Abstract
Background
We report on an outbreak of hepatitis A among men who have sex with men (MSM) in England and its associated healthcare resource burden, the strategies used to control the outbreak and the role of past and current hepatitis A vaccination policy and practice in England.
Methods
National surveillance of hepatitis A, including reference laboratory confirmation and molecular sequencing, and a case questionnaire, was enhanced in 2017 to collect demographic and risk information, disease severity and healthcare utilisation. National Health Service (NHS) data was used to calculate associated healthcare costs.
Results
During the outbreak period (July 2016 to January 2018), 670 confirmed cases were identified in England, caused by three distinct viral strains. The public health response included raising public and professional awareness, reinforcing vaccine recommendations for MSM, contact tracing for post-exposure vaccination, and mass community vaccination where spill-over of infection into the general population occurred. Hepatitis A vaccine was centrally procured to ensure sexual health clinics in England could offer vaccination to MSM. Outbreak associated healthcare costs were estimated to be approximately £1,500,000.
Conclusions
While MSM are at increased risk of hepatitis A infection, inconsistent implementation of MSM vaccination policy in previous years led to an increasingly susceptible MSM population. The large number of cases, hospital admission rate and public health actions contributed to a significant healthcare burden. Recommending hepatitis A vaccination for MSM and clarifying commissioning responsibilities is essential to prevent future outbreaks.
Keywords: Hepatitis A, MSM, Outbreak, Vaccination, Healthcare burden
1. Introduction
Hepatitis A is a liver disease caused by the hepatitis A virus (HAV). HAV is primarily transmitted faecal-orally via ingestion of contaminated food or water or by direct contact with an infectious individual. While not usually life threatening, hospitalisation does occur and infection can lead to fulminant hepatitis, that may occasionally be fatal [1], [2]. A safe and effective vaccine has been available since 1995, and although there is no routine universal hepatitis A vaccination programme in England, selective vaccination of those at risk is recommended, including for travel to endemic countries [3].
A marked decline in hepatitis A incidence in England has been observed since the early 1990s, most likely due to increased vaccination on a background of improvements in hygiene and sanitation earlier in the century. In 1990 almost 1000 cases were observed annually compared to 300–400 annually between 2010 and 2015, with recent international travel the most commonly reported risk factor [3], [4]. Lower levels of endemic transmission in recent decades have resulted in a larger proportion of the population becoming susceptible, with the typical age of infection shifting from children to adults. As a result, unless vaccinated, the majority of adults remain susceptible throughout their lives, increasing the potential for outbreaks to occur [3].
In 2014 in England, approximately 3.1% of the male population were gay or bisexual, that is approximately 646,000 individuals [5], [6]. In the same year, around 140,000 men who have sex with men (MSM) attended sexual health services; such attendance is considered as a proxy for increased risk of acquiring infections through sexual activity [7]. Furthermore, sexual health services have been shown to be the preferred setting for vaccination among MSM, and one where delivering a vaccination programme to this population is feasible. It is also the only setting in England where eligible individuals, and therefore a denominator, can be identified, allowing the monitoring and evaluation of vaccine programme targeting MSM [8].
Faecal-oral transmission through sexual activity is a recognised route of HAV transmission and there have been documented outbreaks of hepatitis A among MSM in recent decades [9], [10]. As MSM risk of hepatitis A was well established, hepatitis A vaccine was recommended and offered in sexual health clinics across England, but it is unclear how uniformly vaccination was implemented, with most London clinics ceasing routine vaccination of MSM several years ago [11]. The exact periods during which the vaccine was offered in different clinic locations is unclear, as is the proportion of MSM vaccinated in different settings for other reasons - such as travel. In addition, the administration of hepatitis A vaccination was not consistently commissioned or routinely monitored in sexual health clinics. As a result, the pre-outbreak susceptibility of MSM in England is difficult to quantify.
