Abstract
Since the 2014 Ebola virus (EBOV) outbreak in West Africa there has been considerable effort towards developing drugs to treat Ebola virus disease and yet to date there is no FDA approved treatment. This is important as at the time of writing there is an ongoing outbreak in the Democratic Republic of the Congo. We have evaluated a small number of natural products, some of which had shown antiviral activity against other pathogens. This is exemplified with eugenol, which is found in high concentrations in multiple essential oils, and has shown antiviral activity against feline calicivirus, tomato yellow leaf curl virus, Influenza A virus, Herpes Simplex virus type 1 and 2, and four airborne phages. Four compounds possessed EC50 values less than or equal to 11 μM. Of these, eugenol, had an EC50 of 1.3 μM against EBOV and is present in several plants including clove, cinnamon, basil and bay. Eugenol is much smaller and structurally unlike any compound that has been previously identified as an inhibitor of EBOV, therefore it may provide new mechanistic insights. This compound is readily accessible in bulk quantities, is inexpensive, and has a long history of human consumption, which endorses the idea for further assessment as an antiviral therapeutic. This work also suggests that a more exhaustive assessment of natural product libraries against EBOV and other viruses is warranted to improve our ability to identify compounds that are so distinct from FDA approved drugs.
Keywords: Antiviral, Drug discovery, Ebola, Eugenol, p-anisaldehyde
The history of natural products as therapeutics is long and varied going back a millennia (1). For example, 175 anticancer drugs were approved between the 1940’s and 2014 and 49% of these were natural products or derived from them (2). The anti-infective drug discovery area is still heavily dependent on natural products and their structures (2). Others have also suggested that approximately 50% of new chemical entities are based on natural products and derivatives. From 1981 to 2010, 64% of FDA approved drugs were natural products or analogs (3). For example, several natural product drugs have been important natural product drugs for decades (4, 5). Marine natural products alone have led to 8 drugs or cosmeceuticals (a product that is a drug, a cosmetic or both) approved by the FDA and EMA. These include FDA approved marine-derived drugs, namely cytarabine, depocyt, vidarabine and ziconotide and at least 10 candidates in clinical trials (6) as well as a large number of marine chemicals in the preclinical pipeline (7). Even scents represent a relatively untapped source of biologically active molecules (8) (e.g. menthol (9–16)).
Since the 2014 Ebola virus (EBOV) outbreak in West Africa responsible for over 11,000 deaths in ten countries (17), no vaccine or drug has been approved. With the most effort applied to EBOV, four other families of filovirus exist (18) therefore this calls for a broad-spectrum antiviral drug for such emerging viruses (19, 20). The 2018 EBOV outbreaks in the Democratic Republic of the Congo has to date led to over 500 deaths. It is likely, that sporadic outbreaks will continue to occur and treatments will be needed to prevent and stop the progression of the next pandemic even as vaccines continue to be tested during these outbreaks.
The search for small molecule drugs for the treatment of EBOV has involved multiple medium and high-throughput screens (HTS) which have identified hundreds of molecules potentially effective against EBOV in vitro. Some of the earliest drugs active against EBOV included clomiphene, toremifene, tamoxifen, and raloxifene and diethylstilbestrol (21). Many other research groups have performed HTS with varying success rates (22–32). The hits identified in these studies belong to several groups of drugs including GPCR antagonists (25), selective estrogen receptor modulators (21), antidepressants (33), L-type calcium channel inhibitors (34) and antimalarials (19, 20).
Our own previous effort in EBOV drug discovery used machine learning to identify three compounds; quinacrine, pyronaridine, and tilorone (35) with the latter showing 100% efficacy in a mouse model of EBOV infection at a dose of 30mg/kg/day (36). This work suggests that promising clinical stage compounds can be identified and progressed through preclinical disease models. A recent study has suggested there are at least 141 drugs with preliminary in vitro and / or in vivo data in animals, most of which are FDA approved drugs (37).
