Abstract
Background
Reducing near‐fatal asthma exacerbations is a critical problem in asthma management.
Objectives
To determine patterns of factors preceding asthma exacerbations in a real‐world setting.
Methods
In a nationwide prospective study of 190 patients who had experienced near‐fatal asthma exacerbation, cluster analysis was performed using asthma symptoms over the 2‐week period before admission.
Results
Three distinct clusters of symptoms were defined employing the self‐reporting of a visual analogue scale. Cluster A (42.1%): rapid worsening within 7.4 hours from moderate attack to admission, young to middle‐aged patients with low Body mass index and tendency to depression who had stopped anti‐asthma medications, smoked, and hypersensitive to environmental triggers and furred pets. Cluster B (40.0%): fairly rapid worsening within 48 hours, mostly middle‐aged and older, relatively good inhaled corticosteroid (ICS) or ICS/long‐acting beta‐agonist (LABA) compliance, and low perception of dyspnea. Cluster C (17.9%): slow worsening over 10 days before admission, high perception of dyspnea, smokers, and chronic daily mild‐moderate symptoms. There were no differences in overuse of short‐acting beta‐agonists, baseline asthma severity, or outcomes after admission for patients in these 3 clusters.
Conclusion
To reduce severe or life‐threatening asthma exacerbation, personalized asthma management plans should be considered for each cluster. Improvement of ICS and ICS/LABA compliance and cessation of smoking are important in cluster A. To compensate for low perception of dyspnea, asthma monitoring of peak expiratory flow rate and/or exhaled nitric oxide would be useful for patients in cluster B. Avoidance of environmental triggers, increase usual therapy, or new anti‐type 2 response‐targeted therapies should be considered for cluster C.
Keywords: cluster analysis, inhaled corticosteroid compliance, near‐fatal asthma, visual analogue scale
Abbreviations
- COPD
chronic obstructive pulmonary disease
- IAA
Innovative Asthma Association
- ICS
inhaled corticosteroid
- IgE
immunoglobulin E
- LABA
inhaled long‐acting beta‐agonist
- PEF
peak expiratory flow
- SABA
short‐acting beta‐agonist
- SpO2
oxygen saturation by pulse oximetry
- VAS
visual analogue scale
1. INTRODUCTION
Risk factors for incipient asthma exacerbation include sex, age, race, socioeconomic status, baseline lung function, smoking history, exposure to viruses, active sinusitis, and symptomatic gastroesophageal reflux disease.1 Frequent asthma exacerbation is a risk factor associated with progression to severe disease and excessive decline in lung function.2 Severe exacerbation is recognized as an indication for evaluating the required level of interventional asthma management to reduce the potential risk of death.3, 4, 5, 6, 7, 8 Using cluster analysis, many studies have described the heterogeneity of severe asthma symptoms during nonexacerbation periods.1, 9, 10, 11, 12 However, to the best of our knowledge, no studies have reported on the heterogeneity of patient factors preceding worsening symptoms leading to severe or life‐threatening exacerbation in a real‐world setting.
Previous reports to date have relied on information extracted by physicians from limited medical histories and on information provided by relatives or friends of patients who died from severe asthma.3, 4, 5 Other studies have shown that short‐acting beta‐agonist (SABA) usage was increased from around 5 days preceding emergency hospitalization.13, 14 However, these previous data were from strictly controlled clinical trials with good compliance to inhaled corticosteroid (ICS) or ICS/long‐acting beta‐agonist (LABA) use, while in general clinical practice, variable asthma drug compliance and smoking habits prevail as confounding factors. To accurately predict and reduce exacerbation, it is necessary to determine the factors influencing the pattern of exacerbation in a real‐world setting and adjust management strategies for each patient accordingly.
We recently performed a nationwide, prospective, multicenter study in which patients admitted for severe or life‐threatening asthma were recruited at emergency hospitals throughout Japan.15 The results demonstrated that significant heterogeneity exists among patients with severe or life‐threatening asthma exacerbation. We further estimated trajectories of asthma symptoms using k‐means for longitudinal data in this study.
Inaccurate assessment of asthma severity often delays the provision of an appropriately intensive treatment, consequently leading to hospital admission or even death. In recent real‐world adult asthma studies, symptoms assessed by visual analogue scale (VAS) scoring have been proposed as potentially reliable tools for managing patients more effectively and for predicting uncontrolled asthma at home or the general practitioner's office.16, 17, 18 To predict, prevent, and manage severe or life‐threatening asthma exacerbation, we herein assessed serial VAS scores from the 14 days before emergency hospitalization and sought to identify predictors of asthma worsening using cluster analysis.
2. METHODS
2.1. Study setting
This study was a secondary analysis of a previously reported prospective, multicenter, observational study of patients with severe or life‐threatening asthma exacerbation.15 In the previous study15, we did not analyze the serial asthma symptom using 10 cm VAS scores. This study was conducted by the Innovative Asthma Association (IAA), a nationwide group performing research across Japan. Patients were recruited from 17 hospitals between October 2011 and December 2012. Briefly, all eligible patients met the following criteria: (i) >16 years of age; (ii) requiring hospitalization due to severe or life‐threatening asthma attacks with SpO2 <90% on room air at the time of hospitalization or emergency transport before treatment, and (iii) no heart failure, pneumonia, pneumothorax, or other pulmonary diseases on X‐ray. Severe or life‐threatening exacerbation was defined according to the Japanese Society of Allergology guidelines,19 that is, an inability to move because of dyspnea, difficulty in speaking, and the following objective findings: peak expiratory flow (PEF) <60%, SpO2 <90%, partial pressure of oxygen in arterial blood (PaO2) ≤60 mm Hg, and partial pressure of carbon dioxide in arterial blood (PaCO2) ≥45 mm Hg.