In July 2016, Public Health England (PHE) detected an increase in hepatitis A cases notified in England, predominantly among MSM, caused by three circulating genotype IA strains [12]. The strains were first reported among MSM in the Netherlands (RIVM-HAV16-090, October 2016), England (VRD_521_206, December 2016) and Germany (V16-25801, January 2017). The strains were identified in the same period in 22 European countries and elsewhere [13], [14]. This paper describes the outbreak in England and its associated healthcare resource burden, the intervention strategies used to control the outbreak and its implications for hepatitis A vaccination policy in England.
2. Methods
2.1. Case definition
A confirmed case was defined as a laboratory (PCR and/or serology) confirmed HAV infection with the specific sequence of VRD_521_206 (Strain 1), RIVM-HAV16-090 (Strain 2) or V16-25801 (Strain 3), with onset of symptoms after 31st June 2016. Probable cases were defined as a laboratory confirmed case of HAV infection (sequence not known) with onset of symptoms after 31st June 2016, and known to be a contact of a confirmed case and/or identifies as MSM. MSM were defined as men who reported having had sexual contact with a man within 8 weeks before the onset of symptoms.
2.2. Data collection
Epidemiological and laboratory data are collected through the enhanced molecular surveillance of hepatitis A in England, which is underpinned by legislation on communicable diseases [15]. As hepatitis A is a notifiable disease, health care professionals are legally obliged to notify their local PHE Health Protection Team, so that prompt public health actions can be taken. Upon notification of a hepatitis A case, the local team collects information from the case on demographic characteristics e.g. address, ethnicity, age, sex, occupation, sexual orientation, recent travel (within 8 weeks of onset of symptoms) as well as disease severity and hospitalisation, using a questionnaire.
During the outbreak period, additional questions for MSM on sexual activities in the 8 weeks prior to onset of symptoms were included, such as sex with anonymous partners, attendance of public sex venues and use of dating apps (downloaded application for a mobile device).
In parallel, diagnostic laboratories are mandated to submit electronic reports of hepatitis A to PHE, prompting a request to the diagnosing laboratory for a residual blood sample to be sent to the PHE Virus Reference Department for serological confirmation, HAV RNA identification and subsequent genotyping and sequencing. Confirmed and probable cases reported to PHE between 1st July 2016 and 31st January 2018 in England were identified through this laboratory based surveillance system.
2.3. Data analysis
In order to characterise the outbreak, we used questionnaire data to describe the distribution of cases over time and by age, sex, sexual orientation, England region, disease status (confirmed, probable) and HAV sequence. We also described travel, sexual behaviour (for MSM only) and HIV co-infection among confirmed cases. We calculated attack ratios by region and age group, using sexual health clinic MSM attendees from 2017 Genitourinary Medicine Clinical Activity Dataset (GUMCAD) data as the population denominator. STATA SE (Version 13.1) was used for statistical analysis.
In order to estimate the burden associated with the outbreak, we described incidents of secondary transmission outside of the MSM population as well as documented incidents where intervention outside the household was required. In addition, the proportion of cases who attended primary care, Accident and Emergency (A&E), the proportion that were admitted to hospital, as well as those with a severe outcome were also calculated with associated healthcare costs. The unit cost of a GP consultation was estimated at £37 [16], A&E attendance at £147.80 [17], a hospitalisation episode at £3,184.84 [17] and a liver transplant, including follow up care at £46,551 (2004 GBP) [18]. Mean length of hospitalisation was assumed to be 4 days, based on Hospital Episode Statistics data [19].
