Abstract
Background
Cognitive dysfunction is highly prevalent in Parkinson’s disease (PD) and a large proportion of patients eventually develops PD-related dementia. Currently, no effective treatment is available. Cognitive training is effective in relieving cognitive dysfunctions in several –neurodegenerative– diseases, and earlier small-scale trials have shown positive results for PD. In this randomized controlled trial, we assess the efficacy of online home-based cognitive training, its long-term effects, as well as the underlying neural correlates in a large group of PD patients.
Methods
In this double-blind randomized controlled trial we will include 140 non-demented patients with idiopathic PD that experience significant subjective cognitive complaints. Participants will be randomized into a cognitive training group and an active control group. In both groups, participants will individually perform an online home-based intervention for eight weeks, three times a week during 45 min. The cognitive training consists of thirteen games that focus on executive functions, attention and processing speed with an adaptive difficulty. The active control comprises three games that keep participants cognitively engaged without a training component. Participants will be subjected to extensive neuropsychological assessments at baseline and after the intervention, and at six months, one year and two years of follow-up. A subset of participants (40 in each treatment condition) will undergo structural and functional magnetic resonance imaging. The primary outcome of this study is the performance on the Tower of London task. Secondary outcomes are objective and subjective cognitive functioning, conversion to PD-related mild cognitive impairment or dementia, functional and structural connectivity and network topological indices measured with magnetic resonance imaging. None of the outcome measures are part of the cognitive training program. Data will be analyzed using multivariate mixed-model analyses and odds ratios.
Discussion
This study is a large-scale cognitive training study in PD patients that evaluates the efficacy in relieving cognitive dysfunction, and the underlying mechanisms. The strengths of this study are the large sample size, the long follow-up period and the use of neuroimaging in a large subsample. The study is expected to have a low attrition and a high compliance rate given the home-based and easily-accessible intervention in both conditions.
Trial registration
ClinicalTrials.gov ID NCT02920632. Registered September 30, 2016.
Electronic supplementary material
The online version of this article (10.1186/s12883-019-1403-6) contains supplementary material, which is available to authorized users.
Keywords: Parkinson’s disease, Cognitive training, Cognitive rehabilitation, Cognitive impairment, Neuropsychological assessment, Neuroimaging, MRI, Network, RCT
Background
Background and rationale
Cognitive impairments are among the plethora of non-motor symptoms associated with Parkinson’s disease (PD) [1, 2]. Approximately 25% of PD patients suffer from significant cognitive impairments already at the time of diagnosis [3, 4], and up to 80% eventually develop PD dementia (PD-D) [5, 6]. Moreover, compared with people without PD, patients with PD have up to 5.9 times the risk to develop dementia [7]. Cognitive impairments have a negative impact on performing the activities of daily living [8, 9] and are an important modulator in the development of neuropsychiatric symptoms, including psychosis [10, 11]. Degeneration of dopaminergic and non-dopaminergic systems is one of the alleged causes of cognitive impairments [12, 13] and have therefore been targets for pharmacological treatments. Although these drugs have modest temporary effects on cognitive symptoms by improving the attentional capacity, they have no proven efficacy in preventing further cognitive decline in PD [14, 15]. Hence, non-pharmacological treatment options must be considered as an alternative treatment for alleviating cognitive dysfunction in PD.
Cognitive training in PD: the gap in knowledge
Cognitive training (CT) was developed after the first brain tumor resections and traumatic brain injury treatment during the World Wars [16], but is currently applied in numerous neurological and psychiatric diseases. Meta-analyses have confirmed its efficacy in relieving cognitive dysfunction in Alzheimer’s disease [17], mild cognitive impairment (MCI) [18], schizophrenia [19], and traumatic brain injury [20, 21]. Furthermore, a recent meta-analysis in PD yielded positive results of CT mainly in relieving ‘frontal’ cognitive dysfunction (i.e. executive dysfunction, and working memory and psychomotor speed impairment) [22]. This meta-analysis, however, included only seven randomized controlled trials (RCTs), with a maximum sample size of 73 PD patients [23]. Consequently, the authors called for larger trials in PD populations – a conclusion that had earlier been stated in a systematic review [24] – although the results cautiously implied cognitive training to be efficacious.
The potential of cognitive training to preserve and protect
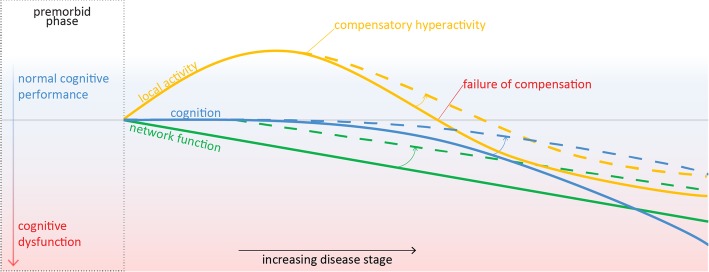
Two study protocols have recently been published, describing a cognitive training intervention in PD [25, 26]. Both interventions are specifically aimed at patients who have already developed PD-related MCI [26] or PD-D [25], respectively. However, neural changes have been demonstrated early on in cognitively preserved PD [27–30]: at this stage compensatory local hyperactivity seems to counteract the progressive buildup of PD pathology that threatens global brain network function [31, 32]. At a later disease stage, this compensatory mechanism gradually fails and ultimately leads to brain-wide network failure and cognitive dysfunction [33–35]. An early-stage intervention to boost the compensatory phase during this window of opportunity is imperative to try and preserve cognitive functions and protect patients from cognitive decline (for a working model, adapted from [36], see Fig. 1).
Fig. 1.
Working model of local compensatory brain activity (in yellow) that preserves intact cognitive functioning (in blue) but fails at later disease stage, while global brain network integrity gradually degenerates (in green). Dashed lines illustrate the hypothesized effects that CT may have on local and global brain infrastructure and on cognitive function. Adapted from [37]
Cognitive training may induce reorganization of structural and functional networks in the brain: it has been proposed that CT leaves a ‘footprint’ on the brain, that prepares the brain for better and faster processing [37]. Multiple studies have provided evidence that CT can induce reorganization of the brain network infrastructure. For example, patients with amnestic MCI showed post-CT normalization of within- and between-network connectivity [38, 39] that correlated with improved performance on memory tasks [39]. In addition, CT can alter resting-state networks in multiple sclerosis [40–42], normalize task-related activity in patients with schizophrenia [43, 44], and enhance functional connectivity [37, 45, 46] and cerebral blood flow [37] in healthy elderly. To date, only a few reports have focused on the underlying neural alterations after CT in PD [47–49] in small and mainly exploratory studies (N = 10–30). Results were mixed, showing increased functional connectivity [48], increased local activation [47, 48], but also decreased local activation [49] in comparison with controls.
In this study we aim to assess the efficacy of CT in a large sample of PD patients using a longitudinal design. Moreover, we aim to establish working mechanisms of CT by visualizing the within- and between-network changes that occur during training and to use the pre-treatment network topology, combined with the demographic and clinical characteristics, to predict who will profit most from CT.