The study was reviewed and approved by the Ethics Committee of Sapporo Medical University (No. 23‐47). The study protocol is registered with the University Hospital Medical Information Network in Japan (number 000006448).
2.2. Questionnaire
A total of 173 variables were acquired consisting of 91 patient‐reported questions and 82 physician‐reported questions during admission period (see Supplementary Appendices 1 and 2 of the Supporting Information in reference 15). The patient‐reported questionnaires were mainly used to obtain baseline information on risk factors, demographics, and asthma at baseline. The physician‐reported questionnaires were mainly used to record clinical and laboratory findings and follow‐up data on admission. The severity of asthma was determined by the physician according to symptomatic evaluation and the daily medication regimen according to the GINA guidelines 2005.20
2.3. Serial VAS
Self‐administered serial VAS of asthma symptoms comprising a 10‐cm‐long vertical segment was completed retrospectively, daily from 14 days before admission (day −14) to day −3, and then at −60, −48, −36, −24, −12, −6, and −3 hours, and finally at 0 hours (on admission). The patient was asked to indicate his/her actual perception of asthma symptoms by marking a point along the segment. In this study, a score of 0 corresponded to optimal symptom‐free breathing, whereas 10 corresponded to the most severe asthmatic symptoms. Mild asthma exacerbation (0 < VAS ≤ 3.3) was defined as “wheeze or cough,” “can lie down,” and “daily life not disturbed”; moderate asthma exacerbation (3.3 < VAS ≤ 6.6) was defined as “cannot lie down,” “mildly disturbed daily life,” and “mild dyspnea sensation”; finally, severe exacerbation (6.6 < VAS ≤ 10) was defined as “cannot move,” “cannot speak,” and “strong dyspnea.” These “mild,” “moderate,” and “severe” categories were depicted by dotted lines on the VAS score sheet (Figures S1 and S2).
2.4. Statistical analysis
Clusters (or trajectories) for the longitudinal VAS scores were estimated using k‐means for longitudinal data.21, 22, 23 This nonparametric procedure partitions cases into clusters. Each case is first assigned arbitrarily to 1 initial cluster. Next, each case is reassigned to the neighborhood cluster based on the center (average) of each cluster. The operation is repeated until that there is no change by mean adjustment in the clusters. This process to convergence is then repeated. The best cluster number is determined by clinical interpretability and a criterion that maximizes a ratio computed by dividing between variance by within variance (ie, maximizing the homogeneity within clusters and maximizing the differences between clusters). Missing VAS scores (<4% of total) were appropriately treated by linear interpolation. The interpolated VAS scores were divided by 10 and subjected to log‐log transformation, and these values were then used in the cluster analysis.
Additionally, differences in characteristics at admission between clusters were tested using the Kruskal‐Wallis test for continuous variables and the chi‐square test for categorical variables. To construct a decision tree for identifying specific clusters, we performed a tree‐based recursive partitioning by conditional inference. Analyses were performed using R version 3.3.2 (R Foundation, Vienna, Austria) with the functions “kml” (package: kml) and “ctree” (package: party).
3. RESULTS
Patients meeting the eligibility criteria during the study period numbered 223 recruited from 17 hospitals throughout Japan.15 Thirty‐three patients were excluded because the attending physicians considered it inappropriate to include them in the study. Thus, cluster analysis was performed on 190 patients (Figure 1). Of these, 50% had a history of prior hospitalization for asthma exacerbation (Table 1), and all of them had been admitted to the hospital emergency room with SpO2 <90%, allowing us to exclude psychosomatic exacerbation and other diseases such as vocal cord dysfunction. The 190 asthmatic patients were clustered into 3 separate groups according to serial VAS scores. These were characterized by rapidly (cluster A), fairly rapidly (cluster B), and slowly (cluster C) worsening asthma symptoms. Trajectories of changes in median VAS values of the 3 clusters in the total of 190 subjects from the day −14 up to hospital admission are shown in Figure 2. Asthma symptoms by VAS scoring gradually worsened from the day −8 to −6 before admission. The median VAS score reached 3.3 cm approximately 24 hours before subsequent admission in all 190 patients. A VAS score of 3.3 cm represents the border between mild and moderate exacerbations. Histograms of the VAS score at day −6, −6 hours, −3 hours before admission, and 0 hours (at admission) for each cluster are depicted in Figure 3. Demographic characteristics, comorbidities, usual symptom triggers, current asthma condition, current triggers, and drug usage of patients in these 3 clusters are shown in Tables 1, 2, and S1.
Figure 1.
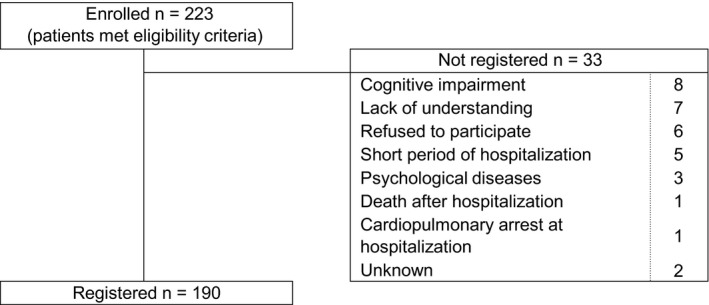
Patient characteristics
Table 1.