3. Results
3.1. Outbreak description
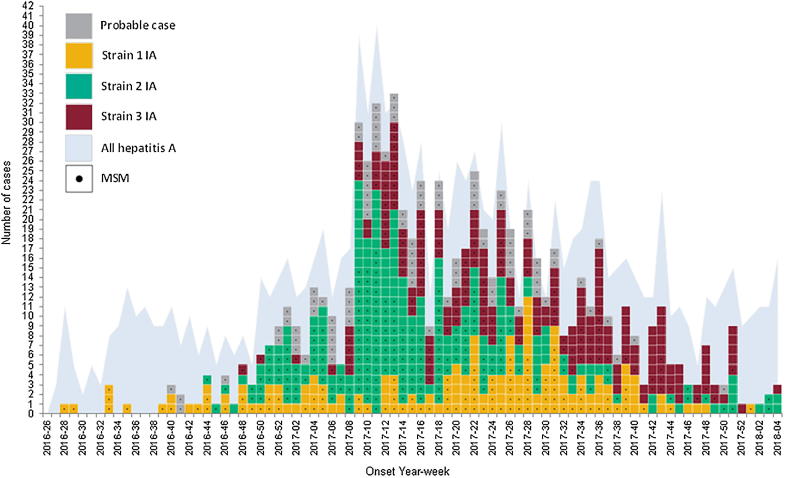
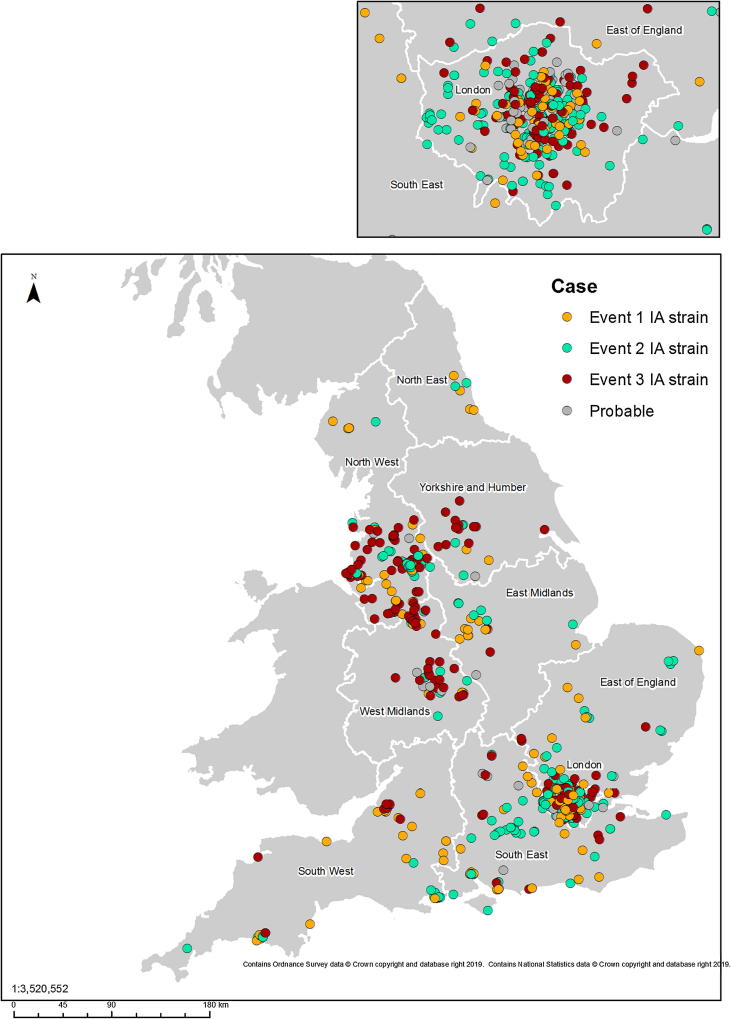
Between July 2016 and January 2018, of the 1243 hepatitis A cases identified in England, 670 (53.9%) matched the outbreak strains. Of these, 168 (25%) were Strain 1, 287 (43%) were Strain 2 and 215 (32%) were Strain 3 (Fig. 1). Additionally, 93 probable cases were identified, bringing the total to 763 cases, representing 61.3% of all hepatitis A cases in England over this period. Strains 1 and 2 were identified in all nine PHE regions across England, with Strain 3 found in all but the North East region (Fig. 2).
Fig. 1.
Confirmed and Probable cases of hepatitis A in England, July 2016 to January 2018 (n = 763) MSM: Men who have sex with men.
Fig. 2.
Geographical distribution of hepatitis A cases, England, July 2016 to January 2018 (n = 763).
