Abstract
Introduction
The World Health Organization recommends vaccination of health workers (HWs) against influenza, but low uptake is intransigent.
We conducted a Rapid Evidence Appraisal on: the risk of influenza in HWs, transmission risk from HWs to patients, the benefit of HW vaccination, and strategies for improving uptake. We aimed to capture a ‘whole-of-system’ perspective to consider possible benefits for HWs, employers and patients.
Methods
We executed a comprehensive search of the available literature published from 2006 to 2018 in the English language. We developed search terms for seven separate questions following the PICO framework (population, intervention, comparators, outcomes) and queried nine databases.
Results
Of 3784 publications identified, 52 met inclusion criteria. Seven addressed HW influenza risk, of which four found increased risk; 15 addressed influenza vaccine benefit to HWs or their employers, of which 10 found benefit; 11 addressed influenza transmission from HWs to patients, of which 6 found evidence for transmission; 12 unique studies addressed whether vaccinating HWs produced patient benefit, of which 9 concluded benefits accrued. Regarding the number of HWs needed to vaccinate (NNV) to deliver patient benefit, NNV estimates ranged from 3 to 36,000 but were in significant disagreement. Fourteen studies provided insights on strategies to improve uptake; the strongest evidence was for mandatory vaccination.
Conclusions
The evidence on most questions related to influenza vaccination in HWs is mixed and often of low-quality. Substantial heterogeneity exists in terms of study designs and settings, making comparison between studies difficult. Notwithstanding these limitations, a majority of studies suggests that influenza vaccination benefit HWs and their employers; and HWs are implicated in transmission events. The effects of vaccinating HWs on patient morbidity and mortality may include reductions in all-cause mortality and influenza-like illness (ILI). Taken together, the evidence suggests that HW vaccination is an important policy for HWs themselves, their employers, and their patients.
Keywords: Influenza, Vaccine, Health worker, Healthcare, Policy, Transmission
Abbreviations: HW, health workers; WHO, World Health organization; ILI, influenza like illness; NNV, number needed to vaccinate; LTCF, long-term care facility(ies); OR, odds ratio; GAVI, the global alliance for vaccines and immunization; RCTs, randomised controlled trials; RR, relative risk; cRCTs, clustered randomised controlled trials
1. Introduction
1.1. Background
In 2012, the World Health Organization (WHO) updated its recommendation on influenza vaccination of health workers (HWs) concluding that “HWs are an important priority group for influenza vaccination, not only to protect the individual and maintain health-care services during influenza epidemics, but also to reduce spread of influenza to vulnerable patient groups” [1], [2]. The WHO Global Influenza Strategy (2019–2030) reinforces this position by supporting countries to “develop and implement national, seasonal immunization policies for HWs and other high-risk groups” [3].
In 2017, 96 of the 194 WHO Member States reported having policies in place for influenza vaccination of HWs [4]. However, the Member States with such policies are unevenly distributed across WHO regions. Aside from mitigating seasonal influenza, influenza vaccine is considered the primary intervention to reduce mortality and morbidity in a severe pandemic. An efficient pandemic response depends largely on how well seasonal vaccine response to annual epidemics is embedded and implemented [5].
The Review Committee on the Functioning of the International Health Regulations (2005) in relation to Pandemic (H1N1) 2009 concluded that experience with comprehensive seasonal influenza programmes would provide valuable preparation in advance of a major pandemic [6]. In November 2018, the Global Alliance for Vaccines and Immunization (GAVI) Board requested that the GAVI secretariat in collaboration with WHO assess the feasibility and impact of routine influenza immunization of HWs to support epidemic and pandemic influenza preparedness [7].
To support these global considerations and considering the poor uptake of influenza vaccination in HWs despite the multiple reasons advocated for it as a policy, we conducted a Rapid Evidence Appraisal [8]. We aimed to capture the breadth of available evidence on influenza in health care settings and the wider health impacts of influenza vaccination in HWs. We critically appraised the literature relating to: influenza risk to HWs and the risk they pose to their patients; the benefit of influenza vaccination in HWs; and influenza vaccine uptake in HWs. By including evidence relevant to multiple stakeholders collectively - HWs, patients, and their employers – we holistically evaluated the evidence in the most policy-relevant framework as possible.
2. Methods
2.1. Systematic literature search
General Search Approach: The search objective was to examine the evidence under three topics using a Rapid Evidence Appraisal approach. Each topic gave rise to 2 or 3 questions for query (see Table 1). Evidence was queried from MEDLINE, EMBASE, CINAHL, Cochrane CENTRAL, Cochrane Reviews, WHO Global Index Medicus, National Health Service (NHS) Evidence, NHS HTA database, and multiple international clinical trials’ registries. We executed three independent searches by topic with distinct search terms to capture literature for all topic questions. Search terms followed the PICO framework (population, intervention, comparators, outcomes) and studies were selected based on pre-determined criteria.
Table 1.
Rapid Evidence Appraisal topics, queried questions, number of studies selected, and their associated references.
| Topic | Description | Questions | Total Studies cited/question | Refs. |
|---|---|---|---|---|
| 1 | The Impact of HW Influenza Vaccination on HWs and their employers |
|
7 | [11], [12], [13], [14], [15], [16], [17] |
|
14 | [11], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30] | ||
| 2 | The Impact of HW Influenza Vaccination on their patients |
|
11 | [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41] |
|
6 | [11], [25], [42], [43], [44], [45] | ||
|
8 | [11], [42], [46], [47], [48], [49], [50], [51] | ||
| 3 | Influenza Vaccine Uptake in HWs- hurdles and solutions |
|
11 | [11], [25], [52], [53], [54], [55], [56], [57], [58], [59], [60] |
|
3 | [11], [61], [62] | ||
Study Types varied by review topic and question but generally included experimental or observational studies, systematic reviews, controlled observational studies, and randomized controlled trials (RCTs). Due to the volume of evidence for Topic 3 (HW vaccine uptake), literature was restricted to systematic reviews, RCTs, and evidence-based policy guidelines or toolkits. Study Outcomes were limited to laboratory-confirmed influenza as a primary outcome measure, when relevant to the queried question. Other outcomes included sickness absence incidence and/or duration, economic or productivity costs due to illness, influenza-like- illness (ILI) or respiratory illness, the number needed to vaccinate (NNV), and vaccination uptake in HWs.
Study Selection, exclusion and inclusion: Studies were selected and extracted onto a standardized template by 3 reviewers (DJ, HM, KM∋) independently, defaulting to a third party (JSN-V-T) as needed. Study selection followed the following process: remove duplicates and unrelated content by title; review abstracts - remove unrelated content, descriptive studies, commentaries, or studies that clearly do not meet the inclusion/exclusion criteria defined for each question; review full text - exclude based on exclusion/inclusion criteria or remove studies that lack a clear study design, methodology, or results section. Studies were then ranked according to the Maryland Scientific Methods (SMS) Quality Scale [9] with 1 being the lowest and 5 being the highest [10]. We limited the inclusion of studies to those scoring 2–5 on the quality scale published from 2006 to 2018 in whole or in summary in the English language.2
2.2. Data handling
Data Extraction: Two reviewers (DJ, KM) executed the search strategy and results were independently screened by three reviewers (DJ, HM, KM) for eligibility using a three-stage sifting approach of title, abstract and full-text. The two reviewers for 2006–2016 (DJ, HM) literature reached consensus on which search hits met the criteria for inclusion at each stage (including exclusion at the full-text stage). Any disagreements were resolved by discussion or involvement of an additional reviewer.
Data analysis: Detailed characteristics of included studies were captured and descriptively summarized in tables and figures identifying study design, population, setting, measured outcomes, results, and limitations (See Table 2, Table 3, Table 4, Table 5).
Table 2.
Rapid Evidence Appraisal included studies and their characteristics for Topic 1 - Questions 1&2.
| Study | Maryland Quality Score and classification | Study Design | Country | Setting | Included Studies/or Population type | # of participants | Intervention or Focus | Outcomes | Are HWs at a greater risk than the general population Yes/No |
|---|---|---|---|---|---|---|---|---|---|
| Dini [11] | 4 High Moderate |
Review | Mixed | Hospital | 2 systematic reviews/ 28 included address this question | 29,358 subjects | H1N1pdm09 vaccine |
Lab-confirmed influenza infection | Yes for H1N1pdm09 |
| Ip [12] | 2 Low Moderate |
OS- RC | Hong Kong | Hospital | Inpatient and outpatient health workers | ∼ 6000 | H1N1pdm09 risk and impact | All cause or Acute Respiratory Illness Related Sickness Absenteeism during influenza epidemics |
Yes during 2009 Inconclusive with seasonal influenza |
| Cooley [13] | No Score-Model Simulation |
Pandemic Simulation Model |
USA | Hospital | Simulation of influenza epidemic and the impact on hospital-based HWs | N/A | Seasonal vaccine |
Secondary attack rate of pandemic influenza among unprotected HWs. | Yes, for pandemic influenza |
| Bellei [14] | 2 Low Moderate |
OS -PC | Brazil | -Outpatients -Community |
Outpatient health workers, outpatients with acute respiratory infection, renal-transplant patients | 203 HWs 140 community 69 renal-transplant |
N/A | Lab-confirmed influenza rates by population, clinical symptoms, and risk factor. | Inconclusive, but vaccination may have confounded results |
| Yiannakoulias [15] | 3 Moderate |
OS-Retrospective CC |
Canada | -Province-wide assessment using billing data | Medical professionals | Exposure to ILI in a patient | Frequency of exposure to patient with ILI in 7 days prior to ILI diagnosis in case providers vs. matched control providers | Yes, but can’t make a clear connection between exposure and ILI using billing data | |
| Sartor [16] | 2 Low Moderate |
OS -PC | France | Internal Medicine Ward | Inpatients and health workers | 23 patients and 22 HWs | N/A | Attack rate of influenza A among patients and HWs | Yes, but patients also at risk during an outbreak |
| Elder [17] | 2 Low Moderate |
OS - CS | Scotland | Acute care hospitals | Inpatient health workers with regular patient contact | 518 | N/A | Serologically-confirmed influenza. Proportion of asymptomatic infection (recall + serology) |
Yes for asymptomatic infection |
| Imai [18] | 5 | SR and meta-analysis | Mixed | 13 studies total 3 RCTs, 2PC, 8RC |
N = 20,282; 5,083 vaccinated 15,199 not |
Seasonal Vaccine only |
Pooled n = RR, Lab-confirmed flu, ILI, absenteeism, mean difference, cost-effectiveness |
Does vaccine benefit HWs or their employers? Yes/No |
|
| High | Yes | ||||||||
| Dini [11] | 4 High Moderate |
Review | Mixed | 6 systematic reviews relevant to this question/ 28 studies | Didn’t pool | Influenza Vaccine |
Lab-confirmed flu, ILI, absenteeism | Yes | |
| Gianino [20] | 2 Low moderate |
