Highlights
-
•
Rotarix® had very good vaccine effectiveness (VE) in UK public health use.
-
•
Two-dose VE against confirmed infection in young children was 77% (95%CI:66–85%)
-
•
The vaccine programme was exceptionally successful (>90% vaccine uptake, high VE)
-
•
Thus, it is highly likely that most acute gastroenteritis (AGE) was no longer due to rotavirus.
-
•
This explains the lack of demonstrable VE against all-cause AGE.
Keywords: Rotavirus, Vaccine effectiveness, Diarrhoea, Gastroenteritis, Electronic health records
Abstract
Background
The monovalent oral rotavirus vaccine Rotarix® was introduced into the UK infant immunisation programme in 2013. We estimated vaccine effectiveness (VE) in the first two years of the programme.
Methods
We used a test-negative case-control design and enhanced national surveillance data for 1869 vaccine-eligible children tested for rotavirus infection to obtain adjusted odds ratios and VE against laboratory-confirmed rotavirus infections. Linked anonymised UK primary care and hospitalisation data from the Clinical Practice Research Datalink (40,723 children) and random-effects Poisson regression were used in a cohort study to estimate VE against all-cause acute gastroenteritis (AGE) and AGE hospitalisations.
Results
VE against laboratory-confirmed infection was 69% (95% Confidence Interval: 40–84%) for one dose and 77% (95%CI: 66–85%) for two doses. Two-dose VE in children aged <12 months and ≥12 months was 85% (95%CI: 74–91%) and 54% (95%CI: 15–75%), respectively. In contrast, we found no evidence that the vaccine was effective against all-cause AGE (VE = −20%, 95%CI: −36% to −5%), or against AGE hospitalisations (VE = 35%, 95% CI: −86% to 77%).
Conclusions
In this first detailed assessment of VE of the Rotarix® vaccine in the English national programme, we show that Rotarix® was highly effective in preventing laboratory-confirmed rotavirus infection in young children. This provides reassurance about the vaccine’s performance in real-life settings and gives key information for future cost-effectiveness analyses. The high VE against rotavirus-specific AGE, and the exceptionally successful implementation of the national rotavirus vaccine programme (with >90% vaccine coverage), explains the lack of VE against all-cause AGE because most AGE in the post-vaccine era would not have been due to rotavirus, although some underestimation of VE could also have occurred due to differential healthcare utilisation by vaccinated and unvaccinated infants. This highlights the importance of using specific vaccine-preventable endpoints for these scenarios.
1. Introduction
Rotavirus is a major cause of severe acute gastroenteritis (AGE) in young children in countries that have not introduced infant rotavirus immunisation programmes [1]. Rotavirus group A is responsible for >90% of human infections and virus genotypes are classified by the virus glycoprotein (G-type) and protease-sensitive protein (P-type), which can independently reassort. To date, 15 G-genotypes and 28 P-genotypes have been described, the most common genotype combinations circulating worldwide being G1P[8], G2P[4], G3P[8], G4P[8], G9P[8] and G12P[8]. In pre-licensure trials, the monovalent (G1P[8]) oral live-attenuated rotavirus vaccine Rotarix® (GlaxoSmithKline Biologicals, Rixensart, Belgium) was shown to have >85% efficacy against severe rotavirus-confirmed gastroenteritis in high- and middle-income settings, and up to 40% efficacy against severe all-cause gastroenteritis [2]. In the UK Rotarix® was introduced as a 2-dose schedule at 2 and 3 months of age in July 2013. High national 2-dose vaccine coverage of >90% by the age of 25 weeks was rapidly attained and sustained [3], [4].
We have shown that, following vaccine introduction in England, there was a substantial decline in hospital admissions for all-cause AGE across all age groups and incident episodes of AGE presenting to primary care, with reductions of 49% and 41% respectively among infants during the rotavirus season [4], [5]. We estimated that these reductions were associated with a £12.5 million reduction in healthcare costs for children aged <5 years in the first year of the rotavirus immunisation programme [5].
Although these ecological studies provide strong evidence of the impact of the rotavirus immunisation programme, it is important to extend the analyses to individual-level data to allow direct assessment of rotavirus vaccine effectiveness (VE) in the public health setting. We therefore investigated the direct effect of the monovalent rotavirus vaccine against two different endpoints during the first two years of the programme in England. The first analysis involved a test-negative case-control design using enhanced laboratory surveillance data to estimate VE against laboratory-confirmed rotavirus infections. The second was a cohort study, using large linked electronic health datasets to estimate VE against all-cause AGE, and severe all-cause AGE resulting in hospitalisation.