Relatively small molecules (MWT 100–200) and natural products have been tested as potential treatments for other viruses. For example nicotinamide (Vitamin B3) was evaluated for activity against HIV (38). Essential oils also have a rich history as potential antivirals and possess numerous pharmacological activities (8, 39).
Our recent research suggests that plant derived natural products and structurally similar small molecules are worthy of closer inspection as a source of novel leads for EBOV drug discovery and will provide more molecular diversity. Seven small natural compounds (MWT range 122.1–164.2) were selected for testing against EBOV (Supplemental Methods) based on their commercial availability and previous testing for biological activity against viruses or bacteria. Eugenol (EC50 = 1.3 μM) and p-anisaldehyde (EC50 = 2.8 μM) were the two most potent of the 7 compounds tested (Table 1 and Figure 1A). Benzyl acetate (EC50 = 10 μM) and phenethyl acetate (EC50 = 10 μM) were less active and L-menthol, nicotinamide and nicotinic acid were inactive (Table 1). The most active compounds did not show appreciable cytotoxicity in HeLa cells, with CC50’s >50 μM (Figure 1B). No EBOV active compounds that were tested in this study had associated Pan Assay INterference compoundS (PAINS) or predicted promiscuity issues issues. p-anisaldehyde does however contain the potentially reactive (i.e., electrophilic) aldehyde (Table S1).
Table 1.
Natural products and other small molecules tested against EBOV. (NA = no activity).
| Molecule | Name: Function | EC50 Activity (μM; ±SD; n=4) |
HeLa CC50 (μM; n=2) |
AlogP | MWT |
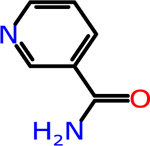 |
Nicotinamide: Active Form of Vitamin B3 | NA | NA | −0.32 | 122.13 |
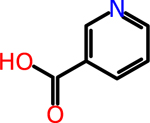 |
Nicotinic acid: Vitamin B3 | NA | NA | 0.31 | 123.11 |
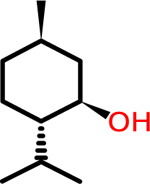 |
L- menthol: Scent | NA | NA | 2.78 | 156.27 |
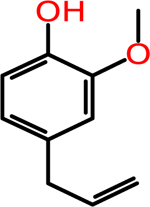 |
Eugenol: Used in Perfumeries, Flavorings, Essential Oils and in Medicine as a Local | 1.3 ± 0.5 | > 50 | 2.58 | 164.20 |
 |
P-anisaldehyde: Flavoring ingredient | 2.9 ± 0.6 | > 50 | 1.57 | 136.15 |
 |
Benzyl acetate: Perfumery and Flavorings | 10.7 ± 5.0 | > 50 | 1.60 | 150.17 |
 |
Phenethyl acetate: Perfumery and Flavorings | 10.4 ± 3.4 | > 50 | 1.93 | 164.20 |
Figure 1.
