Abstract
The human papillomavirus (HPV) 9-valent, recombinant vaccine (Gardasil™9) helps protect young adults (males and females) against anogenital cancers and genital warts caused by certain HPV genotypes (ref. Gardasil™9 insert). This vaccine is administered intramuscularly (IM). The aim of this study was to determine preclinically whether intradermal (ID) vaccination with an unadjuvanted 9-valent recombinant HPV vaccine using a first-generation ID delivery device, the Nanopatch™, could enhance vaccine immunogenicity compared with the traditional ID route (Mantoux technique). IM injection of HPV VLPs formulated with Merck & Co., Inc., Kenilworth, NJ, USA Alum Adjuvant (MAA) were included in the rhesus study for comparison. The Nanopatch™ prototype contains a high-density array comprised of 10,000 microprojections/cm2, each 250 µm long. It was hypothesized the higher density array with shallower ID delivery may be superior to the Mantoux technique. To test this hypothesis, HPV VLPs without adjuvant were coated on the Nanopatch™, stability of the Nanopatch™ with unadjuvanted HPV VLPs were evaluated under accelerated conditions, skin delivery was verified using radiolabelled VLPs or FluoSpheres®, and the immune response and skin site reaction with the Nanopatch™ was evaluated in rhesus macaques. The immune response induced by Nanopatch™ administration, measured as HPV-specific binding antibodies, was similar to that induced using the Mantoux technique. It was also observed that a lower dose of unadjuvanted HPV VLPs delivered with the first-generation Nanopatch™ and applicator or Mantoux technique resulted in an immune response that was significantly lower compared to a higher-dose of alum adjuvanted HPV VLPs delivered IM in rhesus macaques. The study also indicated unadjuvanted HPV VLPs could be delivered with the first-generation Nanopatch™ and applicator to the skin in 15 s with a transfer efficiency of approximately 20%. This study is the first demonstration of patch administration in non-human primates with a vaccine composed of HPV VLPs.
Keywords: Vaccine, Vaccination, Nanopatch™, Microneedles, Patch, Intradermal, Rhesus, HPV, Virus-like particles, Skin, Micro array patch
1. Introduction
Human papillomaviruses (HPV) are the major cause of cervical, vulvar, vaginal, penile, and anal cancers and are also responsible for genital warts [1]. Vaccines to prevent infection by HPV have been available since 2006, the most recent of which is GARDASIL™9, which was approved for prevention of certain cancers by the Food and Drug Administration (FDA) in December 2014, and offers protection from at least 9 HPV genotypes [2], [1]. It has been shown that adjuvanted HPV vaccines have been effective in prevention of HPV infection with genotypes 6, 11, 16, and 18 [3]. A 2-dose regimen of GARDASIL™ and GARDASIL™9 was approved in October 2016 by the FDA for use in boys and girls ages 9–14 years, and the age range for HPV immunization was recently expanded for men and women 27–45 years of age [4], [5]. Despite the effectiveness of the adjuvanted HPV vaccines, access and compliance remains to be improved [6], [7]. For example, uptake of adjuvanted HPV vaccines could be improved in rural areas of the United States and internationally, in less developed areas [8], [9]. As of 2014, in developed regions, 33.6% of females age 10–20 received the full HPV vaccine course as compared to 2.7% of females in the same age group from less developed regions [9].
Adjuvanted HPV vaccines are administered to the muscle using a needle/syringe, and current data supports this route of delivery based on immunogenicity and efficacy studies [1]. In fact, the majority of vaccines are delivered intramuscularly (HepB, dTAP) or subcutaneously (measles, mumps, rubella, varicella).
One alternative to the current routes of vaccine delivery is administration to the skin. The work of Edward Jenner in the late 1700′s demonstrated that percutaneous delivery of smallpox or cowpox to the skin resulted in the development of protective immune responses against smallpox (reviewed in [10]). The success of mounting a strong immune response by delivering vaccines to the skin is due to the population of immune cells present in this tissue. These include Langerhans cells in the epidermis as well as dermal dendritic cells [11]. There have been a number of publications that describe the role of these cells in mounting an immune response via the skin (reviewed in [12]).