2. Methods
2.1. Vaccine effectiveness against rotavirus-confirmed AGE
Rotavirus-positive stool specimens were obtained from enhanced national surveillance for rotavirus gastroenteritis, set up following introduction of Rotarix® into the infant immunisation programme in England. Briefly, NHS hospital laboratories in England and Wales routinely report rotavirus-positive infections electronically to Public Health England (PHE) as part of national infection surveillance. Detection methods vary between hospital sites and may have been performed by antigen detection assays or nucleic acid amplification tests. PHE actively followed up all positive reports in vaccine-eligible infants (born on/after 01 May 2013 and aged ≥6 weeks at diagnosis). Reporting laboratories were asked to submit stool specimens in which rotavirus (antigen or RNA) was identified by any method to the national Virus Reference Department (VRD) for confirmation of the rotavirus diagnosis by reverse-transcription real-time PCR [6], [7]. Specimens in which rotavirus RNA was detected at a cycle threshold (Ct) value of ≤35 were considered rotavirus-positive and were eligible for molecular characterisation. These rotavirus-positive specimens were further analysed to determine rotavirus genotypes according to established binomial classification using the virus VP4 (P) and VP7 (G) sequences (as GxP[x]) [8]. All G1P[8] type viruses were further differentiated as wild-type and vaccine-derived. G1P[8] Rotarix® vaccine-derived strains were defined either where sequences of VP4 and VP7 demonstrated highest homology with Rotarix® sequences (accession numbers JX943612 and JX943614, respectively); and/or through detection of Rotarix® sequence directly using a previously published and validated qRT-PCR assay that specifically targets the NSP2 gene of the Rotarix® strain [9]. Controls (infants with rotavirus-negative gastroenteritis) were obtained via eight sentinel NHS hospital laboratories across England. These laboratories reported results for all patients who had been tested for rotavirus, irrespective of the final test result, with information on the patient’s age and sex [10].
In the second surveillance year (July 2014–June 2015), because of the large number of rotavirus-negative results relative to rotavirus-positive results in vaccine-eligible children, we frequency matched on age (10–24 weeks, 25–52 weeks and >52 weeks) and on month of onset to select approximately 10% of the large pool of potential controls. This was to achieve a similar number of controls as wild type rotavirus-confirmed cases. Vaccination status and details of the illness for cases and controls were obtained from their general practitioner using a standardised questionnaire.
2.1.1. Analysis
All data were entered into a custom Access database and anonymised before analysis. Children for whom the sample was taken ≥28 days after the date of onset of illness were excluded from analyses, due to concerns about misclassification of rotavirus status. We also restricted analyses to children who were ≥10 weeks of age, to allow at least two weeks after the first vaccine dose. Samples with an onset date that was missing or was later than the sample date were reassigned with an onset date of four days prior to the sample date, based on the median value of this difference in the remaining samples. Logistic regression was then used to estimate adjusted odds ratios (aOR) for vaccination, adjusting for age in months and the month and year of symptom onset. Episodes of illness that occurred during the two weeks after each dose of vaccine were analysed separately. VE was calculated as (1-aOR). Analyses were conducted initially using all rotavirus-positive cases, and then repeated after excluding rotavirus-positive cases that were subsequently identified as vaccine-derived rotavirus strains or that could not be confirmed on re-analysis by the VRD. Further analyses were conducted to investigate waning of VE by repeating analyses for infants aged younger than and older than twelve months of age. In sensitivity analyses, we estimated VE after substituting the reported date of AGE onset with the date the sample was taken.
2.2. Vaccine effectiveness against all-cause acute gastroenteritis
This analysis utilised anonymised primary care data from the Clinical Practice Research Datalink (CPRD), which comprises a representative sample of approximately 7% of the UK population [11]. Data include clinical, prescription, vaccination and lifestyle data, referrals to and feedback from secondary care. The data were provided pre-linked at an individual level to data on hospitalisations (Hospital Episode Statistics) and on social deprivation (index of multiple deprivation) [12].
We included infants born after April 2013 who were registered within six weeks of age with a CPRD practice in England that had reached established quality standards and had consented to linkage of patients’ general practice records to hospitalisation data. The subset of infants who had a recorded AGE event in their linked electronic health records during the follow-up period were eligible for AGE severity (hospitalisation) analyses.
The primary outcome of interest was incident AGE, diagnosed in general practice or in hospital. Most AGE in general practice is diagnosed clinically without laboratory confirmation of the causative pathogen, and most hospitalised AGE is not coded using pathogen-specific codes. We therefore used a broad definition of AGE, as described in our previous ecological study [5]. Briefly, we identified AGE diagnoses using Read codes in general practice data, and ICD10 codes in the primary or secondary diagnostic fields in the hospitalisation data, with each code categorised into one of four AGE subtypes, namely infectious gastroenteritis, non-infectious gastroenteritis of specified cause, non-infectious gastroenteritis of unspecified cause and gastroenteritis of unspecified type; all codes and their subtype categorisation are provided in the Supplementary data. We assumed that AGE consultations/hospitalisations within 28 days of each other were part of the same episode. The first consultation within an episode was recorded as the incident date of that episode. The type of AGE episode was defined using the constituent Read or ICD10 codes. We excluded episodes of non-infectious AGE of specified cause from further analyses but included non-infectious AGE of unspecified cause, as previous studies have shown that this is often miscoded infectious AGE [5].
The secondary outcome was severe AGE, defined as a hospitalisation for AGE within 28 days of an incident AGE event. The subset of infants whose AGE diagnosis was made in hospital (bypassing the GP) were included in severity analyses and were all considered to have the outcome of interest (an AGE hospitalisation).
Rotavirus vaccination status was identified using recorded information in the infants’ immunisation files in the general practice data, supplemented by Read and prescription codes in the clinical and therapy files (for code lists, see the Appendix). Vaccination status was considered as a time-varying exposure and we subdivided infants’ person-time into: unvaccinated (Group 0); 0–2 weeks after the first vaccine dose (Group 1); >2 weeks after the first vaccine dose and before receipt of the second dose (Group 2); 0–2 weeks after the second vaccine dose (Group 3); and >2 weeks after the second vaccine dose (Group 4). We considered Group 0 as unvaccinated, Group 2 as having had 1 dose and Group 4 as having had both doses. The two-week windows after each dose (Groups 1 and 3) were considered as “partially vaccinated” (for 1st or 2nd dose) and the person-time was considered separately in analyses.