OS-RC | Italy | Hospital | Hospital health workers | ∼ 5000 | Seasonal vaccine |
Absenteeism during influenza epidemic periods | Yes |
| Pereira [21] | 3 Moderate |
OS- RC | England | 223 health trusts each season | Hospital, primary care, inpatient, outpatient, specialist | ∼800,000 | Seasonal vaccine |
Sickness absence rate, Relationship between sickness absence rate and uptake | Yes |
| Frederick [25] | 3 Moderate |
OS-RC | USA | Hospitals & Outpatient | Health workers | ∼3000 | Mandatory vaccine policy |
Incidence/length of absenteeism mandatory vs. non-mandatory sites | Yes |
| Study | Maryland Quality Score and classification | Study Design | Country | Setting | Included Studies/or Population type | # of participants | Intervention or Focus | Outcomes |
Does vaccine benefit HWs or their employers? Yes/No |
| Riphagen-Dalhuisen [28] | 4 High Moderate |
cRCT | The Netherlands | 6 Tertiary Medical Centres | All hospital HWs during 2009–2011 | HWs = all from | Multi-faceted uptake intervention for H1N1pdm09 and seasonal vaccine | Vaccine uptake; absenteeism rates among HWs in December of each year | No to absenteeism, actually saw an increase |
| Hui [30] | 4 High Moderate |
RCT | Malaysia | Faculty of Dentistry | Staff/students of the faculty of Dentistry Control | .Control = 176 Vaccinated = 179 |
Seasonal Vaccine |
Follow up over a 4-month period from vaccination, via questionnaire to ascertain self-reported outcomes: ILI prevalence; Recurrence of ILI; Visits to doctor due to ILI; Family members or housemates reporting ILI, Days of absenteeism due to ILI, Fever days due to ILI | Yes, to all self-reported outcome measures |
| Nguyen- VanTam [23] | 2 Low moderate |
OS- RC-TS | England | Teaching Hospital, multiple wards | HWs – non-medical staff (nurses/ porters); nursing staff from admissions, intensive therapy and surgical; porters dealing with supplies and waste | N = 271 | N/A | Individual sickness absence contrasted epidemic/ non-epidemic periods in season. Measured: Total time lost/person as % of rostered time.# weeks/ person which absence occurred ‘92-‘93 season non-epidemic yr. comparison, since low flu activity | No |
| Atamna [26] | 3 Moderate |
OS-PC | Israel | Medical Centre | Health workers, vaccinated vs. non-vaccinated during flu seasons and not | 733 vaccinated 908 not (199 in flu season) | Seasonal Vaccine | Rates of PCR confirmed influenza A infection in vaccinated vs. non vaccinated HWs | inconclusive |
| Njuguna [22] | 2 Low moderate |
OS-PC | Kenya | 5 Hospitals with influenza surveillance | Health Workers who were offered free vaccine | 3803 | H1N1pdm09 vaccine |
Vaccine effectiveness in vaccinated vs unvaccinated HCP: Incidence of acute respiratory illness, absenteeism from work due to respiratory illness laboratory-confirmed influenza (using PCR) | No, actually increased absenteeism |
| Saadeh-Navarro [27] | 2 Low moderate |
OS-RC | Mexico | Teaching Hospital | Vaccinated HWs (health care worker defined as all personnel with patient contact) | N = 3636 | Seasonal influenza vaccine |
A reduction in ILI, Absence due to ILI Total days of work lost |
Yes |
| Fujita [24] | 2 Low moderate |
OS-RC | Japan | University hospital | Nursing Staff | 1680 | Seasonal vaccine |
Vaccine effectiveness through self-reported fever and cold-like symptoms. Days absent. |
Yes |
| Preaud [29] | No Score Economic Model |
EM | 8 European Countries | All health workers for those countries | Mixed HWs | N/A | Seasonal trivalent vaccine |
Number of influenza-related events averted (cases, GP visits, hospitalisations, deaths and days of work lost) at current vaccination coverage rates. | Yes, to cases averted |
| Parlevliet [31] | No Score Cost-Benefit Analysis |
CBA | The Netherlands | Hospitals | Health workers | 6251 | Seasonal vaccine |
Retrospective cost-benefit model as employer’s perspective to see costs and benefits. For vaccine programs as workplace absenteeism. | Yes, To cost benefit to employers |
LEGEND: CC– Case Controlled; OS- Observational Study; CS- Cross Sectional; PC- Prospective Cohort; RC- Retrospective Cohort; SR-Systematic Review; EM- Economic Model; CBA- Cost-Benefit Analysis; TS- Time Series; RCT- Randomised Controlled Trial; cRCT- Clustered Randomised Controlled Trial; UC- Unclear; Q1 – Question 1; Q2 – Question 2; RR-Risk Ratio.
Table 3.
Rapid Evidence Appraisal included studies and their characteristics for Topic 2 - Questions 1 – 3.
| Study | Maryland Quality Score and classification | Study Design | Country | Setting | Included Studies/ or Population type | Intervention or Focus | # of participants | Outcomes | Do HWs transmit influenza to patients? Yes/No |
|---|---|---|---|---|---|---|---|---|---|
| Voirin [31] | 4 High Moderate |
OS-PC | France | Geriatric Hospital |
Geriatric patients Nurses, doctors, contacts in geriatric setting |
Wearable sensors for 12 days | patients (n = 37) nurses (n = 32), doctors (n = 15) contacts (n = 18,765) | Sensor data combined with lab-confirmed Influenza A, B, phylogenetic analyses -track transmission | Yes |
| Eibach [32] | 3 Moderate |
OS-RC | France | Geriatric Ward -Acute Care | Geriatric patients and HWs tested for flu | Molecular-based subtyping | Patients (n = 66) HWs (n = 57) |
Nosocomial influenza transmission confirmed with molecular and epidemiologic testing | Yes |
| Vanhems [33] | 3 Moderate |
OS-PC | France | Hosptial in flu season – Acute Care | Patients and HWs | Exposure- contagious persons | Patients (n = 21,519) HWs (n = 2,153) |
RR of hospital-acquired ILI based on source contacts, lab-confirmed influenza | Yes |
| Vanhems [34] | 2 Low Moderate |
OS-PC | France | Geriatric Ward – Short Stay | Geriatric Patients, HWs, contacts | Wearable sensors for 6 days | HWs (n = 46) Patients (n = 29) Contacts (n = 14,037) |
Detection of close contact between HWs, patients, or contacts combined with time in contact to measure risk | Suggests yes, but unclear |
| Pagani [35] | 3 Moderate |
Outbreak | Switzerland | Geriatric Hospital | Suspected cases - Geriatric patients and HWs of nosocomial outbreak | Epidemiology/molecular typing | N = 155 suspected cases | Respiratory virus molecular testing and sequencing to determine nosocomial transmission patterns | UC, HWs not directly cause |
| Voirin [36] | 3 Moderate |
Review | mixed | Hospital | 28 nosocomial influenza outbreak reports | n/a; ORION checklist | 28 outbreaks | Transmission of influenza by HWs to patients and as index cases | Yes in 10 studies |
| Ridgeway [37] | 2 Low Moderate |
OS-PC | USA | University Hospital | Different types of healthcare professionals | Temporary mandatory flu testing in HWs | HWs (n = 449) | % influenza positive HWs by vaccination status; % influenza positive symptomatic HWs vs. asymptomatic | UC, but 50% of flu positives were asymptomatic |
| Kay [38] | 2 Low Moderate |
Outbreak | USA | Hospital retreat facility | HWs attendees and facilitators of a retreat | Epidemiology/viral testing | HWs (n = 32) Facilitators (n = 14) |
Viral load changes and returning to work, shedding and duration, the association with symptoms and fever | UC, but 75% of flu + HWs returned to work whilst ill |
| Rodriguez-Sanchez [39] | 2 Low Moderate |
Outbreak of H1N1 | Spain | Clinical Microbiology/infectious Diseases Unit | Hospitalized patients with confirmed H1N1 influenza (HIV + and non-HIV + ) | n/a; genetic sequencing | HIV + patients (n = 49), Non-HIV+ (n = 37) | Phylogenetic trees, outbreak-specific substitutions, and viral variants in infected patients | UC on the source of outbreak |
| Valley-Omar [40] | 3 Moderate |
OS-PC | South Africa | Hospital pediatric | Nosocomial outbreak of H1N1 and children admitted to pediatric ward over 4 months | Epidemiology/viral sequencing | 14 cases | Nosocomial transmission chains and infection sources using phylogenetic analyses | Yes, transmission with HWs, asymptomatic patients, and visitors |
| Melchior [41] | 2 Low Moderate |
OS-PC | Brazil | University hospital | HIV + patients, children, contacts, and HWs | Flu testing in risk groups | N = 400 | Prevalence of influenza infection by risk group; influenza + asymptomatic; influenza acquisition by contacts | UC |
| Dini [11]* | 4 High Moderate |
Review | Mixed | 6 systematic reviews/28 studies | Influenza Vaccine | Not pooled | Lab-confirmed flu, ILI, absenteeism, NNV | Does vaccination in HWs benefit patients? Yes/No? | |
| UC benefit | |||||||||
| Riphagen-Dalhuisen [25] | 4 High Moderate |
cRCT | The Netherlands | Hospital ward | Internal medicine and pediatric department HWs of cRCT | Interventions to increase uptake | Rates of nosocomial transmission retrospectively as a secondary outcome by internal medicine or pediatric ward | Yes | |
| Seal [42]* | 2 Low Moderate |
Review | Mixed | 1 systematic review of systematic reviews and 2 non-randomised studies | Influenza vaccine in HWs | All-cause mortality, clinically suspected influenza, vaccine efficacy, working days lost, others hospitalization of patients. | No | ||
| Amodio [43] | 2 Low Moderate |
CS-RC | Italy | Hospital | Acute care hospitalized patients | Influenza vaccine in HWs | N = 62,343 patients 185 nosocomial |
Influenza vaccine coverage, nosocomial ILI and the association between the two | Yes |
| Blanco [44] | Can’t score | Model | USA | Hospital | Hypothetical hospital in Michigan | 5 influenza preventions | 700 patients | % reduction of hospitalized flu cases due to each intervention | Yes assumed |
| Shugarman [45] | 2 Low Moderate |
OB-survey | USA | Nursing Facilities | Nursing facilities (nurses = 301) | Influenza vaccine | 301 | Effect of resident and staff influenza immunization rates on the likelihood of ILI clusters. | Yes (ILI) |
| Dionne [46] | 2, Low Moderate | OS-RCS | USA | 550 bed Hospital |
Flu + Hospitalized patients over 5 flu seasons | Influenza vaccine in HWs | 533 influenza + nosocomial cases over 5 years | Proportions of nosocomial cases among HWs and patients .Nosocomial influenza rate with lab-confirmed influenza confirmation |
How many HWs need to be vaccinated if there is a benefit? NNV or range |
| No association; but some reduction in up to 50% vaccine coverage in HWs | |||||||||
| Salgado [47] | 3, Moderate | OS-RCS | USA | 600 bed Hospital | Hospitalized patients in a 600-bed tertiary hospital over 12 years | Influenza vaccine in HWs | N/A | Proportions of nosocomial cases among HWs and patients | Yes, significant proportionate association between vaccine coverage in HWs and a reduction in nosocomial infections |
| Van den Dool ‘09 [48] | Can’t score | Model | The Netherlands | Simulation of 24 bed ward hospital | Nursing home model applied to a general hospital ward patients and HWs | Influenza vaccine in HWs | N/A | Association of HW vaccination and influenza infections prevented in patients | Yes, 40% prevention when 100% of HWs vaccinated. NNV = 3 |
| Van den Dool ’08 [49] | Can’t score | Model | The Netherlands | Simulation of Geriatric nursing home | Nursing home model with 30 beds | Influenza vaccination coverage HWs | N/A | Association of HW vaccination and influenza infections prevented in patients; herd immunity | Yes, 60% prevention when 100% of HWs vaccinated. NNV = 7 |
| Wendelboe 2011 [50] | 3 Moderate |
CS-PC | USA | Geriatric, long-term care facilities | Residents and HWs of 75 LTCFs with and w/o outbreaks over two flu seasons | Vaccination coverage in HWs | N = 21 lab confirmed residents N = 40 ILI residents |
Odds ratio of outbreak LTCFs by vaccination coverage of HWs - Lab-confirmed influenza or ILI symptoms | Yes, proportionate prevention of outbreaks with rising vaccination coverage |
| Wendelboe 2015 [51] | Can’t score | Model | USA | Geriatric, long-term | Residents and HWs of 76 LTCFs over one flu season | Vaccination coverage in HWs | N/A | Herd immunity; HW vaccination and probability of influenza in LTCF residents | Yes, Inverse association |
*Dini, et al and Seal et al relevant for questions 2&3.