Methods and design
Study objectives
In this study protocol we present COGTIPS – the “COGnitive Training In Parkinson Study”. The main research questions of this project are 1) What is the short-term and long-term effect of CT on objective and subjective cognitive functioning in PD? and 2) What are the neural mechanisms underlying the effect of CT in PD?
The study objectives of the COGTIPS study involve assessing an easily-accessible, home-based cognitive function training in individuals with mild subjective cognitive complaints in PD. Our primary objective is to assess the efficacy of an online CT program (compared to an active control condition) on executive functions. Our secondary objectives are to evaluate CT compared with an active control condition (AC) on 1) the efficacy on relieving subjective cognitive complaints; 2) the durability of the effect after 6 months, 1 year and 2 years; 3) the rate of conversion to PD-MCI and PD-D after 1 year and 2 years; 4) the effect on brain network efficiency and connectivity. Furthermore, we aim to identify baseline brain network characteristics that predict treatment outcome.
Based on previous literature on CT in PD and other neurodegenerative diseases, we hypothesize that compared with an active control condition 1) CT alleviates cognitive –mainly executive– dysfunction in PD patients, 2) CT relieves subjective cognitive complaints in daily-life, 3) the CT effect endures for up to 2 years after finishing the intervention, and reduces the risk of conversion to PD-MCI and PD-D, and 4) CT improves brain network efficiency and connectivity.
Study design and setting
COGTIPS is a monocenter phase-III randomized controlled trial that will enroll one-hundred-and-forty (140) PD patients. To assess the superiority of the online CT compared with an AC, participants are randomly appointed to either of the conditions in a 1:1 fashion (70 versus 70). Eighty participants (i.e. 40 in each condition) will undergo pre- and post-training neuroimaging to assess CT-specific effects on functional and structural connectivity. This study was approved by the VU University Medical Center Medical Ethical Committee and this protocol is reported in accordance with SPIRIT guidelines (see SPIRIT checklist in Additional file 2) [50].
The COGTIPS study will be performed at the Amsterdam University Medical Centers (Amsterdam UMC), location VUmc, an academic hospital with expertise in movement disorders located in Amsterdam, the Netherlands. We will enroll Dutch-speaking PD patients that have shown their interest in participation through 1) the outpatient clinic for movement disorders of the Amsterdam UMC, or community or academic hospitals in the area, 2) the PD patient association (“Parkinson Vereniging”), 3) advertisements in media like the Parkinson Magazine and national newspapers, 4) advertisements on participant recruiting websites such as ‘ParkinsonNext’ and ‘Hersenonderzoek.nl’, and 4) a database of PD patients that have previously shown interest in online cognitive training.
Eligibility criteria
Participants will be included on the basis of the presence of subjective cognitive complaints. We will focus on mild-to-moderate disease stage PD patients with mild cognitive complaints, to ensure that these patients are still within the ‘window of opportunity’. An overview of the inclusion and exclusion criteria is depicted in Table 1.
Table 1.
Overview of inclusion and exclusion criteria
| Inclusion criterion | Measured with | Defined by |
| Significant subjective cognitive complaints | Parkinson’s Disease Cognitive Functional Rating Scale | Score > 3 |
| Mild to moderate disease stage | Hoehn & Yahr disease stage | Score < 4 |
| Access to computer or tablet with access to Internet. Capability to use keyboard and computer mouse | Phone interview | – |
| Signed informed consent | – | – |
| General exclusion criterion | Measured with | Defined by |
| Indication for dementia syndrome | Self-administered Gerocognitive Examination | Score < 14 |
| Montreal Cognitive Assessment | Score < 22 | |
| Current drug- or alcohol abuse | CAGE AID-interview | Score > 1 |
| Inability to undergo extensive neuropsychological assessments or eight weeks of home-based cognitive intervention | – | – |
| Moderate to severe depressive symptoms | Beck depression inventory | Score > 18 |
| Presence of one or more impulse control disorders | ICD criteria interview | Positive screening |
| Psychotic symptoms. Benign hallucinations with insight are not an exclusion criterion | Schedule for Assessment of Positive Symptoms – PD | Positive screening |
| Traumatic brain injury | Phone interview | Cerebral contusion with 1) loss of consciousness for > 15 min and 2) posttraumatic amnesia > 1 h |
| Exclusion criterion for participation in magnetic resonance imaging | Measured with | Defined by |
| A space occupying lesion | Assessment by radiologist | – |
| Significant vascular abnormalities | Assessment by radiologist | Fazekas > 1 |
| Severe claustrophobia | MRI safety screening questionnaire | Positive screening |
| Presence of metal in the body (e.g. pacemaker, neurostimulator) | ||
| Pregnancy | ||
| Difficulty with, or shortness of breath during 60 min of lying still |
Participant timeline
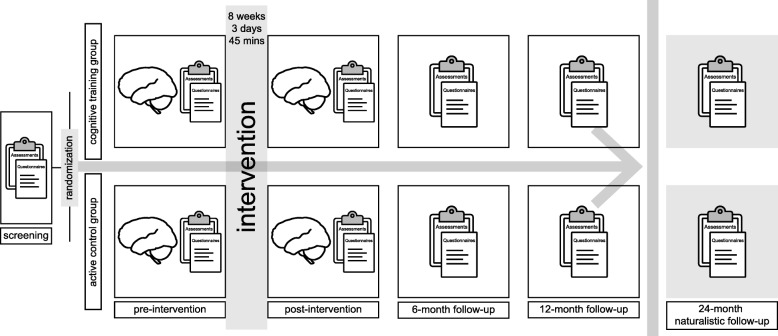
Figure 2 shows a global overview of the time schedule. A detailed description of the participant visits and assessments is shown in Table 2.
Fig. 2.
Global overview of the COGTIPS time schedule
Table 2.