Demographic characteristics, comorbid diseases, and baseline asthma in each cluster
| Variables and characteristics, N = 190 | Cluster A (n = 80) | Cluster B (n = 76) | Cluster C (n = 34) | P‐value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Female, n (%) | 40 (50) | 45 (59) | 23 (68) | .190 |
| Age (y), mean ± SD | 51 ± 17 | 63 ± 18 | 59 ± 17 | <.001 |
| Up to 41 y | 23 (29) | 11 (14) | 5 (15) | <.001 |
| 41 y to <65 y | 38 (48) | 21 (28) | 15 (44) | |
| >65 y | 19 (24) | 44 (58) | 14 (41) | |
| BMI <18.5 kg/m2, n (%) | 20 (25) | 7 (9) | 3 (8) | .015 |
| BMI ≥30.0 kg/m2, n (%) | 8 (10) | 5 (7) | 5 (15) | .285 |
| Lifetime smoker, n (%) | 59 (74) | 42 (55) | 21 (62) | .052 |
| Current smoker, n (%) | 42 (53) | 18 (24) | 11 (32) | <.001 |
| Pack‐year, mean ± SD | 18 (19) | 15 (22) | 18 (25) | .145 |
| Pet ownership, n (%) | 29 (36) | 18 (24) | 11 (32) | .227 |
| Hypersensitivity symptoms to furred pets, n (%) | 26 (33) | 8 (11) | 7 (21) | .004 |
| Comorbid diseases, n (%) | ||||
| Allergic rhinitis | 41 (51) | 36 (47) | 15 (44) | .763 |
| Atopic eczema | 9 (11) | 9 (12) | 2 (6) | .618 |
| Chronic hyperplastic rhinosinusitis/nasal polyposis | 21 (26) | 22 (29) | 10 (29) | .910 |
| Diabetes | 8 (10) | 11 (15) | 4 (12) | .692 |
| Hyperlipidemia | 4 (5) | 19 (25) | 4 (12) | .002 |
| Hypertension | 18 (23) | 23 (30) | 6 (18) | .304 |
| Chronic obstructive pulmonary disease | 8 (10) | 11 (15) | 5 (15) | .648 |
| Inactive pulmonary tuberculosis | 3 (4) | 6 (8) | 2 (6) | .541 |
| Any psychological diseases | 7 (9) | 2 (3) | 0 (0) | .071 |
| Baseline asthma | ||||
| Asthma duration, mean ± SD | 17 ± 14 | 21 ± 20 | 18 ± 18 | .709 |
| Severity, n (%) | ||||
| Intermittent | 29 (36) | 25 (33) | 11 (32) | .803 |
| Mild persistent | 14 (18) | 17 (22) | 4 (12) | |
| Moderate persistent | 22 (28) | 18 (24) | 9 (26) | |
| Severe persistent | 15 (19) | 16 (21) | 10 (29) | |
| Aspirin‐intolerant asthma, n (%) | 3 (3.8) | 0 (0.0) | 2 (5.9) | .146 |
| Previous asthma exacerbation, n (%) | ||||
| Unscheduled visits in the past year (≥once) | 34 (43) | 31 (41) | 15 (44) | .944 |
| History of hospitalization for asthma | 40 (50) | 38 (50) | 17 (50) | 1.000 |
| Hospitalizations for asthma in the past year (≥once) | 16 (20) | 13 (17) | 5 (15) | .775 |
| History of NFA requiring mechanical ventilation | 6 (8) | 6 (8) | 2 (6) | .939 |
| Usual trigger of worsening of symptoms, n (%) | ||||
| Exposure to irritant | 37 (46) | 32 (42) | 21 (62) | .156 |
| Dampness/storm | 25 (31) | 20 (26) | 11 (32) | .733 |
| Alcohol/meal | 18 (23) | 18 (24) | 7 (21) | .937 |
| Cold air/climate change, | 59 (74) | 41 (54) | 25 (74) | .019 |
| Strain/stress | 50 (63) | 40 (53) | 23 (68) | .256 |
| Exposure to furred pets | 17 (21) | 6 (8) | 5 (15) | .063 |
| Exposure to house dust | 31 (39) | 21 (28) | 13 (38) | .295 |
BMI, body mass index; NFA, near‐fatal asthma.
Figure 2.
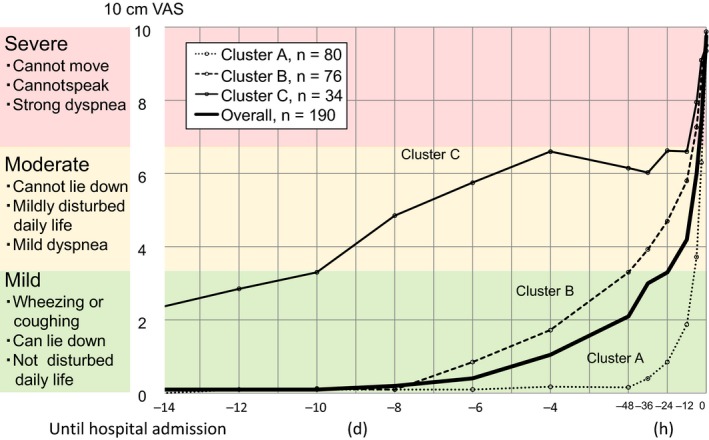
Trajectories of median 10 cm visual analogue scale values from the 14 d before hospital admission for severe or life‐threatening asthma exacerbation
Figure 3.