The outbreak was initially identified as three small distinct clusters in the South West, West Midlands and the North East of England. Up to the end of November 2016, 24 cases were identified, of which 9 (37%) reported recent travel, 8 of which was to Spain. By January 2017 the outbreak had spread to all regions across England. The outbreak peaked in March with 98/129 (76%) of cases that month occurring in London, and over 70% of these cases among MSM. Over the course of the outbreak 336/763 (44%) of cases reported travel within eight weeks of onset of symptoms, 32% of which was to Spain, particularly in the earlier phase of the outbreak.
Table 1 one describes the characteristics of the cases identified during the outbreak period. The median age range among MSM was 33 (18–71) years. Over a third of MSM cases reported one or more high risk sexual behaviours: anonymous sex, sex at public venues and use of electronic dating apps. The attack ratio was highest in London at 3.68 per 1000 MSM attending sexual health services (Table 2). When stratified by age, the 25–34 years range had the highest attack ratio at 4.12 per 1000 MSM attending sexual health services (Table 3).
Table 1.
Characteristics of outbreak cases.
| Strain 1 (n = 168) | Strain 2 (n = 287) | Strain 3 (n = 215) | Probable (n = 93) | Total (n = 763) | |
|---|---|---|---|---|---|
| Median age (range) | 36 (19–87) | 33 (5–80) | 38 (11–70) | 31 (21–73) | 34 (5–87) |
| Male | 148 (88%) | 260 (91%) | 198 (92%) | 93 (100%) | 699/763 (92%) |
| Travel within 8 weeks of onset of symptoms | 83 (49%) | 109 (38%) | 85 (40%) | 59 (63%) | 336/763 (44%) |
| MSM | 100 (68%) | 185 (71%) | 118 (60%) | 93 (100%) | 496/699 (71%) |
| Median age (range) | 33 (18–69) | 32 (18–62) | 35 (19–66) | 30 (20–71) | 33 (18–71) |
| Use of electronic dating app(s) | 30 (30%) | 62 (34%) | 42 (36%) | 43 (46%) | 177/418 (42%) |
| Sex with anonymous partner(s) | 36 (36%) | 64 (35%) | 44 (37%) | 38 (41%) | 182/460 (40%) |
| Sexual contact in public venue(s) | 33 (33%) | 75 (41%) | 45 (38%) | 34 (37%) | 187/417 (45%) |
MSM: Men who have sex with men.
Table 2.
Attack ratio by PHE region.
| PHE Region | Number of MSM cases (confirmed and probable) | MSM Sexual Health Clinic Attendees | Attack ratio (95% CI) |
|---|---|---|---|
| East Midlands | 16 | 5020 | 3.18 (1.82–5.17) |
| East of England | 27 | 7868 | 3.43 (2.26–4.98) |
| London | 285 | 77,362 | 3.68 (3.28–4.15) |
| North East | 5 | 4121 | 1.21 (0.39–2.82) |
| North West | 59 | 16,101 | 3.66 (2.79–4.72) |
| South East | 42 | 16,728 | 2.51 (1.81–3.39) |
| South West | 21 | 9398 | 2.23 (1.38–3.41) |
| West Midlands | 19 | 9816 | 1.93 (1.16–3.02) |
| Yorkshire and Humber | 12 | 7633 | 1.57 (0.81–2.74) |
MSM: Men who have sex with men.
Table 3.
Attack ratio by age group.
| Age Range | Number of MSM cases (confirmed and probable) | MSM Sexual Health Clinic Attendees | Attack ratio (95% CI) |
|---|---|---|---|
| ≤15 | 0 | 190 | 0.00 |
| 16–19 | 4 | 5662 | 0.70 (0.19–1.81) |
| 20–24 | 49 | 23,574 | 2.07 (1.53–2.74) |
| 25–34 | 226 | 54,735 | 4.12 (3.60–4.70) |
| 35–44 | 125 | 34,059 | 3.67 (3.05–4.37) |
| 45–64 | 86 | 31,923 | 2.69 (2.15–3.32) |
| 65+ | 6 | 3590 | 1.67 (0.61–3.63) |
MSM: Men who have sex with men.