Tabular overview of the study time schedule including assessments and visits
| Time-point | T-2 | T-1 | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|---|
| Pre-screening | |||||||
| Informed consent for pre-screening | X | ||||||
| SAGE | X | ||||||
| PD-CFRS | X | X | X | X | X | ||
| MRI safety screening | X | ||||||
| Alcohol abuse screening (CAGE-AID) | X | ||||||
| Eligibility screening | |||||||
| Montreal Cognitive Assessment | X | X | X | X | X | ||
| ICD diagnostic criteria | X | X | |||||
| SAPS-PD† | X | ||||||
| Beck depression inventory | X | X | X | X | X | ||
| Hoehn & Yahr stage | X | X | X | ||||
| Enrolment and allocation | X | ||||||
| Intervention | |||||||
| Cognitive training | ←→ | ||||||
| Active control condition | ←→ | ||||||
| Assessments | |||||||
| Neuropsychological assessment | |||||||
| 1 | Tower of London | X | X | X | X | X | |
| Montreal Cognitive Assessmenta | X | X | X | X | |||
| Pentagon copy | X | X | X | X | X | ||
| 1/2 | Stroop Color Word Test | X | X | X | X | X | |
| 1 | COWAT (‘letter fluency’) a | X | X | X | X | X | |
| 2 | WAIS-III digit span | X | X | X | X | X | |
| 3 | Rey Auditory Verbal Learning Testb | X | X | X | X | X | |
| 3 | Location Learning Testc | X | X | X | X | X | |
| 4 | Boston naming test | X | X | X | X | X | |
| 4 | Category fluency | X | X | X | X | X | |
| 5 | Rey Complex Figure Test | X | X | X | X | X | |
| 5 | Visual Form Discrimination Test | X | X | X | X | X | |
| Questionnaires and interviews | |||||||
| CFQ | X | X | X | X | X | ||
| Apathy scale | X | X | X | X | X | ||
| Parkinson anxiety scale | X | X | X | X | X | ||
| QUIP-RS | X | X | X | X | X | ||
| NZPAQ-SF | X | X | X | X | X | ||
| Credibility/expectancy questionnaire | X | ||||||
| Motor symptom assessments | |||||||
| UPDRS-III - motor score | X | X | X | ||||
| Medication use | |||||||
| Levodopa equivalent daily dosage | X | X | X | X | X | ||
| Neuroimaging* | |||||||
| MP-RAGE | X | X | |||||
| 3D PSIR | X | X | |||||
| fMRI - resting state | X | X | |||||
| DTI | X | X | |||||
Cognitive domains: 1Executive functioning, 2Attention and working memory, 3Memory, 4Language, 5Visuospatial. Abbreviations: CFQ Cognitive Failures Questionnaire, COWAT Controlled Oral Word Association Test, DTI diffusion tensor imaging, MP RAGE magnetization-prepared 180 degrees radio-frequency pulses and rapid gradient-echo; (f) MRI (functional) magnetic resonance imaging, NZPAQ-SF New Zealand Physical Activity Questionnaire – Short Form, PD-CFRS Parkinson’s Disease – Cognitive Functional Rating Scale, PSIR phase-sensitive inversion recovery, QPE Questionnaire for Psychotic Experiences, QUIP-RS Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease – Rating Scale, SAPS-PD Scale for Assessment of Positive Symptoms for Parkinson’s disease, UPDRS Unified Parkinson’s Disease Rating Scale, WAIS Wechsler Adult Intelligence Scale
An overview of cognitive assessments and questionnaires, including references is provided in additional file 1
*in a subsample of N = 80
Parallel forms of the same test are used at consecutive visits if available: aThree parallel forms; bTwo parallel forms; cOne parallel form
Pre-screening, screening and baseline assessment
PD patients that have shown interest in participating in COGTIPS will first undergo pre-screening for which they are required to sign informed consent and send this back by mail or E-mail. Pre-screening consists of a self-administered cognitive screening and questionnaires that are filled out at home (i.e. Self-administered Gerocognitive Examination [51]), and a phone interview. Patients are asked whether they are interested in participating in the subgroup that will undergo neuroimaging and if so, are screened for contraindications. After positive pre-screening, eligible patients are invited for an intake measurement.
At intake, patients will sign informed consent for participation in COGTIPS. They first undergo face-to-face screening of cognitive dysfunction by the Montreal Cognitive Assessment [52, 53], motor impairment by the Unified Parkinson’s Disease – Rating Scale part III [54], psychotic symptoms by the Schedule for Assessment of Positive Symptoms – PD [55], depressive symptoms by the Beck Depression Inventory [56]) and impulse control disorders (ICDs) by an ICD criteria interview. Eligible patients will undergo the baseline assessment (‘T0’) which comprises an extensive neuropsychological assessment, structured interviews and questionnaires. A sub-population will undergo magnetic resonance imaging. Neuroimaging data will be acquired at the Amsterdam UMC, location VUmc, on a Discovery* MR750 3.0 T MRI scanner (General Electric, Milwaukee) with a 32-channel head coil. We will obtain structural imaging (i.e. T1 and diffusion tensor imaging) and functional resting-state imaging. See Additional file 1 for the scan parameters. All assessments are performed by study members that are blinded for the treatment condition. The screening and baseline assessment will be performed during a single visit to the Amsterdam UMC, location VUmc.
Condition allocation and instructions
Following a positive screening for eligibility, a non-blinded study member will allocate the participant to either the CT or AC condition. Participants will be consecutively assigned to either the CT or AC condition on the basis of a randomization sequence. The randomization sequence is generated in Microsoft Excel by using computer-generated random numbers. We will use stratified randomization in which two strata will be generated according to education level. Vocational education level (or lower) defined as an education level of 5 or lower according to a Dutch classification system [57], which is comparable to 11 or less years of education [58]. High education level is defined as level 6 or 7 according to the Verhage classification system, which is comparable to 12 or more years of education.
A non-blinded study member will provide instructions to the participant concerning the log-in procedure for the training, the various training components, and the duration and frequency of training. After instructions, the participant will be asked to fill out a questionnaire concerning the patients’ expectations and credibility regarding the intervention [59]. Participants will additionally receive a hand-out with instructions to take home.
Eight-week intervention period
After the baseline assessment, participants may directly start with the 8-week intervention. A detailed description of the CT and AC interventions is provided below. Compliance will be monitored automatically and will be checked weekly. During the intervention, patients will receive biweekly questionnaires to ensure compliance and check for questions and problems performing the intervention. Non-blinded study members will follow-up on potential problems by phone.
Post-intervention assessments
After 24 intervention sessions, patients are invited for the post-intervention assessment. This assessment will be scheduled as close as possible to the last training session. Participants will first evaluate the intervention with a non-blinded study member. Directly afterwards, participants will undergo a post-intervention assessment (‘T1’). This assessment comprises a neuropsychological assessment and questionnaires (see Table 2). One team member (TB) will be de-blinded after the last T1 visit. All assessments after baseline will make use of parallel versions of neuropsychological tasks, if possible.
After 6 months (‘T2’), 1 year (‘T3’) and 2 years (‘T4’), participants will again undergo an extensive neuropsychological assessment and questionnaires. At T3 and T4, motor symptoms will also be assessed. From T3 onwards will be a naturalistic follow-up.
Blinding
Outcome assessors will be blinded for the full length of their role as assessor, while non-blinded team members will not assess participants at any point in this study. Blinded study members will not have access to the key of the randomization. Trial participants will be blinded for the full length of the study. Participants will be asked not to share any details of their intervention with the outcome assessor at any point in the study. When the participants’ condition is revealed to an outcome assessor, he or she will be replaced by another assessor for this participant.
Drop-outs
Participants that drop out of the study after being allocated to an intervention condition will not be replaced. We expect a low drop-out rate on the basis of our pilot study (one drop-out in 21 participants) and the low burden and short duration of both training conditions. In our sample size calculation, we conservatively account for 10% drop-out.
In case a participant withdraws from the study after 4 weeks of training (or more), we will aim to schedule an exit-measurement to measure the intervention effect.
Medication adjustments
Participants and their neurologist will be requested to retain a stable medication regime during the study period, specifically during the intervention. Patients and their neurologist will be requested to inform the study team if medication changes are clinically necessary.