Covariates of interest included age, index of multiple deprivation (at the individual level when available, otherwise at the general practice level), geographical region, calendar year, calendar month and rotavirus season. The latter was based on historical patterns of rotavirus activity and categorised into low (July-September), medium (October-January and May) and high (February-April) activity [13]. We also examined the effect of whether the infant had been a preterm birth, obtaining this information from the subset of infants that were included in the recently available CPRD/LSHTM Pregnancy Register [14].
2.2.1. Analysis
In primary analyses of incident AGE, the start of follow-up for each infant was at six weeks of age; the end of follow up was the earliest of the date the infant left the practice, died or reached one year of age, the date that the practice last contributed data to CPRD, and 30th September 2015. In AGE hospitalisation analyses, the start of follow-up was the AGE incident date, and the end of follow up was determined as for the primary analyses with the additional criterion of 28 days after the AGE diagnosis.
Incidence of AGE, and of an AGE hospitalisation after AGE, were calculated in a random-effects Poisson model (to allow for multiple events per individual), for vaccinated (one- and two- dose) and unvaccinated infants, adjusted for age in months, IMD, calendar month, calendar year, geographical region and pre-term birth status. The adjusted incidence rate ratio (aIRR) was used to calculate VE (1-aIRR). We expected that VE against all-cause AGE would be higher during periods of higher rotavirus circulation, when most AGE in infants would be due to rotavirus. Thus, in further analyses, VE was calculated against AGE diagnosed during the high-activity rotavirus period.
Data from both studies were analysed using Stata MP v.14.1 (StataCorp, College Station, TX)
2.3. Ethics approval
For the laboratory data study, PHE has legal permission, provided by Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002, to process patient confidential information for national surveillance of communicable diseases [15]. This includes PHE’s responsibility to monitor the safety and effectiveness of vaccines. Approval for the all-cause AGE study was obtained from the Observational Research Ethics Committee of the London School of Hygiene and Tropical Medicine (reference:11843) and from the Independent Scientific Advisory Committee (ISAC) of the Medicines and Healthcare products Regulatory Agency (reference:16_048). The ISAC protocol was made available to the reviewers of this paper.
3. Results
3.1. VE against laboratory-confirmed rotavirus infection
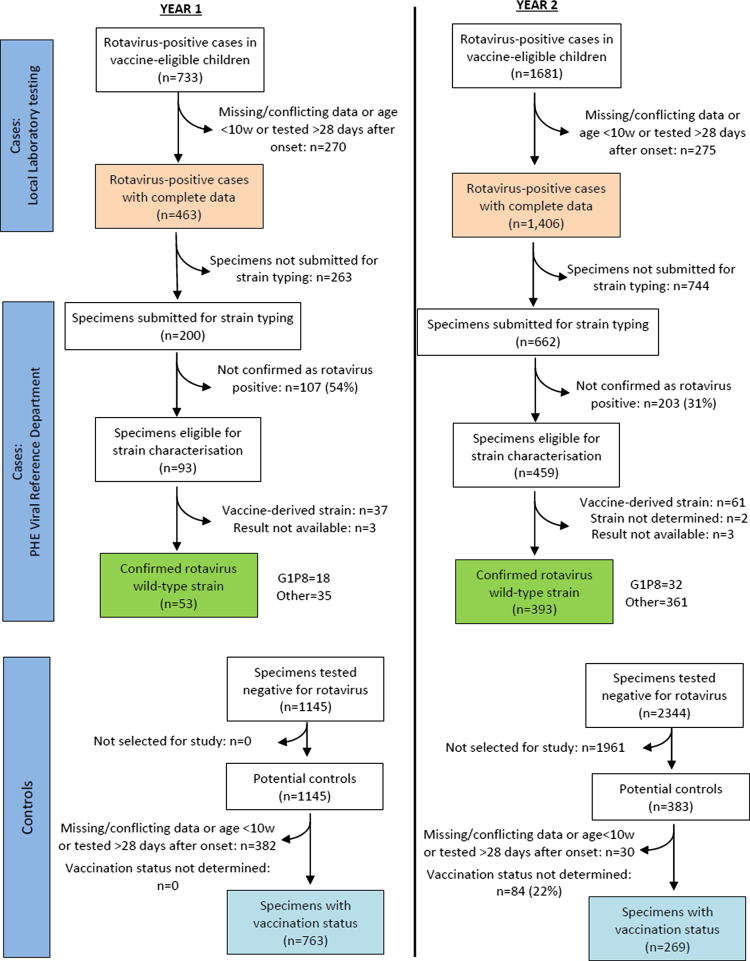
In total, 2414 rotavirus-positive test reports (potential cases) were submitted by local laboratories during the two-year surveillance period, 733 with onset of illness in 2013/14 and 1681 with onset in 2014/15 (Fig. 1). 545 of these potential cases were excluded either because the patient was aged <10 weeks, because the sample was taken >28 days after symptom onset or because of missing or conflicting data. The sentinel laboratories provided 3489 rotavirus-negative test results, from which 1116 were selected as controls. Vaccination status was obtained for all 1869 cases, and for 1032 (92%) controls (763 in 2013/14, and 269 in 2014/15, Fig. 1).
Fig. 1.
Flow chart of results of rotavirus testing of infants (01/07/2013-30/06/2015).