LEGEND: CC– Case Controlled; OS- Observational Study; CS- Cross Sectional; PC- Prospective Cohort; RC- Retrospective Cohort; RCS- Retrospective cross-sectional; SR-Systematic Review; EM- Economic Model; CBA- Cost-Benefit Analysis; TS- Time Series; RCT- Randomised Controlled Trial ; cRCT- Clustered Randomised Controlled Trial; UC- Unclear; Q1 – Question 1; Q2 – Question 2; Q3- Question 3;RR-Risk Ratio; NNV-number needed to vaccinate. Maryland Quality Score (2 – 5; Low Moderate – High).
Table 4.
Rapid Evidence Appraisal included studies and their characteristics for Topic 3 - Questions 1 & 2.
| Study | Quality | Design | # of participants or # of included studies | Country | Population | Intervention |
Question 1 Results What are the successful and practical interventions which increase HW influenza vaccine uptake? |
|---|---|---|---|---|---|---|---|
| Dini 2013 [11] | 4 Moderate High |
Review | 7 systematic reviews | Mixed | 7 systematic reviews evaluating > 200,000 subjects |
Interventions to increase uptake in HWs | Mandatory vaccination is most effective; soft mandates also effective; multi-faceted, complex/integrated programmes effective |
| Riphagen-Dalhuisen 2013 [25] | 5 High |
cRCT | N = 13,830 | The Netherlands | All employees working in 6 University medical centres over two influenza seasons | Pre-intervention survey and model to develop intervention | Influenza vaccine coverage significantly higher in intervention groups (seasonal 32.3% vs. 20.4%; pandemic vaccine 61.7% vs. 38%) |
| Hollmeyer 2013 [52] | 5 High |
Systematic review | 24 included studies | mixed | HWs from acute care hospitals between January 1990-December 2011 | Interventions to increase uptake in HWs | Increases in vaccine uptake due to free and easy access to vaccine; education and behavior modification (education, incentives or reminders); must create culturally-relevant interventions |
| Abramson 2010 [53] | 5 High |
cRCT | 13 clinics, 163 HCWs | Israel | Staff with direct patient contact in primary care community clinics in Jerusalem over 2007–2008 influenza season | HMO recommendation for vaccination of HWs | Immunisation rate 52.8% in the intervention group vs. 26.5% in the control group. Absolute increase since previous year was 25.8% in intervention clinics vs. 6.6% in the control clinics |
| Chambers 2015 [54] | 4 Moderate High |
RCT | Intervention group = 13, controls = 13 | Canada | 26 healthcare organizations across 6 provinces over two flu seasons | A successful influenza guide vs. nothing new | Intervention improved HW vaccination rates, but these rates continued to be sub-optimal and below rates achievable in programs requiring personnel to be immunised |
| Chambers 2012 [55] | 4 Moderate High |
RCT | N = 151 | Canada | Non-vaccinated HWs at 6 weeks of influenza campaign in a healthcare centre (hospital, long-term, mental health) | Ottawa Influenza Decision Aid (OIDA) | It appears that the OIDA increases confidence in vaccination decision but does not increase odds of intending to be immunised |
| ECDC 2013 [56] | 3 Moderate |
Review | 5 included studies | European region | Studies on the drivers and barriers to influenza vaccination/coverage in Europe | Interventions to increase uptake in HWs | Non-hospital settings: campaigns w/ more components, education/promotion and improved vaccine access increased uptake. Only mandatory vaccine could raise uptake above 90% |
| Europe WHO 2015 [57] | Policy toolkit | Evidence-based policy guide/toolkit | 36 included studies | Mixed | Guidance on increasing HW influenza uptake; literature review | Studies of the determinants of vaccine uptake | Development of practical guide-Tailoring Immunization Programmes for Seasonal Influenza (TIP FLU)- categorizes vaccination decision making by behavioural determinants (personal, social/community, environmental, contact with HWs) |
| Europe WHO 2015 [58] | 3 Moderate |
Review | 35 included studies | Mixed | Review to provide examples to countries of successful and replicable interventions to increase uptake in HWs | Interventions to increase uptake in HWs | Most successful interventions include multiple components; important to increase demand through changes in policy, legislation, and legislation; HWs need burden and risk data |
| Macdonald 2013 [59] | 4 High Moderate |
Systematic literature review | 22 included evaluations | European region | Review of 22 evaluation studies in Europe of promotional communication interventions | Promotional communications for flu in Europe | They found that all forms of communication can stimulate HW vaccine uptake; promotional communications that target HWs can also improve uptake among patients |
| Stuart 2012 [60] | 4 High Moderate |
Review | 11 included studies | Australia | 11 studies on seasonal influenza uptake rates of Australian HWs, determinants and strategies to increase uptake | Interventions to increase uptake in HWs/determinants | Factors contributing to immunisation in Australia show only minor variations from international samples. Not enough quality evidence on effective strategies in Australian and globally |
| Study | Quality | Design | # of participants or # of included studies | Country | Population | Intervention |
Question 2 Results What are the sociological, behavioural, and public health policy aspects of influenza vaccine uptake in HWs? |
| Dini 2018 [11] | 4 High Moderate |
Review | 16 included systematic reviews | Mixed | Evaluating vaccination determinants, vaccine adherence or knowledge or beliefs about vaccine in HWs | mixed | Topic has been extensively studied under different categories; higher knowledge among medical doctors/more favorable perception; Risk perception influences uptake; variations in uptake by HW |
| Lorenc 2017 [61] | 4 High Moderate |
Systematic review | 25 included studies | Mixed | Evaluating studies of qualitative evidence on HW vaccine uptake | mixed | Many beliefs are barriers to vaccine uptake- risk beliefs about influenza, vaccine effectiveness, concern about side-effects. Uptake influenced by HWs relationship to employer and feeling autonomous |
| Ofstead 2017 [62] | 4 High Moderate |
Intervention study and survey | N = 726 | USA | Vaccinated and unvaccinated LTCFs’ nursing staff from 4 LTCFs in the Midwest | Ecological model vs. health/belief model to increase uptake | Vaccination rates among LTCF HWs and their family members increased significantly, and absenteeism decreased after the implementation of a multifaceted ecological model |
LEGEND: CC– Case Controlled; OS- Observational Study; CS- Cross Sectional; PC- Prospective Cohort; RC- Retrospective Cohort; RCS- Retrospective cross-sectional; SR-Systematic Review; EM- Economic Model; CBA- Cost-Benefit Analysis; TS- Time Series; RCT- Randomised Controlled Trial ; cRCT- Clustered Randomised Controlled Trial; UC- Unclear; Q2 – Question 2.
Table 5.
Rapid Evidence Appraisal summary of findings table for all questions.
| Review Question | # of individual studies included by question | # of Higher Quality studies (Maryland Quality Score ≥ 4) | Settings Populations or# of studies included | Main findings | Limitations of included studies | Future Considerations |
|---|---|---|---|---|---|---|
|
Topic 1 Question 1 Are HWs at an increased risk of influenza infection? |
7 | 1 study A Comprehensive review which evaluates systematic reviews addressing this question. |
-Hospital settings with different occupations such as physicians, nurses, close patient contact or less patient -H1N1 risk and Seasonal influenza risk -Patients and HWs during outbreaks |
-Yes, in some cases, HWs are at an increased risk, but not always -H1N1pdm09 risk to HWs is clearer than seasonal influenza -Risk can vary by occupation with H1N1pdm09 but unclear for seasonal influenza -Influenza risk appears to vary year to year depending on virus circulation --Difficult to look across multiple studies to draw conclusions unless controlling for study year, circulating virus, patient populations, health worker type or occupation |
--Pooled data may lead to inaccurate conclusions -Publication and selection Bias -Use of indirect measures of influenza -Use of seasonal influenza elements for pandemic models |
-Research to validate variability in risk with influenza by year, virus, occupation, setting, patient populations - Consideration of this variability in risk as an inherent part of research or policy development |
|
Topic 1 Question 2 Does influenza vaccination benefit HWs or their employers? |
14 | 4 studies 2 reviews, 2 RCTs |
-Hospital settings -University medical centres -Staff and students of Dental Faculty -Trivalent vaccine -H1N1pdm09 vaccine |
-Yes, vaccine can be cost-saving and reduce influenza burden, but it isn’t entirely consistent -Several higher-level studies suggest vaccine efficacy in health workers and cost-savings to employers through reduced absenteeism -We identified a total of 5 RCTs to answer this question, 2 not part of recent systematic reviews and had conflicting results -One RCT during 2009–2011 found vaccination to increase absenteeism -One RCT only used ILI as an outcome measure -Vaccine benefit may be greater when considering asymptomatic influenza and the possible impact on transmission |
-Applying vaccine effectiveness, efficacy, or attack rate of healthy adults to HWs. -Self-reported outcomes -Non-specific outcomes -low circulation of virus confounds vaccine effectiveness |
-A need for updated high quality RCTs assessing vaccine benefit over time in multiple settings. --Develop research standards for influenza vaccine benefit --Vaccine type may vary findings |
|
Topic 2 Question 1 Do HWs transmit influenza to patients? |
11 | 1 study Using high resolution contact data detected with sensors |
-Patients and HWs in Elderly care Hospital Wards -In HIV + patients, --In children During Nosocomial outbreaks |
-Yes, HWs have been implicated in some transmission events, most frequently among elderly patients -Transmission and introduction can be directly linked to HWs, patients, and community/visitors- |
-Small sample size in many studies -Most studies of moderate quality |
-Need to better understand the role of asymptomatic infections in transmission -Transmission reduction measures need to consider HWs, patients, and outside contacts as a continuum -Need larger studies over a longer duration using high-resolution contact data |
|
Topic 2 Question 2 Part A Does influenza vaccination in HWs benefit patients |
6 | 2 | -Long-term care facilities in patients -Acute Care facilities -Internal Medicine -Pediatrics |
-Maybe - evidence continues to suggest a benefit on protection against all-cause mortality to LTCF patients -Evidence is mixed in other populations, but one RCT in the Netherlands suggests a reduction in nosocomial infection in internal medicine wards, not pediatrics -Very few newer RCTs evaluating patient benefit -Several studies evaluate ILI rather than lab-confirmed as a measure of benefit |
-Decisions based on 4 highly biased RCTs in long-term care facilities due to attrition rate, no blinding, contamination in control groups, low rates of vaccination coverage | -Need updated cRCTs which span years -Need standardized approaches/population to answering this question -Patient benefit variations need to be understood and appreciated (more benefit to more vulnerable patients) |