Interventions
The intervention in this study aims to train cognitive abilities, with a focus on executive functions, working memory, attention, and processing speed. A modified version of the BrainGymmer online CT platform (https://www.braingymmer.com/en/, a product by Dezzel Media B.V.) is used to provide the training at the patients’ home. We selected this method of cognitive training as it has been evaluated positively in our earlier pilot study in PD patients (see below), it is accessible for patients at home, and previous versions have been used in prior studies [60, 61]. A proof-of-concept in 20 PD patients showed that the experimental condition was evaluated as feasible and enjoyable. Moreover, the CT compared with an active control showed a medium interaction effect size on an executive functioning composite (i.e. Stroop Color Word Test, Trail Making Test and Controlled Oral Word Association Test), with a significantly positive change of executive functioning in the CT group but not in the active controls. Specifically, a large positive interaction effect size of CT on the Stroop color word test was found compared with controls (see Additional file 1 for a visual representation).
Intervention characteristics
In both conditions, 24 training sessions are performed: three times a week for a length of 8 weeks. The training sessions last approximately 45 min, marginally dependent on the participants’ performance. Compliance and training performance data are automatically tracked when a participant performs a training session. Participants can independently schedule the three training sessions per week to ensure flexibility and a low training threshold. The training sessions can be paused at the participants’ discretion but they are advised to try and complete the entire training within 1 hour.
Cognitive training
In the experimental condition, 13 CT games are sequentially performed. The cognitive processes that the training games call upon are similar to processes that are tested during the neuropsychological assessments, but the games are substantially different from the neuropsychological tasks. The training games are equipped with a ‘dynamic difficulty adjustment’: the difficulty of training components is adaptive to the participants’ performance, and will increase or decrease depending on individual performance. This way, participants will be challenged to continuously perform at their maximal ability. Training games, their duration and the hypothesized cognitive loading are shown in Table 3.
Table 3.
Description of training games in the CT condition with their duration and the cognitive loading
| Description | Duration | Cognitive loading |
|---|---|---|
| Repeat a drum rhythm that increases in length | 3 mistakes | Working memory, attention |
| Flanker task | 80 s | Cognitive flexibility |
| Put a sequence in the correct prompted order | 180 s | Visuospatial function, focused attention |
| An ‘N-back’ task using bottles of various shapes and colors | 180 s | Working memory |
| Evaluate if a ‘totem pole’ comprising blocks of different forms and diameters matches a top view | 2 mistakes | Visuospatial function, mental rotation |
| Follow one or more moving targets (i.e. a bunny with a carrot) between several distractors | 4 mistakes | Focused and divided attention |
| Accept or decline stimuli based on switching rules with increasing speed | 90 s | Cognitive flexibility, processing speed |
| Remember an increasing number of colored squares | 120 s | Working memory, attention |
| Click an increasing number of stimuli (i.e. food on a barbeque) at the right time (i.e. when they are well-done) | 180 s | Divided attention, psychomotor and processing speed |
| Search birds with a certain color and form between an increasing number of distractors | 300 s | Visuospatial function, processing speed |
| Stack blocks of numbers that differ by one on top of another to reduce the number of blocks | 180 s | Planning |
| Remember the color and accessories of a penguin and at the same time the location of a fish | 180 s | Working memory, processing speed |
| Finish a puzzle within a limited time | 240 s | Visuospatial function, processing speed |
Active control group
An active control condition is used to correct for the nonspecific cognitive activity that participants in the CT group go through. In the control condition, participants undergo cognitive engagement using three games (i.e. solitaire, trivia questions and hangman) with a total duration of 45 min that will sequentially be performed and are hypothesized not to train specific cognitive functions.
Outcomes
Primary outcome
The primary outcome is the efficacy of CT on executive functions, measured by the percentage correct change score on a previously used computerized self-paced version of the Tower of London (ToL) task [29]. The ToL measures several aspects of executive functions, including planning, inhibition, and working memory [62]. This neuropsychological task consists of a model of three pins with different lengths, and three differently colored beads. In this task, the goal is to get from a starting position to a target position in as minimal steps as possible. There are five planning conditions that range in difficulty, with possible solutions ranging from one to five steps (i.e. task-load S1-S5). After nine exercise items with feedback, 100 pseudo-randomized test trials will be presented with a maximum response duration of 45 s per trial and no feedback on accuracy.
Secondary outcomes
The secondary outcome measures include (i) subjective cognitive complaints, (ii) cognitive function (other than the ToL) and (iii) structural and functional connectivity and brain network characteristics. All outcomes described below are changes after intervention relative to baseline.
-
i
Subjective cognitive dysfunction change after the intervention will be measured by the Parkinson’s Disease Cognitive Functional Rating Scale (PD-CFRS, [63]) score and the Cognitive Failures Questionnaire (CFQ) score at the end of the intervention (T1), and at follow-up (T2, T3, and T4). We use the PD-CFRS questionnaire as a Parkinson-specific and sensitive measurement of subjective cognitive function. This questionnaire will be filled out by the participant and if possible by a caregiver. We will additionally use the CFQ as this measure has been used more frequently and it is more sensitive to small cognitive errors in daily living such as memory problems, absent-mindedness and slips of action [64];
-
iiCognitive function change after the intervention will be measured by
- change on latent underlying cognitive factors in the neuropsychological assessment at T1 and at follow-up (T2, T3, and T4). Participants will undergo an extensive assessment battery of frequently-used and validated neuropsychological tests (see Table 2). See [65] for standard outcome measures of the neuropsychological tests. We will extract latent cognitive traits at baseline and measure training-induced changes on these factors at follow-up (see Analyses for a detailed description);
-
iii
Training-induced neural alterations will be measured with magnetic resonance imaging (MRI). Morphometric brain characteristics will be measured with standard measures (i.e. subcortical volume, cortical thickness, fractional anisotropy). We will measure functional connectivity by extracting independent components of simultaneously fluctuating blood-oxygen level dependent signals that represent resting-state brain networks. Brain network characteristics will be measured by standard topological measures (i.e. modularity, global and local efficiency, betweenness centrality, see [68, 69]).
Exploratory outcomes and covariates
For exploratory purposes, the following outcomes will be collected.
Training-induced cognitive changes on individual neuropsychological tasks (see Table 2) will be assessed to increase comparability with other CT studies, and to increase replicability of the results in future research;
Improvement on the individual CT games will be measured in order to compare potential component-specific transfer effects. Performance on the CT components are collected automatically by the BrainGymmer online training module;
Alterations on psychiatric symptoms of anxiety, depression, apathy, and impulse control disorders, using the Parkinson anxiety scale, Beck depression inventory, Apathy scale, and Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease – Rating Scale, respectively.
Additionally we will collect data on the following potential confounding factors:
Data on physical activity at each visit will be measured by the New Zealand Physical Activity Questionnaire – Short Form, a structured interview on mild, moderate and vigorous physical activity, as physical activity is known to positively influence cognitive function and potentially provide a neuroprotective effect. [70, 71];
We will rate motor symptom severity by the Unified Parkinson’s Disease – Rating Scale part III and assess disease stage by the modified Hoehn & Yahr stage [72];
Medication usage data are collected and transformed into a ‘levodopa equivalent daily dosage’ [33]. Dopamine replacement therapy may influence cognitive functions [73, 74];
Intervention compliance will automatically be monitored by the training module. We will calculate total compliance as the proportion of completed training games out of 24 total sessions: [Ncompleted / Ntotal] × 100%, in which Ntotal is 13 games × 24 sessions in the CT condition, and 3 games × 24 sessions in the AC condition. We define non-compliance as a completion rate lower than 75%, in accordance with Petrelli and colleagues [75].