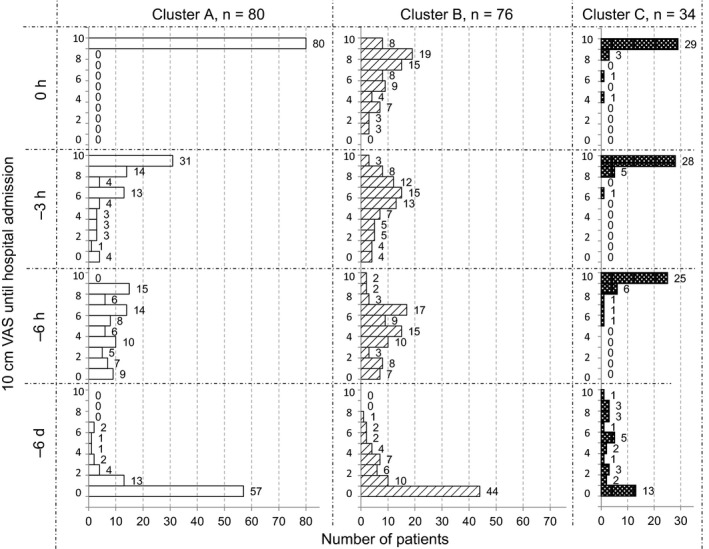
Serial changes in histogram of 10 cm visual analogue scale values at −6 d, −6 h, −3 h, and 0 h before hospital admission in each cluster
Table 2.
Asthma symptoms and medication usage before hospitalization in each cluster
| Variable, N = 190 | Cluster A (n = 80) | Cluster B (n = 76) | Cluster C (n = 34) | P‐value |
|---|---|---|---|---|
| Asthma symptoms in the previous 3 mo | ||||
| Daytime symptoms, n (%) | ||||
| Absent | 18 (23) | 26 (35) | 10 (29) | .093 |
| Less than once a week | 34 (43) | 23 (31) | 7 (21) | |
| Once or more a week, not every day | 20 (25) | 17 (23) | 8 (24) | |
| Every day | 8 (10) | 9 (12) | 9 (27) | |
| Limitation of activities, n (%) | ||||
| Absent | 17 (22) | 26 (35) | 10 (29) | .151 |
| Mild and brief | 42 (53) | 31 (41) | 12 (35) | |
| Disturbs daily life or sleep ≥once a month | 5 (6) | 8 (11) | 2 (6) | |
| Disturbs daily life or sleep ≥once a week | 14 (18) | 7 (9) | 7 (21) | |
| Restricts daily life | 1 (1) | 3 (4) | 3 (9) | |
| Nocturnal symptoms/awakening, n (%) | ||||
| Absent | 39 (51) | 47 (63) | 20 (61) | .117 |
| Less than twice a month | 20 (26) | 11 (15) | 2 (6) | |
| Twice or more a month | 5 (7) | 6 (8) | 2 (6) | |
| Once or more a week | 7 (9) | 4 (5) | 2 (6) | |
| Frequently | 5 (7) | 7 (9) | 7 (21) | |
| Drug use in the previous month | ||||
| ICS, n (%) | ||||
| Do not use | 30 (38) | 25 (33) | 13 (38) | .047 |
| Less than once per week | 10 (13) | 3 (4) | 2 (6) | |
| Occasionally (1‐3 d per week) | 11 (14) | 3 (4) | 4 (12) | |
| Almost every day (≥4 d per week) | 29 (36) | 45 (59) | 15 (44) | |
| SABA, n (%) | ||||
| Do not use | 35 (44) | 38 (50) | 18 (53) | .197 |
| Less than once per week | 13 (16) | 15 (20) | 3 (9) | |
| Occasionally (1‐3 d per week) | 23 (29) | 12 (16) | 5 (15) | |
| Almost every day (≥4 d per week) | 9 (11) | 11 (15) | 8 (24) | |
| OCS regular usea, n (%) | 4 (5) | 9 (12) | 3 (9) | .272 |
| LTRA regular usea, n (%) | 20 (25) | 20 (26) | 13 (38) | .345 |
| LABA regular usea, n (%) | 26 (33) | 34 (45) | 15 (44) | .255 |
| Theophylline regular usea, n (%) | 9 (11) | 19 (26) | 4 (12) | .059 |
| SABA usage in the previous week, n (%) | ||||
| Do not use | 35 (44) | 38 (50) | 18 (53) | .399 |
| Less than once per week | 7 (9) | 4 (5) | 0 (0) | |
| Occasionally (1‐3 d per week) | 17 (21) | 16 (21) | 4 (12) | |
| Almost every day (≥4 d per week) | 21 (26) | 18 (24) | 12 (35) | |
ICS, inhaled corticosteroid; SABA, short‐acting beta‐agonist; OCS, oral corticosteroid; LTRA, leukotriene receptor antagonist; LABA, long‐acting beta‐agonist.
Patients who reported use of the drug almost every day (≥4 d per week).