In addition to smallpox, several other vaccines have been demonstrated to mount an immune response when administered to the skin, and include recombinant hepatitis B virus vaccines (recombinant Hepatitis B surface antigen), influenza, rabies, and varicella [11], [13], [14], [15], [16]. There are several advantages to delivering these vaccines via the intradermal (ID) route. Recombinant Hepatitis B virus vaccines are delivered ID when an individual has not responded (“non-responders”) to intramuscular injection of this vaccine [13]. HepB vaccines contain aluminum adjuvant. It has also been demonstrated that influenza and rabies vaccines can be administered ID at lower doses (i.e. “Dose Sparing”) when compared to IM administration and result in the same or similar immune response. For influenza, a range of 3–9 μg of each serotype (9–27 μg total) delivered ID is as effective as 15 μg (full dose) of each serotype delivered IM (45 μg total) [17].
ID delivery with the rabies vaccine at 1/10th IM dose was as effective as the full-dose delivered IM [15]. With varicella, it was shown using the MicronJet™ intradermal delivery device that an immune response could be elicited in the skin and had the potential for dose sparing [16]. For these viral vaccines, an adjuvant is not required to elicit an immune response in the skin, muscle, or subcutaneously.
The traditional method of administering vaccines intradermally (ID) is the Mantoux technique, which utilizes a needle and syringe and requires a trained health care worker. Vaccine patches, which contain microprojections either coated with the vaccine or made of the vaccine itself, are another means to deliver vaccines to the skin. It has been recently shown in pre-clinical models that vaccines against influenza, measles, HPV, inactivated polio, and others have been administered to skin using various types of microneedle patches and Nanopatches™ [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]. The Nanopatch™ pre-clinical vaccination studies in mouse and rat models demonstrated improved immunogenicity and/or significant dose-sparing (for a comparable immune response). The authors hypothesized that this may have been associated with a “physical immune enhancer,” in which the Nanopatch™ induces local cell disruption, leading to the release of cytokines, danger signals, and other immune-signaling molecules [33]. In the past few years, clinical studies, including one that utilized a next-generation Nanopatch™ and next-generation applicator prototypes delivering conventional influenza vaccines, have demonstrated that immune responses with intradermal patches are comparable with conventional IM delivery [34], [35], [36].
In this report we investigated whether a high-density microprojection vaccine patch, the Nanopatch™, could elicit an immune response in rhesus macaques using an unadjuvanted HPV vaccine as a model system. Previous studies evaluated the Nanopatch™ with unadjuvanted HPV VLPs in mice, which demonstrated an enhanced immune response when compared to the standard intramuscular route [30]. The authors hypothesized that application of a high-density array to the skin would produce a physical enhancer, which is distinct from standard intradermal delivery with a needle/syringe and would result in eliciting a systemic immune response that would have immediate (humoral) and long-term (memory) responses.
In this study, the first-generation Nanopatch™ and first-generation applicator prototypes were designed, tested, and evaluated in rhesus macaques, for the first time in a large animal model. The projections on the Nanopatch™, each 250 µm in length, were designed to deliver vaccine to the epidermis and shallow dermis using an application system designed for use in large animal models. The version of the Nanopatch™ used in this rhesus study was different to that used in the subsequent clinical trials particularly in terms of the material used to manufacture the Nanopatch™, as well as application conditions [34], [37]. To our knowledge, this is the first report of the evaluation of a high density microarray patch with an unadjuvanted HPV vaccine in a large animal model.
2. Materials and methods
2.1. Nanopatch™ design, manufacture, and application
Nanopatches™ for rhesus macaque studies were manufactured using the same method as previously described [38], [39]. Briefly, 6 in. silicon wafers were photolithographically patterned with SU8-2025 photoresist. Wafers were then etched by deep reactive ion etching (DRIE) to yield projections at 10,000 /cm2, each 250 µm in length. Nanopatches™ were diced to 10 X 10 mm and the edges bevelled to 45°, then Nanopatches™ were bonded to a polycarbonate backing, and quartered. A Nanopatch™ dosage form is a single 10 X 10 mm Nanopatch™. The Nanopatches™ were quartered to ensure that any flex of the skin could be balanced with some flex of the Nanopatch™. In the absence of quartering, the Nanopatches™ may have had the risk of cracking, and therefore quartering allowed the most efficient transfer of the antigen.