Data-analyses
Data-analyses will be performed on the Modified-Intention-To-Treat population, which comprises the compliant participants that underwent at least 75% of the intervention and at least one post-training assessment. We will compare the baseline characteristics of this sample to the Intention-to-Treat population (all randomized subjects). Secondary Per Protocol-analyses will be performed comprising the population that underwent the complete study protocol. Analyses will be performed with IBM SPSS version 22 (Armonk, NY, USA) and in R [76]. We will employ a statistical threshold of α = .05.
The primary outcome will be analyzed using a multivariate mixed-model analysis using the accuracy on the five separate task-loadings (S1-S5) of the ToL at post-training visit (T1) as dependent measures, the training condition (CT vs. AC) as independent measure and baseline score of the outcome measures as covariates. We will construct a separate adjusted model with age, sex and years of education as additional covariates of no-interest. No imputation of missing values will be performed as this is not needed in linear mixed models.
The secondary outcome measures will also be analyzed with linear mixed-models with baseline score of the outcome measures as covariates. Subjective cognitive dysfunction will be modeled with the total score of the PD-CFRS (both self-report and caregiver) and the CFQ a) at post-training (T1) and b) at all follow-up assessments (T2, T3 and T4) as dependent variables. We will perform a factor analysis on all neuropsychological assessment outcomes (see Table 2) at baseline using a factor analysis with regularized maximum likelihood estimation to produce latent cognitive traits. We will compute baseline trait scores (i.e. factor scores), and compute trait scores at follow-up measurements based on the baseline factor analysis. The effect of CT on cognitive functions will be assessed with a multivariate mixed-model comparable to the above, using the trait scores as dependent variables. The effect of CT relative to AC on neuropsychiatric symptoms will be analyzed using similar multivariate mixed-models with as dependent variables the Beck Depression Inventory, the Parkinson Anxiety Scale, the Apathy Scale and the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease – Rating Scale. Covariates will be added to the regression model based on a change-in-estimate method if there is a change of ≥10% of the regression coefficient for the intervention variable.
In order to analyze between-group differences in conversion to PD-MCI or PD-D, we will first classify patients at baseline, T3 and T4 as having normal cognition, PD-MCI or PD-D. We define conversion ‘down’ as conversion to a milder cognitive dysfunction classification, no conversion as classification in the same category at a later assessment visit and conversion ‘up’ as conversion to a worse cognitive function classification. We will assess the association between the intervention and conversion rate with a Fisher’s exact test. Odds ratios and confidence intervals of the conversion ‘down’ and no conversion groups versus the conversion ‘up’ group will be computed as a measure of effect size.
We will perform Fisher’s exact tests to verify if the demographic and clinical characteristics of the MRI subsample are similar to those of the full study sample. Functional MRI and diffusion tensor imaging data will be (pre) processed and analyzed with Statistical Parametric Mapping (SPM) software, FMRIB Software Library (FSL) and in-house Matlab (The MathWorks, Inc., Natick, MA, USA), scripts in combination with open-source toolboxes for (dynamic) network analysis [68, 69] to study the effects of cognitive training on the functional and structural brain network, respectively. We will also employ typical independent component analysis in combination with dual regression for resting-state functional connectivity and morphometric (e.g. cortical thickness) analysis on T1-weighted structural MRI to study within and between group-effects of our intervention. Moreover, to establish treatment response at the individual level, Multivariate Pattern classification (‘machine learning’) analyses will be performed to identify predictive markers (clinical, neuropsychological and neuroimaging) to be able to predict (in future patients) who is most likely to benefit from cognitive training.
Sample size
The sample size calculation is performed on the basis of a previous meta-analysis on the effects of CT on cognitive function [22]. This study showed an effect size of Hedges g = .23 (i.e. f = .12), based on the effect of CT on improving global cognitive function. The sample size needed to detect this effect is 112, based on a repeated-measures analysis of variance, corrected for a moderate correlation between pre- and post-treatment measures (i.e. r ≈ .6). This sample size estimation also provides a good indicator for the power of our multivariate mixed-model regression analysis with adjustment for baseline measures.
To ensure adequate power for the secondary study parameters, i.e. the development of PD-MCI and PD-D at one and 2 years follow-up, with an α = .05 and β = .8, and based on a small drop-out (~ 10%) given the home-based, easily-accessible training, we will include 140 participants.
Discussion
The aim of the “COGnitive Training In Parkinson Study” (COGTIPS) is to assess the efficacy of an eight-week, online cognitive training program on alleviating cognitive dysfunction and subjective cognitive complaints, on delaying long-term cognitive deterioration and on increasing brain network connectivity and efficiency. COGTIPS is the first study in PD in a large group of PD patients –in accordance with recommendations from an earlier meta-analysis and review [22, 24]– that combines extensive clinical assessments with neuroimaging. We focus on PD patients in the ‘window of opportunity’, i.e., non-demented PD patients with mild subjective cognitive complaints that are expected to have the opportunity to employ significant neural plasticity in response to cognitive training. With the use of up to two-year follow-up assessments, this study can shed more light on the long-term effects of CT and its value in delaying conversion to PD-MCI and PD-D. The large subsample that will undergo MRI may show insight in the working mechanism of CT and baseline neuroimaging may additionally provide network organization characteristics that can predict individual training response.
The target population of COGTIPS consist of Dutch PD patients in the mild to moderate disease stage who experience significant subjective cognitive complaints but are not suspected of having PD-D. In this population that is often still active in work or social life, disease progression and cognitive decline provoke substantial worrying and are therefore an important subject of research [77]. The target population is large as about 50.000 Dutch individuals have PD, roughly 50% of whom have cognitive impairments [3], which does not include the even more prevalent subjective cognitive complaints that do not formally meet ‘impairment’ criteria [78]. However, the population is potentially heterogeneous given the large variety in age and degree of cognitive dysfunction. We may also expect ceiling scores on some of the neuropsychological assessment tasks in this non-demented PD population. We are, however, able to adhere to the level II criteria for PD-MCI and the criteria for probable PD-D using an extensive neuropsychological assessment battery [66, 67].
We will compare the CT adapted from the BrainGymmer environment to an active control condition based on ‘crystallized intelligence’ tasks. We thus correct for the cognitive engagement that participants are subjected to, to allow for any placebo effect mainly on subjective cognitive improvement and training effect on repeated cognitive assessment. Any CT-specific results will therefore be due to the training components. In the CT condition we will use an individually-based difficulty adaptation to adjust the training to the patients’ abilities. This ensures that participants are continuously stimulated at their own cognitive level and do not get frustrated or anxious by a training that is too difficult or bored by one that is too easy. Considering that we apply a home-based intervention and subjects can schedule their own training days, we expect a low attrition rate.
