane alone as at least superior to CMF as the minimum condition guaranteeing efficacy. Thus, the upper limit of the HR for the use of a taxane alone compared with AC plus a taxane is set at ≤1.321 (1/0.88 × 0.86). If Δ = 1.321, the true HRs are 1.0 (taxane alone is equivalent) and 1.06 (taxane alone is slightly inferior), with an α of .05 (1‐sided, 90% confidence interval [CI]), β = .20), and the number of events required for validation based on a normal approximation of the log HR estimate is 320 and 480, respectively, in the 2 groups. To observe 320 or 480 events, 800 to 1200 patients would be required for this study, assuming a minimum follow‐up period of 5 years. Trial outcomes were analyzed by a biostatistician according to the initial plan.
Interim analyses were planned so that when the expected number of events was reached, the trial would be stopped and the null hypothesis would be rejected if the upper limit of the 99.0% CI was ≤ 1.321 for noninferiority or if the upper limit of the 99.5% CI for either taxane did not exceed 1.0 for superiority.
Cumulative survival curves for DFS and OS were estimated using the Kaplan‐Meier method. HRs were estimated using the Cox regression model. Subgroup analyses were preplanned to compare each DFS and/or OS in the 4 trial groups in terms of ER‐ and HER2 status, respectively. All statistical analyses were conducted using SAS statistical software (version 9.2; SAS Institute Inc, Cary, NC).
RESULTS
Between December 2001 and April 2006, a total of 1060 patients were enrolled at 84 participating institutions in Japan. Figure 2 shows the trial profile. A total of 904 patients (85.2%) completed the protocol treatment. Table 1 provides the baseline characteristics of the 1049 patients whose data were included in the intention‐to‐treat analysis.
Table 1.
Baseline Characteristics
| Characteristic | ACpT N=262 (%) | ACdT N=263 (%) | PTx N=263 (%) | DTx N=261 (%) | Total N=1049 (%) |
|---|---|---|---|---|---|
| Mean age (SD), y | 52.8 ± 8.3 | 52.7 ± 9.5 | 52.4 ± 8.9 | 51.9 ± 8.6 | 52.4 ± 8.8 |
| UICC Stage I | 42 (16.0) | 18 (6.9) | 29 (11.0) | 35 (13.5) | 124 (11.8) |
| IIA | 95 (36.3) | 115 (43.9) | 102 (38.8) | 104 (40.0) | 416 (39.7) |
| IIB | 86 (32.8) | 106 (40.5) | 109 (41.4) | 97 (37.3) | 398 (38.0) |
| IIIA | 39 (14.9) | 23 (8.8) | 23 (8.7) | 24 (9.2) | 109 (10.4) |
| Tumor size, mm | |||||
| <30 | 168 (64.1) | 168 (63.9) | 168 (63.9) | 166 (63.6) | 670 (63.9) |
| ≥30 | 94 (35.9) | 95 (36.1) | 95 (36.1) | 95 (36.4) | 379 (36.1) |
| No. of positive lymph nodes | |||||
| 1–3 | 156 (59.5) | 159 (60.5) | 156 (59.3) | 156 (59.8) | 627 (59.8) |
| 4–9 | 63 (24.0) | 61 (23.2) | 64 (24.3) | 64 (24.5) | 252 (24.0) |
| ≥10 | 43 (16.4) | 43 (16.3) | 43 (16.3) | 41 (15.7) | 170 (16.2) |
| ER status | |||||
| Positive | 148 (56.5) | 146 (55.5) | 150 (57.0) | 147 (56.3) | 591 (56.3) |
| Negative | 111 (42.4) | 116 (44.1) | 112 (42.6) | 112 (42.9) | 451 (43.0) |
| Unknown | 3 (1.1) | 1 (0.4) | 1 (0.4) | 2 (0.8) | 7 (0.7) |
| PgR status | |||||
| Positive | 109 (41.6) | 124 (47.1) | 111 (42.2) | 114 (43.7) | 458 (43.7) |