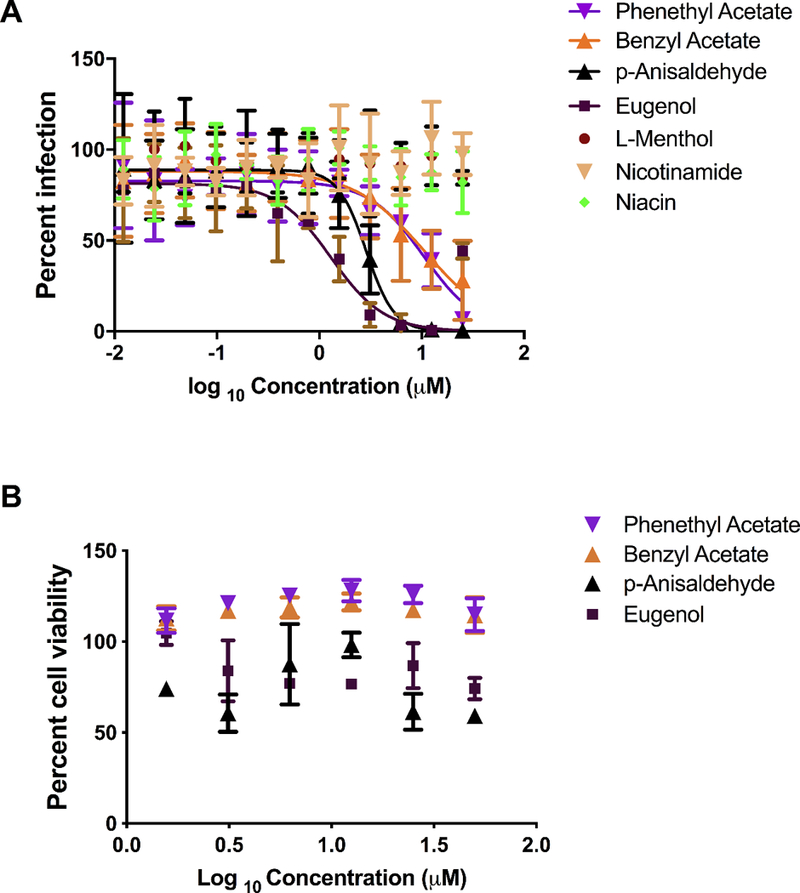
A. EBOV activity of various compounds using EBOV infected HeLa cells 24 hours post infection. Error bars represent the SD. B. Cytotoxicity of natural product compounds using HeLa cells. Error bars represent the SD.
It is common for antivirals against one disease to be effective against another that may be closely related. Nicotinamide is active against HIV and HBV (40), but was found to be inactive against EBOV in this study, suggesting a potentially different mechanism for these other viruses. Extracts from plants have been found to contain varied pharmacological activities over the long history of medicine (41) and some suggest that the flora of various countries are still untapped sources for potential drugs or starting points for drug discovery (42). Surprisingly several of the very common essential oils are still finding new activities, such as acting as antimicrobials and antivirals, with the latter being demonstrated in this study.
Eugenol is one such essential oil commonly found in cloves, cinnamon, basil and Bay with diverse biological activities. Eugenol has shown promising activity against feline calicivirus (43), tomato yellow leaf curl virus (44, 45), Influenza A virus (46), Herpes Simplex virus type 1 (47), Herpes Simplex virus 2 (48, 49), four airborne phages (50) as well as larvicidal activity against Aedes Aegypti (51). Dai et al (46) showed that Eugenol inhibits autophagy and influenza A virus replication by interfering with the ERK, p38MAPK and IKK/NF-κB signal pathways. Eugenol also displays broad antimicrobial, antifungal and anti-inflammatory activity (52). it has also been shown to be antiproliferative and have anti-metastatic effects (53). It is likely bioactivated via O-dealkylation of the O-alkoxy group resulting in catechol which is further oxidized to o-quinone (54). To our knowledge this is the first time eugenol has been tested against EBOV, likely because it is very small, more like a drug-fragment (55–57) and therefore very structurally different to the many Ebola active compounds tested to date (22–32). Because it is so small, eugenol provides plenty of scope for medicinal chemistry optimization. It is also present in several foods and has a long history of use by humans, therefore it may represent a faster path to regulatory approval if it possesses in vivo activity in an animal model infected with EBOV.
A second promising compound we identified is p-anisaldehyde which is also present in fragrances and yet the literature is silent on its potential antiviral potential, although it has been used to deter pests (58, 59). Benzyl acetate is another essential oil widely used in perfumes and cosmetics as well as pesticide applications (60). Phenethyl acetate is a volatile flavor present in many fruits and other foods as well as in fungal infected honeybee larvae, where it induces hygienic behavior (61). Menthol was the only scent or essential oil that we tested that had no appreciable activity against EBOV.
