Abstract
Paternal environment can induce detrimental developmental origins of health and disease (DOHaD) effects in resulting offspring and even future descendants. Such paternal-induced DOHaD effects might originate from alterations in a possible seminal fluid microbiome (SFM) and composite metabolome. Seminal vesicles secrete a slightly basic product enriched with fructose and other carbohydrates, providing an ideal habitat for microorganisms. Past studies confirm the existence of a SFM that is influenced by genetic and nutritional status. Herein, we sought to determine whether treatment of male mice with a combination of antibiotics designed to target SFM induces metabolic alterations in seminal vesicle gland secretions (seminal fluid) and histopathological changes in testes and epididymides. Adult (10- to 12-week-old) National Institutes of Health (NIH) Swiss males (n = 10 per group) were treated with Clindamycin 0.06 mg/kg day, Unasyn (ampicillin/sulbactam) 40 mg/kg day and Baytril (enrofloxacin) 50 mg/kg day designed to target the primary bacteria within the SFM or saline vehicle alone. Fourteen-day antibiotic treatment of males induced metabolomic changes in seminal vesicles with inosine, xanthine and L-glutamic acid decreased but D-fructose increased in glandular secretions. While spermatogenesis was not affected in treated males, increased number of epididymal tubules showed cribriform growth in this group (7 antibiotic-treated males: 3 saline control males; P = 0.01). Antibiotic-treated males showed more severe cribriform cysts. Current findings suggest antibiotic treatment of male mice results in seminal fluid metabolome and epididymal histopathological alterations. It remains to be determined whether such changes compromise male reproductive function or lead to DOHaD effects in resulting offspring.
Introduction
The pre- and post-natal environment can permanently shape future health, for better or worse, of an individual (Chow & Lee 1964, Roeder & Chow 1972, Dorner 1977, Gram et al. 1995). The late Sir David Barker was the first to formulate this concept of ‘fetal origin of adult disease’ (Barker 1990). This paradigm later morphed into the final term of ‘developmental origins of adult health and disease (DOHaD)’ to reflect the premise that extrinsic factors encountered by the developing fetus/neonate might also affect later health (Gillman et al. 2007, Hanson et al. 2011, Barouki et al. 2012, Hanson & Gluckman 2014, Hanson 2015). The original DOHaD concept assumed that only the in utero or maternal environmental condition could influence health or disease risk of resulting offspring. However, it is increasingly becoming apparent that paternal state prior to fertilization may be equally if not more important.
Paternal condition can lead to DOHaD-based diseases in his offspring and even potential transgenerational effects (Binder et al. 2012a,b, Rando 2012, Rodgers et al. 2013, Bromfield 2014, Bromfield et al. 2014, Gapp et al. 2014, Sharma & Rando 2014, Binder et al. 2015, Faure et al. 2015). Paternal obesity in mice has been linked with F1 embryo and offspring disruptions, including delayed development, carbohydrate utilization, mitochondrial disturbances, metabolic disorders and reduced sperm motility (Binder et al. 2012a,b, Fullston et al. 2015). Offspring of male mice fed a low-protein diet show DNA methylation and gene expression changes associated with cholesterol and lipid metabolism and leads to cardio-metabolic disorders (Carone et al. 2010, Watkins & Sinclair 2014). Paternal exposure to environmental chemicals results in transcriptomic and miRNA changes in resulting embryos (Brevik et al. 2012a,b).
The well-documented birth records kept by the parish of Överkalix in northern Sweden from the late 1800s to the 1900s has allowed for assessments on how grandparents’ nutritional state affects their grandoffspring. The combined studies suggest that disease predilection in granddaughters was primarily associated with overall health of their grandmothers. In contrast, physical condition of grandfathers correlated with risk of cardio-metabolic disorders in their grandsons (Bygren et al. 2001, Kaati et al. 2002, Pembrey et al. 2006).
Paternal-induced DOHaD effects may also originate due to fluctuations in seminal vesicle fluid contents, in particular, metabolites and cytokines, and/or female immune response to these compounds (Bromfield 2014, Bromfield et al. 2014, Binder et al. 2015). We have discovered that a novel microbiome resides in the seminal vesicles, and the microorganism inhabitants are influenced by genetic status and nutritional status of the male (Javurek et al. 2016, 2017). It is yet to be determined whether changes in the seminal fluid microbiome (SFM) affect the conceptus environment or programming. These findings though raise the concern as to how antibiotic treatment prior to fertilization might affect the microbiota within the seminal vesicles.
While past studies have not considered the potential effects of antibiotic treatment on the resident seminal vesicle microbiome, the effects of several bacteriostatic and bactericidal antibiotics on the testes and epididymides have been examined. Short-term antibiotic treatment of rodent models and amphibians with antibiotics, including tetracycline, doxycycline, gentamycin, ciprofloxacin, enrofloxacin, ofloxacin, salinomycin, streptomycin, ceftazidime and cefmetazole, results in impaired spermatogenesis, decreased sperm motility, suppression of androgen production by Leydig cells and generation of oxygen-free radicals within the male reproductive tract (Moe et al. 1989, Crotty et al. 1995, Demir et al. 2007, Aral et al. 2008, Farombi et al. 2008, El-Harouny et al. 2010, Antohi et al. 2011, Alp et al. 2012, Elzeinova et al. 2013, Ojo et al. 2013, Silla et al. 2015). Some of these changes are reversible after cessation of the antibiotic treatment (Moe et al. 1989, Ojo et al. 2013). It is not clear though if these pathologies are due to direct antibiotic-induced testicular toxicity or secondary to dysbiosis and associated bacterial metabolic imbalances in the seminal vesicles. The underlying hypothesis thus tested in the current studies was that short-term administration of antibiotics designed to target resident microorganisms in the seminal vesicles results in metabolome imbalances in this organ and histopathological changes in the testes and epididymides. To test this possibility, NIH Swiss male mice were treated for 14 days with the ‘CUB’ antibiotic protocol – Clindamycin, Unasyn (ampicillin/sulbactam) and Baytril (enrofloxacin) or vehicle saline solution. This combination of antibiotics is designed to target the primary bacteria identified previously in the SFM (Javurek et al. 2016, 2017). Metabolomic assessments were then performed on the seminal vesicle gland secretions (seminal fluid), and testes and epididymides were histologically analyzed.
Materials and methods
Animals and treatments
The animal experiments were approved by the University of Missouri Animal Care and Use Committee (Protocol #8693) and performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Ten-to-twelve-week-old, NIH Swiss male mice were purchased from Envigo (Madison, WI, USA) and shipped to the University of Missouri Animal Sciences Research Center (ASRC). They were then placed on a control diet (7.2% fat, TD.140790, Envigo) and provided access to water ad libitum. Mice were maintained on a 12-h light:12-h darkness cycle with the lights on at 7:30 h and off at 19:30 h. Mice were habituated to the ASRC animal facility for 1 week. Males were then randomly assigned to one of two daily treatment groups: combination antibiotic group (CUB antibiotic protocol – Clindamycin (0.06 mg/kg day; Hospira, Inc. – acquired by Pfizer), Unasyn (ampicillin/sulbactam, 40 mg/kg day, Pfizer, Inc.) and Baytril (enrofloxacin, 50 mg/kg day; Bayer Animal Health, Whippany, NJ, USA) or saline vehicle alone (n = 10 per group). Mice were injected intraperitoneally daily for 2 weeks with a volume of 0.2 mL for the CUB or saline treatment and monitored daily for any evidence of infection, pain or distress. No such signs were observed. After 2 weeks of the respective treatments, males were killed and whole seminal fluid collected from the seminal vesicles in a sterile aseptic manner, as described previously (Javurek et al. 2016, 2017). Testes with attached epididymides were fixed in Bouin’s fixative for 24 h and then changed over daily for 3 days to 70% ethanol solution.