Of the 665 confirmed cases with complete identifiers that could be matched against a national HIV database, 48 (7.2%) were co-infected with HIV.
As the outbreak progressed, the infection spread to the wider community, reducing the proportion of cases identifying as MSM. Twenty-five incidents were identified where public health action was required outside of the household setting. Vaccination of contacts outside the household was undertaken in 11 of the 25 incidents, ranging from vaccination of 3 kitchen staff to 1800 students/teaching staff. There were three incidents where secondary transmission is known to have occurred outside of the MSM population, resulting in 4 non-MSM cases. In one of these incidents, the index case identified as MSM.
3.2. Outbreak response
Immediate public health action included the follow up of cases and vaccination of their contacts, as recommended in national guidance [1]. Where the contact was identified and consent given, sexual partner notification was performed. To raise awareness of the increased transmission among the MSM population, PHE engaged with local and national LGBT charities to disseminate public health information. PHE worked alongside sexual health charities on awareness campaigns, co-developing leaflets and posters for distribution to sexual health/HIV clinics and other relevant settings [20]. The messaging focused on minimising risk during sexual activity and promotion of hepatitis A vaccination among MSM.
As the outbreak increased in size and involved an increasing number of regions, in June 2017, PHE issued a joint briefing note with the British Association for Sexual Health and HIV (BASHH) and British HIV Association, recommending that all MSM attending sexual health and HIV clinics should be opportunistically offered hepatitis A vaccine. The outbreak coincided with a global shortage of hepatitis A vaccine, and in order to mitigate supply issues PHE procured a central stockpile of hepatitis A vaccine in July 2017, which was made available to all sexual health clinics across England for use in MSM. Concurrently, PHE, with endorsement from the Joint Committee for Vaccination and Immunisation (JCVI), an independent scientific expert group on vaccines, issued temporary dose-sparing recommendations. These recommendations included off-label use of paediatric vaccines in adults to maximise the number of at risk individuals who could be immunised [21]. From July 2017-January 2018, over 16,000 doses of adult and paediatric vaccine were distributed to sexual health clinics across England. In addition to this national response, some local services in London responded with tailored approaches such as pop up vaccination clinics in gay bars and clubs [22].
3.3. Healthcare burden and cost of the outbreak
Of the 763 confirmed and probable cases, 395 (52%) reported attendance to their GP. 435 (57%) reported hospital admission. Of the 220 confirmed and probable cases asked, 127 (58%) reported A&E attendance. One instance of a non-MSM case with fulminant hepatitis requiring a liver transplant was documented.
As A&E attendance was only recorded from June 2017 onwards, we applied the proportion who attended A&E (58%) during that period to the total number of cases during the outbreak period (7 6 3), estimating approximately 442 cases would have attended A&E during the outbreak. Based on these figures, we estimated the direct healthcare costs of the outbreak at approximately £1,500,000, largely driven by the high proportion of hospital admissions (Table 4).
Table 4.
Outbreak associated healthcare costs.
| Resource | Unit Cost | n | Total Cost |
|---|---|---|---|
| GP Appointment | £37 | 395 | £14,615 |
| A&E Attendance | £147.8 | 442 | £65,327 |
| Hospital Admission | £3184.84 | 435 | £1,385,405 |
| Liver Transplant | £46,551 | 1 | £46,551 |
| Total | £1,511,898 | ||
4. Discussion
Between July 2016 and January 2018, 670 confirmed HAV infections with three sequences (VRD_521_206 [Strain 1], RIVM-HAV16-090 [Strain 2] and V16-25801 [Strain 3]) were identified in nine regions across England with an additional 93 probable cases identified. This outbreak predominantly affected the MSM population, more than doubling the background rate of all hepatitis A cases, with faecal-oral transmission during sex likely to be the primary route of transmission. Cases appear to have been originally imported multiple times from neighbouring European countries also experiencing recent increases in hepatitis A cases in men, leading to sustained virus circulation among the MSM population in England, and eventually affecting the wider population. The similar features among patients infected with the three co-circulating strains strengthened the decision to manage this incident as a single outbreak.