| Negative | 149 (56.9) | 138 (52.5) | 149 (56.7) | 144 (55.2) | 580 (55.3) |
| Unknown | 4 (1.5) | 1 (0.4) | 3 (1.1) | 3 (1.1) | 11 (1.0) |
| Surgery | |||||
| Partial mastectomy | 122 (46.6) | 121 (46.0) | 122 (46.4) | 122 (46.7) | 487 (46.4) |
| Modified radical mastectomya | 136 (51.9) | 140 (53.2) | 139 (52.9) | 137 (52.5) | 552 (52.6) |
| Radical mastectomy (Halsted) | 4 (1.5) | 2 (0.8) | 2 (0.8) | 2 (0.8) | 10 (1.0) |
| HER2 status | |||||
| Negative | 91 (34.7) | 85 (32.3) | 94 (35.7) | 94 (36.0) | 364 (34.7) |
| 1+ | 85 (32.4) | 75 (28.5) | 71 (27.0) | 69 (26.4) | 300 (28.8) |
| 2+ | 24 (9.2) | 28 (10.6) | 29 (11.0) | 27 (10.3) | 108 (10.3) |
| 3+ | 42 (16.0) | 44 (16.7) | 39 (14.8) | 43 (16.5) | 168 (16.0) |
| Unknown | 20 (7.6) | 31 (11.8) | 30 (11.4) | 28 (10.7) | 109 (10.4) |
Abbreviations: ACdT, 4 cycles of the combination of doxorubicin plus cyclophosphamide followed by 4 cycles of docetaxel; ACpT, 4 cycles of the combination of doxorubicin plus cyclophosphamide followed by 4 cycles of paclitaxel; DTx, docetaxel alone; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PgR, progesterone receptor; PTx, paclitaxel alone; SD, standard deviation.
Modified (muscle‐preserving) radical mastectomy.
The interim analysis was conducted on June 15, 2008, when the number of the latest group of patients reached 75% but did not meet the criteria to stop the trial. For the final analysis, noninferiority required the upper limit of the 90.3% CI to be ≤ 1.321, and superiority required a 95.2% CI that did not exceed 1.0.
As of December 2013, the median follow‐up was 84.5 months. There were 192 cases of disease recurrence among patients in the AC‐containing treatment arms and 219 among those in the AC‐free treatment arms. For the results of DFS analysis, the HR in the AC‐free treatment arms and the AC‐containing treatment arms was 1.19 (90.3% CI, 1.012‐1.405), and did not satisfy the initial setting for noninferiority (P noninferiority = .30) (Fig. 3A). Disease recurrence in the docetaxel‐containing treatment arms (ACdT and DTx) and paclitaxel‐containing treatment arms (ACpT and PTx) were 178 and 233, respectively (Fig. 3B). The median DFS in the ACpT (reference) treatment arm was 84.4 months (95% CI, 79.3‐93.1 months). Other median DFS values were 85.7 months (95% CI, 82.6‐95.5 months) in the ACdT arm, 87.9 months (95% CI, 84.1‐94.8 months) in the DTx arm, and 78.0 months (95% CI, 60.5‐84.1 months) in the PTx arm (Fig. 3C).
Figure 3.
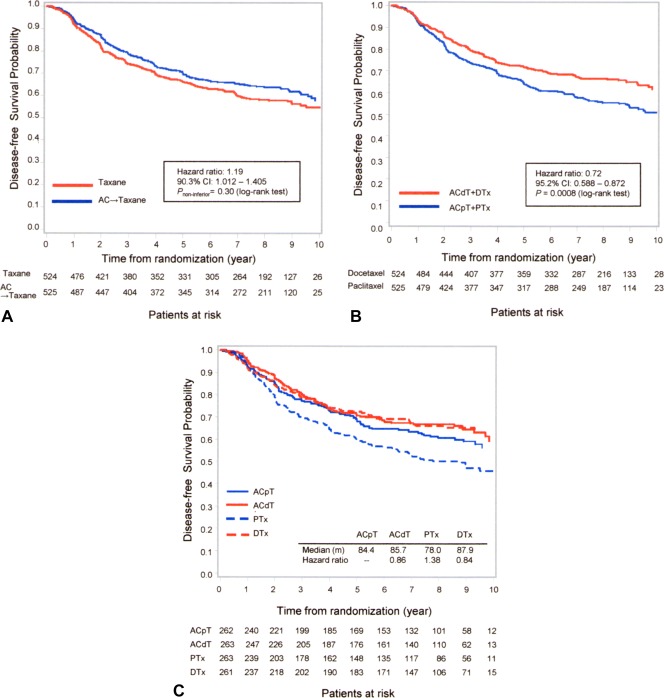
(A) Disease‐free survival for the combination of a doxorubicin plus cyclophosphamide (AC)‐containing versus AC‐free (taxane) regimen using the Kaplan‐Meier method. (B) Disease‐free survival of docetaxel regimens (4 cycles of AC followed by 4 cycles of docetaxel [ACdT] plus docetaxel) versus paclitaxel regimens (4 cycles of AC followed by 4 cycles of paclitaxel [ACpT] plus paclitaxel) using the Kaplan‐Meier method. (C) Disease‐free survival of all regimens using the Kaplan‐Meier method. CI indicates confidence interval; DTx, 8 cycles of docetaxel without AC; PTx, 8 cycles of paclitaxel without AC.