The current assessment is admittedly far from an exhaustive analysis of all natural products with inhibitory activity against EBOV. Interestingly when we used our previously successful Ebola machine learning models they scored these small molecules poorly to borderline active according to these Bayesian models (0.33–0.54, Table S2, Supplemental References) which may be representative of the fact that there are few compounds like these natural products in the models tested to date and further indicative of the need for model retraining with smaller compounds before future virtual screening of natural product libraries. Interestingly, menthol clearly scores the poorest of these compounds. Very few of these smaller sized molecules have previously been identified with activity against EBOV. For example, a recent screen of compounds inhibiting the VP35-nucleoprotein interface identified a small drug (MWT 273.2) tolcapone (IC50 = 2μM) which is a catechol-O-methyltransferase inhibitor (62). It remains to be seen if eugenol and p-anisaldehyde are targeting VP35 as well.
Further efforts to characterize these natural product in vitro EBOV actives could include analyzing their effects on: virus and cellular protein levels, EBOV genomic RNA and mRNA, primary human cells like macrophages or dendritic cells. Time-course experiments to show at which stage of infection the two compounds are active as antivirals and assessment of their activity against other human pathogenic RNA viruses would also be of interest to discern their EBOV mechanism.
Serendipity has played an important role in drug discovery over its history (41, 63–70) but we cannot rely on this solely to discover drugs. We can utilize simple, abundant natural products, such as essential oils, as a starting point for new anti-EBOV drug discovery. While the discovery and development of new drugs and chemical probes from natural products is an active area (71), there are several well-known challenges, such as only small amounts of compound are available, analog development is difficult, complexity of synthesis, identification of potential targets/diseases, etc. (72) and avoiding re-isolating known compounds (73). Advantages of natural products include their high potency (74), predisposition to biological activity due to evolution and selection and history of therapeutic success. There has been much recent discussion of the importance of using natural products to increase the diversity of high throughput screens (71). Additionally, the need for further collaborations with chemists who focus on natural product isolation, characterization, and synthesis, cannot be underestimated in these efforts.
Supplementary Material
Acknowledgments
Funding: SE kindly acknowledges NIH funding: R21TR001718 from NCATS (PI – Sean Ekins).
Ms. Kimberley M. Zorn, Dr. Mary A. Lingerfelt and Dr. Alex M. Clark, are kindly acknowledged for their assistance.
Footnotes
Competing interests
T.L., K.M.Z., and S.E. work for Collaborations Pharmaceuticals, Inc.
Supplementary data
Supplementary data related to this article can be found at:
References
- 1.Spainhour CB. Naturla products In: Gad SC, editor. Drug Discovery Handbook. Hoboken: John Wiley and Sons; 2005. p. 11–72. [Google Scholar]
- 2.Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. [DOI] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo). 1975;28(10):721–726. [DOI] [PubMed] [Google Scholar]
- 5.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–260; second page, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li R Marinopyrroles: Unique Drug Discoveries Based on Marine Natural Products. Med Res Rev. 2016;36(1):169–189. [DOI] [PubMed] [Google Scholar]