Detailed sexual history questions in the case surveillance questionnaire enable a better understanding of transmission in this population. However, over a third of MSM cases reported anonymous sexual contacts, and as a result it was not possible to establish sexual networks or direct epidemiological links with a high level of confidence. The number of incidents in the wider community that are likely to be linked to MSM are therefore undoubtedly underestimated. The high proportion of cases engaging in high risk behaviour suggests these have played a role in the outbreak. The use of sex-on-premises venues in particular, where anonymous encounters are common, provided a tenuous link between some cases and supported the hypothesis that these venues were key in the propagation of the virus in a high-risk subset of the MSM population. Due to logistical and resource constraints, it was not possible to recruit controls in the context of this outbreak, and it was therefore not possible to show a statistical association between being a case and using these venues.
Prior to this outbreak, guidance around vaccinating MSM was inconsistent, with PHE recommending that immunisation should be offered to MSM with multiple sexual partners, particularly during outbreaks [3]. Whereas BASHH guidance, which is widely followed by sexual health services and local authority commissioners, stated that “universal vaccination in this group cannot be strongly recommended” [23]. As a result, vaccination practice varied from region to region, with different regions having offered the vaccine at different times, likely contributing to the varying attack ratios reported across England. Detailed information about past vaccination policy in different regions was not available and it was therefore difficult to estimate hepatitis A susceptibility in the MSM population, which complicated planning vaccine procurement and delivery. In a London survey of hepatitis A vaccination provision in sexual health clinics, 20/23 clinics reported stopping vaccination among MSM at some time in the past, and therefore could not confidently estimate the background immunity in their MSM clinic population [11]. The variation in vaccination practice in London sexual health clinics may partly explain the very high attack ratio seen in London. In addition, regional variation of hepatitis A vaccination in England meant that some MSM populations were likely better protected against infection. For example, Brighton and Hove continued to offer vaccination through sexual health/HIV services until 2017 and saw no MSM cases despite a large MSM population (S Nicholson, personal communication). This suggests that historical routine offer of hepatitis A vaccine to MSM may have mitigated or prevented the outbreak in certain areas.
The discrepancies in vaccine recommendations, compounded by a lack of consensus on commissioning responsibilities for vaccination during the outbreak, likely led to a delay in initiating vaccination in all areas. Sexual health services are commissioned by local government in England and are therefore vulnerable to local shifts in priorities and budget restrictions, which do not lend themselves to consistent implementation and achievement of high vaccine uptake among MSM. The budget pressure was temporarily mitigated by the exceptional national procurement of vaccine stock, which was the only option to ensure sufficient supply. The central supply therefore allowed sexual health clinics to rapidly kick-start their MSM vaccination programmes.
In November 2017, as a result of the outbreak, BASSH updated their hepatitis A vaccination guidelines to be more aligned with those of PHE/JCVI [3], and recommended that all MSM attending sexual health clinics should be screened for HAV antibody and vaccinated if anti-HAV IgG negative. In an outbreak situation BASSH now recommend that all MSM should be offered vaccination at the first visit unless there is confirmed evidence of prior vaccination/immunity [24]. A study in the North East suggested that, based on a HAV IgG seroprevalence of 42% [25], screening prior to vaccination of MSM may be a cost neutral strategy. A similar statement was made by CDC [26]. Surveys are therefore underway to estimate the age-specific seroprevalence of HAV in MSM attending clinics in England and new GUMCAD codes for monitoring vaccine uptake have been introduced which may inform and evaluate vaccine strategies going forward. The HPV vaccination programme for MSM in England, piloted in 2016–18 and rolled out nationally in April 2018, has demonstrated that vaccination programmes can be successfully delivered to MSM through Sexual Health services. In the case of hepatitis A however, commissioning and procuring the vaccine is a local decision and it is therefore likely that the national recommendation to vaccinate all MSM attending Sexual Health services will not be uniformly implemented.