2.2. Nanopatch™ evaluation by Scanning Electron Microscopy
Nanopatches™ were analyzed by scanning electron microscopy (SEM) as previously described [42]. SEM of coated and uncoated Nanopatches™, and Nanopatches™ following application to rhesus skin was performed on a Philips XL30 SEM (45° tilt, 20 kV).
2.3. Nanopatch™ Proof-of-Principle 4 Applicator (PoP4)
Each Nanopatch™ was delivered using a custom applicator, PoP4 (Proof of Principle 4). PoP4 pushed the patches onto the site at a high speed (∼15 m/s) to ensure full engagement of the Nanopatch™ surface with the skin [40], [41]. For Nanopatch™ delivery studies, the PoP4 applicator is first attached to the Nanopatch™ with holder, the Nanopatch™ is removed from the holder, placed against the skin, and the plunger activated to administer the Nanopatch™ to the skin. The Nanopatch™ is then removed. This first-generation applicator was designed only for preclinical use.
2.4. Nanopatch™ vaccine coating
Single, individual 10 × 10 mm Nanopatches™ were coated with 41 μL of coating solution containing HPV 9-valent (Genotype 6, 11, 16, 18, 31, 33, 45, 52, and 58) using the gas-jet drying approach that has been described previously, in combination with a formulation containing methylcellulose and trehalose [38], [42]. For shipping, transfer efficiency, and stability studies, 54 μg of unadjuvanted HPV VLPs were applied to the patch in a volume of 41 μL (1.32 mg/mL final concentration). For the rhesus immunogenicity study, 70 μg of unadjuvanted HPV VLPs were applied to the patch in the same volume (1.71 mg/mL final concentration). Each HPV genotype 6, 11, 16, 18, 31, 33, 45, 52, and 58 had individual concentrations of 0.19, 0.25, 0.38, 0.25, 0.13, 0.13, 0.13, 0.13, and 0.13 mg/mL, respectively. The entire volume of the unadjuvanted HPV VLP-containing formulation was coated and dried onto each Nanopatch™, which included the projections and base of the Nanopatch™. Following the coating procedure, Nanopatches™ were protected from the environment by placement into hinged boxes containing desiccant and then sealed in foil pouches with temperature monitors.
2.5. Nanopatch™ cold shipping studies
Nanopatches™ were placed into individual hinged plastic containers containing dessicant, snap closed, and placed into foil pouches. The foil pouches contained both the hinged plastic containers and temperature monitoring devices (as described, Nanopatch™ vaccine coating). The foil pouches were placed with cold packs and shipped internationally. The temperature log therefore reflected the condition of the interior of the foil bags. Because the cold shipment was made over the course of approximately 2 weeks, and the temperature loggers were adjacent to the containers holding the Nanopatches™, the temperature of the Nanopatches™ may have reached 2-8C during the shipment.
2.6. Nanopatch™ transfer efficiency studies in pig cadaver skin (Pig Ears)
To evaluate the transfer efficiency of unadjuvanted HPV VLPs that were coated on the Nanopatch™, 54 μg of 14C -labeled unadjuvanted HPV VLP genotype 11 was coated onto Nanopatches™ and applied using a PoP4 applicator to pig ears. This applicator was tested on pig ears prior to being utilized with rhesus macaques. To visually confirm that the Nanopatches™ engaged firmly and penetrated the surface of the skin, Coomassie blue was added to some of the Nanopatches™. Following administration, the site of administration was swabbed to remove any material left on the skin surface, then cut out using a scalpel, digested overnight, placed in scintillation fluid, and counted on a scintillation counter.