- 7.Mayer AM, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, McIntosh JM, Newman DJ, Potts BC, Shuster DE. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci. 2010;31(6):255–265. [DOI] [PubMed] [Google Scholar]
- 8.Kaiser R Meaningful scents around the world. Zurich: Wiley, VCH; 2006. [Google Scholar]
- 9.Tokunaga T, Sugawara H, Tadano C, Muro M. Effect of stimulation of cold receptors with menthol on EMG activity of quadriceps muscle during low load contraction. Somatosens Mot Res. 2017;34(2):85–91. [DOI] [PubMed] [Google Scholar]
- 10.Suchodolski J, Feder-Kubis J, Krasowska A. Antifungal activity of ionic liquids based on (−)-menthol: a mechanism study. Microbiol Res. 2017;197:56–64. [DOI] [PubMed] [Google Scholar]
- 11.Wondergem R, Bartley JW. Menthol increases human glioblastoma intracellular Ca2+, BK channel activity and cell migration. J Biomed Sci. 2009;16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park EJ, Kim SH, Kim BJ, Kim SY, So I, Jeon JH. Menthol Enhances an Antiproliferative Activity of 1alpha,25-Dihydroxyvitamin D(3) in LNCaP Cells. J Clin Biochem Nutr. 2009;44(2):125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watt EE, Betts BA, Kotey FO, Humbert DJ, Griffith TN, Kelly EW, Veneskey KC, Gill N, Rowan KC, Jenkins A, Hall AC. Menthol shares general anesthetic activity and sites of action on the GABA(A) receptor with the intravenous agent, propofol. Eur J Pharmacol. 2008;590(1–3):120–126. [DOI] [PubMed] [Google Scholar]
- 14.Shahverdi AR, Fazeli MR, Rafii F, Kakavand M, Jamalifar H, Hamedi J . Inhibition of nitrofurantoin reduction by menthol leads to enhanced antimicrobial activity. J Chemother. 2003;15(5):449–453. [DOI] [PubMed] [Google Scholar]
- 15.Juergens UR, Stober M, Vetter H. The anti-inflammatory activity of L-menthol compared to mint oil in human monocytes in vitro: a novel perspective for its therapeutic use in inflammatory diseases. Eur J Med Res. 1998;3(12):539–545. [PubMed] [Google Scholar]
- 16.Zimmermann M, Preac-Mursic V. In vitro activity of taurolidine, chlorophenol-camphor-menthol and chlorhexidine against oral pathogenic microorganisms. Arzneimittelforschung. 1992;42(9):1157–1159. [PubMed] [Google Scholar]
- 17.CDC. 2014 ebola outbreak in West Africa - case counts 2016. Available from: http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html.
- 18.Hoenen T, Feldmann H. Reverse genetics systems as tools for the development of novel therapies against filoviruses. Expert Rev Anti Infect Ther. 2014;12(10):1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansen LM, Brannan JM, Delos SE, Shoemaker CJ, Stossel A, Lear C, Hoffstrom BG, DeWald LE, Schornberg KL, Scully C, Lehár J, Hensley LE, White JM, Olinger GG. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med. 2013;5(190):190ra179–190ra179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madrid PB, Chopra S, Manger ID, Gilfillan L, Keepers TR, Shurtleff AC, Green CE, Iyer LV, Dilks HH, Davey RA, Kolokoltsov AA, Carrion R Jr, Patterson JL, Bavari S, Panchal RG, Warren TK, Wells JB, Moos WH, Burke RL, Tanga MJ. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One. 2013;8(4):e60579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansen LM, Brannan JM, Delos SE, Shoemaker CJ, Stossel A, Lear C, Hoffstrom BG, Dewald LE, Schornberg KL, Scully C, Lehar J, Hensley LE, White JM, Olinger GG. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med. 2013;5(190):190ra179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansen LM, DeWald LE, Shoemaker CJ, Hoffstrom BG, Lear-Rooney CM, Stossel A, Nelson E, Delos SE, Simmons JA, Grenier JM, Pierce LT, Pajouhesh H, Lehar J, Hensley LE, Glass PJ, White JM, Olinger GG. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci Transl Med. 2015;7(290):290ra289. [DOI] [PubMed] [Google Scholar]