Cases were similar in age to controls in both the first surveillance year (median age of cases versus controls: 19.4 weeks (range: 10.0–58.1 weeks) versus 20.4 weeks (range: 10.0–50.7 weeks)) and in the second year (median age 50 weeks (10.0–110.7 weeks) vs 54.7 weeks (10.4–108.0 weeks)). Cases and controls were also similar with respect to sex in both years (cases: 53.7% male versus controls: 53.5% male), and in the timing of specimen collection (5.9% of cases vs. 5.4% of controls had a gap of >14 days between specimen collection and testing). Among those with available information, 42% of cases (425/1017) and 45% of controls (158/349) were hospitalised. After adjusting for age and for the month and year of illness onset, the estimated VE was modest, at 20% (95% CI: −14% to 43%) for one dose and 40% (23–53%) for two doses (Table 1a).
Table 1.
Rotavirus vaccine effectiveness against rotavirus infection.
| Vaccine dose | Cases | Controls | Adjusted ORa (95% CI) | Vaccine effectiveness (95% CI) |
|---|---|---|---|---|
| (a) All cases | ||||
| 0 | 390 | 173 | Ref | Ref |
| 1 | 186 | 130 | 0.80 (0.57, 1.14) | 20% (−14%, 43%) |
| 2 | 1126 | 653 | 0.60 (0.47, 0.77) | 40% (23%, 53%) |
| TOTAL | 1702b | 956b | ||
| (b) Virus Reference Department confirmed wild-type cases | ||||
| 0 | 133 | 173 | Ref | Ref |
| 1 | 26 | 130 | 0.31 (0.16, 0.60) | 69% (40%, 84%) |
| 2 | 277 | 653 | 0.23 (0.15, 0.34) | 77% (66%, 85%) |
| TOTAL | 436c | 956b | ||
Odds ratio, adjusted for age in months, month of onset of AGE and year of onset of AGE.
Data not shown for 167 cases and 76 controls with AGE onset in the 2 weeks after rotavirus vaccination.
Data not shown for 10 cases with AGE onset in the 2 weeks after rotavirus vaccination.
From the 1869 rotavirus positive cases used in the initial analysis, 862 stool specimens were submitted to the reference laboratory for confirmation of the rotavirus diagnosis and strain typing. A total of 310 (36%) specimens were not confirmed as rotavirus positive and were excluded (Fig. 1). A further 106 specimens were determined to contain vaccine-derived rotavirus strains (n = 98) or had results that were undetermined or unavailable (n = 8). The commonest wild-type rotavirus genotypes detected in the remaining 446 specimens were G1P[8] (n = 50), G2P[4] (n = 106) and G12P[8] (n = 113). VE was appreciably higher when cases were restricted to those with confirmed wild-type infection, at 69% (95% CI:40–84%) for one dose and 77% (95% CI:66–85%) for two doses (Table 1b).
When data were stratified by the age of the child, there was evidence that VE was higher in children aged <12 months for the analysis restricted to confirmed cases, at 85% (95% CI:74–91%) compared to 54% (95% CI:15–75%) for children aged ≥12 months (Table 2).
Table 2.
Rotavirus vaccine effectiveness against rotavirus infection, stratified by age at onset of illness.
| Vaccine dose | Cases | Controls | Adjusted ORa (95% CI) | Vaccine effectiveness (95% CI) |
|---|---|---|---|---|
| (a) Age <12 months: All cases | ||||
| 0 | 255 | 155 | ref | ref |
| 1 | 153 | 125 | 0.81 (0.56, 1.17) | 19% (-17%, 44%) |
| 2 | 624 | 524 | 0.62 (0.47, 0.83) | 38% (17%, 53%) |
| TOTAL | 1032b | 804b | ||
| (b) Age <12 months: Virus Reference Lab confirmed wild-type cases | ||||
| 0 | 79 | 155 | ref | ref |
| 1 | 14 | 125 | 0.20 (0.09, 0.46) | 80% (54%, 91%) |
| 2 | 91 | 524 | 0.15 (0.09, 0.26) | 85% (74%, 91%) |
| TOTAL | 184c | 804b | ||
| (c) Age ≥12 months: All cases | ||||
| 0 | 135 | 18 | ref | ref |
| 1 | 33 | 5 | 0.92 (0.31, 2.72) | 8% (−172%, 69%) |
| 2 | 502 | 129 | 0.54 (0.31, 0.93) | 46% (7%, 69%) |
| TOTAL | 670 | 152d | ||
| (d) Age ≥12 months: Virus Reference Department confirmed wild-type cases | ||||
| 0 | 54 | 18 | ref | ref |
| 1 | 12 | 5 | 0.81 (0.23, 2.86) | 19% (−186%, 77%) |
| 2 | 186 | 129 | 0.46 (0.25, 0.85) | 54% (15%, 75%) |
| TOTAL | 252e | 152d | ||
Odds ratio, adjusted for age in months, month of onset of AGE and year of onset of AGE.
Data not shown for 166 cases and 75 controls with AGE onset in the 2 weeks after rotavirus vaccination.
Data not shown for 10 cases with AGE onset in the 2 weeks after rotavirus vaccination.
Data not shown for 1 control with AGE onset in the 2 weeks after rotavirus vaccination.
No case had AGE onset in the 2 weeks after rotavirus vaccination.