Over half of the cases were admitted to hospital, with an admission rate higher than previously documented [27]. The burden placed on the NHS and associated healthcare costs due to the outbreak are therefore also important and relevant. The cost of the outbreak to healthcare was estimated at over £1.5 million, which is likely to be an underestimate as some admissions will have required intensive care, and costs to sexual health services were not included. Moreover, staff time associated with health protection activities in PHE, supporting incident response in NHS England local immunisation teams and undertaking contact tracing and post-exposure vaccination of contacts in GP and sexual health clinics were not included. This outbreak caused unnecessary additional pressures on hospitals that could have been avoided had vaccination of the MSM population been routinely offered. Hepatitis A vaccines are safe and effective, costing approximately £20 per dose for adult monovalent vaccine. With the calculated healthcare costs considered to be an underestimate, the cost of vaccination in this outbreak is likely to be cost neutral, and potentially cost saving. A full cost effectiveness analysis of hepatitis A vaccination strategies in MSM is ongoing and will inform vaccination strategies going forward to prevent future hepatitis A outbreaks in this at-risk population.
The scale of this outbreak has highlighted that the MSM population is a population susceptible to HAV infection and should be prioritised for vaccination. The lack of consistent policy and practice to vaccinate MSM against HAV likely led to an accumulation of individuals susceptible to the disease over time, allowing the outbreak to emerge and rapidly spread through complex and interlinked sexual networks across England and beyond. Although this outbreak was largely controlled through reactive vaccination and reassertion of vaccine recommendations, without a clear long-term vaccination strategy and commissioning arrangements that ensure successful delivery of a hepatitis A vaccination programme in MSM across England, a susceptible population of MSM will likely re-accumulate, creating the conditions for future outbreaks. Commissioners should translate the national recommendation to vaccinate all MSM against hepatitis A into local commissioning arrangements in order to prevent these from occurring.
Acknowledgments
Acknowledgements
We would like to thank all PHE Centre local and regional teams involved in the investigation and management of the outbreak. We would also like to thank Hester Allen and Hamish Mohammed for supplying the GUMCAD data used in the analysis.
Conflicts of interest
None of the authors have conflicts of interest relevant to this work. The Immunisation, Hepatitis and Blood Safety Department have provided pharmaceutical manufacturers with post-marketing surveillance reports. A cost recovery charge is made for these reports.
Authors’ contributions
MR, SM and ME conceived and designed the study and contributed to the interpretation of the findings. JP and KB collated the data. JP analysed the data and drafted the manuscript. ME and SM provided critical input into the manuscript. All authors reviewed, edited and approved the final manuscript.
Footnotes
Summary: An outbreak of hepatitis A occurring among MSM resulted in 670 confirmed cases between 2016 and 2018. Health promotion, vaccination and updated guidelines were used to prevent re-accumulation of susceptible individuals. Outbreak associated healthcare costs were estimated at £1,500,000.
References
- 1.Public health England . 2017. Public health control and management of hepatitis A, 2017 Guidelines. London, UK. [Google Scholar]
- 2.Heymann D.L. 20th ed. APHA Press; 2014. Control of communicable diseases manual. [Google Scholar]
- 3.Public Health England . Immunisation against infectious disease. London, UK. 2017. pp. 143–144. [Google Scholar]
- 4.Public Health England . 2015. Laboratory reports of hepatitis A infection, and hepatitis C: 2015. Health Protection Report. London, UK. [Google Scholar]
- 5.Public Health England . 2017. Producing modelled estimates of the size of the lesbian, gay and bisexual (LGB) population of England. [Google Scholar]
- 6.Office for National Statistics . 2014. Annual mid-year population estimates, UK: 2014. [Google Scholar]
- 7.Bayley J., Mesher D., Nadarzynski T., Hughes G., Soldan K. Attendance of MSM at Genitourinary Medicine services in England: implications for selective HPV vaccination programme (a short communication) Sex Transm Infect. 2017 doi: 10.1136/sextrans-2016-052912. [DOI] [PubMed] [Google Scholar]
- 8.Public Health England. Human papillomavirus (HPV) vaccination for Men who have sex with Men (MSM) 2016/17 pilot evaluation; February 2018. https://www.gov.uk/government/publications/hpv-vaccination-for-men-who-have-sex-with-men-year-1-pilot [accessed 31/12/2018].