Metabolome analyses
Seminal fluid samples were first partitioned into two immiscible extracts by adding chloroform (1.5 mL) and 1.5 mL of HPLC-grade water containing 25 μg/mL ribitol (as internal standard). The mixture was vortexed and centrifuged to facilitated phase partitioning. The upper aqueous phase was recovered and analyzed, as described previously (Broeckling et al. 2005). Briefly, the aqueous phase was dried under gaseous nitrogen stream, methoximated in pyridine with 120 μL of 15 mg/mL methoxyamine hydrochloride, and then trimethylsilylated with 120 μL MSTFA (N-methyl-N-(trimethylsilyl)trifluoroacetamide) + 1%TMCS (chlorotrimethylsilane) reagent. Metabolic profiling was performed using an Agilent 6890N GC coupled to a 5973N single quadrupole mass spectrometer with a scan range from m/z 50 to 650 (Agilent Technologies, Inc.). Separation was achieved with a temperature program of 80°C for 2 min, then ramped at 5°C/min to 315°C and held at 315°C for 12 min, a 30 m DB-5MS column (J&W Scientific, 0.25 mm ID, 0.25 μm film thickness) and a constant flow of 1.0 mL/min. A standard alkane mix was used for gas chromatography coupled to mass spectrometry (GCMS) quality control and retention index calculations. The data were deconvoluted using Automated Mass Spectral Deconvolution and Identification Software (AMDIS) and annotated through mass spectral and retention index matching to an in-house constructed spectra library. The unidentified components were then identified using spectral matching to a commercial NIST17 mass spectral library. The combined identifications were in .ELU file format, and the abundance of the ions were extracted using custom MET-IDEA software (Lei 2012). The abundances were then normalized to the internal standard, ribitol, and the normalized values were used for statistics. Statistics such as Partial Least Squares Discriminant Analysis (PLS-DA) and volcano plots were performed with the MetaboAnalyst 3.0 program, after the data were normalized to sum, log transformation and Pareto scaling. (http://www.metaboanalyst.ca/, Date Accessed March 21, 2018).
Testes and epididymides histopathology
The testes and epididymides were sectioned under a Nikon SMZ2B stereomicroscope (Nikon Instruments Inc., Melville, NY, USA) and placed in a cassette with foam sponges for histological analysis. The areas sampled for the testes and epididymides are depicted in Fig. 1. From each animal, five to ten 3–5 μm sections at 50 μm apart were cut and stained with periodic acid-Schiff (PAS)/hematoxylin to visualize the acrosome formation in spermatids. Histopathology of testes involved an analysis of all stages of spermatogenesis (Hess & de Franca 2008) and an examination of acrosome formation in the spermatids. In the epididymides, portions of caput, corpus and cauda regions were examined for potential differences. The only major pathological change observed in the epididymides was the formation of cribriform cysts (Butterworth & Bisset 1992, Kempinas & Klinefelter 2014); therefore, this observation received further investigation. We defined mature cribriform cysts as those having epithelial changes that included at least three major features: intraepithelial lumens surrounded by epithelial cells forming a bridge over the apical aspect of the cyst, secretory material within the lumen and microvilli having grown into the cystic lumen (Nistal et al. 1990, Butterworth & Bisset 1992). Immature cysts were defined as those having only one of the three major identifying characteristics or showing only a large vacuole along the basement membrane.
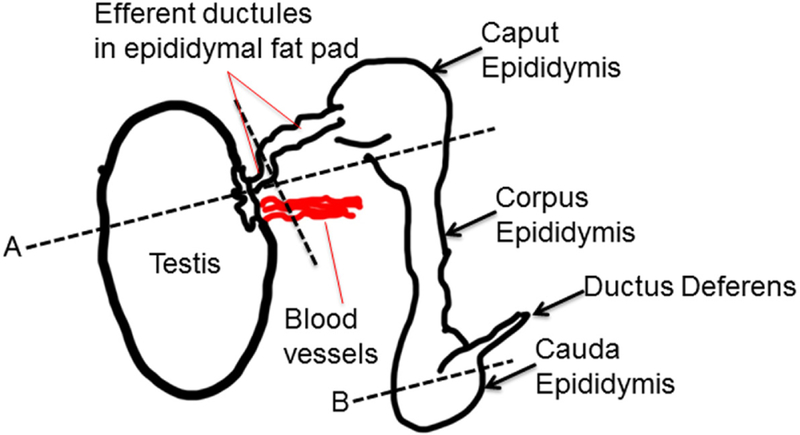
Figure 1.
Diagram of where the testes and epididymis were grossly sectioned. Section (A) included the testes, efferent ductules and caput epididymis. Section (B) permitted examination of the cauda epididymis.
Statistical analyses
Histomorphological changes in the epididymides antibiotic-treated vs saline control males were analyzed with the chi-square function in Graphpad Prism 7 Program (GraphPad Software, Inc.).