When analyses were repeated using the sample date instead of the reported AGE onset date, results were broadly similar, with two-dose VE against confirmed infection of 72% (95% CI: 59–81%, data not shown). We also carried out a post-hoc analysis, restricted to cases and controls who had specimens tested ≤14 days after symptom onset: VE estimates were very similar to those derived from all cases and controls (Supplementary Table 1).
3.2. VE against all-cause AGE
In total, 40,723 eligible infants were included in the all-cause AGE analysis. Vaccine coverage was high, with 88% receiving one dose and 79% receiving two doses of vaccine.
Over the two-year study period, 4742 infants had ≥1AGE episode (4266 infants had one episode, 438 had two episodes, 34 had three episodes and 4 had four episodes), of which 4845 episodes occurred outside the two weeks after vaccination. Rates of all-cause AGE during non-vaccinated periods, and after receipt of one dose and two doses of vaccine were 160.1, 167.2 and 228.6 cases/1000 person-years respectively (Table 3). After adjusting for age, deprivation, month and year, there was no evidence that the vaccine protected against all-cause AGE, with effectiveness of 0% (95% CI: −16 to 13%) for one dose and −20% (95% CI: −36 to −5%) for two doses. Further adjustment for geographical region made little difference to the results (data not shown). Only 166 AGE episodes (3.4%) among 164 infants resulted in hospitalisation. Although the point estimates for VE against hospitalised AGE were greater than zero, with a 47% and 35% reduction in AGE hospitalisations after one and two doses respectively, 95% confidence intervals were wide and included the null value (Table 3).
Table 3.
Rotavirus vaccine effectiveness against (a) all-cause acute gastroenteritis (AGE), and (b) hospitalisation after AGE.
| Vaccine dose | Person-years | AGE cases | Rate/1000py | Adjusted RRa(95% CI) | Vaccine effectiveness (95% CI) |
|---|---|---|---|---|---|
| (a) Against any AGE | |||||
| 0 | 3534 | 566 | 160.1 | Ref | Ref |
| 1 | 2701 | 452 | 167.2 | 1.00 (0.87, 1.16) | 0 (−16, 13) |
| 2 | 16,733 | 3827 | 228.6 | 1.20 (1.05, 1.36) | −20 (−36, −5) |
| TOTAL | 22,968 | 4845 | |||
| (b) Against hospitalised AGE | Hospitalisations | ||||
| 0 | 95.2 | 26 | 272.9 | ref | ref |
| 1 | 114.7 | 17 | 147.13 | 0.53 (0.19, 1.45) | 47 (-45, 81) |
| 2 | 1533.7 | 106 | 69.07 | 0.65 (0.23, 1.86) | 35 (−86, 77) |
| TOTAL | 1743.6 | 149 | |||
Incidence rate ratio, adjusted for age, deprivation, month of onset of AGE and year of onset of AGE.
Similar results were found after stratifying by year, and after restricting to AGE in high rotavirus season (Supplementary Table 2). Among the subset of 34,561 infants who were included in the CPRD pregnancy register, additional adjustment for prematurity gave an estimated effectiveness against all-cause AGE of 0% (95% CI: −15 to 26%) for one dose, and −18% (95% CI: −36 to −2%) for 2 doses.
After seeing these results, we considered whether health-seeking behaviour might have resulted in increased AGE ascertainment among vaccinated infants, resulting in underestimation of VE. We therefore carried out a further analysis, restricting to infants who had at least one other general practice consultation after four months of age. In this revised analysis, there remained no evidence of effectiveness against all-cause AGE (effectiveness for one dose: 3% (95% CI: −12 to 16%); for two doses: −17% (95% CI: −33 to −3%)
4. Discussion
In these two studies of a highly vaccinated infant population, a strong protective effect of Rotarix® was demonstrated against laboratory-confirmed rotavirus infection in the first two years of the programme; estimated effectiveness was 77% overall, and >80% in infants in their first year of vaccine receipt. In contrast, we did not demonstrate VE against all-cause AGE; as discussed below, this is likely to be due to the successful implementation of the vaccination programme, with high and sustained vaccine coverage and a substantial impact of the vaccine.
Inclusion of all cases initially classified as rotavirus-positive by local laboratories led to lower VE, and demonstrates the importance of confirming rotavirus diagnoses and strain characterisation, particularly to distinguish wild-type infection from excreted vaccine. Methods employed for primary detection of rotavirus infection across frontline diagnostic laboratories differs from site-to-site. Whilst there is a general trend to move toward molecular assays, some laboratories continue to utilise immunochromatographic methods which can have low diagnostic specificity compared to enzyme immnoassays and nucleic acid detection [16]. Laboratory methods for detection of rotavirus RNA based on PCR approaches are highly sensitive and can detect the presence of viral RNA in both clinical and subclinical infection. Studies have attempted to identify Ct cutoff values which discriminate clinical from subclinical infection, for which Ct values around Ct 24–27 have been recommended [17], [18]. In this study, we selected a higher Ct cutoff of 35 because alongside confirming rotavirus infection associated with clinical disease, we were interested to monitor where G1P[8] Rotarix®-vaccine-derived viruses were being detected in infants, and we anticipated that RNA levels in such specimens would be low and hence associated with Ct values >27. It is likely that among cases in whom vaccine-derived virus was detected, AGE was caused by other pathogens or non-infectious aetiologies in recently rotavirus-vaccinated children, with the vaccine strain found coincidently (thus misclassifying cases). Clinicians managing infants in primary and secondary care need to be aware that infants vaccinated with the oral rotavirus vaccine may secrete the vaccine strain in their stools for up to three weeks after vaccination; submitting stool samples for rotavirus testing during this period may, therefore, lead to a misdiagnosis of rotavirus gastroenteritis. Whilst the attenuated vaccine strain may cause mild symptoms including diarrhoea in some immunised infants, other causes need to be considered in unwell infants with more severe symptoms. Nearly all vaccine-derived strains are identified in 2- and 3-month old infants (i.e. when they are due the oral rotavirus vaccine) and do not constitute vaccine failure, unless the strain is confirmed by the reference laboratory as wild-type, which is now rare in the UK in this age group in particular. The increasing use of molecular testing by local laboratories highlights the importance of referral of specimens for differentiating wild-type rotavirus infections from vaccine derived strains, and continued work to establish cut offs to distinguish clinically relevant infections.