An important issue to overcome will be the medication use of participants, as the full study period will be more than 2 years. It is not realistic to expect stable medication over such a long period of time, although we will try to minimize medication changes as much possible in the first year by checking medication stability before subject participation and asking both the subject and neurologist to try and keep the medication regime stable. We will additionally correct for medication changes in our analyses and use a levodopa-equivalent daily dosage to aggregate the different types of PD medication.
There are substantial indications that cognitive training may provide an effective, non-pharmacological intervention to improve cognitive function in PD and delay cognitive decline, but evidence from large-scale RCTs is lacking. The aim of COGTIPS is to provide evidence for the efficacy of an easily-accessible, home-based online cognitive training, to validate the potential long-term effects and to shed more light on the underlying neural mechanism that mediate the beneficial effect of CT on cognitive function.
Additional files
Additional information. (PDF 237 kb)
SPIRIT checklist of the COGnitive Training In Parkinson Study (PDF 183 kb)
Acknowledgments
The authors thank E.H. Koenen, M. de Meijer, E.E.L. Buimer, C. Adegeest for their hard work in the data acquisition of the pilot study. We thank dr. J.I.V. Buitenweg for her advice concerning the cognitive training. We thank prof. dr. B.A. Schmand for his advice in setting up the neuropsychological assessment protocol.
Abbreviations
- AC
Active control condition
- COGTIPS
COGnitive Training In Parkinson Study
- CT
Cognitive training
- FSL
FMRIB Software Library
- ICD
Impulse control disorder
- MCI
Mild cognitive impairment
- MRI
magnetic resonance imaging
- PD
Parkinson’s disease
- PD-CFRS
Parkinson’s Disease Cognitive Functional Rating Scale
- PD-D
Parkinson’s disease dementia
- RCT
Randomized controlled trial
- SPM
Statistical Parametric Mapping
- SPSS
Statistical Package for the Social Sciences
- ToL
Tower of London
Authors’ contributions
TvB, OvdH and CV contributed to the conception and design of the study, pilot data acquisition and statistical analyses, and to drafting the study protocol. HB and YvdW contributed to the design of the study, the statistical analyses, and to substantively revising the work. JT contributed to the statistical analyses. IZ contributed to pilot data acquisition and pilot data analyses. RH and TB contributed to the design of the study and acquisition of pilot data. All authors read and approved the final manuscript.
Funding
This study was funded by the Dutch Parkinson’s Disease Association (‘Parkinson Vereniging’ fellowship 19–2015/MvhH; k€ 40 to van den Heuvel / Vriend) and the Brain Foundation of the Netherlands (‘Hersenstichting’ fellowship HA-2017-00227; k€ 300 to Vriend / van den Heuvel). The Brain Foundation of the Netherlands (‘Hersenstichting’) peer reviewed the design of this study. Two members of the Dutch Parkinson patient Association (‘Parkinson Vereniging’) made a contribution to the design of the study. The funding bodies had no role in the collection, analysis, and interpretation of data or in writing the manuscript. Dezzel Media B.V. did not sponsor this study, nor contributed to the design of the study, collection of outcome data, the analysis and interpretation of data or in writing the manuscript.
Availability of data and materials
The datasets generated, used and analyzed during the COGTIPS trial and its preceding pilot trial are or will be available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Approval by the Medical Ethical Committee (METc) of the VU University Medical Center was obtained for the COGTIPS trial (METc registration number: 2016.543/NL58750.029.16). Participants in this study will provide written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chris Vriend and Odile A. van den Heuvel shared last author
Contributor Information
Tim D. van Balkom, Email: t.vanbalkom@amsterdamumc.nl
Henk W. Berendse, Email: h.berendse@amsterdamumc.nl
Ysbrand D. van der Werf, Email: yd.vanderwerf@amsterdamumc.nl
Jos W. R. Twisk, Email: jwr.twisk@vumc.nl
Iris Zijlstra, Email: iriszijlstra11@gmail.com.
Rob H. Hagen, Email: rob027@kpnmail.nl
Tanja Berk, Email: tanjaberk@kpnmail.nl.
Chris Vriend, Email: c.vriend@amsterdamumc.nl.
Odile A. van den Heuvel, Email: oa.vandenheuvel@amsterdamumc.nl
References
- 1.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord. 2009;24:2175–2186. doi: 10.1002/mds.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Troster AI, Weintraub D. MDS task force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord. 2011;26(10):1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weintraub D, Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Siderowf A, Aarsland D, Barone P, Burn D, Chahine LM, et al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease. Mov Disord. 2015;30(7):919–927. doi: 10.1002/mds.26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 6.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 7.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Klepac N, Trkulja V, Relja M, Babic T. Is quality of life in non-demented Parkinson's disease patients related to cognitive performance? A clinic-based cross-sectional study. Eur J Neurol. 2008;15(2):128–133. doi: 10.1111/j.1468-1331.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal E, Brennan L, Xie S, Hurtig H, Milber J, Weintraub D, Karlawish J, Siderowf A. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord. 2010;25(9):1170–1176. doi: 10.1002/mds.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurt CS, Landau S, Burn DJ, Hindle JV, Samuel M, Wilson K, Brown RG, Group P-PS Cognition, coping, and outcome in Parkinson's disease. Int Psychogeriatr. 2012;24(10):1656–1663. doi: 10.1017/S1041610212000749. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher P, Leake A, Marion MH. Patients with Parkinson's disease dementia stay in the hospital twice as long as those without dementia. Mov Disord. 2011;26(5):919. doi: 10.1002/mds.23573. [DOI] [PubMed] [Google Scholar]
- 12.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9(12):1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 13.Emre M. Dementia in Parkinson's disease: cause and treatment. Curr Opin Neurol. 2004;17(4):399–404. doi: 10.1097/01.wco.0000137529.30750.ab. [DOI] [PubMed] [Google Scholar]
- 14.Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, Hametner EM, Poewe W, Rascol O, Goetz CG, et al. The Movement Disorder Society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson's disease. Mov Disord. 2011;26(Suppl 3):S42–S80. doi: 10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, Durif F, Kulisevsky J, van Laar T, Lees A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med. 2004;351(24):2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- 16.Prigatano GP. A history of cognitive rehabilitation. In: Halligan PW, Wade DT, editors. The Effectiveness of Rehabilitation for Cognitive Deficits. Oxford: Oxford University Press; 2005. pp. 3–10. [Google Scholar]