Results
Seminal fluid metabolome
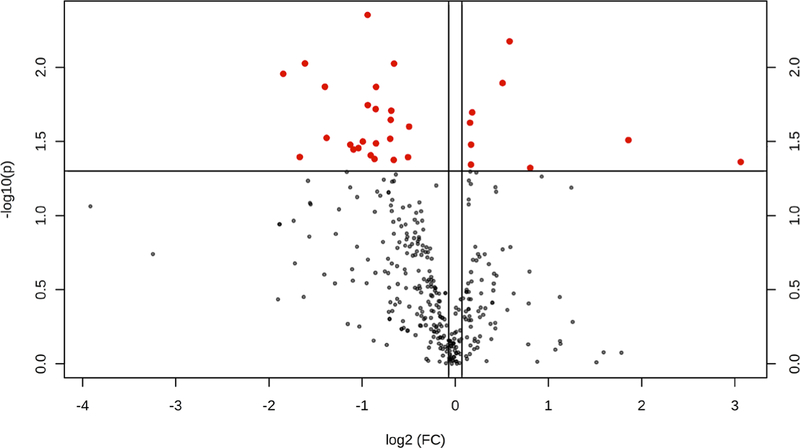
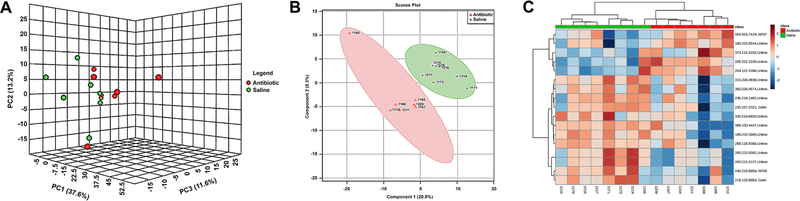
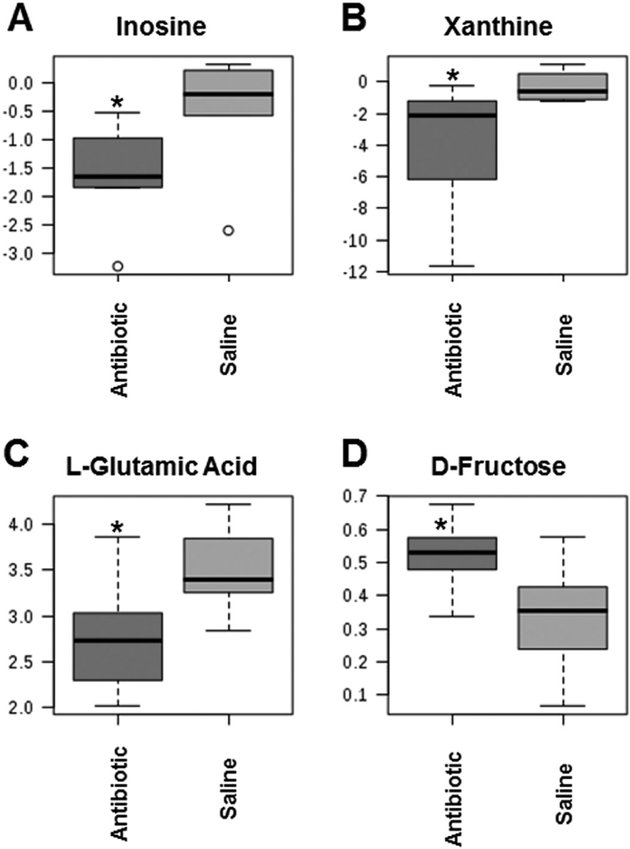
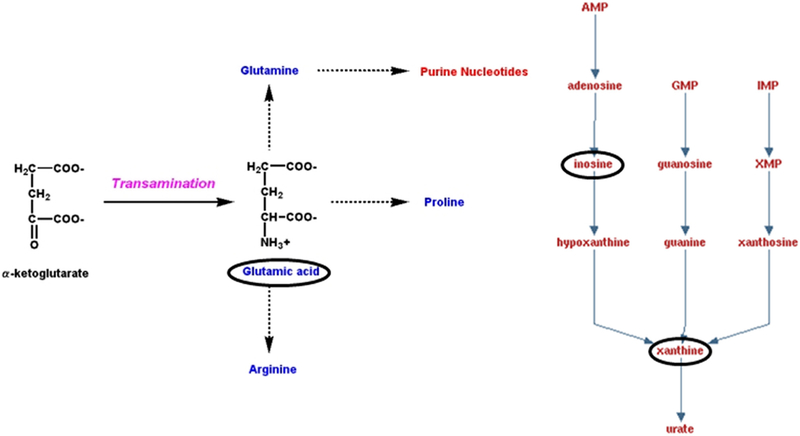
The metabolomics data preprocessing were performed using the R programming language (https://www.r-project.org/, Date Accessed March 21, 2018). Initial preprocessing revealed that several samples had abnormal variables as determined by three times its first and third quartiles (first quartiles − 3*IQR, third quartiles + 3*IQR, IQR: interquartile range). The samples were then analyzed using principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA). Three samples from the antibiotic group and two samples from the saline control group were clearly segregated from the rest of the samples on PCA (PC1: 85.3%, PC2: 3.6%) and orthogonal partial least squares discriminant analysis (oPLS-DA; T score: 68.7%, orthogonal T score: 7.5%, P < 0.001 from 1000 permutation tests in oPLS-DA). These samples were considered as outliers and excluded from the data analyses. Data in a .Raw file format, which shows the instrument response of ions including the internal standard, ribitol, are listed in Supplementary data 1 (see section on supplementary data given at the end of this article). Figure 2 illustrates the volcano plot after exclusion of the above described five outlier samples. Metabolites showing statistical differences (P < 0.05) are provided in Supplementary data 3 (Volcano Plot Results). Visualization of the metabolomics data with a 3D PCA score plot reveals that the CUB antibiotic-treated mice samples show distinct separation, in particular, on the PC2 axis (Fig. 3A). PLS-DA was also used to visualize the data (Fig. 3B). Similarly, heat map analyses based on the top differentially expressed metabolites reveal distinct separation between CUB antibiotic- and saline-treated males (Fig. 3C). The primary metabolites that were significantly different in the seminal fluid in CUB antibiotic-treated males versus saline control males are shown in Fig. 4. Inosine, xanthine and L-glutamic acid were decreased in CUB-treated males, whereas, D-fructose was elevated in this group. There were also several unidentified metabolites that differed between the two groups. The raw metabolomics data are included in Supplementary data 1. Pathway analyses of the metabolomics data indicate that the metabolites that differ between the two groups are primarily involved in purine degradation and potentially urate metabolism (Fig. 5).
Figure 2.
Volcano plot after exclusion of the five outlier samples that includes the remaining seven replicates in the CUB antibiotic group and eight replicates in the saline vehicle control group. The red circles indicate that the metabolites are significantly different between two groups (P < 0.05) while the black ones are not statistically different.
Figure 3.
Metabolome data from CUB antibiotic-treated (red) and saline control (green) males. (A) 3D PCA score plot for metabolome data from CUB antibiotic treated (red) and saline control (green) males that show overall metabolomics profile of the two groups separated especially on the PC2 axis. (B) Partial least squares discriminant analysis (PLS-DA) plot of the samples with outliers removed. The red triangle represents samples treated with antibiotics while the green cross represents saline controls. (C) Heat map based on the top differentially expressed metabolites between CUB antibiotic and saline-treated males.
Figure 4.
Select metabolites (A-D) that were differentially expressed in the seminal fluid in CUB antibiotic-treated males versus saline control males. *P ≤ 0.05.
Figure 5.
Purine degradation and urate metabolism pathway. The circled metabolites were decreased in the seminal fluid of males treated with the CUB antibiotic protocol regimen. Purines require glutamic acid for their original synthesis.