Our estimated VE against laboratory-confirmed rotavirus infection is consistent with findings from pre-licensure trials in high-income settings and with other European post-licensure studies of vaccine-eligible age groups (estimating the direct effect of vaccination); the latter have reported effectiveness against healthcare visits for rotavirus-related illness ranging from 68 to 98% [2], [19]. Evidence of waning VE of the monovalent vaccine over the first 2–3 years of life has been inconsistent. A recent systematic review reported evidence of similar effectiveness among children aged <12 months versus >12 months in low-mortality settings, but lower effectiveness in the second year of life in studies from medium- and high-mortality settings [20]. However, a study in Germany, where both the monovalent and pentavalent rotavirus vaccines are available, reported lower VE against outpatient visits for rotavirus diarrhoea among children aged 18–29 months compared to children aged 6–17 months (57% vs 74% respectively) [21].
Our findings of no VE against all-cause AGE is in contrast to the results of our previous vaccine impact studies, which showed marked reductions in all-cause AGE at the population level across healthcare settings after rotavirus vaccine introduction, for infants and for older children not targeted for vaccination [4], [5]. Other studies of the monovalent vaccine in populations with appreciably lower vaccination coverage than in the UK have found higher VE against AGE [22]. For example, in Israeli infants, VE against AGE healthcare consultations was 50.1% (95% CI:47.5–52.6%), and in Australian children aged <60 months, effectiveness against AGE hospitalisations was 77.7% (95% CI:40.2–91.7%) [23], [24]. Conversely, there was no evidence of effectiveness against community cases of AGE in a study of Brazilian infants with high vaccine uptake, with similar incidence of AGE in unvaccinated and unvaccinated children [25].
The divergent findings of a strong impact of rotavirus vaccine against all-cause AGE but little evidence of VE is likely to be due to the highly effective implementation of the vaccination progamme, with rapid attainment of >90% vaccine coverage by 25 weeks of age, together with high VE against rotavirus-specific AGE [3], [4]. As a result, almost all AGE in the study population in the post-vaccine era was likely to have been due to non-rotavirus organisms (or non-infectious causes). This also explains the higher VE against all-cause AGE found in previous studies of rotavirus in populations with lower vaccine coverage, in which there would have been more circulating rotavirus among infants compared to our study population.
Our two studies have several strengths. Both were population-based with results that should be generalizable to the UK population, and the cohort study was large. We used detailed diagnostic algorithms to identify episodes of all-cause AGE, and multivariable analyses to adjust for important potential confounders. Samples in the laboratory-based study underwent additional testing to confirm or refute rotavirus diagnoses and, importantly, to exclude cases with positive rotavirus results due to detection of excreted vaccine virus rather than wild-type rotavirus infection. Also, local laboratory staff were unaware of the vaccination status of the children tested for rotavirus, minimising the risk of bias arising from preferential sending of samples to Public Health England.
Some limitations also need consideration. There were relatively small numbers of laboratory-confirmed cases, due to the effectiveness of the vaccine and high vaccine coverage, resulting in relatively wide confidence intervals for some estimates. The limited number of confirmed cases also prevented separate assessment of VE against homotypic and heterotypic rotavirus strains, although the high effectiveness estimate obtained is consistent with strong cross-protection against non-G1P[8] rotavirus strains shown in the pre-licensure trials [26], [27]. Information on geographical region and on socio-economic status was not available in the laboratory dataset, and so these variables were not adjusted for in the test negative analysis. However, national rotavirus vaccine coverage data indicate that there is little variation in vaccine uptake regionally [28]. Furthermore, the cases and controls in the test negative study all had AGE, had sought healthcare, and from the available evidence appear to have had similar severity of illness. Thus, socioeconomic status should not have been a major confounder of the rotavirus-confirmed VE estimate; cases and controls are also likely to have been similar with respect to other risk factors for an acute gastrointestinal illness. Not all general practices responded to the request for vaccination data for the controls in the second year of the study, but response rates were 100% for cases (in both study years) and for controls in the first year, and we have no reason to believe that infants from non-responding practices had different vaccine uptake to those that responded. Similarly, it seems unlikely that GPs would under-report vaccination history in cases or controls – if any under-reporting did occur it would most likely be random with respect to case/control status, which would result in a (probable small) underestimation of VE.