|
Topic 2 question 2 Part B How many HWs need to be vaccinated (NNV) to ensure a benefit to patients? |
6 | 0 | -Long term care facilities -Medical Centre hospital -Tertiary Hospital |
-NNV range from 3 to 50 -Appears to be a benefit in LTCFs when 100% of HWs are vaccinated with the possible prevention of 60% of infections -Appears to be a proportional benefit with a greater reduction in nosocomial transmission with higher coverage -Appears to be a curvilinear relationship between rising coverage and reduction in nosocomial infections that can be impacted by visitors or other contact elements |
-Vaccine effectiveness for HWs based on that for healthy adults -Other assumptions in models may lead to inaccurate conclusions -Outcome definitions in models significantly impacted whether herd immunity could be induced |
-NNV estimates need to factor in differences in outcome measures used -Need to expand body of knowledge beyond geriatric populations which may skew the total findings -Need to develop HW vaccine efficacy and attack rate standards for models |
|
Topic 3 Question 1 What are the successful and practical interventions which increase HW influenza vaccine uptake? |
10 | 8 | -Primary care community clinics -Acute care hospital staff -HWs in tertiary hospitals -HWs with and w/o patient contact -All personnel of a health institution |
-Mandatory Vaccination to be able to work remains the most successful intervention to increase uptake -Other successful interventions are:
|
Publication bias considering studies that were not effective may not be published -Selection bias as some may not have reported baseline vaccine uptake before intervention -Lack of blinding in RCTs -Hard to separate the benefit of one intervention as distinct from the others when a part of multi-faceted interventions |
-Clear guidance needed on vaccine program implementation in HWs which clarify the options of mandatory policy options vs. other. -Develop a standardized checklist of components critical for vaccine uptake success - Develop guidance on how to develop targeted interventions catering to the individual context for implementation |
|
Topic 3 Question 2 What are the sociological, behavioural, and public health policy aspects of influenza vaccine uptake in HWs? |
3 | 3 | -Long-term care facilities - Mixed hospital settings of HWs -Mixed outpatient settings of HWs |
-Strongest barriers to uptake were HW’s lack of confidence about disease severity or vaccine effectiveness and a lack of professional or ethical obligation to get vaccinated. -Strongest reason to vaccinate was to protect oneself and not patients -Success of a vaccination program may be influenced by the complex relationship between HWs and the organization and management of the health care system within which they work. -Ecological model shows promise an alternate to the widely used health belief model |
-Limited use of models other than Health Beliefs Model | -Greater diversity of study approaches to reframe issues through alternative lenses -Need to integrate HW influenza vaccine programs into existing programs to ensure long term -Need to expand discussion on the importance of top-down support for vaccine programs in HWs to develop a culture of vaccination -Need to have a better understanding of the influenza of management /organizationl structures where implementation will take place |
3. Literature search results
3.1. Identified literature
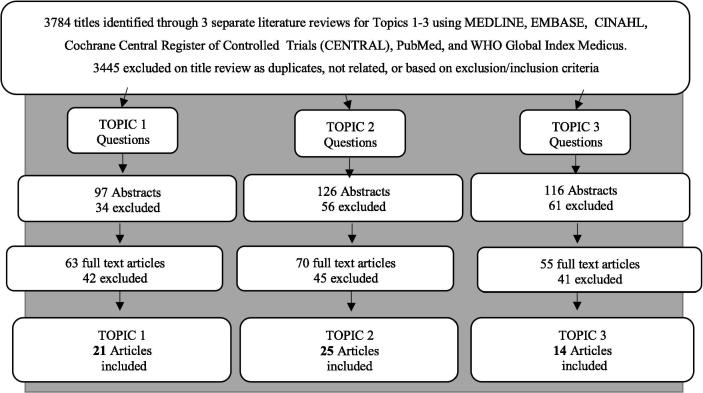
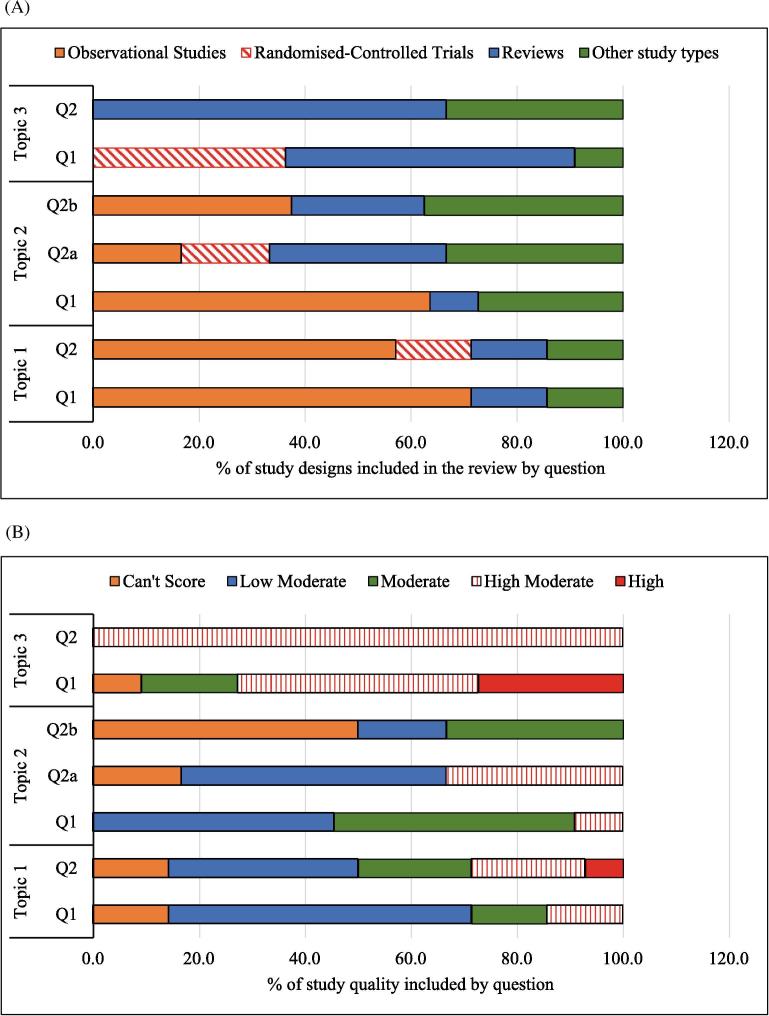
The collective literature search identified 3,784 publications, of which 52 individual publications met the inclusion criteria (see Fig. 1). Some publications addressed multiple questions resulting in a total of 60 references cited for all questions combined (see table 1). Of the 60 included studies, we identified 7 RCTs, 24 observational studies, 16 reviews (non-systematic or systematic), and 13 “other” study types (see Fig. 2A and B). Individual studies and systematic reviews were excluded from the final list of included studies (Table 2, Table 3, Table 4) when they formed part of a more recent systematic review. These removed studies were still considered in the text if they are of moderate to high quality.
Fig. 1.
Flow Diagram of the literature search strategy, study removal and selection by topic.
Fig. 2.
(A and B). % Distribution of different study types included in our review and % distribution of included studies by Maryland Scientific Methods Quality Scale. (A) % Distribution of Included Study Types by Topic and Question illustrates the limited number of randomized-controlled trials. (B.) % Distribution of Included Studies by Maryland Scientific Methods Quality Scale illustrates the limited number of high-quality studies identified.
3.2. Evidence by topic and question (Table 2, Table 3, Table 4)
Topic 1 Question 1 - Are HWs at an increased risk of influenza infection?
We identified only 1 higher quality study (4 or higher score) for this question. The review by Dini et al. [11] included 28 studies (12 systematic reviews, 13 meta-analyses, 3 appraisals) and addressed several aspects relevant to influenza vaccination in HWs, including laboratory-confirmed incidence and risk compared to controls. The review included a higher level systematic review and meta-analysis on the occupational risk of pandemic H1N1 in HWs compared to the general population or across occupations. This meta-analysis [63] evaluated 15 studies (29,358 subjects), including 11 high quality studies of laboratory-confirmed influenza, and showed a significantly increased OR = 2.08 (95% CI, 1.73–2.51) in HWs with a higher risk in physicians {OR = 6.03 (95% CI, 2.11–17.8)}.
Kuster et al. [64], a systematic review also included in Dini et al, compared data on influenza infection rates in HWs and healthy adults from 29 surveys (n = 58,245) over 97 influenza seasons [11]. The review calculated a pooled annual incidence of 18.7% (95% CI, 15.80–22.11) per season in unvaccinated HWs versus 5.44% (95% CI, 3.01–9.84) per season in unvaccinated adults and 6.49% (95% CI, 4.63–9.09) for vaccinated HWs versus 1.20% (95% CI, 0.86–1.68) for vaccinated adults. However, this review [64] concluded the overall effect was driven by asymptomatic rather than symptomatic infections. A German study included as part of Dini et al evaluated ILI incidence combined with pre and post-season influenza serology in HWs and non-HWs [65] and concluded there was no difference in risk of symptomatic or asymptomatic influenza infection in HWs compared to non-HWs [11]. However, HWs were found to have an increased risk of clinically diagnosed acute respiratory infection (OR = 3.0, p = 0.04), and were more likely to have a pre-season antibody titre of ≥ 40 to influenza A/H3N2, indicating the possibility of asymptomatic or subclinical influenza infections.
Five additional studies of differing methodological approach and quality provided conflicting conclusions. Three studies pointed to a higher risk [13], [13], [16] including a pandemic simulation modelling study [13] which suggested a plausible attack rate as high as 60% greater in HWs and a moderate-low quality retrospective study in Hong Kong which suggested a higher H1N1 risk in HWs and an inconclusive result when applied to seasonal influenza [12]. Sartor et al. [16] found HWs at an increased risk of influenza infection in a geriatric hospital, although the study contained fewer than 50 subjects. A moderate quality case-control study in Canada found inconclusive results on the risk of ILI in exposed HWs as did a prospective cohort study in Brazil using lab-confirmed influenza [14], [15].
Topic 1, Question 2 - Does influenza vaccination benefit HWs or their employers?
We identified several higher quality studies for this question. Two reviews compared lab-confirmed influenza in vaccinated HWs vs. controls and found a vaccine efficacy of ∼50–90% and a significant protective effect {Relative Risk(RR) = 0.40 (95% CI, 0.23–0.69)} [18], [66] for HWs. The high quality systematic review by Imai et al. [18] further found a reduced incidence of absenteeism due to ILI {RR = 0.62 (95%CI, 0.45–0.85)}, and a significantly shorter sick leave in the vaccinated group {RR = 0.46 (ILI + lab-confirmed), RR = 0.60 (lab-confirmed)}. The study concluded that vaccination provided a protective effect in HWs and deemed vaccination programs as cost-saving. The widely-cited studies by Kliner et al. [66] and Kuster et al. [64] were included in the appraisal by Dini et al who identified 6 reviews relevant to this question [64], [66], [67], [68], [69], [70] and also concluded that vaccination was protective and potentially cost-saving in HWs [11].
Two contradictory RCTs included a Malaysian study that [28] found reduced absenteeism (12 days vs. 52 in the control group, p = 0.002) and ILI (reduced by 52.6%, p = 0.002) due to vaccination, using all self-reported outcomes. A Dutch RCT [25] actually found an increase in absenteeism in the vaccinated groups {2009/10 (4.6% vs 3.4%); 2010/11 (4.6% vs 3.9%)}, although they attributed the increase to more frequent testing for influenza in the intervention cluster and the study was performed during periods of H1N1pdm09 activity in 2009–2011.
We identified 8 observational studies of moderate to lower moderate quality [19], [20], [21], [22], [23], [26], [27], [30], 5 of which found a vaccine benefit to HWs or employers and 3 which found no benefift or inconclusive results. A study in a large Italian hospital found an increase in absenteeism [19] from 2.99 days/person (outside of flu season) to 5.06 days/person (during flu season), which translated to 11,000 attributable absent days/year. The study also found that vaccinated HWs had fewer excess sick days compared to non-vaccinated HWs (1.45 days/person vs. 2.09). A retrospective study in the United States (4063 subjects) compared absenteeism in mandatory vs. non-mandatory vaccination sites [21] and found that vaccinated HWs had fewer symptomatic days absent {2012/2013, OR = 0.82 (95% CI, 0.72–0.93); 2014/2015, OR = 0.81 (95% CI, 0.69–0.95)} and concluded that mandatory vaccination would reduce symptomatic absenteeism.
Three studies suggested no benefit including a prospective cohort study in Israel [23] which found HWs less likely to develop lab-confirmed influenza, although this finding was not significant. A study in Kenya [26] found vaccinated participants were more likely to develop acute respiratory illness (ARI) and more likely to miss work after vaccination. A large retrospective ecological study [20] evaluated ∼800,000 HWs per year over 4 flu seasons in England and found that a 10% increase in vaccine uptake would lead to a decrease in approximately 0.43 percentage points in the absolute sickness absence rate (from 4.5% to 4.07%), or a 10% relative decrease in the sickness rate. Studies looking at the cost-benefit of vaccination generally found HW vaccination to be cost-saving and of economic benefit from the perspective of the employer [18], [24], [28], [29].
Topic 2, Question 1 – Do HWs transmit influenza to patients?