- 23.Kouznetsova J, Sun W, Martinez-Romero C, Tawa G, Shinn P, Chen CZ, Schimmer A, Sanderson P, McKew JC, Zheng W, Garcia-Sastre A. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg Microbes Infect. 2014;3(12):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards MR, Pietzsch C, Vausselin T, Shaw ML, Bukreyev A, Basler CF. High-Throughput Minigenome System for Identifying Small-Molecule Inhibitors of Ebola Virus Replication. ACS Infect Dis. 2015;1(8):380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng H, Lear-Rooney CM, Johansen L, Varhegyi E, Chen ZW, Olinger GG, Rong L. Inhibition of Ebola and Marburg Virus Entry by G Protein-Coupled Receptor Antagonists. J Virol. 2015;89(19):9932–9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anantpadma M, Kouznetsova J, Wang H, Huang R, Kolokoltsov A, Guha R, Lindstrom AR, Shtanko O, Simeonov A, Maloney DJ, Maury W, LaCount DJ, Jadhav A, Davey RA. Large-Scale Screening and Identification of Novel Ebola Virus and Marburg Virus Entry Inhibitors. Antimicrob Agents Chemother. 2016;60(8):4471–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Cui R, Li G, Gao Q, Yuan S, Altmeyer R, Zou G. Teicoplanin inhibits Ebola pseudovirus infection in cell culture. Antiviral Res. 2016;125:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luthra P, Liang J, Pietzsch CA, Khadka S, Edwards MR, Wei S, De S, Posner B, Bukreyev A, Ready JM, Basler CF. A high throughput screen identifies benzoquinoline compounds as inhibitors of Ebola virus replication. Antiviral Res. 2018;150:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litterman NK, Lipinski CA, Ekins S. Small molecules with antiviral activity against the Ebola Virus. F1000Res. 2015;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picazo E, Giordanetto F. Small molecule inhibitors of ebola virus infection. Drug Discov Today. 2014;20:277–286. [DOI] [PubMed] [Google Scholar]
- 31.Basu A, Mills DM, Mitchell D, Ndungo E, Williams JD, Herbert AS, Dye JM, Moir DT, Chandran K, Patterson JL, Rong L, Bowlin TL. Novel Small Molecule Entry Inhibitors of Ebola Virus. J Infect Dis. 2015;212 Suppl 2:S425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long J, Wright E, Molesti E, Temperton N, Barclay W. Antiviral therapies against Ebola and other emerging viral diseases using existing medicines that block virus entry. F1000Res. 2015;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, Dal Cin P, Dye JM, Whelan SP, Chandran K, Brummelkamp TR. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477(7364):340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhein BA, Maury WJ. Ebola virus entry into host cells: identifying therapeutic strategies. Curr Clin Microbiol Rep. 2015;2(3):115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekins S, Freundlich JS, Clark AM, Anantpadma M, Davey RA, P M. Machine learning models identify molecules active against the Ebola virus in vitro. F1000Res. 2016;4:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekins S, Lingerfelt MA, Comer JE, Freiberg AN, Mirsalis JC, O’Loughlin K, Harutyunyan A, McFarlane C, Green CE, Madrid PB. Efficacy of Tilorone Dihydrochloride against Ebola Virus Infection. Antimicrob Agents Chemother. 2017;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai JPF, Hsu CW. Drug repurposing for Ebola virus disease: principles of consideration and the Animal Rule. J Pharm Sci. 2018:doi: 10.1016/j.xphs.2018.1009.1010. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38.Murray MF. Nicotinamide: an oral antimicrobial agent with activity against both Mycobacterium tuberculosis and human immunodeficiency virus. Clin Infect Dis. 2003;36(4):453–460. [DOI] [PubMed] [Google Scholar]