The characteristics of cluster A were a rapidly worsening state (n = 80, 42.1%); median VAS score reached 3.3 or 6.6 cm by 7.4 or 2.7 hours before admission, respectively (Figure 2). The VAS score at admission was 10 cm in 100% of patients in this cluster (Figure 3). The trigger for current exacerbation given as “stopped using anti‐asthma medication” was most frequent in cluster A relative to the other 2 clusters (P = .003, Table S1). Patients in cluster A were lean (25% with a body mass index <18.5), young or middle‐aged (mean 51 year), had poor compliance with anti‐asthma medication (only 36% of patients used their ICS 4 or more days a week), were current smokers (53%), and were allergic to furred pets, mites, and moths, sensitive to cold air or other climate variations (Tables 1, 2 and S1). It was founded a tendency (P = .071) for cluster A to have more psychological diseases; 6 of depression and 1 anxiety, as compared with cluster B and C (Table 1). A tree diagram separating cluster A from B and C is shown in Figure S3.
In contrast, patients in cluster B had rapid worsening (n = 76, 40.0%) with the median duration of 48 and 8.7 hours from the time of the VAS score reaching 3.3 and 6.6 cm, respectively, to the time of admission (Figure 2). They were middle‐aged or older (63 year), with comorbidities including hyperlipidemia (25%) and high hemoglobin A1c (P = .022), and had relatively good ICS or ICS/LABA compliance, but with a reduced sensation of dyspneic deterioration (Tables 1 and S2). Even at admission, 45% perceived mild‐to‐moderate dyspnea (Figure 3), with the lowest arterial blood oxygen concentration of all 3 clusters, at a mean PaO2 of 72 Torr (Table S1).
Finally, cluster C (n = 34, 17.9%) consisted of patients with slowly worsening symptoms, with periods of approximately 10 days and 12 hours from the time of the VAS score reaching 3.3 and 6.6 cm, respectively, until admission (Figure 2). They were predominantly middle‐aged (59 year) women (68%) of whom 32% were current smokers. The most characteristic features of this cluster were mild‐to‐moderate asthma symptoms manifesting daily, despite quite good compliance with ICS use. Severe asthmatic symptoms (VAS > 6.6) were already present in 94% patients 6 hours before admission, in contrast to 25% in cluster A, and only 8% in cluster B (Figure 3).
These characteristics and the exacerbation status of patients in the 3 clusters are summarized in Table 3. Additionally, it can be stated that there were no differences among these patients regarding maximum oxygen inhalation dose, duration of supplemental oxygen, or duration of hospitalization (data not shown). There was no seasonal variation of the frequency of each cluster (data not shown).
Table 3.
Summary of the characteristics of patients in each cluster
| Variable, N = 190 | Cluster A | Cluster B | Cluster C | |
|---|---|---|---|---|
| Rapid exacerbation | Fairly rapid exacerbation | Slow exacerbation | ||
| Demographics | Age, mean, years | 51 | 63 | 59 |
| up to 41 y | ++ | + | + | |
| 41 y to <65 y | +++ | ++ | ++ | |
| ≥65 y | + | +++ | ++ | |
| BMI <18.5 kg/m2 | +++ | − | − | |
| Current smoker | +++ | + | ++ | |
| Hypersensitivity symptoms to furred pets | ++ | + | + | |
| Comorbidity | Hyperlipidemia | − | ++ | + |
| Usual trigger of worsening of symptoms | Cold air/climate change, (%) | +++ (74) | +++ (54) | +++ (74) |
| Drug usage in the previous month | ICS regular usea | ++ | +++ | ++ |
| Trigger of the current exacerbation | Stop using anti‐asthma medication | + | − | − |
| Time to hospitalization | From VAS3.3, median, hour | 7.4 | 48.0 | 240.0 |
| From VAS6.6, median, hour | 2.7 | 8.7 | 12.0 | |
ICS, inhaled corticosteroid; BMI, body mass index; VAS, visual analogue scale.
−, <10%, + 10‐<25%; ++, 25‐<50%; +++, 50‐<75%, ++++, 75%‐100%.
Patients who reported use of the drug almost every day (≥4 d per week) of ICS use in the previous month in Table 2.
4. DISCUSSION
The work presented here is a secondary analysis of our previous study,15 representing details of the events that occur shortly before and during severe or life‐threatening asthma exacerbation. Our results document a gradual increase in the VAS score for asthmatic symptoms over 6‐8 days before hospital admission, to hospital, leading to moderate severity (VAS > 3.3) at 24 hours before admission in all 190 patients surveyed (Figure 2). These results are consistent with a previous study by Tattersfield et al.13 reported a gradual decline PEF over 5‐7 days associated with severe exacerbation. These investigators also reported that changes in PEF, asthma symptoms, and use of rescue beta‐agonists before exacerbation occurred in a parallel manner. Herein, using serial VAS scores of asthma symptoms, we have identified 3 groups of patients with diverse backgrounds but showing distinct characteristics leading up to severe or life‐threatening exacerbation. As assessed in a real‐world setting rather than a controlled trial, these patients fell into 3 groups with rapid, fairly rapid, or slow progression. We found that there were no differences among these 3 clusters in overuse of SABA before admission, baseline asthma severity, comorbid chronic obstructive pulmonary disease (COPD), or outcome after hospital admission. Interestingly, there were differences in ICS or ICS/LABA compliance, perception of dyspnea, and baseline hypersensitivity to environmental stimuli. These findings imply that optimal personalized management regimens, aimed at preventing severe or life‐threatening asthma exacerbation in normal daily life, would differ even among patients within these clusters.