Evidence varied widely in methodological approach, yet some findings suggest HWs are at least one of the sources of transmission. We identified moderate to higher quality studies which implicated HWs in some transmission events, particularly in geriatric patients, in acute care settings during flu season [31], [32], [33] and during hospital outbreaks [36]. These studies utilized technologies such as wearable sensors [31], [34], molecular-based subtyping methods [32], or phylogenetic analyses combined with case studies or epidemiology [35], [40]. One retrospective cohort study analyzed routes of transmission among HWs and geriatric patients using molecular-based subtyping methods. The study identified three nosocomial outbreak clusters within one ‘outbreak’ and found a higher influenza incidence in patients (24%) than in HWs (11%) [32]. A large nosocomial outbreak in a geriatric hospital [35] used detailed case studies combined with phylogenetic analysis to reveal 5 clusters of cases and multiple introductions of community strains into the hospital. Similarly, a study by Valley-Omar et al. [40] in South Africa combined epidemiological investigation with phylogenetic analysis to study influenza transmission chains and the extent of nosocomial transmission over a four-month period within a pediatric hospital. This analysis found that most potential nosocomial infections resulted from multiple introductions of Influenza A into the hospital and suggested transmission between asymptomatic patients, HWs, and visitors.
Topic 2, Question 2, Part A – Does influenza vaccination in HWs benefit patients?
We identified two higher quality studies relevant to this question [11], [25], and several lower quality studies using ILI [43], [44], [45] as an outcome measure. The comprehensive review by Dini et al. [11] evaluated 6 systematic reviews [66], [69], [72], [73], [74], [76] and found overall inconclusive results as studies were of moderate but mostly lower quality, evaluated the same 4 RCTs [77], [78], [79], [80], and came to different conclusions. The review included the latest version of a Cochrane Systematic Review by Thomas et al. [74] which evaluated these 4 highly cited RCTs in long term care facilities (LTCFs) [77], [78], [79], [80]. However, different from prior reviews by the same author, this time they excluded all-cause mortality as an outcome measure (only influenza-related outcomes) and found no conclusive evidence of the benefit of HW vaccination to LTCF residents. They also found a low quality of evidence with a high risk of bias. Another systematic review and meta-analysis [76] included in Dini et al pooled data from 4 RCTs and suggested a benefit to all-cause mortality {RR = 0.71 (95% CI, 0.59 – 0.85)} and ILI {0.58 (95% CI, 0.46 – 0.73)}, but all-cause hospitalization and laboratory-confirmed influenza were not statistically significantly altered [11].
Although we identified 5 higher quality clustered randomized controlled trials (cRCTs) [25], [77], [78], [79], [80] which address this question, only the Dutch trial [25] was not included in prior reviews (per our exclusion criteria), other than in de Serres et al. [73] in the next section, illustrating the need for updated cRCTS. This pragmatic cRCT [25] measured rates of nosocomial infection as a secondary outcome in 2 high risk departments – pediatrics and internal medicine. Within the higher vaccine coverage groups of the internal medicine departments, nosocomial influenza and/or pneumonia was recorded in 3.9% compared to 9.7% in the control hospitals, respectively (p = 0.015). An increase in vaccine coverage was associated with decreased inpatient morbidity from influenza and/or pneumonia in internal medicine settings, but not in pediatrics.
Topic 2, question 2, Part B - How many HWs need to be vaccinated (NNV) to ensure a benefit to patients?
We found a limited number of moderate quality observational and modelling studies [46], [47], [48], [49], [50], [51] which suggest that a proportionate effect of HW vaccination is likely, where nosocomial influenza infections decrease with increasing HW vaccination coverage. Salgado et al. [47] conducted a retrospective cross sectional study in a USA tertiary care hospital over 12 consecutive years. HW vaccination rates increased from 4% in 1987–1988 to 67% in 1999–2000 (P < 0.0001). Proportions of nosocomial influenza cases among employees or patients both declined significantly (P < 0.0001). Logistic regression analysis revealed a significant inverse association between HW compliance with vaccination and the rate of nosocomial influenza among patients (P < 0 0.001). The authors reported that surveillance of nosocomial spread and isolation policies remained constant during the study period, but concomitant increase in patient influenza vaccination rates may have contributed to the decrease in nosocomial infections. A nested-case control study [75] included in the review by Seal et al. [42] found that a vaccinated proportion of ≥ 35% of HWs in short-stay units appeared to protect against nosocomial influenza among patients (OR = 0.07; 95% CI, 0.005–0.98) independent of patient age, influenza season and potential influenza source. The authors concluded that a minimum of 35% vaccination coverage in HWs would be required to have a potential protective impact on hospital-acquired influenza infection.
Dini et al. [11] also included a review by de Serres et al. [73] who used the mathematical principal of dilution as the basis for their re-examination of the main 4 cRCTs used as evidence for mandatory vaccine policies in HW [77], [78], [79], [80]. They disputed the NNV (number needed to vaccinate) of 8 found by Hayward in 2006 in LTCF settings [79] and recalculated it to be 36,000, which was quickly disputed by Hayward [81]. Hayward stressed that results from their study in LTCFs were not necessarily applicable outside of the most frail and vulnerable patients.
In two modelling studies by van den Dool et al. [48], [49], one model in LTCFs predicted “a robust linear relationship between the number of HWs vaccinated and the expected number of influenza infections in patients, preventing ∼ 60% of influenza virus infections among patients” (NNV = 7). Comparing these results in LTCFs to a hospital or short stay setting, they found an equivalent or higher estimate of impact in hospital patients (NNV = 3) and that vaccination of 100% of HWs would potentially prevent 40% of inpatient nosocomially acquired influenza infections.
Topic 3, Question 1– What are the successful and practical interventions which increase HW influenza vaccine uptake?
We identified several higher quality RCTs and systematic reviews addressing this question and there is consensus that no single intervention component has been found to rapidly and substantially raise influenza vaccination rates in HWs, aside from mandatory vaccination [82]. However, several systematic reviews [52], [59], [83] and tool kits [57], [58] have highlighted that multifaceted approaches which sustain over time can see increases > 90% [47]. Dini et al. [11] evaluated 7 systematic reviews which together evaluated strategies to increase uptake in HWs in > 200,000 subjects. They found that some successful alternatives to mandatory vaccination included “soft-mandates”, such as masks, “opt-out”, or declinations statements, and multi-faceted programmes which take into consideration the local context, include incentives, education, advertising, and easy vaccine access as efforts to enhance behavior change.
Higher level systematic reviews had similar findings including a meta-regression analysis that [83] found that the single most successful strategies after mandatory vaccination were “soft” mandate strategies and a policy excluding non-vaccinated HWs from working with highly vulnerable patient groups. Another systematic review [52] found that successful interventions contained the following critical components: free and easy access to vaccine; knowledge and behavior change through educational activities; reminders and incentives; management/organizational approaches including personnel charged with implementing the programme; and a long-term strategy. An additional study of 121 publications also concluded that all interventions increased uptake to some extent with the most successful being those which required vaccination as a condition of being allowed to work [84].
Several randomised trials [85] have successfully used a pre-intervention survey as a basis for subsequent intervention mapping [25], [86] in the development of multi-faceted interventions. This is particularly important as the reasons for vaccine hesitancy are complex and heterogeneous [52], making local, social, cultural, institutional and logistical factors all relevant to the development of educational or knowledge-based interventions. Other RCTs failed to dramatically raise uptake using educational tools or manuals and a decision aide intervention which changed views but not actions [54], [55], [56].
Topic 3, Question 2 – What are the sociological, behavioural, and public health policy aspects of influenza vaccine uptake in HWs?
We identified 3 higher moderate studies on this topic, including two reviews [11], [61]. One review [11] evaluated 16 systematic reviews on vaccination determinants in HWs, adherence to vaccination, and risk perceptions or beliefs about vaccination. The study broadly found the following: knowledge about influenza varied by occupation, not necessarily occupational level (doctors vs. dentists for example); many misconceptions about influenza persist, even though influenza knowledge has improved over time; the relationship between HWs’ perception and mitigation of risk is complex and multi-factorial and needs to be better understood. Included as part of this review was a large systematic review by Schmid [86] which included 470 studies, of which 117 looked at HW barriers to influenza vaccine uptake. Their review found that micro-level determinants included age, gender, additional risk factors, and past behavior as the most reported factors to influence uptake. They further evaluated the determinants based on the 4C model for vaccine hesitancy (complacency, confidence, convenience, and calculation) and found that the strongest barrier to uptake was a HWs lack of confidence about disease severity or vaccine effectiveness and “a lack of professional or ethical obligation to get vaccinated”. This was in line with the systematic review by Hollmeyer which found that HWs are motivated to be immunized against influenza more often for their own benefit than for the benefit of their patients [52].
Lorenc et al. [61] conducted a systematic review of 25 studies on HWs’ beliefs and perceptions about vaccination within different contexts and found that many participants are unsure of the real value of vaccination programs. This study highlighted that the success of a vaccination program may be influenced by the complex relationship between HWs and the organization and management of the health care system within which they work. Ofstead and colleagues [62] executed a three-part evidence-based intervention study in LTCFs which compared a multi-faceted ecological model and the health belief model. They raised uptake from 50% to 85%, decreased respiratory illness-related absenteeism by 12% and concluded that an ecological model was more effective than the health belief model at increasing uptake as it includes broader policy or organizational aspects relevant to program implementation.
4. Discussion
We executed a Rapid Evidence Appraisal [8] of the literature from 2006 to 2018 to evaluate the evidence relevant to the ongoing discussions about seasonal influenza vaccination in HWs. Influenza infection and vaccination in HWs poses a complex policy challenge due to the general lack of high quality evidence, the inherent complexity of influenza, and the number of potential benefactors: HWs themselves by avoiding influenza infection, their patients by virtue of reduced influenza transmission in health facilities, their employers in terms of business continuity, and potentially their families and wider community contacts.
4.1. Influenza risk to health workers
The evidence on whether HWs are at increased risk of influenza infection (symptomatic and asymptomatic) in HWs is mixed. Recent studies suggest a higher risk with pH1N1 [11], [12], [13], [63], a high attack rate, and variations in risk which are connected to occupational exposure. Other studies find a risk to patients and HWs during an outbreak [16] and following contact with ILI patients [15]. Additional studies suggest only a risk of asymptomatic infection [17], [64]. Taken together, these studies highlight that setting [88], occupation [89] or patient contact level, risk procedure [90], circulating virus, and existing immunity in the population (i.e. H1N1) can all vary risk [63]. However, respiratory illness has been reported as one of the main causes of sickness absence in HWs [92], [91], pandemics and epidemics can be associated with concurrent increased rates of HW absenteeism [12], [92], [93], and HWs do overall appear to be at risk of contracting influenza.
4.2. Vaccine benefit to health workers or their employers
Recent higher level evidence [11], [18] evaluating RCTs, cohort studies, and systematic reviews does suggest a vaccine benefit to HWs and their employers including a protective effect against laboratory-confirmed influenza, shortened sick leave, and a reduced incidence of absenteeism (due to ILI). However, vaccine benefit studies during the years of pH1N1, including an RCT in the Netherlands, found the opposite effect, an increase in absenteeism in the vaccination arms. Different authors draw contradictory conclusions from the use of the same RCTs [94], [95], [96], indicating a need for updated, large scale studies of higher quality. Observational studies also draw different conclusions and are constrained by poor study design, non-specific outcomes, a high risk of bias, and failure to adjust for confounding factors. Absence due to ILI is frequently used as an outcome measure when ILI itself has relatively poor predictive value for influenza infection [97], [98]. Although studies may provide contradictory conclusions, vaccine efficacy in HWs has been shown to be as high as 90% for well-matched seasonal influenza vaccines [66], [68], and high for pandemic vaccine [99]. Therefore, it seems reasonable that vaccinating HWs against influenza will reduce influenza burden in HWs, related work absenteeism, and possibly influence subclinical infection and/or “presenteeism” – attending work whilst ill.