- 39.Andrade-Ochoa S, Nevarez-Moorillon GV, Sanchez-Torres LE, Villanueva-Garcia M, Sanchez-Ramirez BE, Rodriguez-Valdez LM, Rivera-Chavira BE. Quantitative structure-activity relationship of molecules constituent of different essential oils with antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complement Altern Med. 2015;15:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li WY, Ren JH, Tao NN, Ran LK, Chen X, Zhou HZ, Liu B, Li XS, Huang AL, Chen J. The SIRT1 inhibitor, nicotinamide, inhibits hepatitis B virus replication in vitro and in vivo. Arch Virol. 2016;161(3):621–630. [DOI] [PubMed] [Google Scholar]
- 41.Sneader W Drug Discovery A history. Cheppenham: Wiley; 2005. [Google Scholar]
- 42.Ntie-Kang F, Zofou D, Babiaka SB, Meudom R, Scharfe M, Lifongo LL, Mbah JA, Mbaze LM, Sippl W, Efange SM. AfroDb: a select highly potent and diverse natural product library from African medicinal plants. PLoS One. 2013;8(10):e78085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aboubakr HA, Nauertz A, Luong NT, Agrawal S, El-Sohaimy SA, Youssef MM, Goyal SM. In Vitro Antiviral Activity of Clove and Ginger Aqueous Extracts against Feline Calicivirus, a Surrogate for Human Norovirus. J Food Prot. 2016;79(6):1001–1012. [DOI] [PubMed] [Google Scholar]
- 44.Sun WJ, Lv WJ, Li LN, Yin G, Hang X, Xue Y, Chen J, Shi Z. Eugenol confers resistance to Tomato yellow leaf curl virus (TYLCV) by regulating the expression of SlPer1 in tomato plants. N Biotechnol. 2016;33(3):345–354. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Fan Y. Eugenol enhances the resistance of tomato against tomato yellow leaf curl virus. J Sci Food Agric. 2014;94(4):677–682. [DOI] [PubMed] [Google Scholar]
- 46.Dai JP, Zhao XF, Zeng J, Wan QY, Yang JC, Li WZ, Chen XX, Wang GF, Li KS. Drug screening for autophagy inhibitors based on the dissociation of Beclin1-Bcl2 complex using BiFC technique and mechanism of eugenol on anti-influenza A virus activity. PLoS One. 2013;8(4):e61026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Astani A, Reichling J, Schnitzler P. Screening for antiviral activities of isolated compounds from essential oils. Evid Based Complement Alternat Med. 2011;2011:253643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benencia F, Courreges MC. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother Res. 2000;14(7):495–500. [DOI] [PubMed] [Google Scholar]
- 49.Bourne KZ, Bourne N, Reising SF, Stanberry LR. Plant products as topical microbicide candidates: assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antiviral Res. 1999;42(3):219–226. [DOI] [PubMed] [Google Scholar]
- 50.Turgeon N, Michel K, Ha TL, Robine E, Moineau S, Duchaine C. Resistance of Aerosolized Bacterial Viruses to Four Germicidal Products. PLoS One. 2016;11(12):e0168815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandey SK, Tandon S, Ahmad A, Singh AK, Tripathi AK. Structure-activity relationships of monoterpenes and acetyl derivatives against Aedes aegypti (Diptera: Culicidae) larvae. Pest Manag Sci. 2013;69(11):1235–1238. [DOI] [PubMed] [Google Scholar]
- 52.Marchese A, Barbieri R, Coppo E, Orhan IE, Daglia M, Nabavi SF, Izadi M, Abdollahi M, Nabavi SM, Ajami M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit Rev Microbiol. 2017;43(6):668–689. [DOI] [PubMed] [Google Scholar]
- 53.Abdullah ML, Hafez MM, Al-Hoshani A, Al-Shabanah O. Anti-metastatic and anti-proliferative activity of eugenol against triple negative and HER2 positive breast cancer cells. BMC Complement Altern Med. 2018;18(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolton JL, Dunlap TL, Dietz BM. Formation and biological targets of botanical o-quinones. Food Chem Toxicol. 2018;120:700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murray CW, Blundell TL. Structural biology in fragment-based drug design. Curr Opin Struct Biol. 2010;20(4):497–507. [DOI] [PubMed] [Google Scholar]