It is possible that some of the apparent lack of effect of vaccination against all-cause AGE (and the slight protective effect seen for two doses) was due to health-seeking behaviour among parents of vaccinated children. However, estimates were very similar when analyses were restricted to infants that were being brought to the GP for other reasons, and health-seeking behaviour is unlikely to explain the lack of effectiveness against severe AGE resulting in hospitalisation. Nevertheless, some underestimation of VE against all-cause AGE could have occurred as a result of differential healthcare utilisation by vaccinated and unvaccinated infants. A recent UK study applied novel methods to estimate propensity for vaccine uptake in a primary care birth cohort of infants and applied this to estimate rotavirus VE against all-cause AGE seen in general practice [29]. After adjusting for propensity to be vaccinated, the direct effectiveness of rotavirus vaccine shifted from a negative value (VE = −26%, 95%CI: −48%, −7%) to no clear evidence of effectiveness (VE = 11%, 95%CI: −11%, 29%); when further restricted to rotavirus season (defined as January-May), the propensity-adjusted direct VE was positive, although with very wide confidence intervals (VE = 26%, 95%CI = 1%, 45%). A strength of the test-negative design is that the caregivers of all the children (cases and controls) sought care for their child, and thus if a control had developed rotavirus infection they would have been likely to be included as a case.
In conclusion, we have demonstrated high effectiveness of monovalent rotavirus vaccine against rotavirus-confirmed infections in the first two years of the programme. This provides reassurance that the vaccine is effective in routine use in England, and provides key information for future cost effectiveness analyses, helping to ensure the financial sustainability of the rotavirus vaccination programme. As data accumulate in the post-vaccination era, more detailed assessment of waning of effectiveness over time can be undertaken, and investigation of rotavirus strain-specific protection. Our study also highlights the key importance of using specific outcomes when vaccine coverage and vaccine effectiveness are both very high.
Acknowledgments
Acknowledgements
We would like to thank the PHE Rotavirus Surveillance Network: Dr Rohini Manuel and Dr Duncan Clark of the Public Health Laboratory London, Barts Health NHS Trust, London; Dr John Magee of the Public Health Laboratory Newcastle, Freeman Hospital, Newcastle; Dr Husam Osman of the Public Health Laboratory Birmingham, Heart of England NHS Foundation Trust, Birmingham; Dr Anthony D Hale of the Public Health Laboratory Leeds, Leeds Teaching Hospitals NHS Trust, Leeds; Dr Hamid Jalal, Dr Mark Farrington and Dr Martin L’Estrange of the Public Health Laboratory Cambridge, Addenbrooke’s Hospital, Cambridge; Dr Ed Kaczmarski and Dr Andrew Turner of the Public Health Laboratory Manchester, Manchester Royal Infirmary, Manchester; Dr Emanuela Pelosi and Dr Peter Hawtin of the Public Health Laboratory Southampton, Southampton General Hospital, Southampton; Dr David Carrington and Dr Adam Finn of the Public Health Laboratory Bristol, Bristol. We would also like to thank the other laboratories who submitted rotavirus-positive specimens to the national Virus Reference Department for confirmation of the diagnosis and strain characterisation, the general practices who provided details of the gastrointestinal illness and vaccination status of cases and controls in the laboratory-based study, and Sameena Nawaz, formerly of VRD, PHE, Colindale, for the work in confirming and genotyping rotavirus strains.
Author contributions
All authors were involved in the design of the studies. JLW carried out the statistical analyses and co-wrote the first draft with SLT. All authors contributed to further drafts and approved the final manuscript.
Funding
This research was funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Immunisation at the London School of Hygiene and Tropical Medicine in partnership with Public Health England (PHE). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England. The funders had no role in the study design, data collection, analysis, or interpretation.
Conflict of Interest
The Immunisation and Countermeasures Division of Public Health England has provided vaccine manufacturers with post-marketing surveillance reports which the Marketing Authorisation Holders are required to submit to the UK Licensing Authority in compliance with their Risk Management Strategy. A cost recovery charge is made for these reports. The authors report no other relationships or activities that could appear to have influenced the submitted work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2019.100005.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Giaquinto C., van Damme P. Age distribution of paediatric rotavirus gastroenteritis cases in Europe: the REVEAL study. Scand J Infect Dis. 2010;42:142–147. doi: 10.3109/00365540903380495. [DOI] [PubMed] [Google Scholar]
- 2.Soares-Weiser K., Maclehose H., Bergman H. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2012;11:CD008521. doi: 10.1002/14651858.CD008521.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Public Health England. Rotavirus infant immunisation programme 2014/15: Vaccine uptake report on the temporary sentinel data collection for England (PHE publications gateway number 2015141). Available at https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/440456/RotavirusGatewayFinalVersion.pdf [Accessed 17/12/2017].