2.7. Delivery of 14C-labeled HPV genotype 11 into rhesus macaque skin (Rhesus Study #1)
All procedures with rhesus macaques were approved through the Institutional Animal Care and Use Committee (IACUC) of Merck & Co., Inc., Kenilworth, NJ, USA and followed Animal Procedure Statements (APS). The transfer efficiency of unadjuvanted HPV VLPs coated on Nanopatches™ to rhesus skin was performed with 54 μg 14C-labeled HPV genotype 11 per Nanopatch™. The sites selected were the quadriceps (thigh), forearm, and inguinal areas. Rhesus macaques were sedated using Ketamine HCL (100 mg/mL) (Fort Dodge) prior to Nanopatch™ administration. Nanopatches™ were applied from fifteen seconds to two minutes and skin samples were then collected from the Nanopatch™ application area within 5 min after the Nanopatch™ was removed from the skin, and in sections that were marginally larger (several mm around the perimeter) than the Nanopatch™ application area. The two-minute application had been established in previous applications to the mouse ear (data not shown), consistent with previous mouse studies with Nanopatches™ and non-adjuvanted HPV vaccine [30]. Transfer efficiency was determined by placing the tissue in scintillation fluid followed by counting using a scintillation counter.
2.8. Delivery of FluoSpheres® into rhesus macaque skin (Rhesus study #2)
To determine the depth of penetration of the microprojections into the skin of rhesus macaques, a FluoSphere® (ThermoFisher) coating was used on the Nanopatches™. The FluoSpheres® are carboxylate-modified microspheres, 0.22 μm diameter, yellow-green fluorescent (505/515 nm), 2.0% solids (Cat No. F8811), and were diluted 1:10 in MilliQ water. The FluoSpheres® were utilized to image the depth of delivery of material to the skin. The FluoSpheres® are 220 nm in diameter and HPV VLPs are approximately 3-fold smaller in diameter when compared to FluoSpheres®. Rhesus macaques were sedated using Ketamine HCL (100 mg/mL) (Fort Dodge) prior to Nanopatch™ administration. Nanopatches™ were applied for two minutes. Skin samples were then collected as described for Nanopatches™ coated with 14C-labeled HPV genotype 11. Histological samples of the skin were prepared. Fluorescent microscopy images of skin sections following Nanopatch™ application were obtained using a 488 nm wavelength of light with a Aperio ScanScope slide scanner. Measurements in the skin were taken to determine the depth of delivery of FluoSpheres® at four different sites: the quadriceps, forearm, ear, and inguinal areas.
2.9. HPV VLP stability on Nanopatches™
Recombinant HPV VLPs were generated at Merck & Co., Inc., West Point, PA, USA. Unadjuvanted HPV VLPs were coated in Australia onto Nanopatches™ and were placed in holding containers and incubated at 25 °C or 37 °C. Potency of the HPV VLPs was determined by surface plasmon resonance (SPR) using the Biacore 2000 (GE Healthcare). The SPR method measures the amount of intact VLPs that bind to specific neutralizing monoclonal antibodies and is represented as a percent relative to a reference VLP lot. These antibodies recognize HPV VLPs that are intact and have the correct structure to elicit an immune response. The SPR method has been previously described [43].
2.10. Rhesus macaque vaccination (Rhesus Study #3)
Rhesus macaque studies were performed in accordance with all animal ethics guidelines, and animal procedures were approved under the IACUC of Merck & Co., Inc., Kenilworth, NJ, USA. Animals were 4–5 years old and placed in groups of 6 that were gender- and weight- balanced, with individual weights ranging from 4.6 to 10.2 kg. Six animals per group were selected based on previous studies performed with HPV VLPs and rhesus macaques (data not shown). All animals had a negative titer for HPV prior to vaccination. Intradermal site preparation was performed by first using an Oster clipper with #40 blade to remove the coarse hair. Following this, an electric Remington shaver was used to remove hair from the surface prior to Nanopatch™ application or ID administration (Mantoux). Quadriceps sites were selected for immunization due to the ability to obtain consistent Nanopatch™ administration and large surface area. Vaccination was performed at 0 and 4 weeks and blood collected at 0, 4, and 8 weeks. Table 1 indicates the route, dose, method, and dose delivered of HPV VLPs utilized. Animals were sedated using Ketamine HCL (100 mg/mL) (Fort Dodge) prior to vaccine administration and blood collection. The Nanopatches™ supplied for the rhesus macaque study were coated with 70 μg of unadjuvanted HPV VLPs at the same ratio as described in the stability procedure. Following delivery, this yielded approximately 14 mg which is 1/5 the dose given IM.