4.3. Influenza risk to patients
The expanding volume of studies combining epidemiological and phylogenetic analysis, among other new technologies [71], [72], suggest that HWs are implicated in at least some transmission during nosocomial outbreaks of influenza. However, the complexity of transmission in health facilities is also driven by various factors including patient to patient and visitor to patient contact, varying levels of possible infectiousness with asymptomatic vs. symptomatic transmission, and high patient turnover, particularly in acute care settings [37], [38], [40]. Nevertheless, Pagani et al. [35] identified multiple introductions of strains into the hospital from the community during an outbreak and noted that unvaccinated HWs may have played an important role in sustaining the outbreak. Other factors which may increase or decrease the extent to which HWs are implicated in transmission are individual (immune status, severity of disease, viral shedding), pathogen (virulence, infective dose), and environmental factors (infection control practices, ward layout, frequency of patient, staff and visitor contacts), including contact with HWs of varying degrees of risk exposure themselves. Notwithstanding, the evidence does suggest that HWs can clearly pose a transmission risk to patients, at least some of the time.
4.4. Vaccine benefit to patients
The benefit of HW influenza vaccination to patients is not clear or consistent in the literature and continues to be debated as numerous systematic reviews using mostly the same RCTs [77], [78], [79], [80] all draw different conclusions [11], [66], [73], [76]. Research in LTCFs has consistently shown a benefit to patients, at least for all-cause mortality. Variations across study populations or studies, such as patient populations, level of HW to patient contact, patient vulnerability or susceptibility, or levels of risk exposure for HWs themselves, limit comparability between findings. This may explain why studies in LTCFs consistently find vaccination of HWs beneficial to elderly patients, where the stability of the setting, patient characteristics, and high level of contact between HWs and patients are all consistent factors. Whereas, a larger body of evidence suggests an unclear association between HW vaccination and patient benefit in hospital settings, which may be due to variations in study design, HW or patient characteristics, their individual risk or susceptibility, difference in vaccine type or diagnostic testing, or even the time of year. LTCF patients do however appear to benefit, at least regarding mortality, which demonstrates that vaccine benefit to patient warrants efforts to increase vaccination in HWs caring for the elderly [100].
4.5. Influenza vaccine uptake
The factors that feed into a HWs decision-making pathway for influenza vaccination are diverse and individually-driven [52]. Therefore, any efforts must be as equally diverse as the HWs they target. Mandatory vaccination is debated extensively from many perspectives including the reasons for [21] and against it [73]; however, no amount of debate can refute that mandatory vaccine policies do raise uptake quickly, usually to levels in excess of 94–96% [82]. Other successful interventions are multi-faceted, sustained over time [47], and evolve over time as data is collected on the target population [85]. These make vaccine easily accessible, maintain strong organizational support, develop education which varies by targeted groups, and may include “soft-mandate” policies, such as declination statements, or alternatives such as masks [52], [83].
One area which deserves more consideration, is the use of different types of models in the development of interventions to improve uptake. Traditionally, the health belief model is most commonly used. However, studies exploring other possibilities [62], [86] have shown promise with an ecological model, which takes into consideration the contextual, organizational, policy or background components which are relevant to a vaccine program and henceforth to a HWs decision to take the vaccine. These models incorporate the growing understanding that HW vaccine uptake involves much more than individual knowledge, attitudes, and beliefs. The greater context of the HW and their relationship to the organization, other HWs, and broader cultural factors are all important to vaccine uptake.
4.6. Study limitations
Although we made every effort to execute an unbiased and systematic approach to our search and selection of included literature, our study was not without limitations. Due to resource and time constraints, we limited the search to English language abstract or full-text articles, did not include grey literature, and removed publications that were included as part of a more recent systematic review. This may have led to some selection bias and limited our exposure to all relevant articles. However, due to the breadth of questions included in the search and the execution of three distinct searches, we felt that this diversification may have added strength to the study design to compensate for this weakness.
The majority of included studies were observational studies, which limits the strength of our findings as observational studies are susceptible to bias and there is limited ability to infer causality. Additionally, many studies were executed in different types of settings, with different target populations, using different vaccines and with varying levels of risk or exposure to the populations being studies. This makes comparing across studies very difficult.
Our methodology lacks the robustness of a formal systematic review or meta-analysis and does not report on effect size. Systematic reviews have very defined limitations for study selection and inclusion and typically do not query so many questions. We executed a Rapid Evidence Appraisal - a time-limited effort of assessing the evidence to draw conclusions, a process similar to what might happen in a ‘real-life’ policy environment. To compensate, we approached the literature search in a systematic way to add robustness to our study design and the breadth of our included questions and consideration of multiple elements relevant to the decision-making process, offers a more realistic approach for policy makers. Finally, our study was adapted and updated from a white paper originally intended to advise and inform WHO when establishing the influenza vaccine research agenda. The objective was to support global policy recommendations.
5. Conclusion (see summary of findings Table 5)
In recent years, several comprehensive reviews [11], [18], [42], [61], [64], [66], [67], [73], [74], [76], [83], [86], [87] have attempted to rigorously address different aspects of influenza vaccination in health workers. Paradoxically, the number of reviews far outweighs the number of studies that are of high quality or give definitive answers. Similarly, our review included only RCTs that have not already been evaluated as part of a more updated systematic review. It is therefore hardly surprising that it has proved difficult to draw universal and emphatic conclusions for policy makers, government leaders, and healthcare managers; and that implementation is poor and acceptance low.
Nevertheless:
-
•
Nosocomial influenza is a recognized problem and it is clear there is a problem to solve or a case to answer.
-
•
There is adequate evidence that HWs contract influenza and data suggests they are at risk of continuing to work whilst infected.
-
•
There are sufficient data to conclude that influenza vaccines are as effective in HWs as in other adults of similar age.
-
•
Data are emerging that capture the complexity of influenza transmission in health facilities and new molecular evidence implicates HWs in transmission events.
-
•
Data on whether vaccinating HWs protects patients is of mixed quality and does not universally favor a positive (protective) outcome. However, there are strong signals from studies in LTCFs that HW vaccination protects patients, especially with regard to mortality.
The evidence base requires improvement and future research should aim to evaluate the impact of HW vaccination on clearly defined and standardized outcome measures in specific cohorts of beneficiaries. A better understanding of transmission dynamics across a diversity of HW and patient risk groups is needed to facilitate a more nuanced and useful estimation of the wider benefits of HW influenza vaccination. The large body of knowledge on effective ways to increase vaccine uptake in HWs needs to be incorporated consistently within vaccination policies and practices. Programmes will need to consider the importance of organizational and contextual factors as drivers alongside the individual perceptions of HWs. The evidence demonstrating the effectiveness of mandation in improving uptake is however emphatic.
Influenza is likely to persist as a unique methodological challenge for researchers to produce reliable and robust results which lead to definitive conclusions about HW vaccination. Influenza burden, infectiousness, and severity vary yearly, seasonally, and spatially. Influenza risk also varies by setting, such as in proximity to young children or immune-compromised individuals, and by occupation or activity (such as performing respiratory procedures on infected patients). Additionally, influenza vaccine effectiveness is equally variable by season and subtype, making comparisons between years and populations challenging.
Due to the inherent complexity of influenza, waiting for the perfect results with influenza risks decision paralysis. When considering the evidence with a ‘whole-of-system’ approach, rather than considering specific evidence questions in isolation, we argue that the case for vaccinating HWs against influenza is maintained. Furthermore, to ensure the success and likelihood of expansion of seasonal influenza programs globally, guidance is needed for policy makers and implementers on how to best integrate influenza vaccination to HWs within existing vaccination and occupational health frameworks.
Declaration of Competing Interest
Author JSN-V-T declares no conflicts of interest in the last 5 years. Between 2000 and 2004 he was, at times, employed by SmithKline Beecham (now a part of Glaxo SmithKline) and Aventis Pasteur MSD, both of whom manufacture and/or distribute influenza vaccines; arising from this period he has no outstanding shares, share options, or accrued pension rights pertaining to either company.
Authors have no further conflict of interests to declare. All authors attest they meet the ICMJE criteria for authorship.
Acknowledgments
Acknowledgements
N/A.
Disclosure
All authors have approved the final article.
JSN-V-T is currently working on secondment at the Department of Health and Social Care, England (DHSC).
Philipp Lambach works for the World Health Organization (WHO). Hamid Mahgoub works for East of England Health Protection Team, Public Health England. Dawn C. Jenkin works for Nottinghamshire County Council.
The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization, DHSC, Nottinghamshire County Council, and Public Health England.
Funding
This study was funded by a grant from the World Health Organization's Initiative for Vaccine Research. The authors would like to acknowledge the contributions of the Centers for Disease Control and Prevention (CDC), which provides financial support to the World Health Organization Initiative for Vaccine Research (U50CK000431).
Contributors
-
-
Dawn C. Jenkin: Literature search 2006-2016, interpretation of results, drafting of original report which served as the basis for this manuscript, editing final manuscript.
-
-
Hamid Mahgoub: Literature search 2006-2016, interpretation of results, drafting of original report which served as the basis for this manuscript, editing final manuscript.
-
-
Kathleen F. Morales: Main writer, editor, preparer of the manuscript; literature search 2016-2018, interpretation of results, development of all tables and figures in the manuscript.
-
-
Jonathan S. Nguyen-Van-Tam: Study design, interpretation of results, drafting and finalizing the manuscript.
-
-
Philipp Lambach: Study design, interpretation of results, drafting and finalizing the manuscript.
Footnotes
See supplementary materials for full protocol with search criteria for each topic and exclusion and inclusion criteria by topic and question. KM executed search strategy/screened the literature for 2016–2018 and reevaluated inclusion/exclusion to total search 2006–2018.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2019.100036.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Organization WH. Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012; 87: pp. 461–476. [PubMed]
- 2.Organization WH. Summary of WHO Position Papers - Immunization of Health Care Workers, September 2016. 2016.
- 3.Organization WH. Global Influenza Strategy 2019-2030. http://www.who.int/iris/handle/10665/311184: World Health Organization; 2019.
- 4.WHO/Unicef Joint Reporting Form. 2017.
- 5.Zhang W., Hirve S., Kieny M.P. Seasonal vaccines - critical path to pandemic influenza response. Vaccine. 2017;35:851–852. doi: 10.1016/j.vaccine.2016.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.General D. Implementation of the International Health Regulations (2005). Report of the Review Committee on the Functioning of the International Health Regulations (2005) in relation to Pandemic (H1N1) 2009. 2011.
- 7.Gavi Board starts framing Alliance's approach to 2021-2025 period. https://www.gavi.org/library/news/press-releases/2018/gavi-board-starts-framing-alliance-s-approach-to-2021-2025-period/2018.
- 8.Service GSR. How to do a REA. Rapid Evidence Assessment Toolkit. online: The National Archives of the United Kingdom; 2014.
- 9.Farrington D GD, Sherman L, Welsh B. The Maryland Scientific Methods Scale. In: Farrington D, MacKenzie D, Sherman L, Welsh L (eds) Evidence based crime prevention. London: Routledge; 2002.
- 10.https://whatworksgrowth.org/resources/the-scientific-maryland-scale/.