- 4.Atchison C.J., Stowe J., Andrews N. Rapid declines in age group-specific rotavirus infection and acute gastroenteritis among vaccinated and unvaccinated individuals within 1 year of rotavirus vaccine introduction in England and Wales. J Infect Dis. 2016;213:243–249. doi: 10.1093/infdis/jiv398. [DOI] [PubMed] [Google Scholar]
- 5.Thomas S.L., Walker J.L., Fenty J. Impact of the national rotavirus vaccination programme on acute gastroenteritis in England and associated costs averted. Vaccine. 2017;35:680–686. doi: 10.1016/j.vaccine.2016.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iturriza Gómara M., Wong C., Blome S., Desselberger U., Gray J. Molecular characterization of VP6 genes of human rotavirus isolates: correlation of genogroups with subgroups and evidence of independent segregation. J Virol. 2002;76:6596–6601. doi: 10.1128/JVI.76.13.6596-6601.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iturriza Gómara M., Simpson R., Perault A.M. Structured surveillance of infantile gastroenteritis in East Anglia, UK: incidence of infection with common viral gastroenteric pathogens. Epidemiol Infect. 2008;136:23–33. doi: 10.1017/S0950268807008059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iturriza-Gómara M., Dallman T., Bányai K. Rotavirus genotypes co-circulating in Europe between 2006 and 2009 as determined by EuroRotaNet, a pan-European collaborative strain surveillance network. Epidemiol Infect. 2011;139:895–909. doi: 10.1017/S0950268810001810. [DOI] [PubMed] [Google Scholar]
- 9.Gautam R., Esona M.D., Mijatovic-Rustempasic S., Ian Tam K., Gentsch J.R., Bowen M.D. Real-time RT-PCR assays to differentiate wild-type group A rotavirus strains from Rotarix(®) and RotaTeq(®) vaccine strains in stool samples. Hum Vaccin Immunother. 2014;10:767–777. doi: 10.4161/hv.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atchison C., Collins S., Brown D., Ramsay M.E., Ladhani S.L. Reduction in rotavirus disease due to the infant immunisation programme in England; evidence from national surveillance. J Infection. 2015;71:128–131. doi: 10.1016/j.jinf.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Herrett E., Gallagher A.M., Bhaskaran K. Data resource profile: clinical practice research datalink (CPRD) Int J Epidemiol. 2015;44:827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anon. CPRD linked data. Available at https://www.cprd.com/dataaccess/LinkedData.asp [Accessed 17/12/2017].
- 13.Public Health England. Rotavirus: Guidance, data and analysis. The characteristics, diagnosis, surveillance and epidemiology of rotavirus. April 2013. Available from: https://www.gov.uk/government/collections/rotavirus-guidance-data-and-analysis [Accessed 14/12/2018].
- 14.Minassian C, Thomas SL, Williams R, Meeraus W. CPRD Pregnancy Register (Version 1.0). CPRD, December 2016.
- 15.National Health Service England and Wales. The Health Service (Control of Patient Information) Regulations 2002 (2002 No. 1438m Regulation 3). Available at http://www.legislation.gov.uk/uksi/2002/1438/regulation/3/made [Accessed 17/12/2017].
- 16.Ye S., Lambert S.B., Grimwood K. Comparison of test specificities of commercial antigen-based assays and in-house PCR methods for detection of rotavirus in stool specimens. J Clin Microbiol. 2015;53:295–297. doi: 10.1128/JCM.02251-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips G., Lopman B., Tam C.C., Iturriza-Gomara M., Brown D., Gray J. Diagnosing rotavirus A associated IID: using ELISA to identify a cut-off for real time RT-PCR. J Clin Virol. 2009;44:242–245. doi: 10.1016/j.jcv.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Bennett A., Bar-Zeev N., Jere K.C. Determination of a viral load threshold to distinguish symptomatic versus asymptomatic rotavirus infection in a high-disease-burden African population. J Clin Microbiol. 2015;53:1951–1954. doi: 10.1128/JCM.00875-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karafillakis E., Hassounah S., Atchison C. Effectiveness and impact of rotavirus vaccines in Europe, 2006–2014. Vaccine. 2015;33:2097–2107. doi: 10.1016/j.vaccine.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Jonesteller C.L., Burnett E., Yen C., Tate J.E., Parashar U.D. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006–2016. Clin Infect Dis. 2017;65:840–850. doi: 10.1093/cid/cix369. [DOI] [PubMed] [Google Scholar]
- 21.Adlhoch C., Hoehne M., Littmann M. Rotavirus vaccine effectiveness and case-control study on risk factors for breakthrough infections in Germany: 2010–2011. Pediatr Infect Dis J. 2013;32:e82–e89. doi: 10.1097/INF.0b013e3182720b71. [DOI] [PubMed] [Google Scholar]
- 22.Hungerford D., Smith K., Tucker A. Population effectiveness of the pentavalent and monovalent rotavirus vaccines: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2017;17:569. doi: 10.1186/s12879-017-2613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muhsen K., Chodick G., Goren S., Shalev V., Cohen D. The uptake of rotavirus vaccine and its effectiveness in preventing acute gastroenteritis in the community. Vaccine. 2010;29:91–94. doi: 10.1016/j.vaccine.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Snelling T.L., Schultz R., Graham J. Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis. 2009;49:428–431. doi: 10.1086/600395. [DOI] [PubMed] [Google Scholar]
- 25.Fontes Vieira S.C., Queiroz Gurgel R., Kirby A. Acute diarrhoea in a community cohort of children who received an oral rotavirus vaccine in Northeast Brazil. Mem Inst Oswaldo Cruz. 2011;106:330–334. doi: 10.1590/s0074-02762011000300012. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Palacios G.M., Pérez-Schael I., Velázquez F.R. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 27.Vesikari T., Karvonen A., Prymula R. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 28.Public Health England. Rotavirus immunisation programme: vaccine coverage estimates. Available at https://www.gov.uk/government/publications/rotavirus-immunisation-programme-vaccine-coverage-estimates [Accessed 14/12/2018].
- 29.Hungerford D., Vivancos R., Read J.M. Mitigating bias in observational vaccine effectiveness studies using simulated comparator populations: applications to rotavirus vaccination in the UK. Vaccine. 2018;36(45):6674–6682. doi: 10.1016/j.vaccine.2018.09.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.