Testes and epididymides histopathology
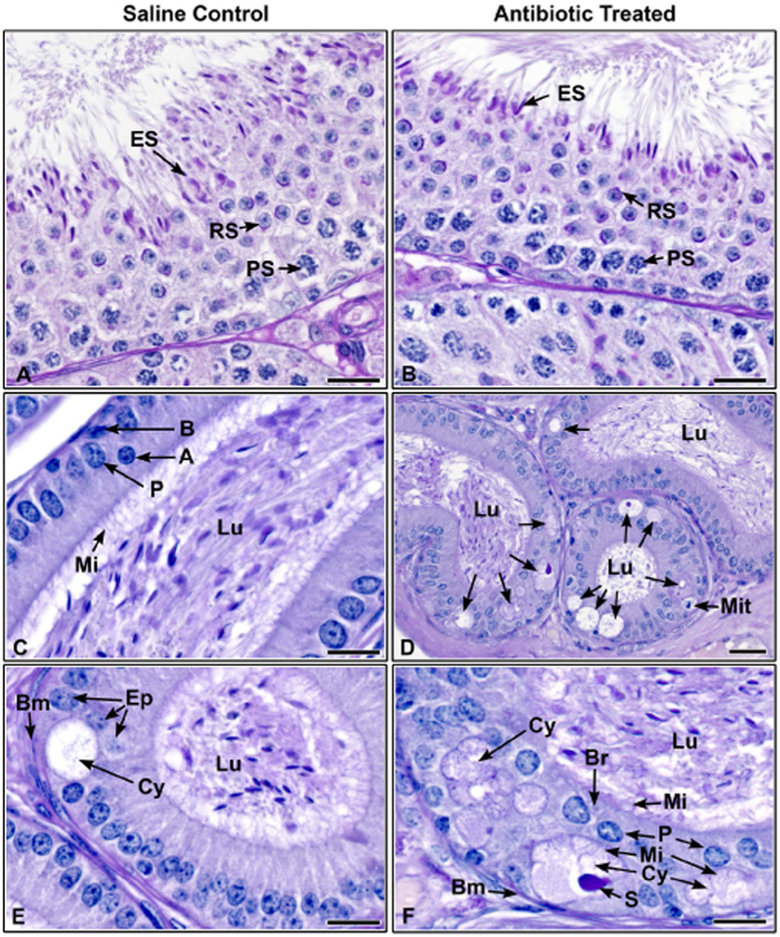
The 2-week CUB antibiotic protocol did not affect spermatogenesis, as the seminiferous epithelium was normal in both treated and control testes (Fig. 6A and B). Sperm structure and acrosome formation were normal, as indicated by PAS and a normal number of spermatozoa were observed in the epididymal lumen (Fig. 6A and B). However, in antibiotic-treated males the epididymides had an increased incidence of cribriform, cyst-like growths of the epithelium (Fig. 6C and D), which were always located in the caput/corpus and/or the corpus/cauda epididymides junctions. In the antibiotic-treated group, 70% (7/10) of the males had cribriform changes in the epithelium, while in the control group, only 30% (3/10) of the males had these changes and they appeared to be less severe (P = 0.01). To delineate further this pathological change between the groups, the sections were reanalyzed to determine the number of males in each group that showed well-defined and mature cribriform cysts with 50% (5/10) of the antibiotic-treated males (Fig. 6D and F), but only one control male (10%) possessed this change (Fig. 6E, P = 0.02). Mitotic figures were also observed in the epididymal epithelium of three antibiotic-treated males (Fig. 6D), which is very rare in adult mice, and none were observed in epididymides of saline control males (P = 0.03). Table 1 provides a summary of the quantitative histopathological changes observed in the epididymides.
Figure 6.
Histological section of testes and epididymides from saline control and CUB antibiotic-treated males. (A) Testis from saline control male showing normal spermatogenesis, Stage VII. Elongated spermatids (ES) line the epithelium with tails extending into the lumen. Round spermatids (RS) with normal acrosomal formations, as seen with periodic acid-Schiff’s staining (PAS) are found in the central region of the epithelium (PS, pachytene spermatocytes). (B) Testis from CUB antibiotic-treated male, late Stage VII. Normal elongated spermatids (ES) line the epithelium as in the control. Round spermatids (RS) with normal acrosomal formations are present, as well as pachytene spermatocytes (PS). (C) Proximal corpus epididymis from a control male. Note the concentration of sperm in the lumen (Lu) and a columnar epithelium consisting of principal (P), apical (A) and basal (B) cells. Long microvilli (Mi) extend into the lumen. (D) Proximal corpus epididymis from a CUB antibiotic-treated male showing cribriform cystic changes (arrows) in the epithelium. These abnormal formations appear as large vacuoles in the basal region that develops into a cystic luminal space, surrounded by shortened epithelial cells. Sperm are found in the epididymal lumen (Lu). A mitotic figure (Mit) is seen in the basal region. (E) Proximal corpus epididymis from a control male showing a rare cribriform cyst in the epithelium. This cyst (Cy) appears less developed than in the treated samples, because there is a sparse amount of secretory material and microvilli are lacking within the cystic lumen (Bm, basement membrane; Lu, lumen of the epididymis). (F) CUB antibiotic-treated proximal corpus epididymis. This his higher magnification of an area from D, showing more well-developed cribriform cysts (Cy), with some showing epithelial cells underneath on the basement membrane (Bm), as well as a well-developed ‘bridge’ of epithelial cells (Br). These cysts have well-defined secretory material (S) in the cystic lumen and the lining epithelial cells show microvilli (Mi) extending into both the cyst as well as the lumen (Lu) of the epididymis. Bars = 20 μm.
Table 1.
Histomorphometric changes in the epididymis of antibiotic-treated and saline control male mice.
| Group | # of males having cribriform changes in the epididymal epithelium | # of males with mature cribriform cysts in the epididymal epithelium | # of males with mitotic figures in the epididymal epithelium |
|---|---|---|---|
| Antibiotic treated | 7/10* | 5/10** | 3/10*** |
| Control | 3/10 | 1/10 | 0/10 |
P value comparison of antibiotic-treated to control males = 0.01;
P value comparison of antibiotic-treated to control males = 0.02;
P value comparison of antibiotic-treated to control males = 0.03.
Discussion
The primary goal of this study was to determine whether short-term antibiotic treatment for 2 weeks might disrupt the normal balance of resident microflora within the seminal vesicles leading to metabolome changes in this organ. An ancillary goal was to determine whether this combined antibiotic regimen would affect spermatogenesis and result in histopathological changes in the testes and epididymides. Several characterized and uncharacterized metabolites differed in CUB antibiotic-treated males compared to controls. Of the characterized ones, inosine, xanthine and L-glutamic acid were reduced in CUB-treated males, but D-fructose was increased in this group.
Pathway analysis demonstrates that the three known metabolites decreased in CUB antibiotic-treated males are involved in purine degradation and urate pathway metabolism. Propionibacterium acnes has been previously identified to be a resident bacterium within the seminal fluid (Javurek et al. 2016, 2017). This bacterium can metabolize purines. Both P. acnes and an increase in urate production, i.e. gout, are associated with chronic prostatitis and prostate cancer (Shannon et al. 2006, Fassi Fehri et al. 2011, Perry & Lambert 2011, Kuo et al. 2012, Olsson et al. 2012, Shinohara et al. 2013, Bae et al. 2014, Chen et al. 2014). Chronic treatment with allopurinol reduces the risk of prostate cancer in gout-afflicted patients (Shih et al. 2017). The current findings suggest that at least some of the urate production might originate within the seminal vesicles. Moreover, urate might influence antioxidant capacity that could affect oxidative stress in the epididymides or semen (Potts et al. 1999, Rhemrev et al. 2000, Guz et al. 2013). However, the current metabolomics analyses did not reveal any direct changes in urate levels, as this metabolite was below the level of detection and/or other signature reactive oxygen species molecules such as H2O2. Future studies should measure mitochondrial function and potential oxidative stress in the epididymides and sperm with the various techniques currently available to examine for such perturbations (Brand & Nicholls 2011).