- 17.Sitzer DI, Twamley EW, Jeste DV. Cognitive training in Alzheimer's disease: a meta-analysis of the literature. Acta Psychiatr Scand. 2006;114(2):75–90. doi: 10.1111/j.1600-0447.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 18.Chandler MJ, Parks AC, Marsiske M, Rotblatt LJ, Smith GE. Everyday impact of cognitive interventions in mild cognitive impairment: a systematic review and meta-analysis. Neuropsychol Rev. 2016;26(3):225–251. doi: 10.1007/s11065-016-9330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- 20.Elliott M, Parente F. Efficacy of memory rehabilitation therapy: a meta-analysis of TBI and stroke cognitive rehabilitation literature. Brain Inj. 2014;28(12):1610–1616. doi: 10.3109/02699052.2014.934921. [DOI] [PubMed] [Google Scholar]
- 21.Rohling ML, Faust ME, Beverly B, Demakis G. Effectiveness of cognitive rehabilitation following acquired brain injury: a meta-analytic re-examination of Cicerone et al.'s (2000, 2005) systematic reviews. Neuropsychology. 2009;23(1):20–39. doi: 10.1037/a0013659. [DOI] [PubMed] [Google Scholar]
- 22.Leung IH, Walton CC, Hallock H, Lewis SJ, Valenzuela M, Lampit A. Cognitive training in Parkinson disease: a systematic review and meta-analysis. Neurology. 2015;85(21):1843–1851. doi: 10.1212/WNL.0000000000002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards JD, Hauser RA, O'Connor ML, Valdes EG, Zesiewicz TA, Uc EY. Randomized trial of cognitive speed of processing training in Parkinson disease. Neurology. 2013;81(15):1284–1290. doi: 10.1212/WNL.0b013e3182a823ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hindle JV, Petrelli A, Clare L, Kalbe E. Nonpharmacological enhancement of cognitive function in Parkinson's disease: a systematic review. Mov Disord. 2013;28(8):1034–1049. doi: 10.1002/mds.25377. [DOI] [PubMed] [Google Scholar]
- 25.Hindle JV, Watermeyer TJ, Roberts J, Martyr A, Lloyd-Williams H, Brand A, Gutting P, Hoare Z, Edwards RT, Clare L. Cognitive rehabiliation for Parkinson's disease demantia: a study protocol for a pilot randomised controlled trial. Trials. 2016;17:152. doi: 10.1186/s13063-016-1253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Weijer SC, Duits AA, Bloem BR, Kessels RP, Jansen JF, Kohler S, Tissingh G, Kuijf ML. The Parkin'Play study: protocol of a phase II randomized controlled trial to assess the effects of a health game on cognition in Parkinson's disease. BMC Neurol. 2016;16(1):209. doi: 10.1186/s12883-016-0731-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerrits NJ, van der Werf YD, Verhoef KM, Veltman DJ, Groenewegen HJ, Berendse HW, van den Heuvel OA. Compensatory fronto-parietal hyperactivation during set-shifting in unmedicated patients with Parkinson's disease. Neuropsychologia. 2015. [DOI] [PubMed]
- 28.Trujillo JP, Gerrits NJ, Veltman DJ, Berendse HW, van der Werf YD, van den Heuvel OA. Reduced neural connectivity but increased task-related activity during working memory in de novo Parkinson patients. Hum Brain Mapp. 2014;36(4):1554–1566. doi: 10.1002/hbm.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trujillo JP, Gerrits NJ, Vriend C, Berendse HW, van den Heuvel OA, van der Werf YD. Impaired planning in Parkinson's disease is reflected by reduced brain activation and connectivity. Hum Brain Mapp. 2015;36(9):3703–3715. doi: 10.1002/hbm.22873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vriend C, Gerrits NJ, Berendse HW, Veltman DJ, van den Heuvel OA, van der Werf YD. Failure of stop and go in de novo Parkinson's disease-a functional magnetic resonance imaging study. Neurobiol Aging. 2015;36(1):470–475. doi: 10.1016/j.neurobiolaging.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci. 2014;15(10):683–695. doi: 10.1038/nrn3801. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 33.Olde Dubbelink KT, Stoffers D, Deijen JB, Twisk JW, Stam CJ, Berendse HW. Cognitive decline in Parkinson's disease is associated with slowing of resting-state brain activity: a longitudinal study. Neurobiol Aging. 2013;34(2):408–418. doi: 10.1016/j.neurobiolaging.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Baggio HC, Sala-Llonch R, Segura B, Marti MJ, Valldeoriola F, Compta Y, Tolosa E, Junque C. Functional brain networks and cognitive deficits in Parkinson’s disease. Hum Brain Mapp. 2014;35(9):4620–4634. doi: 10.1002/hbm.22499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olde Dubbelink KT, Hillebrand A, Stoffers D, Deijen JB, Twisk JW, Stam CJ, Berendse HW. Disrupted brain network topology in Parkinson’s disease: a longitudinal magnetoencephalography study. Brain. 2014;137(Pt 1):197–207. doi: 10.1093/brain/awt316. [DOI] [PubMed] [Google Scholar]
- 36.Gerrits NJHM. Understanding cognitive heterogeneity in Parkinson's disease:: An imaging approach. 2015. [Google Scholar]
- 37.Chapman SB, Aslan S, Spence JS, Hart JJ, Jr, Bartz EK, Didehbani N, Keebler MW, Gardner CM, Strain JF, DeFina LF, et al. Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cereb Cortex. 2015;25(2):396–405. doi: 10.1093/cercor/bht234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klados MA, Styliadis C, Frantzidis CA, Paraskevopoulos E, Bamidis PD. Beta-band functional connectivity is reorganized in mild cognitive impairment after combined computerized physical and cognitive training. Front Neurosci. 2016;10:55. doi: 10.3389/fnins.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suo C, Singh M F, Gates N, Wen W, Sachdev P, Brodaty H, Saigal N, Wilson G C, Meiklejohn J, Singh N, Baune B T, Baker M, Foroughi N, Wang Y, Mavros Y, Lampit A, Leung I, Valenzuela M J. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Molecular Psychiatry. 2016;21(11):1633–1642. doi: 10.1038/mp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonavita S, Sacco R, Della Corte M, Esposito S, Sparaco M, d'Ambrosio A, Docimo R, Bisecco A, Lavorgna L, Corbo D, et al. Computer-aided cognitive rehabilitation improves cognitive performances and induces brain functional connectivity changes in relapsing remitting multiple sclerosis patients: an exploratory study. J Neurol. 2015;262(1):91–100. doi: 10.1007/s00415-014-7528-z. [DOI] [PubMed] [Google Scholar]
- 41.De Giglio L, Tona F, De Luca F, Petsas N, Prosperini L, Bianchi V, Pozzilli C, Pantano P. Multiple sclerosis: changes in thalamic resting-state functional connectivity induced by a home-based cognitive rehabilitation program. Radiology. 2016;280(1):202–211. doi: 10.1148/radiol.2016150710. [DOI] [PubMed] [Google Scholar]
- 42.Parisi L, Rocca MA, Valsasina P, Panicari L, Mattioli F, Filippi M. Cognitive rehabilitation correlates with the functional connectivity of the anterior cingulate cortex in patients with multiple sclerosis. Brain Imaging Behav. 2014;8(3):387–393. doi: 10.1007/s11682-012-9160-9. [DOI] [PubMed] [Google Scholar]