Table 1.
Group, route, method, dose, and dose delivered for immunogenicity studies.
| Group | Route | Method | Dose | Dose Delivered |
|---|---|---|---|---|
| 1 | Intramuscular | Intramuscular Needle/syringe |
70 μg HPV VLPs with aluminium adjuvant | 70 μg |
| 2 | Intradermal | Nanopatch™ | 70 μg loaded on Nanopatch™ | 14 μg* |
| 3 | Intradermal | Nanopatch™ | 140 μg with two 70 μg Nanopatches™ | 28 μg* |
| 4 | Intradermal | Mantoux Needle/Syringe |
70 μg | 70 μg |
| 5 | Intradermal | Mantoux Needle/Syringe |
14 μg | 14 μg |
Note: Delivery efficiency was at worst-case 20% (i.e. 70 μg/Nanopatch™ X 0.20 = 14 μg), and % of potent HPV VLPs were recovered from the Nanopatch™ following elution under in vitro laboratory conditions.
2.11. HPV-specific IgG ELISA
For assessing HPV-specific humoral immune responses, sera were collected from immunized monkeys at 4 weeks (4 weeks post dose 1) and 8 weeks (4 weeks post dose 2). Endpoint ELISA was utilized to measure HPV specific total IgG titres. Briefly, 96 well plates were coated overnight at 4 °C with a 4-valent mix of HPV-VLP (genotypes 6, 11, 16, 18) diluted in histidine buffer at 0.2 μg/ml each (total VLP concentration = 0.8 μg/ml). The four HPV genotypes that were tested were considered representative of the 9-valent vaccine that was administered. Plates were then washed and blocked with PBS + 1% BSA. Serum samples were then added to the plates starting with a 1:100 dilution followed by 3-fold serial dilutions and the plates incubated for 1.5 h at room temperature. After washing, peroxidase conjugated affinity-purified goat anti-monkey IgG (gamma chain) (Rockland) was used as a secondary antibody at a 1:5000 dilution and incubated for 1 h at room temperature. Following a final wash, TMB substrate (Virolabs, VA) was added for 3–5 min, stopped with H2SO4, and plates were read using a PerkinElmer Envision plate reader that measured absorbance at 450 nm.
2.12. Statistical analysis
IgG titer values at 4 and 8 weeks were collected and log10-transformed with Graphpad Prism (Version 6.01, Graphpad Software, Inc.). Statistical analysis was performed using Tukey’s multiple comparison’s test with P-values obtained. A P-value < 0.05 is statistically significant.
2.13. Evaluation of skin following Nanopatch™ application
Prior to and following the application of Nanopatches™, photographs of the sites were taken to assess any impact the application had on the surface of the rhesus skin.