Noninferiority between the AC‐containing and AC‐free treatment arms was demonstrated in terms of OS (Fig. 4A). There was a significant difference in OS observed between the docetaxel‐containing treatment arms and the paclitaxel‐containing treatment arms (Fig. 4B). In the subgroup analysis, there was a stronger treatment effect for ACpT compared with PTx for patients with HER2‐positive disease (HR, 1.85; 95% CI, 1.15‐2.98) due to hormone receptors and/or HER2 status (Fig. 5).
Figure 4.
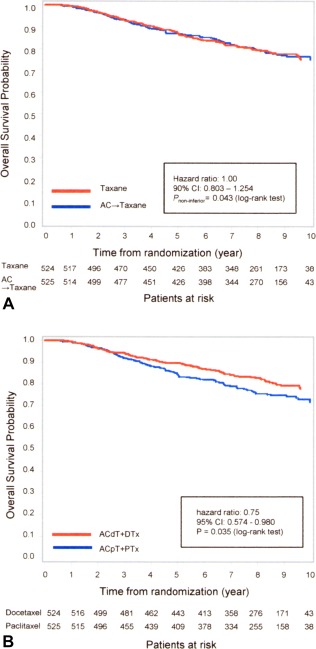
(A) Overall survival for doxorubicin plus cyclophosphamide (AC)‐containing versus AC‐free (taxane) regimens using the Kaplan‐Meier method. (B) Overall survival of docetaxel (4 cycles of AC followed by 4 cycles of docetaxel [ACdT] plus docetaxel) versus paclitaxel (4 cycles of AC followed by 4 cycles of paclitaxel [ACpT] plus paclitaxel) regimens using the Kaplan‐Meier method. CI indicates confidence interval; DTx, 8 cycles of docetaxel without AC; PTx, 8 cycles of paclitaxel without AC.
Figure 5.
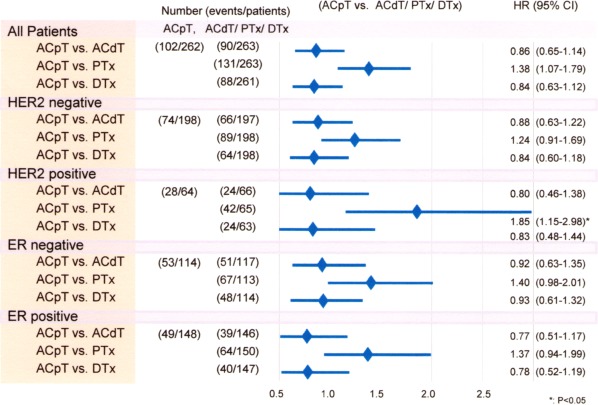
Subgroup analysis of disease‐free survival according to hormone and human epidermal growth factor receptor 2 (HER2) status in the 4 cycles of the combination of doxorubicin plus cyclophosphamide (AC) followed by 4 cycles of paclitaxel (ACpT), 4 cycles of AC followed by 4 cycles of docetaxel (ACdT), paclitaxel alone (PTx), and docetaxel alone (DTx) regimens using the Cox regression model. Abbreviations: CI, confidence interval; ER, estrogen receptor; HR, hazard ratio.
The most frequent grade 3 to 4 adverse event was neutropenia, which occurred more frequently in the AC‐containing treatment arms: 17.2% in the ACpT treatment arm and 19.5% in the ACdT treatment arm versus 1.9% in the PTx treatment arm and 6.9% in the DTx treatment arm (Table 2). The incidence of febrile neutropenia also was found to be higher in the AC‐containing treatment arms: 5.7% in the ACpT treatment arm and 11.1% in the ACdT treatment arm versus 0.4% in the PTx treatment arm and 8.1% in the DTx treatment arm.