We found that patients assigned to cluster A showed rapid worsening just hours before moderate asthma attack and required emergency admission. Nonetheless, their subjective perception of asthma symptoms was essentially accurate, as reflected by their VAS score being 10 cm on admission for all patients in cluster A (Figure 3). They were lean young or middle‐aged patients with poor anti‐asthma medication compliance, were smokers, and were allergic to furred pets, mites, and moths, sensitive to cold air and other climate changes (Tables 1, 2 and S2). This cluster is thus characterized by rapidly worsening airway inflammation associated with smoking and cessation of ICS or ICS/LABA, with consequently enhanced responses to environmental triggers, potentially leading to rapid deterioration and asthma exacerbation. Not surprisingly, ICS or ICS/LABA noncompliance and smoking both increase asthma exacerbations.11, 12, 24 Williams et al.10 reported that ICS or ICS/LABA compliance rates exceeding 75% of the prescribed dose over 6 months appeared to represent a threshold above which moderate asthma exacerbations were significantly reduced. Nonetheless, this was not associated with a decrease in the number of asthma‐related emergency department visits and hospitalizations. Not only better ICS or ICS/LABA compliance but also education to encourage avoidance of environmental triggers would, therefore, be important for decreasing severe or life‐threatening exacerbation in cluster A patients.
Cluster B patients, with a fairly rapid worsening before admission within 48 hours of a moderate asthma attack (VAS = 3.3) or within 8.7 hours of a severe attack (VAS = 6.6) were middle‐aged or older, with hyperlipidemia, relatively good ICS or ICS/LABA compliance, and less perceived dyspnea. The most distinctive feature of patients in cluster B was their low perception of dyspnea during the exacerbation. VAS scores were lower than in clusters A or C even at hospital admission (Figure 3). A low degree of the sensation of dyspnea is recognized as a crucial problem in severe asthma frequently developing into a near‐fatal attack. Our present results are therefore fully consistent with the literature.25, 26 For these patients, objective measurements, that is, daily monitoring of PEF27 or fractional exhaled nitric oxide concentration, may be useful for early detection of exacerbation and recognizing the patient′s actual objective asthmatic status.
Finally, cluster C consisted of patients gradually worsening from VAS = 3.3 and VAS = 6.6 over approximately 10 days and 12 hours, respectively. The most characteristic features of this cluster were mild‐to‐moderate asthmatic symptoms present daily despite relatively good compliance with ICS. Severe symptoms (VAS > 6.6) were already present in 94% of patients 6 hours before admission (Figure 3). Patients falling into this cluster are unique in that although they had strong perceptions of dyspnea, they failed to seek medical attention before respiratory failure, likely because they had become accustomed to experiencing mild‐to‐moderate symptoms every day. Patients in this cluster shared hypersensitivity to environmental triggers and furred pets with cluster A, as well as smoking. As shown in Table 1, baseline severity of asthma in cluster C patients was not different from the other 2 clusters. Only 29% of cluster C patients had severe persistent asthma poorly controlled by the current standard of care medication; for this subgroup, T‐helper type 2 response‐targeting therapies or other new drugs28 might be considered. However, in most patients in this cluster, avoidance of environmental triggers, education on the necessity for even better ICS or ICS/LABA compliance, and changing to higher dose ICS or ICS/LABA may be effective.
Baseline disease severity of the patients in our study was mild intermittent in 34%, mild persistent in 18%, moderate persistent in 26%, and severe persistent in the remaining 22%, as shown in Table 1. Consistent with previous reports, these findings indicate that severe exacerbation or near‐fatal asthma is not related to baseline asthma severity.29, 30 Surprisingly, 52% of the adult patients in this real‐life setting had mild asthma, and their baseline disease severities did not differ among the 3 clusters (Table 1). In a Japanese study of asthma deaths in adults,20 the most frequent degree of baseline asthma severity was “severe” in 39.2% of cases, followed by “moderate” in 33.0%. On the other hand, in a UK pediatric cohort study, half of asthma deaths were in patients with mild or moderate asthma.31 It was also reported that near‐fatal or severe asthma exacerbations show age‐related differences in clinical presentation.32, 33 In our present study, relative to younger adults (<41 years), there was a trend toward increasing VAS symptom scores in middle‐aged and elderly patients (≥41 years) between day −8 days and −24 hours before hospitalization. However, there were no differences over the period 12 hours before admission among our 3 groups (data not shown). The clustering was not affected by baseline disease severity or age.
We also found a tendency for patients in cluster A to have more psychological diseases; depression and anxiety, as comparing with cluster B and C (Table 1). As cluster A patients have poor ICS or ICS/LABA compliance, the psychological state might be associated with this poor compliance. Depression and anxiety are known to be important risk factors for severe or near‐fatal asthma.34, 35, 36 Psychosocial stress is well known to evoke asthma exacerbation, either as triggers or as covariates of worse outcomes,34 and strain or stress is also believed to predispose to asthma. In our study, there is no difference in usual triggers of worsening asthma due to strain or stress among 3 clusters (Table 1). Psychosocial problems are very complex, and the relationship between perception of dyspnea sensations and actual asthma exacerbation is especially unclear, indicating the need for further research.