- 43.Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73(4):842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramaniam K, Luks TL, Garrett C, Chung C, Fisher M, Nagarajan S, Vinogradov S. Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. Neuroimage. 2014;99:281–292. doi: 10.1016/j.neuroimage.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao W, Cao X, Hou C, Li T, Cheng Y, Jiang L, Luo C, Li C, Yao D. Effects of cognitive training on resting-state functional connectivity of default mode, salience, and central executive networks. Front Aging Neurosci. 2016;8:70. doi: 10.3389/fnagi.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Marco M, Meneghello F, Duzzi D, Rigon J, Pilosio C, Venneri A. Cognitive stimulation of the default-mode network modulates functional connectivity in healthy aging. Brain Res Bull. 2016;121:26–41. doi: 10.1016/j.brainresbull.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Cerasa A, Gioia MC, Salsone M, Donzuso G, Chiriaco C, Realmuto S, Nicoletti A, Bellavia G, Banco A, D'Amelio M, et al. Neurofunctional correlates of attention rehabilitation in Parkinson's disease: an explorative study. Neurol Sci. 2014;35(8):1173–1180. doi: 10.1007/s10072-014-1666-z. [DOI] [PubMed] [Google Scholar]
- 48.Diez-Cirarda M, Ojeda N, Pena J, Cabrera-Zubizarreta A, Lucas-Jimenez O, Gomez-Esteban JC, Gomez-Beldarrain MA, Ibarretxe-Bilbao N. Increased brain connectivity and activation after cognitive rehabilitation in Parkinson's disease: a randomized controlled trial. Brain Imaging Behav. 2016. [DOI] [PMC free article] [PubMed]
- 49.Nombela C, Bustillo PJ, Castell PF, Sanchez L, Medina V, Herrero MT. Cognitive rehabilitation in Parkinson's disease: evidence from neuroimaging. Front Neurol. 2011;2:82. doi: 10.3389/fneur.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan AW, Tetzlaff JM, Altman DG, Dickersin K, Moher D. SPIRIT 2013: new guidance for content of clinical trial protocols. Lancet. 2013;381(9861):91–92. doi: 10.1016/S0140-6736(12)62160-6. [DOI] [PubMed] [Google Scholar]
- 51.Scharre DW, Chang SI, Murden RA, Lamb J, Beversdorf DQ, Kataki M, Nagaraja HN, Bornstein RA. Self-administered Gerocognitive examination (SAGE): a brief cognitive assessment instrument for mild cognitive impairment (MCI) and early dementia. Alzheimer Dis Assoc Disord. 2010;24(1):64–71. doi: 10.1097/WAD.0b013e3181b03277. [DOI] [PubMed] [Google Scholar]
- 52.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 53.Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, Melzer TR, Kirwan J, Keenan R, Wells S, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 54.Fahn S, Elton RL. UPDRS Development Committee A: Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Florham Park GM, editors. Recent developments in Parkinson's disease. Volume 2. NJ: Macmillian Healthcare Information; 1987. pp. 293–304. [Google Scholar]
- 55.Voss T, Bahr D, Cummings J, Mills R, Ravina B, Williams H. Performance of a shortened scale for assessment of positive symptoms for Parkinson's disease psychosis. Parkinsonism Relat Disord. 2013;19(3):295–299. doi: 10.1016/j.parkreldis.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 56.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 57.Verhage F. Intelligentie en leeftijd: Onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar: Van Gorcum Assen. 1964. [Google Scholar]
- 58.Hochstenbach JB, den Otter R, Mulder TW. Cognitive recovery after stroke: a 2-year follow-up. Arch Phys Med Rehabil. 2003;84(10):1499–1504. doi: 10.1016/s0003-9993(03)00370-8. [DOI] [PubMed] [Google Scholar]
- 59.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 60.Buitenweg JIV, van de Ven RM, Prinssen S, Murre JMJ, Ridderinkhof KR. Cognitive flexibility training: a large-scale multimodal adaptive active-control intervention study in healthy older adults. Front Hum Neurosci. 2017;11:529. doi: 10.3389/fnhum.2017.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van de Ven RM, Buitenweg JI, Schmand B, Veltman DJ, Aaronson JA, Nijboer TC, Kruiper-Doesborgh SJ, van Bennekom CA, Rasquin SM, Ridderinkhof KR, et al. Brain training improves recovery after stroke but waiting list improves equally: a multicenter randomized controlled trial of a computer-based cognitive flexibility training. PLoS One. 2017;12(3):e0172993. doi: 10.1371/journal.pone.0172993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouma A, Mulder J, Lindeboom J, Schmand B. Handboek neuropsychologische diagnostiek.-2e herz. dr: Pearson. 2012. [Google Scholar]
- 63.Kulisevsky J, Fernandez de Bobadilla R, Pagonabarraga J, Martinez-Horta S, Campolongo A, Garcia-Sanchez C, Pascual-Sedano B, Ribosa-Nogue R, Villa-Bonomo C. Measuring functional impact of cognitive impairment: validation of the Parkinson's disease cognitive functional rating scale. Parkinsonism Relat Disord. 2013;19(9):812–817. doi: 10.1016/j.parkreldis.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(Pt 1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 65.Lezak MD, Howieson DB, Loring DW, Fischer JS. Neuropsychological assessment. USA: Oxford University press; 2004. [Google Scholar]
- 66.Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society task force guidelines. Mov Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 68.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Sizemore AE, Bassett DS. Dynamic graph metrics: Tutorial, toolbox, and tale. Neuroimage. 2018;180(Pt B):417–427. doi: 10.1016/j.neuroimage.2017.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77(3):288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ratey JJ, Loehr JE. The positive impact of physical activity on cognition during adulthood: a review of underlying mechanisms, evidence and recommendations. Rev Neurosci. 2011;22(2):171–185. doi: 10.1515/RNS.2011.017. [DOI] [PubMed] [Google Scholar]
- 72.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 73.Miah IP, Olde Dubbelink KT, Stoffers D, Deijen JB, Berendse HW. Early-stage cognitive impairment in Parkinson's disease and the influence of dopamine replacement therapy. Eur J Neurol. 2012;19(3):510–516. doi: 10.1111/j.1468-1331.2011.03578.x. [DOI] [PubMed] [Google Scholar]
- 74.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 75.Petrelli A, Kaesberg S, Barbe MT, Timmermann L, Fink GR, Kessler J, Kalbe E. Effects of cognitive training in Parkinson's disease: a randomized controlled trial. Parkinsonism Relat Disord. 2014;20(11):1196–1202. doi: 10.1016/j.parkreldis.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 76.Team RC . R: a language and environment for statistical computing. R Foundation for Statistical Computing: Vienna; 2014. [Google Scholar]
- 77.Dauwerse L, Hendrikx A, van de Moosdijk L, Leedekerken W, Dellebeke H, Tulp H, van het Hoofd M, Schipper K, Abma T. Bewogen door onderzoek. Amsterdam: VU medisch centrum; 2012. [Google Scholar]
- 78.Bosboom JL, Stoffers D, Wolters E. Cognitive dysfunction and dementia in Parkinson's disease. J Neural Transm. 2004;111(10–11):1303–1315. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information. (PDF 237 kb)
SPIRIT checklist of the COGnitive Training In Parkinson Study (PDF 183 kb)
Data Availability Statement
The datasets generated, used and analyzed during the COGTIPS trial and its preceding pilot trial are or will be available from the corresponding author upon reasonable request.