Table 2.
Selected Adverse Events (Grades 3 and 4) Noted Among the Patients in the Current Study According to Treatment Groupsa
| Adverse Events | ACpT, % | ACdT, % | PTx, % | DTx, % |
|---|---|---|---|---|
| Neutropenia | 17.2 | 19.5 | 1.9 | 6.9 |
| Febrile neutropenia | 5.7 | 11.1 | 0.4 | 8.1 |
| Leukopenia | 4.2 | 8.0 | 0.4 | 3.1 |
| Thrombocytopenia | 0.4 | 0.4 | 0.0 | 0.0 |
| Anemia | 0.8 | 0.4 | 0.4 | 0.0 |
| Elevated AST or ALT | 1.9 | 1.1 | 3.1 | 0.4 |
| Elevated bilirubin | 0.0 | 0.4 | 0.4 | 0.0 |
| Edema | 0.0 | 1.1 | 0.0 | 12.6 |
| Pleural effusion | 0.0 | 0.0 | 0.0 | 0.0 |
| Ascites | 0.0 | 0.0 | 0.0 | 0.0 |
| Body weight gain | 0.0 | 0.0 | 0.0 | 0.0 |
| Hair loss | 0.0 | 0.0 | 0.0 | 0.0 |
| Phlebitis at injection site | 0.0 | 0.0 | 0.0 | 0.0 |
| Nail changes | 0.0 | 0.0 | 0.0 | 0.0 |
| Stomatitis | 0.8 | 1.1 | 0.0 | 0.0 |
| Nausea | 4.6 | 3.4 | 0.4 | 1.2 |
| Vomiting | 3.1 | 2.7 | 0.0 | 0.8 |
| Constipation | 1.1 | 0.8 | 0.4 | 0.4 |
| Diarrhea | 0.0 | 1.1 | 0.4 | 1.9 |
| Urinary urgency | 0.0 | 0.6 | 0.0 | 0.2 |
| Hematuria | 0.0 | 0.4 | 0.0 | 0.0 |
| Fatigue | 3.8 | 3.1 | 1.9 | 1.9 |
| Lacrimation | 0.0 | 0.0 | 0.0 | 0.4 |
| Rash, desquamation | 1.5 | 0.8 | 0.0 | 0.8 |
| Sensory neuropathy | 4.2 | 0.4 | 5.7 | 3.8 |
| Motor neuropathy | 0.8 | 0.8 | 0.4 | 1.3 |
| Joint pain (arthralgia) | 6.1 | 3.8 | 7.5 | 1.6 |
| Muscle pain (myalgia) | 3.8 | 3.1 | 5.3 | 0.8 |
| Secondary cancerb | 1.9 | 2.8 | 1.4 | 1.0 |
| Endometrial cancer | 0.0 | 0.5 | 0.0 | 0.5 |
| Cardiac arrhythmia | 1.0 | 0.0 | 0.5 | 1.1 |
Abbreviations: ACdT, 4 cycles of the combination of doxorubicin plus cyclophosphamide followed by 4 cycles of docetaxel; ACpT, 4 cycles of the combination of doxorubicin plus cyclophosphamide followed by 4 cycles of paclitaxel; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DTx, docetaxel alone; PTx, paclitaxel alone.
Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria (version 2.0).
Cancer other than endometrial.
Gastrointestinal adverse events were more frequently observed among patients in the AC‐containing treatment arms than those in the AC‐free treatment arms. Although edema was nearly unnoticeable in patients in the ACpT, ACdT, and PTx treatment arms, approximately 12.6% of patients in the DTx treatment arm reported this event. Neuropathy was reported in all treatment arms; however, sensory neuropathy was infrequently observed in patients randomized to the ACdT treatment arm. Joint pain and muscle pain were common in all 3 treatment arms except the DTx arm.
DISCUSSION
To compare the clinical difference between AC‐taxane and AC‐free regimens and/or 2 taxanes, this randomized trial adopted a 2 × 2 factorial design of postoperative chemotherapy in patients with lymph node‐positive breast cancer.