The increase in D-fructose in the CUB antibiotic-treated males suggests that there might be fewer commensal bacteria present in this organ to utilize this energy source resulting in the accumulation of this nutrient within the seminal fluid. To date, there has been one other study examining how paternal state, in this case, diet-induced obesity (DiO) affects the metabolome profiles in seminal fluid (Binder et al. 2015). DiO males had elevations in fructose and taurine within the seminal vesicle fluid. However, myo-inositol, glycerol phosphate, glycine, isoleucine, glutamic acid, unmethoxymated hexose and threonine were decreased in this group. Thus, this paternal environmental change resulted in opposing effects on fructose within the seminal fluid relative to antibiotic treatment, which is likely because DiO males were provisioned with greater nutrient substrates. It is interesting to note that both DiO and CUB antibiotic-treated males had lower glutamic acid within the seminal fluid, but the significance of this finding is uncertain.
In contrast to previous studies that tested similar antibiotics and duration of treatment (Moe et al. 1989, Crotty et al. 1995, Demir et al. 2007, Aral et al. 2008, Farombi et al. 2008, El-Harouny et al. 2010, Antohi et al. 2011, Alp et al. 2012, Elzeinova et al. 2013, Ojo et al. 2013, Silla et al. 2015), no effects on spermatogenesis were evident in the current studies. Careful examination of prior reports of antibiotic-induced testicular effects (Aral et al. 2008, Farombi et al. 2008, Elzeinova et al. 2013, Ojo et al. 2013), however, revealed critical concerns in the histopathological interpretation that might be attributed to usage of methods that are sub-optimal for testicular toxicological studies. Such methods can lead to improper fixation resulting in significant artifact, namely sloughing of the seminiferous epithelium (Creasy 2003). In two of the aforementioned studies, epididymal effects were noted, but histopathological images were not provided (Farombi et al. 2008, Elzeinova et al. 2013); however, in both studies, there were additional effects on sperm motility. Although we did not determine sperm motility in the current study, histopathological changes were observed in the epididymides of antibiotic-treated males, which were more severe in antibiotic-treated males.
In the epididymides of CUB antibiotic-treated males, cribriform cysts were observed growing primarily in the caput-corpus and corpus-cauda epididymal junctions. Significantly more of these epithelial changes appeared in treated males than in the controls, and the cysts were dramatically more severe in the antibiotic-treated samples. Epididymal cribriform cysts are associated with glandular-like growths that form a lumen within the epithelium of the tubular system. Although there is no unified description for this type of cyst, some common features are reported. These include the formation of epithelial bridges over the cystic lumen within the epithelium, which forms a pitted pattern, secretions into the lumen and growth of microvilli into the cystic lumen from the surrounding epithelial cells (Nistal et al. 1990, Butterworth & Bisset 1992). This anomaly has been identified in various taxa, including humans, rodents, chimpanzees and dogs (James & Heywood 1979, Butterworth & Bisset 1992, Abbott 1993, Smithwick & Young 1997, Shah et al. 1998, Jones et al. 2000, La Perle et al. 2002). Although some investigators propose these cysts to be a variation of normal epithelium, others speculate they may represent pre-adenomatous growth.
It is uncertain though if this pathological change alters male reproductive function. However, an increased presence of cribriform cysts has been associated with several diseases and pathological conditions in men (Vazquez et al. 1986, Nistal et al. 1990, Glasker et al. 2006), in knockout and transgenic mice (Zhao et al. 1998, 2001, La Perle et al. 2002, Lupien et al. 2006, Krutskikh et al. 2011, Wild et al. 2012, Wang et al. 2017), as well as following chemical-induced male reproductive effects (Dunn & Green 1963, McIntyre et al. 2000, Sawamoto et al. 2003, Shin et al. 2010, Ramos-Ibeas et al. 2013, Aghaei et al. 2014, Miyaso et al. 2014, Lan et al. 2015). While we did not measure serum testosterone concentrations, no histological changes were evident in the Leydig cells. However, future studies should determine potential effects on testosterone and pituitary hormones. Postulated causes of inducing this formation include reduced testosterone concentrations (Itoh et al. 1999), sloughing of germ cells (Aruldhas et al. 2004), occlusion of the efferent ductules and epididymides (Aruldhas et al. 2004) and necrosis of epididymal epithelium (Itoh et al. 1999). Nevertheless, much remains to be elucidated in the underlying mechanisms leading to this unusual cystic formation (Kempinas & Klinefelter 2014).
In conclusion, short-term treatment of male mice with antibiotics designed to target the primary microorganisms within the seminal vesicle fluid induced metabolomics changes with inosine, xanthine and L-glutamic acid reduced but D-fructose elevated in the glandular secretions. While spermatogenesis was apparently normal in antibiotic-treated males, greater number of epididymal tubules showed cribriform growth and mitotic figures in this group, suggesting a potential effect that could influence sperm maturation in the epididymides. Current findings reveal short-term CUB antibiotic treatment of male mice results in seminal fluid metabolome and histopathological changes in the epididymides. It remains to be determined whether such disturbances affect male reproductive function and lead to DOHaD effects in resulting offspring.
Supplementary Material
Acknowledgements
Gratitude is expressed to the several undergraduate students who assisted with these studies.
Funding
The University of Missouri, Office of Research provided initial instrumental and personnel funding for the MU Metabolomics Center. L W S is supported in part by NSF awards 1340058 and 1139489. C S R is supported in part by NIEHS 1R01ES025547-A1, NIEHS 5R21ES023150 and NIEHS U01 ES020929. Studies were partially funded by the MU Bond Life Sciences Center. The MU Metabolomics Center is a recent initiative funded through the University of Missouri.