- 11.Dini G., Toletone A., Sticchi L., Orsi A., Bragazzi N.L., Durando P. Influenza vaccination in healthcare workers: a comprehensive critical appraisal of the literature. Hum Vaccin Immunother. 2018;14:772–789. doi: 10.1080/21645515.2017.1348442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ip D.K., Lau E.H., Tam Y.H., So H.C., Cowling B.J., Kwok H.K. Increases in absenteeism among health care workers in Hong Kong during influenza epidemics, 2004–2009. BMC Infect Dis. 2015;15:586. doi: 10.1186/s12879-015-1316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooley P., Lee B.Y., Brown S., Cajka J., Chasteen B., Ganapathi L. Protecting health care workers: a pandemic simulation based on Allegheny County. Influenza Other Respir Viruses. 2010;4:61–72. doi: 10.1111/j.1750-2659.2009.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellei N., Carraro E., Perosa A.H., Benfica D., Granato C.F. Influenza and rhinovirus infections among health-care workers. Respirology. 2007;12:100–103. doi: 10.1111/j.1440-1843.2006.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yiannakoulias N., Russell M.L., Svenson L.W., Schopflocher D.P. Doctors, patients and influenza-like illness: clinicians or patients at risk? Public Health. 2004;118:527–531. doi: 10.1016/j.puhe.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Sartor C., Zandotti C., Romain F., Jacomo V., Xe Ronique. Disruption of services in an internal medicine unit due to a nosocomial influenza outbreak. Infect Control Hosp Epidemiol. 2002;23:615–619. doi: 10.1086/501981. [DOI] [PubMed] [Google Scholar]
- 17.Elder A.G., O'Donnell B., McCruden E.A., Symington I.S., Carman W.F. Incidence and recall of influenza in a cohort of Glasgow healthcare workers during the 1993–4 epidemic: results of serum testing and questionnaire. BMJ. 1996;313:1241–1242. doi: 10.1136/bmj.313.7067.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai C., Toizumi M., Hall L., Lambert S., Halton K., Merollini K. A systematic review and meta-analysis of the direct epidemiological and economic effects of seasonal influenza vaccination on healthcare workers. PLoS ONE. 2018;13:e0198685. doi: 10.1371/journal.pone.0198685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianino M.M., Politano G., Scarmozzino A., Charrier L., Testa M., Giacomelli S. Estimation of sickness absenteeism among Italian healthcare workers during seasonal influenza epidemics. PLoS ONE. 2017;12:e0182510. doi: 10.1371/journal.pone.0182510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira M., Williams S., Restrick L., Cullinan P., Hopkinson N.S. Healthcare worker influenza vaccination and sickness absence - an ecological study. Clin Med (Lond) 2017;17:484–489. doi: 10.7861/clinmedicine.17-6-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frederick J., Brown A.C., Cummings D.A., Gaydos C.A., Gibert C.L., Gorse G.J. Protecting healthcare personnel in outpatient settings: the influence of mandatory versus nonmandatory influenza vaccination policies on workplace absenteeism during multiple respiratory virus seasons. Infect Control Hosp Epidemiol. 2018;39:452–461. doi: 10.1017/ice.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saadeh-Navarro E., Garza-González E., Salazar-Montalvo R.G., Rodríguez-López J.M., Mendoza-Flores L., Camacho-Ortiz A. Association between early influenza vaccination and the reduction of influenza-like syndromes in health care providers. Am J Infect Control. 2016;44:250–252. doi: 10.1016/j.ajic.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Atamna Z., Chazan B., Nitzan O., Colodner R., Kfir H., Strauss M. Seasonal influenza vaccination effectiveness and compliance among hospital health care workers. Israel Med Assoc J: IMAJ. 2016;18:5–9. [PubMed] [Google Scholar]
- 24.Preaud E., Durand L., Macabeo B., Farkas N., Sloesen B., Palache A. Annual public health and economic benefits of seasonal influenza vaccination: a European estimate. BMC Public Health. 2014;14:813. doi: 10.1186/1471-2458-14-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riphagen-Dalhuisen J., Burgerhof J.G., Frijstein G., van der Geest-Blankert A.D., Danhof-Pont M.B., de Jager H.J. Euro Surveillance: Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin. 2013. Hospital-based cluster randomised controlled trial to assess effects of a multi-faceted programme on influenza vaccine coverage among hospital healthcare workers and nosocomial influenza in the Netherlands, 2009 to 2011; p. 20512. [DOI] [PubMed] [Google Scholar]
- 26.Njuguna H., Ahmed J., Oria P.A., Arunga G., Williamson J., Kosgey A. Uptake and effectiveness of monovalent influenza A (H1N1) pandemic 2009 vaccine among healthcare personnel in Kenya, 2010. Vaccine. 2013;31:4662–4667. doi: 10.1016/j.vaccine.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Fujita Y., Okada T., Mugishima H., Kumasaka K., Sawa M., Tachihara S. Trial of influenza HA vaccination for healthcare workers in consecutive years. Jpn J Infect Dis. 2009;62:464–466. [PubMed] [Google Scholar]
- 28.Hui L.S., Rashwan H., bin Jaafar M.H., Hussaini H.M., Isahak D.I. Effectiveness of influenza vaccine in preventing influenza-like illness among faculty of dentistry staff and students in Universiti Kebangsaan Malaysia. Healthcare Infect. 2008;13:4–9. [Google Scholar]
- 29.Parlevliet W., de Borgie C., Frijstein G., Guchelaar H.-J. Cost-benefit analysis of vaccination against influenza of employees from an academic medical centre. Dis Manage Health Outcomes. 2002;10:579–587. [Google Scholar]
- 30.Nguyen-Van-Tam J., Granfield R., Pearson J., Fleming D., Keating N. Do influenza epidemics affect patterns of sickness absence among British hospital staff? Infect Control Hosp Epidemiol. 1999;20:691–694. doi: 10.1086/501568. [DOI] [PubMed] [Google Scholar]
- 31.Voirin N., Payet C., Barrat A., Cattuto C., Khanafer N., Regis C. Combining high-resolution contact data with virological data to investigate influenza transmission in a tertiary care hospital. Infect Control Hosp Epidemiol. 2015;36:254–260. doi: 10.1017/ice.2014.53. [DOI] [PubMed] [Google Scholar]
- 32.Eibach D., Casalegno J.S., Bouscambert M., Benet T., Regis C., Comte B. Routes of transmission during a nosocomial influenza A(H3N2) outbreak among geriatric patients and healthcare workers. J Hosp Infect. 2014;86:188–193. doi: 10.1016/j.jhin.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Vanhems P., Voirin N., Roche S., Escuret V., Regis C., Gorain C. Risk of influenza-like illness in an acute health care setting during community influenza epidemics in 2004–2005, 2005–2006, and 2006–2007: a prospective study. Arch Intern Med. 2011;171:151–157. doi: 10.1001/archinternmed.2010.500. [DOI] [PubMed] [Google Scholar]
- 34.Vanhems P., Barrat A., Cattuto C., Pinton J.F., Khanafer N., Regis C. Estimating potential infection transmission routes in hospital wards using wearable proximity sensors. PLoS ONE. 2013;8:e73970. doi: 10.1371/journal.pone.0073970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagani L., Thomas Y., Huttner B., Sauvan V., Notaridis G., Kaiser L. Transmission and effect of multiple clusters of seasonal influenza in a Swiss geriatric hospital. J Am Geriatr Soc. 2015;63:739–744. doi: 10.1111/jgs.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voirin N., Barret B., Metzger M.H., Vanhems P. Hospital-acquired influenza: a synthesis using the outbreak reports and intervention studies of nosocomial infection (ORION) statement. J Hosp Infect. 2009;71:1–14. doi: 10.1016/j.jhin.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Ridgway J.P., Bartlett A.H., Garcia-Houchins S., Carino S., Enriquez A., Marrs R. Influenza among afebrile and vaccinated healthcare workers. Clin Infect Dis. 2015;60:1591–1595. doi: 10.1093/cid/civ163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kay M., Zerr D.M., Englund J.A., Cadwell B.L., Kuypers J., Swenson P. Shedding of pandemic (H1N1) 2009 virus among health care personnel, Seattle, Washington, USA. Emerg Infect Dis. 2011;17:639–644. doi: 10.3201/eid1704.100866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Sanchez B., Alonso M., Catalan P., Sanchez Conde M., Gonzalez-Candelas F., Giannella M. Genotyping of a nosocomial outbreak of pandemic influenza A/H1N1 2009. J Clin Virol. 2011;52:129–132. doi: 10.1016/j.jcv.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Valley-Omar Z., Nindo F., Mudau M., Hsiao M., Martin D.P. Phylogenetic exploration of nosocomial transmission chains of 2009 influenza A/H1N1 among children admitted at red cross war memorial children's hospital, Cape Town, South Africa in 2011. PLoS ONE [Electron Res] 2015;10:e0141744. doi: 10.1371/journal.pone.0141744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melchior T.B., Perosa A.H., Camargo C.N., Granato C., Bellei N. Influenza virus prevalence in asymptomatic and symptomatic subjects during pandemic and postpandemic periods. Am J Infect Control. 2015;43:460–464. doi: 10.1016/j.ajic.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 42.Seal K., Spry C. CADTH Rapid Response Reports. Ottawa (ON) 2017. Seasonal Influenza Immunization of Health Care Workers for the Prevention of Influenza in Patients: A Review of the Clinical Effectiveness. [PubMed] [Google Scholar]
- 43.Amodio E., Restivo V., Firenze A., Mammina C., Tramuto F., Vitale F. Can influenza vaccination coverage among healthcare workers influence the risk of nosocomial influenza-like illness in hospitalized patients? J Hosp Infect. 2014;86:182–187. doi: 10.1016/j.jhin.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Blanco N., Eisenberg M.C., Stillwell T., Foxman B. What transmission precautions best control influenza spread in a hospital? Am J Epidemiol. 2016;183:1045–1054. doi: 10.1093/aje/kwv293. [DOI] [PubMed] [Google Scholar]
- 45.Shugarman L.R., Hales C., Setodji C.M., Bardenheier B., Lynn J. The influence of staff and resident immunization rates on influenza-like illness outbreaks in nursing homes. J Am Med Direct Assoc. 2006;7:562–567. doi: 10.1016/j.jamda.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Dionne B., Brett M., Culbreath K., Mercier R.-C. Potential ceiling effect of healthcare worker influenza vaccination on the incidence of nosocomial influenza infection. Infect Control Hosp Epidemiol. 2016;37:840–844. doi: 10.1017/ice.2016.77. [DOI] [PubMed] [Google Scholar]
- 47.Salgado C.D., Giannetta E.T., Hayden F.G., Farr B.M. Preventing nosocomial influenza by improving the vaccine acceptance rate of clinicians. Infect Control Hosp Epidemiol. 2004;25:923–928. doi: 10.1086/502321. [DOI] [PubMed] [Google Scholar]
- 48.van den Dool C., Bonten M.J.M., Hak E., Wallinga J. Modeling the effects of influenza vaccination of health care workers in hospital departments. Vaccine. 2009;27:6261–6267. doi: 10.1016/j.vaccine.2009.07.104. [DOI] [PubMed] [Google Scholar]
- 49.Van Den Dool C., Bonten M.J.M., Hak E., Heijne J.C.M., Wallinga J. The effects of influenza vaccination of health care workers in nursing homes: Insights from a mathematical model. PLoS Med. 2008;5:1453–1460. doi: 10.1371/journal.pmed.0050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wendelboe A.M., Avery C., Andrade B., Baumbach J., Landen M.G. Importance of employee vaccination against influenza in preventing cases in long-term care facilities. Infect Control Hosp Epidemiol. 2011;32:990–997. doi: 10.1086/661916. [DOI] [PubMed] [Google Scholar]
- 51.Wendelboe A.M., Grafe C., McCumber M., Anderson M.P. Inducing herd immunity against seasonal influenza in long-term care facilities through employee vaccination coverage: a transmission dynamics model. Comput Math Methods Med. 2015;2015:178247. doi: 10.1155/2015/178247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hollmeyer H., Hayden F., Mounts A., Buchholz U. Review: interventions to increase influenza vaccination among healthcare workers in hospitals. Influenza Other Respir Viruses. 2013;7:604–621. doi: 10.1111/irv.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abramson Z.H., Avni O., Levi O., Miskin I.N. Randomized trial of a program to increase staff influenza vaccination in primary care clinics. Ann Family Med. 2010;8:293–298. doi: 10.1370/afm.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chambers L.W., Crowe L., Lam P.P., MacDougall D., McNeil S., Roth V. A new approach to improving healthcare personnel influenza immunization programs: a randomized controlled trial. PLoS ONE [Electron Res] 2015;10:e0118368. doi: 10.1371/journal.pone.0118368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chambers L.W., Wilson K., Hawken S., Puxty J., Crowe L., Lam P.P. Impact of the Ottawa influenza decision aid on healthcare personnel's influenza immunization decision: a randomized trial. J Hosp Infect. 2012;82:194–202. doi: 10.1016/j.jhin.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 56.ECDC. Review of the scientific literature on drivers and barriers of seasonal influenza vaccination coverage in the EU/EEA. Stockholm: ECDC; 2013.