The current study could not demonstrate noninferiority between AC followed by a taxane and an AC‐free regimen in terms of DFS. This may be a function of the PTx regimen (175 mg/m2 every 3 weeks for 8 cycles) having resulted in the worst HR and median survival noted among the 4 regimens tested. Outcomes were better with the ACpT regimen compared with the PTx regimen, possibly because of an additive effect. Conversely, docetaxel alone (at a dose of 75 mg/m2 every 3 weeks for 8 cycles) yielded the best outcome in terms of median survival and HR compared with the standard ACpT treatment arm. Thus, noninferiority could not be demonstrated when the 2 taxanes were analyzed jointly. The findings of the current study suggest that the combination of AC and a taxane cannot be replaced by taxane alone.
In terms of our second clinical question, the results of the current study did not demonstrate the equivalence or superiority of the 2 taxanes with regard to DFS and OS. The DTx and ACdT treatment arms proved superior to the standard ACpT treatment arm. This previously was demonstrated in a phase 3 study by Jones et al in which docetaxel (at a dose of 100 mg /m2 every 3 weeks) was found to be superior to paclitaxel (at a dose of 175 mg/m2 every 3 weeks) in terms of median survival and time to disease progression among patients with metastatic breast cancer.8 At least in the current phase 3 study, the docetaxel‐containing treatment arms appeared to be superior to the paclitaxel‐containing treatment arms when administered every 3 weeks in an adjuvant regimen for patients with lymph node‐positive breast cancer.
However, the dosing schedule between the 2 taxanes is at issue. Sparano et al compared the 2 taxanes with weekly and triweekly adjuvant treatment for breast cancer. Weekly paclitaxel proved superior over a triweekly dosing schedule.9 This finding was corroborated in a randomized phase 3 trial by Seidman et al.10 Schedule dependency (weekly dose‐dense paclitaxel plus carboplatin every 3 weeks) of paclitaxel also was observed in patients with ovarian cancer.11 Thus, paclitaxel appears to be more effective when administered weekly and docetaxel appears to be more effective when administered in a triweekly regimen.8, 9 Triweekly administration of DTx would be a viable alternative to standard ACpT in the current study. However, we were unable to investigate the possibility of weekly administration with paclitaxel. This is a possible limitation to the current study.
It was not a standard clinical practice in the early 2000s, when this trial was designed, to select specific chemotherapeutic regimens for different biological subgroups of patients with breast cancer. An exploratory subset analysis demonstrated that the PTx regimen was notably inferior to the ACpT regimen in patients with HER2 overexpression. Similarly, HER2 positivity in breast cancer cells has since been associated with clinical responsiveness to anthracycline‐containing chemotherapy12; however, to the best of our knowledge, the precise biological mechanism of this observation remains unknown.
The incidence of neutropenic fever and gastrointestinal toxicity, such as nausea, vomiting, and mucositis, associated with docetaxel is notably low and acceptable. Peripheral edema was observed in 13% of patients in the DTx treatment arm, but this complication is manageable in the majority of patients with the use of corticosteroids and diuretics.13 Although chemotherapy‐induced peripheral neuropathy has been reported after treatment with taxanes, a patient‐reported health‐related quality of life assessment found that peripheral neuropathy after treatment with taxane alone was tolerable.14
We conclude that AC cannot be omitted from the chemotherapeutic regimen for patients with lymph node‐positive breast cancer and that when docetaxel and paclitaxel are administered in a triweekly schedule, docetaxel results in better DFS. These results suggest that 8 cycles of triweekly administered docetaxel may offer some benefit for patients with lymph node‐positive breast cancer. Further confirmatory trials are warranted to determine which subset of patients will benefit the most.
FUNDING SUPPORT
Corporate and individual sponsors of the current study are provided at http://www.csp.or.jp/cspor/kyousan_e.html. Supported by the Public Health Research Foundation (PHRF). The PHRF had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The Comprehensive Support Project for Oncology Research (CSPOR) Data Center, which handled and stored all data generated from this trial, is an outsourced third party, entirely independent from PHRF.