Footnotes
Supplementary data
This is linked to the online version of the paper at https://doi.org/10.1530/REP-18-0072.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Abbott DP 1993. Cribriform intra-tubular epididymal change and testicular atrophy. Histopathology 23 293 ( 10.1111/j.1365-2559.1993.tb01208.x) [DOI] [PubMed] [Google Scholar]
- Aghaei S, Nikzad H, Taghizadeh M, Tameh AA, Taherian A & Moravveji A 2014. Protective effect of pumpkin seed extract on sperm characteristics, biochemical parameters and epididymal histology in adult male rats treated with cyclophosphamide. Andrologia 46 927–935. ( 10.1111/and.12175) [DOI] [PubMed] [Google Scholar]
- Alp H, Cirit U, Tas M, Rifaioglu MM, Hatipoglu NK, Aytekin I, Yucel M, Firat U, Ozmen MF, Seker U & Eren LB 2012. Effects of sildenafil citrate, isoniazid, and streptomycin on testicular tissue and epididymal semen quality in rats. Urology 80 953.e9–953.e14. ( 10.1016/j.urology.2012.05.016) [DOI] [PubMed] [Google Scholar]
- Antohi E, Gales C & Nechifor M 2011. Pharmacological agents that affect sperm motility. Revista Medico-Chirurgicala a Societatii De Medici Si Naturalisti Din Iasi 115 1183–1188. [PubMed] [Google Scholar]
- Aral F, Karacal F & Baba F 2008. The effect of enrofloxacin on sperm quality in male mice. Research in Veterinary Science 84 95–99. ( 10.1016/j.rvsc.2007.04.007) [DOI] [PubMed] [Google Scholar]
- Aruldhas MM, Subramanian S, Sekhar P, Hasan GC, Govindarajulu P & Akbarsha MA 2004. Microcanalization in the epididymis to overcome ductal obstruction caused by chronic exposure to chromium – a study in the mature bonnet monkey (Macaca radiata Geoffroy). Reproduction 128 127–137. ( 10.1530/rep.1.00067) [DOI] [PubMed] [Google Scholar]
- Bae Y, Ito T, Iida T, Uchida K, Sekine M, Nakajima Y, Kumagai J, Yokoyama T, Kawachi H, Akashi T et al. 2014. Intracellular Propionibacterium acnes infection in glandular epithelium and stromal macrophages of the prostate with or without cancer. PLoS ONE 9 e90324 ( 10.1371/journal.pone.0090324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ 1990. The fetal and infant origins of adult disease. BMJ 301 1111 ( 10.1136/bmj.301.6761.1111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R, Gluckman PD, Grandjean P, Hanson M & Heindel JJ 2012. Developmental origins of non-communicable disease: implications for research and public health. Environmental Health 11 42 ( 10.1186/1476-069X-11-42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder NK, Hannan NJ & Gardner DK 2012a. Paternal diet-induced obesity retards early mouse embryo development, mitochondrial activity and pregnancy health. PLoS ONE 7 e52304 ( 10.1371/journal.pone.0052304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder NK, Mitchell M & Gardner DK 2012b. Parental diet-induced obesity leads to retarded early mouse embryo development and altered carbohydrate utilisation by the blastocyst. Reproduction, Fertility, and Development 24 804–812. ( 10.1071/RD11256) [DOI] [PubMed] [Google Scholar]
- Binder NK, Sheedy JR, Hannan NJ & Gardner DK 2015. Male obesity is associated with changed spermatozoa Cox4i1 mRNA level and altered seminal vesicle fluid composition in a mouse model. Molecular Human Reproduction 21 424–434. ( 10.1093/molehr/gav010) [DOI] [PubMed] [Google Scholar]
- Brand MD & Nicholls DG 2011. Assessing mitochondrial dysfunction in cells. Biochemical Journal 435 297–312. ( 10.1042/BJ20110162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevik A, Lindeman B, Brunborg G & Duale N 2012a. Paternal benzo[a] pyrene exposure modulates microRNA expression patterns in the developing mouse embryo. International Journal of Cell Biology 2012 407431 ( 10.1155/2012/407431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevik A, Lindeman B, Rusnakova V, Olsen AK, Brunborg G & Duale N 2012b. Paternal benzo[a]pyrene exposure affects gene expression in the early developing mouse embryo. Toxicological Sciences 129 157–165. ( 10.1093/toxsci/kfs187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckling CD, Huhman DV, Farag MA, Smith JT, May GD, Mendes P, Dixon RA & Sumner LW 2005. Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. Journal of Experimental Botany 56 323–336. ( 10.1093/jxb/eri058) [DOI] [PubMed] [Google Scholar]
- Bromfield JJ 2014. Seminal fluid and reproduction: much more than previously thought. Journal of Assisted Reproduction and Genetics 31 627–636. ( 10.1007/s10815-014-0243-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ & Robertson SA 2014. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. PNAS 111 2200–2205. ( 10.1073/pnas.1305609111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth DM & Bisset DL 1992. Cribriform intra-tubular epididymal change and adenomatous hyperplasia of the rete testis – a consequence of testicular atrophy? Histopathology 21 435–438. ( 10.1111/j.1365-2559.1992.tb00427.x) [DOI] [PubMed] [Google Scholar]
- Bygren LO, Kaati G & Edvinsson S 2001. Longevity determined by paternal ancestors’ nutrition during their slow growth period. Acta Biotheoretica 49 53–59. ( 10.1023/A:1010241825519) [DOI] [PubMed] [Google Scholar]
- Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD et al. 2010. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143 1084–1096. ( 10.1016/j.cell.2010.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Yen JH & Chang SJ 2014. Gout patients have an increased risk of developing most cancers, especially urological cancers. Scandinavian Journal of Rheumatology 43 385–390. ( 10.3109/03009742.2013.878387) [DOI] [PubMed] [Google Scholar]
- Chow BF & Lee CJ 1964. Effect of dietary restriction of pregnant rats on body weight gain of the offspring. Journal of Nutrition 82 10–18. ( 10.1093/jn/82.1.10) [DOI] [PubMed] [Google Scholar]
- Creasy DM 2003. Evaluation of testicular toxicology: a synopsis and discussion of the recommendations proposed by the Society of Toxicologic Pathology. Birth Defects Research: Part B Developmental and Reproductive Toxicology 68 408–415. ( 10.1002/bdrb.10041) [DOI] [PubMed] [Google Scholar]
- Crotty KL, May R, Kulvicki A, Kumar D & Neal DE Jr 1995. The effect of antimicrobial therapy on testicular aspirate flow cytometry. Journal of Urology 153 835–838. ( 10.1016/S0022-5347(01)67731-0) [DOI] [PubMed] [Google Scholar]
- Demir A, Turker P, Onol FF, Sirvanci S, Findik A & Tarcan T 2007. Effect of experimentally induced Escherichia coli epididymo-orchitis and ciprofloxacin treatment on rat spermatogenesis. International Journal of Urology 14 268–272. ( 10.1111/j.1442-2042.2007.01682.x) [DOI] [PubMed] [Google Scholar]
- Dorner G 1977. Hormones, brain differentiation and fundamental processes of life. Journal of Steroid Biochemistry 8 531–536. ( 10.1016/0022-4731(77)90258-8) [DOI] [PubMed] [Google Scholar]
- Dunn TB & Green AW 1963. Cysts of the epidididymis, cancer of the cervix, granular cell myoblastoma, and other lesions after estrogen injection in newborn mice. Journal of the National Cancer Institute 31 425–455. [PubMed] [Google Scholar]