This study does have potential limitations. First, assessment of exacerbation was only carried out once per patient, such that whether a second or third exacerbation would have had the same predictors in each patient could not be determined. However, this study aimed to recognize factors leading to severe or life‐threatening exacerbation. As half of our study population had already experienced the previous hospitalization for severe or life‐threatening exacerbation, detailed medical histories of asthma exacerbations may partially compensate for this limitation. Therefore, long‐term follow‐up of this cohort is needed. Second, our study depends on the reliability of retrospective self‐reporting asthma symptom VAS scoring used in the cluster analysis. Low perception of dyspnea in patients with near‐fatal asthma has been reported in the literature,25, 26 suggesting questionnaire‐based evaluations of symptoms may not necessarily be reliable. Certainly, in this study low perception of dyspnea was prominent in cluster B and high perception of dyspnea in cluster C. Objective measurements of lung function including PEF or fractional exhaled nitric oxide would be helpful for validating our results, but that is not practical in an actual clinical setting. It has also been reported that changes in asthma symptom scores, PEF, and use of rescue beta‐agonists before exacerbation showed parallels.13 Third, in this study we could not address the influence of ethnicity upon our cluster, because all participants are one race of Japanese.
In conclusion, we herein identified 3 distinct clusters of worsening patterns and factors associated with those patterns leading to severe or life‐threatening asthma exacerbation in a real‐life setting rather than in a clinical trial. The most important factors contributing to being assigned to one of these 3 clusters were degree of ICS or ICS/LABA compliance, low or high perception of dyspnea, and baseline hypersensitivity to environmental stimuli, as well as smoking, and sensitivity to furred pets. Combinations of these factors were different for each cluster, such that daily‐life asthma education and treatment action plans should also be explicitly tailored to the patients in each cluster. Another strength of our study is the finding that not only low perception but also high perception of dyspnea is a critical characteristic of incipient severe or life‐threatening asthma exacerbation. Further studies are necessary to assess the usefulness of suggesting phenotypic interventions for the prevention of recurrent severe or life‐threatening asthma exacerbation.
AUTHOR CONTRIBUTIONS
Hiroshi Tanaka, MD, Eiji Nakatani, PhD, Yuma Fukutomi, MD, Kiyoshi Sekiya, MD, and Masami Taniguchi, MD, designed this study and contributed to the interpretation of data. Eiji Nakatani, PhD, and Hideaki Kaneta, MD, performed the statistical data analysis. All other authors have participated in completing case cards. The original manuscript was drafted by Hiroshi Tanaka, MD, and Eiji Nakatani, PhD, and all authors have participated in revision of the final submitted draft of this manuscript.
CONFLICT OF INTERESTS
All authors declare that they have no relevant conflict of interests.
Supporting information
ACKNOWLEDGMENTS
The authors present this publication on behalf of the IAA research investigators and the Foundation for Biomedical Research and Innovation (FBRI). We thank all the collaborative IAA group physicians throughout Japan who contributed to this survey. The authors appreciate the careful review of this manuscript by Shinsuke Kojima, MD, PhD; Mikio Yoshitomi, PhD; and Atsuhiko Kawamoto, MD, PhD, at the Translational Research Informatics (TRI) Center, FBRI, Kobe, Japan. We also thank Masanori Fukushima, MD, PhD, as the director of the TRI Center for their assistance.
Tanaka H, Nakatani E, Fukutomi Y, et al. Identification of patterns of factors preceding severe or life‐threatening asthma exacerbations in a nationwide study. Allergy. 2018;73:1110–1118. 10.1111/all.13374
Funding information
This work was performed by a collaboration between the IAA research investigators and FBRI in Japan. The FBRI is a public interest incorporated foundation, committed to the promotion of translational and clinical research in Japan, receiving financial resources from pharmaceutical/medical device companies. This was an investigator‐initiated clinical study with operational and technical support provided by the FBRI.