- 9.Bell A., Ncube F., Hansell A. An outbreak of hepatitis A among young men associated with having sex in public venues. Commun Dis Public Health. 2001;4(3):163–170. [PubMed] [Google Scholar]
- 10.Tortajada C., de Olalla P.G., Diez E. Hepatitis A among men who have sex with men in Barcelona, 1989–2010: insufficient control and need for new approaches. BMC Infect Dis. 2012;12:11. doi: 10.1186/1471-2334-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brook G., Mindlin M., Menon-Johansson A. P195 How prepared are gum and hiv clinics in london to respond to the hepatitis a outbreak? A survey of vaccination policy and logistics. Sex Transm Infect. 2017;93:A80. [Google Scholar]
- 12.Beebeejaun K., Degala S., Balogun K. Outbreak of hepatitis A associated with men who have sex with men (MSM), England, July 2016 to January 2017. Euro Surveill. 2017;22(5) doi: 10.2807/1560-7917.ES.2017.22.5.30454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Centre for Disease Prevention and Control. Epidemiological update: hepatitis A outbreak in the EU/EEA mostly affecting men who have sex with men. Available at: https://ecdc.europa.eu/en/news-events/epidemiological-update-hepatitis-outbreak-eueea-mostly-affecting-men-who-have-sex-men-0 [accessed 09/03/2018].
- 14.Victoria State Government. Important vaccination update for MSM. Available at: https://www2.health.vic.gov.au/about/news-and-events/healthalerts/important-vaccination-update-for-msm-january-2018 [accessed 23/05/2018].
- 15.Department of Health. Health Protection Legislation (England) Guidance 2010. London, UK; 2010.
- 16.Curtis L., Burns A. University of Kent; Canterbury: 2017. Unit costs of health & social care 2017: personal social services research unit. [Google Scholar]
- 17.National Health Service. National schedule of reference costs. Available at: https://improvement.nhs.uk/resources/reference-costs/ [accessed 04/06/2018].
- 18.Shepherd J., Brodin H., Cave C., Waugh N., Price A., Gabbay J. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2004;8(39) doi: 10.3310/hta8390. iii–iv, 1–125. [DOI] [PubMed] [Google Scholar]
- 19.National Health Service. Hospital admitted patient care activity, 2016–17. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2016-17 [accessed 05/06/2018].
- 20.Public Health England. Promotional material. Hepatitis A: preventing infection in men who have sex with men. Available at: https://www.gov.uk/government/publications/hepatitis-a-preventing-infection-in-men-who-have-sex-with-men [accessed 01/06/2018].
- 21.Public Health England. Hepatitis A vaccination in adults – temporary recommendations. London, UK; 2017.
- 22.Mindlin M., Crook P., King M. P207 Outbreak of hep a affecting msm: the london response. Sex Transm Infect. 2017;93:A84. [Google Scholar]
- 23.Brook G., Bhagani S., Kulasegaram R. United Kingdom National Guideline on the Management of the viral hepatitides A, B and C 2015. Int J STD AIDS. 2016;27(7):501–525. doi: 10.1177/0956462415624250. [DOI] [PubMed] [Google Scholar]
- 24.British Association for Sexual Health & HIV. 2017 interim update of the 2015 BASHH National Guidelines for the Management of the Viral Hepatitides; 2017.
- 25.Bhagey A., Foster K., Ralph S. High prevalence of anti-hepatitis A IgG in a cohort of UK HIV-negative men who have sex with men: implications for local hepatitis A vaccine policy. Int J STD AIDS. 2018 doi: 10.1177/0956462418770008. 956462418770008. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunzation Practices (ACIP). Antlanta, Georgia; 1999. [PubMed]
- 27.Ly K.N., Klevens R.M. Trends in disease and complications of hepatitis A virus infection in the United States, 1999–2011: a new concern for adults. J Infect Dis. 2015;212(2):176–182. doi: 10.1093/infdis/jiu834. [DOI] [PMC free article] [PubMed] [Google Scholar]