CONFLICT OF INTEREST DISCLOSURES
Toru Watanabe was supported by a grant from the Public Health Research Foundation for work performed outside of the current study; received consulting fees from Taiho Pharmaceutical, Yakult Honsya, and Kowa; and received honoraria from Chugai Pharmaceutical, Kyowa Hakko Kirin, Nippon Chemiphar, Novartis Pharma, Daiichi Sankyo, Takeda Pharmaceutical, Genomic Health, Taiho Pharmaceutical, AstraZeneca, Pfizer Japan, and Eisai for work performed outside of the current study. Masaru Kuranami was supported by a grant from the Public Health Research Foundation for work performed outside of the current study. Kenichi Inoue was supported by a grant from the Public Health Research Foundation for work performed outside of the current study; received honoraria from Chugai Pharmaceutical, Eisai, Sanofi, AstraZeneca, Taiho Pharmaceutical, Novartis Pharma, GlaxoSmithKline, Daiichi Sankyo, Takeda Pharmaceutical, Pfizer Japan, and Shionogi; and received research funds from Chugai Pharmaceutical, Eisai, Taiho Pharmaceutical, Novartis Pharma, GlaxoSmithKline, Daiichi Sankyo, and Pfizer Japan for work performed outside of the current study. Norikazu Masuda was supported by a grant from the Public Health Research Foundation for work performed outside of the current study; received honoraria from Chugai Pharmaceutical, Eisai, Sanofi, AstraZeneca, Taiho Pharmaceutical, Kyowa Hakko Kirin, Novartis Pharma, GlaxoSmithKline, Daiichi Sankyo, Takeda Pharmaceutical, Pfizer Japan, Boehringer Ingelheim Japan, Ono Pharmaceutical, Otsuka Pharmaceutical, and Eli Lilly Japan; and received research funds from Chugai Pharmaceutical and Eisai for work performed outside of the current study. Kenjiro Aogi was supported by a grant from the Public Health Research Foundation for work performed outside of the current study; received honoraria from Chugai Pharmaceutical, Eisai, Sanofi, SRL, AstraZeneca, Taiho Pharmaceutical, Novartis Pharma, Daiichi Sankyo, Mochida Pharmaceutical, Ono Pharmaceutical, Otsuka Pharmaceutical, Nihon Medi‐Physics, and Eli Lilly Japan; and received research funding from Chugai Pharmaceutical, Eisai, and Sanofi for work performed outside of the current study. Shinji Ohno was supported by a grant from the Public Health Research Foundation for work performed outside of the current study; received honoraria from Chugai Pharmaceutical, Eisai, Sanofi, AstraZeneca, Taiho Pharmaceutical, Kyowa Hakko Kirin, Novartis Pharma, GlaxoSmithKline, Daiichi Sankyo, Takeda Pharmaceutical, Pfizer Japan, Otsuka Pharmaceutical, and Eli Lilly Japan; and received research funding from Chugai Pharmaceutical, Eisai, GlaxoSmithKline, Daiichi Sankyo, Yakult Honsya, Nippon Kayaku, and Novartis Pharma for work performed outside of the current study. Hiroji Iwata was supported by a grant from the Public Health Research Foundation for work performed outside of the current study; received consulting fees from Daiichi Sankyo; received honoraria from Chugai Pharmaceutical, AstraZeneca, GlaxoSmithKline, Daiichi Sankyo, Novartis Pharma, Taiho Pharmaceutical, Pfizer Japan, Sanofi, Takeda Pharmaceutical, Bristol‐Myers Squibb, Nippon Kayaku, and Eisai; and received research funds from GlaxoSmithKline, Novartis Pharma, Pfizer Japan, Daiichi Sankyo, Bayer Yakuhin, Nippon Kayaku, and Chugai Pharmaceutical. Hirofumi Mukai was supported by a grant from the Public Health Research Foundation for work performed outside of the current study; received honoraria from AstraZeneca, Eisai, Novartis Pharma, and Taiho Pharmaceutical; and received research funds from the Japanese government, Chugai Pharmaceutical, Daiichi Sanyko, Eisai, Nippon Kayaku, Novartis Pharma, Pfizer Japan, and Sanofi for work performed outside of the current study. Yukari Uemura was supported by a grant from the Public Health Research Foundation for work performed outside of the current study and received honoraria from Chugai Pharmaceutical and Teijin Pharma for work performed outside of the current study. Yasuo Ohashi was supported by a grant from the Public Health Research Foundation for work performed outside of the current study; received personal fees from Statcom Co. Ltd and Sanofi KK; received a grant from Eisai Co. Ltd; received nonfinancial support from Takeda Pharmaceutical Co. Ltd and Yakult Honsha Co. Ltd; and received consulting fees from Takeda Pharmaceutical, Chugai Pharmaceutical, and Shionogi for work performed outside of the current study.