- 56.Erlanson DA, McDowell RS, O’Brien T. Fragment-based drug discovery. J Med Chem. 2004;47(14):3463–3482. [DOI] [PubMed] [Google Scholar]
- 57.Bembenek SD, Tounge BA, Reynolds CH. Ligand efficiency and fragment-based drug discovery. Drug Discov Today. 2009;14(5–6):278–283. [DOI] [PubMed] [Google Scholar]
- 58.Showler AT, Harlien JL. Effects of the Botanical Compound p-Anisaldehyde on Horn Fly (Diptera: Muscidae) Repellency, Mortality, and Reproduction. J Med Entomol. 2018;55(1):183–192. [DOI] [PubMed] [Google Scholar]
- 59.Showler AT, Harlien JL. Botanical Compound p-Anisaldehyde Repels Larval Lone Star Tick (Acari: Ixodidae), and Halts Reproduction by Gravid Adults. J Med Entomol. 2018;55(1):200–209. [DOI] [PubMed] [Google Scholar]
- 60.Zhang QH, Schneidmiller RG, Hoover DR. Essential oils and their compositions as spatial repellents for pestiferous social wasps. Pest Manag Sci. 2013;69(4):542–552. [DOI] [PubMed] [Google Scholar]
- 61.Swanson JA, Torto B, Kells SA, Mesce KA, Tumlinson JH, Spivak M. Odorants that induce hygienic behavior in honeybees: identification of volatile compounds in chalkbrood-infected honeybee larvae. J Chem Ecol. 2009;35(9):1108–1116. [DOI] [PubMed] [Google Scholar]
- 62.Liu G, Nash PJ, Johnson B, Pietzsch C, Ilagan MX, Bukreyev A, Basler CF, Bowlin TL, Moir DT, Leung DW, Amarasinghe GK. A Sensitive in Vitro High-Throughput Screen To Identify Pan-filoviral Replication Inhibitors Targeting the VP35-NP Interface. ACS Infect Dis. 2017;3(3):190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reeves WC. Partners: serendipity in arbovirus research. J Vector Ecol. 2001;26(1):1–6. [PubMed] [Google Scholar]
- 64.Smyth JF. Science and serendipity in cancer research. Eur J Cancer. 2001;37(1):8. [DOI] [PubMed] [Google Scholar]
- 65.Duffin J Poisoning the spindle: serendipity and discovery of the anti-tumor properties of the Vinca alkaloids. Pharm Hist. 2002;44(2):64–76. [PubMed] [Google Scholar]
- 66.Mao J, Yuan H, Wang Y, Wan B, Pieroni M, Huang Q, van Breemen RB, Kozikowski AP, Franzblau SG. From serendipity to rational antituberculosis drug discovery of mefloquine-isoxazole carboxylic acid esters. J Med Chem. 2009;52(22):6966–6978. [DOI] [PubMed] [Google Scholar]
- 67.Howland RH. Serendipity and psychopharmacology. J Psychosoc Nurs Ment Health Serv. 2010;48(10):9–12. [DOI] [PubMed] [Google Scholar]
- 68.Monneret C Platinum anticancer drugs. From serendipity to rational design. Ann Pharm Fr. 2011;69(6):286–295. [DOI] [PubMed] [Google Scholar]
- 69.Bolgar B, Arany A, Temesi G, Balogh B, Antal P, Matyus P. Drug repositioning for treatment of movement disorders: from serendipity to rational discovery strategies. Curr Top Med Chem. 2013;13(18):2337–2363. [DOI] [PubMed] [Google Scholar]
- 70.Ekins S, Diaz N, Chung J, Mathews P, McMurtray A. Enabling Anyone to Translate Clinically Relevant Ideas to Therapies. Pharm Res. 2017;34(1):1–6. [DOI] [PubMed] [Google Scholar]
- 71.Young DW. Considerations related to small-molecule screening collections In: Bittker JA, Ross NT, editors. High Throughput Screening Methods: Evolution and Refinement. Cambridge: The Royal Society of Chemistry; 2017. p. 16–36. [Google Scholar]
- 72.Rodrigues T Harnessing the potential of natural products in drug discovery from a cheminformatics vantage point. Org Biomol Chem. 2017;15(44):9275–9282. [DOI] [PubMed] [Google Scholar]
- 73.Mohamed A, Nguyen CH, Mamitsuka H. Current status and prospects of computational resources for natural product dereplication: a review. Brief Bioinform. 2016;17(2):309–321. [DOI] [PubMed] [Google Scholar]
- 74.Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14(2):111–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