REFERENCES
- 1. Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and co‐morbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195:302‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbation predict excess lung function decline in asthma. Eur Respir J. 2007;30:452‐456. [DOI] [PubMed] [Google Scholar]
- 3. Fraser PM, Speizer FE, Waters SD, Doll R, Mann NM. The circumstances preceding death from asthma in young people in 1968 to 1969. Br J Dis Chest. 1971;65:71‐84. [PubMed] [Google Scholar]
- 4. Sears MR, Rea HH. Patients at risk for dying of asthma: New Zealand experience. J Allergy Clin Immunol. 1987;80:477‐481. [DOI] [PubMed] [Google Scholar]
- 5. Beasley R, Pearce N, Crane J, Burgess C. Beta‐agonists: what is the evidence that their use increases the risk of asthma morbidity and mortality? J Allergy Clin Immunol. 1999;104:518‐530. [DOI] [PubMed] [Google Scholar]
- 6. Royal College of Physicians . Why Asthma Kills: The National Review of Asthma Deaths (NRAD) Confidential Enquiry Report. London, UK: RCP; 2014. [Google Scholar]
- 7. Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. Npj Prim Care Respir Med. 2014;24:14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ichinose M, Sugiura H, Nagase H, et al. Japanese guidelines for adult asthma 2017. Allergol Int. 2017;66:163‐189. [DOI] [PubMed] [Google Scholar]
- 9. Ministry of Health, Labour and Welfare . Japanese population Survey 2015. http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei15/dl/00_all.pdf. Accessed on 5 December 2016
- 10. Williams LK, Peterson EL, Wells K, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid noncompliance. J Allergy Clin Immunol. 2011;128:1185‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suissa S, Ernest P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalization for asthma. Thorax. 2002;57:880‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams LK, Pladevall M, Xi H, et al. Relationship between compliance to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114:1288‐1293. [DOI] [PubMed] [Google Scholar]
- 13. Tattersfield AE, Postma DS, Barnes PJ, et al. Exacerbation of Asthma: a descriptive study of 425 severe asthma exacerbations. The FACET International Study Group. Am J Respir Crit Care Med. 1999;160:594‐599. [DOI] [PubMed] [Google Scholar]
- 14. Patel M, Pilcher J, Hancox RJ, et al. The use of β2‐agonist therapy before hospital attendance for severe asthma exacerbation: a post hoc analysis. Npj Prim Care Respir Med. 2015;25:14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sekiya K, Nakatani E, Fukutomi Y, et al. Severe or life‐threatening asthma exacerbation: patient heterogeneity identified by cluster analysis. Clin Exp Allergy. 2016;46:1043‐1055. [DOI] [PubMed] [Google Scholar]
- 16. Ciprandi G, Schiavetti I, Ricciardolo FL. Symptom perception and asthma control. Postgrad Med. 2015;127:738‐743. [DOI] [PubMed] [Google Scholar]
- 17. Ciprandi G, Schiavetti I, Sorbello V, Ricciardolo FL. Perception of asthma symptoms as assessed on the visual analog scale in subjects with asthma: a real‐life study. Respir Care. 2016;61:23‐29. [DOI] [PubMed] [Google Scholar]
- 18. Dhand R, Kalra S, Malik SK. Use of visual analogue scales for assessment of the severity of asthma. Respiration. 1988;54:255‐262. [DOI] [PubMed] [Google Scholar]
- 19. Nakazawa T, Dobashi K. Current asthma deaths among adults in Japan. Allergol Int. 2004;53:205‐209. [Google Scholar]
- 20. GINA report, global strategy for asthma management and prevention. http://www.ginaasthma.org/. [DOI] [PubMed]
- 21. Genolini C, Falissard B. KmL: a package to cluster longitudinal data. Comput Methods Programs Biomed. 2011;104:e112‐e121. [DOI] [PubMed] [Google Scholar]
- 22. Hartigan JA, Wong MA. Algorithm AS 136: a k‐means clustering algorithm. J R Stat Soc Ser C Appl Stat. 1979;28:100‐108. [Google Scholar]
- 23. Genolini C, Falissard B. KmL: k‐means for longitudinal data. Comput Statistics. 2010;25:317‐328. [Google Scholar]
- 24. Bender B, Zhang L. Negative affect, medication compliance, and asthma control in children. J Allergy Clin Immunol. 2008;122:490‐495. [DOI] [PubMed] [Google Scholar]
- 25. Magadle R, Berar‐Yanay N, Weiner P. The risk of hospitalization and near‐fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002;121:329‐333. [DOI] [PubMed] [Google Scholar]
- 26. Kikuchi Y, Okabe S, Tamura G, et al. Chemosensitivity and perception of dyspnea in patients with a history of near‐fatal asthma. N Engl J Med. 1994;330:1329‐1334. [DOI] [PubMed] [Google Scholar]
- 27. Mendoza GR. Peak flow monitoring. J Asthma. 1991;28:161‐177. [DOI] [PubMed] [Google Scholar]
- 28. Ray A, Raunhal M, Oriss TB, Ray P, Wenzel SE. Current concepts of severe asthma. J Clin Invest. 2016;126:2394‐2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kunitoh H, Yahikozawa H, Kakuta T, et al. Fatal and near fatal asthma. Ann Allergy. 1992;69:111‐115. [PubMed] [Google Scholar]
- 30. Romagnoli M, Caramori G, Braccioni F, et al. Near‐fatal asthma phenotype in the ENFMOSA cohort. Clin Exp Allergy. 2007;37:552‐557. [DOI] [PubMed] [Google Scholar]
- 31. Anagnostou K, Harrison B, Iles R, Nasser S. Risk factors for childhood asthma deaths from the UK Eastern Region Confidential Enquiry 2001–2006. Prim Care Respir J. 2012;21:71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsai CL, Lee WY, Hanania NA, Camargo CA Jr. Age‐related differences in clinical outcomes for acute asthma in the United State, 2006–2008. J Allergy Clin Immunol. 2012;129:1252‐1258. [DOI] [PubMed] [Google Scholar]
- 33. Sekiya K, Taniguchi M, Fukutomi Y, et al. Age‐specific characteristics of inpatients with severe asthma exacerbation. Allergol Int. 2013;62:331‐336. [DOI] [PubMed] [Google Scholar]
- 34. Huovinen E, Kaprio J, Koskenvuo M. Asthma in relation to personality traits, life satisfaction, and stress: a prospective study among 11,000 adults. Allergy. 2001;56:971‐977. [DOI] [PubMed] [Google Scholar]
- 35. Serano J, Plaza V, Sureda B, et al. Alexithymia: a relevant psychological variable in near‐fatal asthma. Eur Respir J. 2006;28:296‐302. [DOI] [PubMed] [Google Scholar]
- 36. Amelink M, Hashimoto S, Spinhoven P, et al. Anxiety, depression and personality traits in severe, prednisone‐dependent asthma. Respir Med. 2014;108:438‐444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