- El-Harouny MA, Zalata AA, Naser ME, Abo El-Atta HM, El-Shawaf IM & Mostafa T 2010. Long-term ofloxacin testicular toxicity: an experimental study. Andrologia 42 92–96. ( 10.1111/j.1439-0272.2009.00961.x) [DOI] [PubMed] [Google Scholar]
- Elzeinova F, Peknicova J, Ded L, Kubatova A, Margaryan H, Dorosh A, Makovicky P & Rajmon R 2013. Adverse effect of tetracycline and doxycycline on testicular tissue and sperm parameters in CD1 outbred mice. Experimental and Toxicologic Pathology 65 911–917. ( 10.1016/j.etp.2013.01.004) [DOI] [PubMed] [Google Scholar]
- Farombi EO, Ugwuezunmba MC, Ezenwadu TT, Oyeyemi MO & Ekor M 2008. Tetracycline-induced reproductive toxicity in male rats: effects of vitamin C and N-acetylcysteine. Experimental and Toxicologic Pathology 60 77–85. ( 10.1016/j.etp.2008.02.002) [DOI] [PubMed] [Google Scholar]
- Fassi Fehri L, Mak TN, Laube B, Brinkmann V, Ogilvie LA, Mollenkopf H, Lein M, Schmidt T, Meyer TF & Bruggemann H 2011. Prevalence of Propionibacterium acnes in diseased prostates and its inflammatory and transforming activity on prostate epithelial cells. International Journal of Medical Microbiology 301 69–78. ( 10.1016/j.ijmm.2010.08.014) [DOI] [PubMed] [Google Scholar]
- Faure C, Dupont C, Chavatte-Palmer P, Gautier B & Levy R 2015. Are semen parameters related to birth weight? Fertility and Sterility 103 6–10. ( 10.1016/j.fertnstert.2014.11.027) [DOI] [PubMed] [Google Scholar]
- Fullston T, McPherson NO, Owens JA, Kang WX, Sandeman LY & Lane M 2015. Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an ‘obesogenic’ diet. Physiological Reports 3 e12336 ( 10.14814/phy2.12336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E & Mansuy IM 2014. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nature Neuroscience 17 667–669. ( 10.1038/nn.3695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW, Barker D, Bier D, Cagampang F, Challis J, Fall C, Godfrey K, Gluckman P, Hanson M, Kuh D et al. 2007. Meeting report on the 3rd international congress on Developmental Origins of Health and Disease (DOHaD). Pediatric Research 61 625–629. ( 10.1203/pdr.0b013e3180459fcd) [DOI] [PubMed] [Google Scholar]
- Glasker S, Tran MG, Shively SB, Ikejiri B, Lonser RR, Maxwell PH, Zhuang Z, Oldfield EH & Vortmeyer AO 2006. Epididymal cystadenomas and epithelial tumourlets: effects of VHL deficiency on the human epididymis. Journal of Pathology 210 32–41. ( 10.1002/path.2029) [DOI] [PubMed] [Google Scholar]
- Gram IT, Jacobsen BK, Straume B, Arnesen E, Løchen ML & Lund E 1995. Early origin of coronary heart disease. Earlier published work supports the ‘Barker hypothesis’. BMJ 310 1468–1468. ( 10.1136/bmj.310.6992.1468b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guz J, Gackowski D, Foksinski M, Rozalski R, Zarakowska E, Siomek A, Szpila A, Kotzbach M, Kotzbach R & Olinski R 2013. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS ONE 8 e68490 ( 10.1371/journal.pone.0068490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M 2015. The birth and future health of DOHaD. Journal of Developmental Origins of Health and Disease 6 434–437. ( 10.1017/s2040174415001129) [DOI] [PubMed] [Google Scholar]
- Hanson MA & Gluckman PD 2014. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiological Reviews 94 1027–1076. ( 10.1152/physrev.00029.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M, Godfrey KM, Lillycrop KA, Burdge GC & Gluckman PD 2011. Developmental plasticity and developmental origins of noncommunicable disease: theoretical considerations and epigenetic mechanisms. Progress in Biophysics and Molecular Biology 106 272–280. ( 10.1016/j.pbiomolbio.2010.12.008) [DOI] [PubMed] [Google Scholar]
- Hess RA & de Franca LR 2008. Spermatogenesis and cycle of the seminiferous epithelium. Advances in Experimental Medicine and Biology 636 1–15. ( 10.1007/978-0-387-09597-4_1) [DOI] [PubMed] [Google Scholar]
- Itoh M, Miyamoto K, Satriotomo I & Takeuchi Y 1999. Spermatic granulomata are experimentally induced in epididymides of mice receiving high-dose testosterone implants. I. A light-microscopical study. Journal of Andrology 20 551–558. ( 10.1002/j.1939-4640.1999.tb02555.x) [DOI] [PubMed] [Google Scholar]
- James RW & Heywood R 1979. Age-related variations in the testes and prostate of beagle dogs. Toxicology 12 273–279. ( 10.1016/0300-483X(79)90073-8) [DOI] [PubMed] [Google Scholar]
- Javurek AB, Spollen WG, Ali AM, Johnson SA, Lubahn DB, Bivens NJ, Bromert KH, Ellersieck MR, Givan SA & Rosenfeld CS 2016. Discovery of a novel seminal fluid microbiome and influence of estrogen receptor alpha genetic status. Scientific Reports 6 23027 ( 10.1038/srep23027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javurek AB, Spollen WG, Johnson SA, Bivens NJ, Bromert KH, Givan SA & Rosenfeld CS 2017. Consumption of a high-fat diet alters the seminal fluid and gut microbiomes in male mice. Reproduction, Fertility, and Development 29 1602–1612. ( 10.1071/RD16119) [DOI] [PubMed] [Google Scholar]
- Jones EC, Murray SK & Young RH 2000. Cysts and epithelial proliferations of the testicular collecting system (including rete testis). Seminars in Diagnostic Pathology 17 270–293. [PubMed] [Google Scholar]
- Kaati G, Bygren LO & Edvinsson S 2002. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. European Journal of Human Genetics 10 682–688. ( 10.1038/sj.ejhg.5200859) [DOI] [PubMed] [Google Scholar]
- Kempinas WG & Klinefelter GR 2014. Interpreting histopathology in the epididymis. Spermatogenesis 4 e979114 ( 10.4161/21565562.2014.979114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutskikh A, De Gendt K, Sharp V, Verhoeven G, Poutanen M & Huhtaniemi I 2011. Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia. Endocrinology 152 689–696. ( 10.1210/en.2010-0768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CF, Luo SF, See LC, Chou IJ, Fang YF & Yu KH 2012. Increased risk of cancer among gout patients: a nationwide population study. Joint Bone Spine 79 375–378. ( 10.1016/j.jbspin.2011.09.011) [DOI] [PubMed] [Google Scholar]
- La Perle KM, Blomme EA, Sagartz JE & Capen CC 2002. Epididymal cribriform hyperplasia with nuclear atypia in p53 homozygous knockout mice on a mixed 129/Sv-FVB/N background. Comparative Medicine 52 568–571. [PubMed] [Google Scholar]
- Lan Z, Hyung Kim T, Shun Bi K, Hui Chen X & Sik Kim H 2015. Triclosan exhibits a tendency to accumulate in the epididymis and shows sperm toxicity in male Sprague-Dawley rats. Environmental Toxicology 30 83–91. ( 10.1002/tox.21897) [DOI] [PubMed] [Google Scholar]
- Lei Z, Li H, Chang J, Zhao PX & Sumner LW 2012. MET-IDEA version 2.06; improved efficiency and additional functions for mass spectrometry-based metabolomics data processing. Metabolomics 8 105–110. ( 10.1007/s11306-012-0397-5) [DOI] [Google Scholar]
- Lupien M, Dievart A, Morales CR, Hermo L, Calvo E, Kay DG, Hu C & Jolicoeur P 2006. Expression of constitutively active Notch1 in male genital tracts results in ectopic growth and blockage of efferent ducts, epididymal hyperplasia and sterility. Developmental Biology 300 497–511. ( 10.1016/j.ydbio.2006.09.010) [DOI] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Wallace DG, Maness SC, Gaido KW & Foster PM 2000. Effects of in utero exposure to linuron on androgen-dependent reproductive development in the male Crl:CD(SD)BR rat. Toxicology and Applied Pharmacology 167 87–99. ( 10.1006/taap.2000.8998) [DOI] [PubMed] [Google Scholar]