AUTHOR CONTRIBUTIONS
Toru Watanabe: Conceptualization, methodology, validation, investigation, resources, data curation, writing–original draft, writing–review and editing, supervision, and project administration. Masaru Kuranami: Investigation and resources. Kenichi Inoue: Investigation and resources. Norikazu Masuda: Investigation and resources. Kenjiro Aogi: Investigation and resources. Shinji Ohno: Investigation and resources. Hiroji Iwata: Conceptualization, investigation, and resources. Hirofumi Mukai: Investigation and resources. Yukari Uemura: Software, formal analysis, and data curation. Yasuo Ohashi: Conceptualization, methodology, formal analysis, data curation supervision, project administration, and funding acquisition.
The data regarding health‐related quality of life as a secondary endpoint in the National Surgical Adjuvant Study of Breast Cancer 02 have been published previously in Ohsumi S, Shimozuma K, Ohashi Y, et al. Subjective and objective assessment of edema during adjuvant chemotherapy for breast cancer using taxane‐containing regimens in a randomized controlled trial: the National Surgical Adjuvant Study of Breast Cancer 02. Oncology. 2012;82:131‐138; and Shimozuma K, Ohashi Y, Takeuchi A, et al. Taxane‐induced peripheral neuropathy and health‐related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N‐SAS BC 02, a randomized clinical trial. Support Care Cancer. 2012;20:3355‐3364.
We thank all patients, investigators, and clinical research specialists who participated in the current study (for a list of participating hospitals, please see http://www.csp.or.jp/ [in Japanese]). We also thank the pharmaceutical sponsor who provided guidance regarding the proper use of the study drugs and comments on the study. We deeply appreciate the late Kaoru Abe, MD, PhD, President Emeritus of the National Cancer Center, for his supreme mentorship.
REFERENCES
- 1. Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node‐positive primary breast cancer. J Clin Oncol. 2003;21:976–983. [DOI] [PubMed] [Google Scholar]
- 2. Buzdar AU, Singletary SE, Valero V, et al. Evaluation of paclitaxel in adjuvant chemotherapy for patients with operable breast cancer: preliminary data of a prospective randomized trial. Clin Cancer Res. 2002;8:1073–1079. [PubMed] [Google Scholar]
- 3. Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node‐positive breast cancer: results from NSABP B‐28. J Clin Oncol. 2005;23:3686–3696. [DOI] [PubMed] [Google Scholar]
- 4. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) , Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta‐analyses of long‐term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin‐induced congestive heart failure. Ann Intern Med. 1979;91:710–717. [DOI] [PubMed] [Google Scholar]
- 6. Ando M, Narabayashi M, Watanabe T, et al. Therapy‐related leukemia and myelodysplastic syndrome in breast cancer patients treated with cyclophosphamide or anthracyclines. Jpn J Clin Oncol. 1999;29:28–32. [DOI] [PubMed] [Google Scholar]
- 7. Aapro M, Bernard‐Marty C, Brain EG, et al. Anthracycline cardiotoxicity in the elderly cancer patient: a SIOG expert position paper. Ann Oncol. 2011;22:257–267. [DOI] [PubMed] [Google Scholar]
- 8. Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542–5551. [DOI] [PubMed] [Google Scholar]
- 9. Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every‐3‐weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER‐2 overexpressors and random assignment to trastuzumab or not in HER‐2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. [DOI] [PubMed] [Google Scholar]
- 11. Katsumata N, Yasuda M, Takahashi F, et al; Japanese Gynecologic Oncology Group . Dose‐dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open‐label, randomised controlled trial. Lancet. 2009;374:1331–1338. [DOI] [PubMed] [Google Scholar]
- 12. Pritchard KI, Shepherd LE, O'Malley FP, et al; National Cancer Institute of Canada Clinical Trials Group . HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–2111. [DOI] [PubMed] [Google Scholar]
- 13. Ohsumi S, Shimozuma K, Ohashi Y, et al. Subjective and objective assessment of edema during adjuvant chemotherapy for breast cancer using taxane‐containing regimens in a randomized controlled trial: the National Surgical Adjuvant Study of Breast Cancer 02. Oncology. 2012;82:131–138. [DOI] [PubMed] [Google Scholar]
- 14. Shimozuma K, Ohashi Y, Takeuchi A, et al. Taxane‐induced peripheral neuropathy and health‐related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N‐SAS BC 02, a randomized clinical trial. Support Care Cancer. 2012;20:3355–3364. [DOI] [PubMed] [Google Scholar]