- 57.WHO. A rapid review of interventions with positive evaluation outcomes was conducted by the WHO Regional Office for Europ. WHO Regional Office for Europe; 2015.
- 58.WHO. Tailoring Immunization Programmes for Seasonal Influenza (TIP FLU) A guide for increasing health care workers’ uptake of seasonal influenza vaccination.: WHO Regional Office for Europe; 2015.
- 59.Macdonald L., Cairns G., Angus K., de Andrade M. Promotional communications for influenza vaccination: a systematic review. J Health Commun. 2013;18:1523–1549. doi: 10.1080/10810730.2013.840697. [DOI] [PubMed] [Google Scholar]
- 60.Stuart M.J. Review of strategies to enhance the uptake of seasonal influenza vaccination by Australian healthcare workers. Commun Dis Intell Quart Rep. 2012;36:E268–E276. [PubMed] [Google Scholar]
- 61.Lorenc T., Marshall D., Wright K., Sutcliffe K., Sowden A. Seasonal influenza vaccination of healthcare workers: systematic review of qualitative evidence. BMC Health Serv Res. 2017;17:732. doi: 10.1186/s12913-017-2703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ofstead C.L., Amelang M.R., Wetzler H.P., Tan L. Moving the needle on nursing staff influenza vaccination in long-term care: Results of an evidence-based intervention. Vaccine. 2017;35:2390–2395. doi: 10.1016/j.vaccine.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 63.Lietz J., Westermann C., Nienhaus A., Schablon A. The occupational risk of influenza A (H1N1) infection among healthcare personnel during the 2009 pandemic: a systematic review and meta-analysis of observational studies. PLoS ONE. 2016;11:e0162061. doi: 10.1371/journal.pone.0162061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuster S.P., Shah P.S., Coleman B.L., Lam P.P., Tong A., Wormsbecker A. Incidence of influenza in healthy adults and healthcare workers: a systematic review and meta-analysis. PLoS ONE. 2011;6:e26239. doi: 10.1371/journal.pone.0026239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams C.J., Schweiger B., Diner G., Gerlach F., Haaman F., Krause G. Seasonal influenza risk in hospital healthcare workers is more strongly associated with household than occupational exposures: results from a prospective cohort study in Berlin, Germany, 2006/07. BMC Infect Dis. 2010;10:8. doi: 10.1186/1471-2334-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kliner M., Keenan A., Sinclair D., Ghebrehewet S., Garner P. Influenza vaccination for healthcare workers in the UK: appraisal of systematic reviews and policy options. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Restivo V., Costantino C., Bono S., Maniglia M., Marchese V., Ventura G. Influenza vaccine effectiveness among high-risk groups: a systematic literature review and meta-analysis of case-control and cohort studies. Hum Vaccin Immunother. 2018;14:724–735. doi: 10.1080/21645515.2017.1321722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ng A.N., Lai C.K. Effectiveness of seasonal influenza vaccination in healthcare workers: a systematic review. J Hosp Infect. 2011;79:279–286. doi: 10.1016/j.jhin.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Michiels B., Govaerts F., Remmen R., Vermeire E., Coenen S. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine. 2011;29:9159–9170. doi: 10.1016/j.vaccine.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 70.Burls A., Jordan R., Barton P., Olowokure B., Wake B., Albon E. Vaccinating healthcare workers against influenza to protect the vulnerable–is it a good use of healthcare resources? a systematic review of the evidence and an economic evaluation. Vaccine. 2006;24:4212–4221. doi: 10.1016/j.vaccine.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 71.Dumigan D., Conrad E., Kuncas M., Cortes L., Tyler L., Santerre A. Implementing a mandatory influenza vaccination program in a university-affiliated teaching hospital. Am J Infect Control. 2012;40(5):e75. [Google Scholar]
- 72.Dolan G.P., Harris R.C., Clarkson M., Sokal R., Morgan G., Mukaigawara M. Vaccination of health care workers to protect patients at increased risk for acute respiratory disease. Emerg Infect Dis. 2012;18:1225–1234. doi: 10.3201/eid1808.111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Serres G., Skowronski D.M., Ward B.J., Gardam M., Lemieux C., Yassi A. Influenza vaccination of healthcare workers: critical analysis of the evidence for patient benefit underpinning policies of enforcement. PLoS ONE. 2017;12:e0163586. doi: 10.1371/journal.pone.0163586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomas R.E., Jefferson T., Lasserson T.J. Influenza vaccination for healthcare workers who care for people aged 60 or older living in long-term care institutions. Cochrane Database Syst Rev. 2016:CD005187. doi: 10.1002/14651858.CD005187.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benet T., Regis C., Voirin N., Robert O., Lina B., Cronenberger S. Influenza vaccination of healthcare workers in acute-care hospitals: a case-control study of its effect on hospital-acquired influenza among patients. BMC Infect Dis. 2012;12:30. doi: 10.1186/1471-2334-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed F., Lindley M.C., Allred N., Weinbaum C.M., Grohskopf L. Effect of influenza vaccination of healthcare personnel on morbidity and mortality among patients: systematic review and grading of evidence. Clin Infect Dis. 2014;58:50–57. doi: 10.1093/cid/cit580. [DOI] [PubMed] [Google Scholar]
- 77.Potter J., Stott D.J., Roberts M.A., Elder A.G., O'Donnell B., Knight P.V. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis. 1997;175:1–6. doi: 10.1093/infdis/175.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lemaitre M., Meret T., Rothan-Tondeur M., Belmin J., Lejonc J.L., Luquel L. Effect of influenza vaccination of nursing home staff on mortality of residents: a cluster-randomized trial. J Am Geriatr Soc. 2009;57:1580–1586. doi: 10.1111/j.1532-5415.2009.02402.x. [DOI] [PubMed] [Google Scholar]
- 79.Hayward A.C., Harling R., Wetten S., Johnson A.M., Munro S., Smedley J. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ. 2006;333:1241. doi: 10.1136/bmj.39010.581354.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carman W.F., Elder A.G., Wallace L.A., McAulay K., Walker A., Murray G.D. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet. 2000;355:93–97. doi: 10.1016/S0140-6736(99)05190-9. [DOI] [PubMed] [Google Scholar]
- 81.Hayward A.C. Influenza vaccination of healthcare workers is an important approach for reducing transmission of influenza from staff to vulnerable patients. PLoS ONE. 2017;12:e0169023. doi: 10.1371/journal.pone.0169023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pitts S.I., Maruthur N.M., Millar K.R., Perl T.M., Segal J. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330–340. doi: 10.1016/j.amepre.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 83.Lytras T., Kopsachilis F., Mouratidou E., Papamichail D., Bonovas S. Interventions to increase seasonal influenza vaccine coverage in healthcare workers: a systematic review and meta-regression analysis. Human Vacc Immunotherap. 2016;12:671–681. doi: 10.1080/21645515.2015.1106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siemieniuk R, Coleman B, Shafiz S, Al-Den A, Bornsten S, Kean R, McGeer A, Goodliffe L. Interventions to increase healthcare worker influenza vaccination: a meta-analysis. Poster ID1713 presented at the IDWeek2014. Advancing Science, Improving Care. October 8-12 2014, Philadelphia, PA, USA. Available at https://idsa.confex.com/idsa/2014/webprogram/Paper47456.html.
- 85.Rothan-Tondeur M., Filali-Zegzouti Y., Golmard J.L., De Wazieres B., Piette F., Carrat F. Randomised active programs on healthcare workers' flu vaccination in geriatric health care settings in France: the VESTA study. J Nutrit, Health Aging. 2011;15:126–132. doi: 10.1007/s12603-011-0025-5. [DOI] [PubMed] [Google Scholar]
- 86.Schmid P., Rauber D., Betsch C., Lidolt G., Denker M.L. Barriers of influenza vaccination intention and behavior - a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS ONE. 2017;12:e0170550. doi: 10.1371/journal.pone.0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corace K.M., Srigley J.A., Hargadon D.P., Yu D., MacDonald T.K., Fabrigar L.R. Using behavior change frameworks to improve healthcare worker influenza vaccination rates: a systematic review. Vaccine. 2016;34:3235–3242. doi: 10.1016/j.vaccine.2016.04.071. [DOI] [PubMed] [Google Scholar]
- 88.Sandoval C., Barrera A., Ferres M., Cerda J., Retamal J., Garcia-Sastre A. Infection in health personnel with high and low levels of exposure in a hospital setting during the H1N1 2009 influenza A pandemic. PLoS ONE. 2016;11:e0147271. doi: 10.1371/journal.pone.0147271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olalla J., de Ory F., Casas I., Benitez N. Vaccination against influenza a virus (H1N1) among Spanish healthcare workers. Eur J Intern Med. 2012;23:e69–e70. doi: 10.1016/j.ejim.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 90.MacIntyre R., Dwyer D., Seale H., Quanyi W., Yi Z., Yang P. High risk procedures and respiratory infections in hospital health care workers - quantifying the risk. Int J Infect Dis. 2012;16:e379. [Google Scholar]
- 91.Donovan T.L., Moore K.M., VanDenKerkhof E.G. Employee absenteeism based on occupational health visits in an urban tertiary care Canadian hospital. Public Health Nurs (Boston, Mass) 2008;25:565–575. doi: 10.1111/j.1525-1446.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 92.Bhadelia N., Sonti R., McCarthy J.W., Vorenkamp J., Jia H., Saiman L. Impact of the 2009 influenza A (H1N1) pandemic on healthcare workers at a tertiary care center in New York City. Infect Control Hosp Epidemiol. 2013;34:825–831. doi: 10.1086/671271. [DOI] [PubMed] [Google Scholar]
- 93.Hammond G.W., Cheang M. Absenteeism among hospital staff during an influenza epidemic: implications for immunoprophylaxis. Can Med Assoc J. 1984;131:449–452. [PMC free article] [PubMed] [Google Scholar]
- 94.Wilde J.A., McMillan J.A., Serwint J., Butta J., O'Riordan M.A., Steinhoff M.C. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA. 1999;281:908–913. doi: 10.1001/jama.281.10.908. [DOI] [PubMed] [Google Scholar]
- 95.Weingarten S., Staniloff H., Ault M., Miles P., Bamberger M., Meyer R.D. Do hospital employees benefit from the influenza vaccine? a placebo-controlled clinical trial. J Gen Intern Med. 1988;3:32–37. doi: 10.1007/BF02595754. [DOI] [PubMed] [Google Scholar]
- 96.Saxen H., Virtanen M. Randomized, placebo-controlled double blind study on the efficacy of influenza immunization on absenteeism of health care workers. Pediat Infect Dis J. 1999;18:779–783. doi: 10.1097/00006454-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 97.Thomas R.E. Is influenza-like illness a useful concept and an appropriate test of influenza vaccine effectiveness? Vaccine. 2014;32:2143–2149. doi: 10.1016/j.vaccine.2014.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chughtai A.A., Wang Q., Dung T.C., Macintyre C.R. The presence of fever in adults with influenza and other viral respiratory infections. Epidemiol Infect. 2017;145:148–155. doi: 10.1017/S0950268816002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lansbury L.E., Smith S., Beyer W., Karamehic E., Pasic-Juhas E., Sikira H. Effectiveness of 2009 pandemic influenza A(H1N1) vaccines: a systematic review and meta-analysis. Vaccine. 2017;35:1996–2006. doi: 10.1016/j.vaccine.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 100.Black C.L., Yue X., Ball S.W., Fink R.V., de Perio M.A., Laney A.S. Influenza vaccination coverage among health care personnel - united states, 2017–18 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:1050–1054. doi: 10.15585/mmwr.mm6738a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bellei N., Carraro E., Perosa A., Granato C. Patterns of influenza infections among different risk groups in Brazil. Brazil J Infect Dis. 2007;11:399–402. doi: 10.1590/s1413-86702007000400005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.