- Miyaso H, Naito M, Hirai S, Matsuno Y, Komiyama M, Itoh M & Mori C 2014. Neonatal exposure to diethylstilbestrol causes granulomatous orchitis via epididymal inflammation. Anatomical Science International 89 215–223. ( 10.1007/s12565-013-0225-7) [DOI] [PubMed] [Google Scholar]
- Moe JB, Sotani K, Manabe J, Ikegami N, Tanase H, Lohrberg SM, Larsen ER & Piper RC 1989. Differential effects of cefmetazole sodium on the reproductive system of infant and pubertal male rats. Fundamental and Applied Toxicology 13 146–155. ( 10.1016/0272-0590(89)90314-X) [DOI] [PubMed] [Google Scholar]
- Nistal M, Iniguez L & Paniagua R 1990. Pitted pattern in the human epididymis. Journal of Reproduction and Fertility 89 655–661. ( 10.1530/jrf.0.0890655) [DOI] [PubMed] [Google Scholar]
- Ojo OO, Bhadauria S & Rath SK 2013. Dose-dependent adverse effects of salinomycin on male reproductive organs and fertility in mice. PLoS ONE 8 e69086 ( 10.1371/journal.pone.0069086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J, Drott JB, Laurantzon L, Laurantzon O, Bergh A & Elgh F 2012. Chronic prostatic infection and inflammation by Propionibacterium acnes in a rat prostate infection model. PLoS ONE 7 e51434 ( 10.1371/journal.pone.0051434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M & Golding J 2006. Sex-specific, male-line transgenerational responses in humans. European Journal of Human Genetics 14 159–166. ( 10.1038/sj.ejhg.5201538) [DOI] [PubMed] [Google Scholar]
- Perry A & Lambert P 2011. Propionibacterium acnes: infection beyond the skin. Expert Review of Anti-Infective Therapy 9 1149–1156. ( 10.1586/eri.11.137) [DOI] [PubMed] [Google Scholar]
- Potts RJ, Jefferies TM & Notarianni LJ 1999. Antioxidant capacity of the epididymis. Human Reproduction 14 2513–2516. ( 10.1093/humrep/14.10.2513) [DOI] [PubMed] [Google Scholar]
- Ramos-Ibeas P, Pericuesta E, Fernandez-Gonzalez R, Ramirez MA & Gutierrez-Adan A 2013. Most regions of mouse epididymis are able to phagocytose immature germ cells. Reproduction 146 481–489. ( 10.1530/REP-13-0145) [DOI] [PubMed] [Google Scholar]
- Rando OJ 2012. Daddy issues: paternal effects on phenotype. Cell 151 702–708. ( 10.1016/j.cell.2012.10.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhemrev JP, van Overveld FW, Haenen GR, Teerlink T, Bast A & Vermeiden JP 2000. Quantification of the nonenzymatic fast and slow TRAP in a postaddition assay in human seminal plasma and the antioxidant contributions of various seminal compounds. Journal of Andrology 21 913–920. ( 10.1002/j.1939-4640.2000.tb03422.x) [DOI] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S & Bale TL 2013. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. Journal of Neuroscience 33 9003–9012. ( 10.1523/JNEUROSCI.0914-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder LM & Chow BF 1972. Maternal undernutrition and its long-term effects on the offspring. American Journal of Clinical Nutrition 25 812–821. ( 10.1093/ajcn/25.8.812) [DOI] [PubMed] [Google Scholar]
- Sawamoto O, Yamate J, Kuwamura M, Kotani T & Kurisu K 2003. Development of sperm granulomas in the epididymides of L-cysteine-treated rats. Toxicologic Pathology 31 281–289. ( 10.1080/01926230390204315) [DOI] [PubMed] [Google Scholar]
- Shah VI, Ro JY, Amin MB, Mullick S, Nazeer T & Ayala AG 1998. Histologic variations in the epididymis: findings in 167 orchiectomy specimens. American Journal of Surgical Pathology 22 990–996. ( 10.1097/00000478-199808000-00009) [DOI] [PubMed] [Google Scholar]
- Shannon BA, Garrett KL & Cohen RJ 2006. Links between Propionibacterium acnes and prostate cancer. Future Oncology 2 225–232. ( 10.2217/14796694.2.2.225) [DOI] [PubMed] [Google Scholar]
- Sharma U & Rando OJ 2014. Father-son chats: inheriting stress through sperm RNA. Cell Metabolism 19 894–895. ( 10.1016/j.cmet.2014.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HJ, Kao MC, Tsai PS, Fan YC & Huang CJ 2017. Long-term allopurinol use decreases the risk of prostate cancer in patients with gout: a population-based study. Prostate Cancer and Prostatic Diseases 20 328–333. ( 10.1038/pcan.2017.14) [DOI] [PubMed] [Google Scholar]
- Shin IS, Park NH, Lee JC, Kim KH, Moon C, Kim SH, Shin DH, Park SC, Kim HY & Kim JC 2010. One-generation reproductive toxicity study of epichlorohydrin in Sprague-Dawley rats. Drug and Chemical Toxicology 33 291–301. ( 10.3109/01480541003734030) [DOI] [PubMed] [Google Scholar]
- Shinohara DB, Vaghasia AM, Yu SH, Mak TN, Bruggemann H, Nelson WG, De Marzo AM, Yegnasubramanian S & Sfanos KS 2013. A mouse model of chronic prostatic inflammation using a human prostate cancer-derived isolate of Propionibacterium acnes. Prostate 73 1007–1015. ( 10.1002/pros.22648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silla AJ, Keogh LM & Byrne PG 2015. Antibiotics and oxygen availability affect the short-term storage of spermatozoa from the critically endangered booroolong frog, Litoria booroolongensis. Reproduction, Fertility, and Development 27 1147–1153. ( 10.1071/RD14062) [DOI] [PubMed] [Google Scholar]
- Smithwick EB & Young LG 1997. Sequential histology of the adult chimpanzee epididymis. Tissue and Cell 29 383–412. ( 10.1016/S0040-8166(97)80026-2) [DOI] [PubMed] [Google Scholar]
- Vazquez MH, de Larminat MA, Gurpide E, Scorticati C & Blaquier JA 1986. Androgen metabolism in the human epididymis. Effect of in vivo estrogen administration. Journal of Steroid Biochemistry 25 239–244. ( 10.1016/0022-4731(86)90422-X) [DOI] [PubMed] [Google Scholar]
- Wang YY, Lin YH, Wu YN, Chen YL, Lin YC, Cheng CY & Chiang HS 2017. Loss of SLC9A3 decrease CFTR protein and causes obstructed azoospermia in mice. PLoS Genetics 13 e1006715 ( 10.1371/journal.pgen.1006715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ & Sinclair KD 2014. Paternal low protein diet affects adult offspring cardiovascular and metabolic function in mice. American Journal of Physiology: Heart and Circulatory Physiology 306 H1444–H1452. ( 10.1152/ajpheart.00981.2013) [DOI] [PubMed] [Google Scholar]
- Wild PJ, Ikenberg K, Fuchs TJ, Rechsteiner M, Georgiev S, Fankhauser N, Noske A, Roessle M, Caduff R, Dellas A et al. 2012. p53 suppresses type II endometrial carcinomas in mice and governs endometrial tumour aggressiveness in humans. EMBO Molecular Medicine 4 808–824. ( 10.1002/emmm.201101063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Liaw L & Hogan BL 1998. Bone morphogenetic protein 8A plays a role in the maintenance of spermatogenesis and the integrity of the epididymis. Development 125 1103–1112. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Chen YX, Liu XM, Xu Z & Qi X 2001. Mutation in Bmp7 exacerbates the phenotype of Bmp8a mutants in spermatogenesis and epididymis. Developmental Biology 240 212–222. ( 10.1006/dbio.2001.0448) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.