3. Results
3.1. Nanopatches™ and vaccine coating
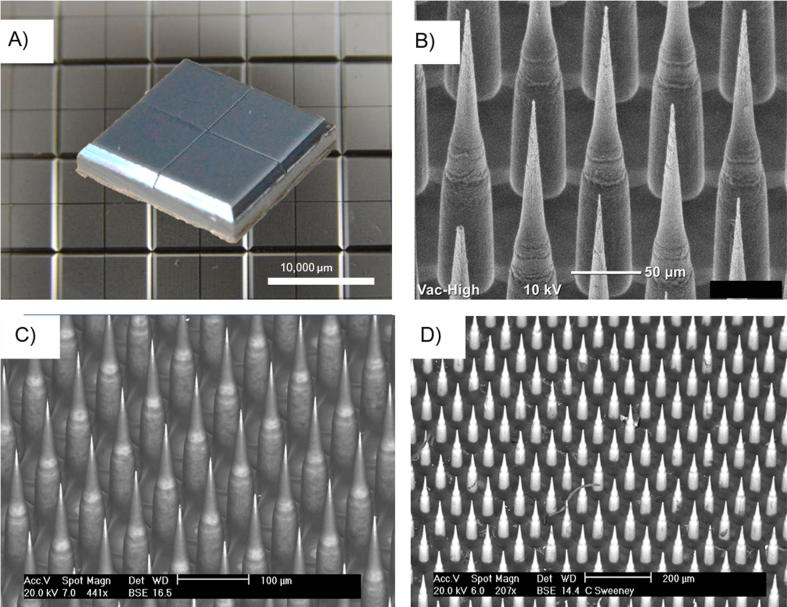
In preparation for the evaluation of an unadjuvanted HPV vaccine delivered ID in rhesus macaques, Nanopatches™ were fabricated using the deep reactive ion etching process and evaluated using scanning electron microscopy (SEM) with backscatter (Fig. 1A, B). These results demonstrated that consistent projections could be generated using this process (Fig. 1B). Next, a few representative Nanopatches™ were coated using a gas-jet drying procedure with the coating solution and visualized by SEM with backscatter both prior to and following administration to rhesus skin (Fig. 1C, D). The coating solution was the same as the one utilized with the 9-valent formulation for administration of the unadjuvanted HPV vaccine to rhesus macaques. This solution did not contain aluminum adjuvant. Fig. 1D depicts the Nanopatch™ following administration to rhesus skin, with light areas where vaccine is no longer present on the projections. Organic material such as the presence of a hair is shown remaining on the Nanopatch™ in darker contrast (Fig. 1D).
Fig. 1.
Nanopatches™ to deliver HPV vaccine to Rhesus macaques. (A) A single Nanopatch™, measuring 10 X 10 mm, providing a single dose of vaccine, shown prior to coating or application, on a backdrop of multiple patches post-manufacture. Each Nanopatch™ contains 4 quadrants, as depicted, (B) Nanopatch™ projections showing 250 µm long projections, (C) Nanopatch™ projections following coating of the HPV vaccine on their surface (Uncoated areas of the projections are light contrast, whereas areas coated with vaccine appear dark), (D) Nanopatch™ projections shown post-application to rhesus skin, Scale bars a = 10 mm (10,000 µm), b = 50 µm, c = 100 µm, d = 200 µm.
For coating the Nanopatch™, a volume of 41 μL containing the targeted amount of antigen was applied to the Nanopatch™ and dried using the gas-jet approach (see Materials and Methods). All of the antigen was dried on the Nanopatch™, which included both the projections and base of the Nanopatch™. Following the coating and drying of unadjuvanted HPV VLPs in a formulation on individual Nanopatches™, it was then determined whether the HPV VLPs maintained their potency. There was 73–93% recovery of HPV VLPs from Nanopatches™ following hydration of the patch surface with water. The concentration of the material eluted from the Nanopatch™ was also determined.
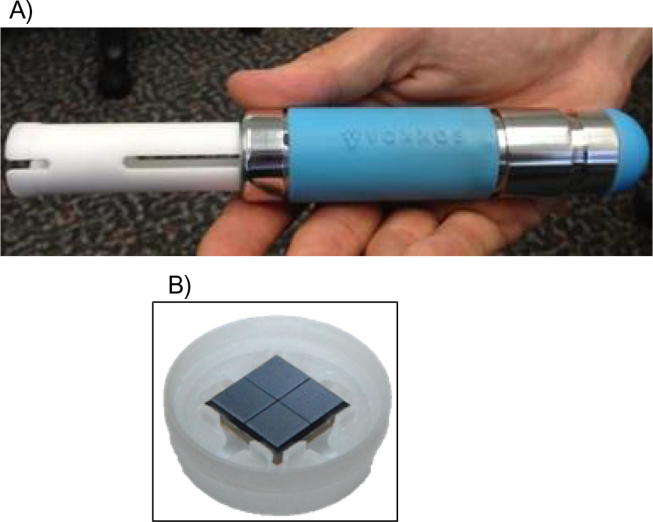
The applicator, referred to as the Proof of Principle 4 (PoP4) applicator, used to administer the Nanopatch™ is shown in Fig. 2A. It consists of a spring-loaded plunger that connects to a Nanopatch™ which is held in a plastic holder. A Nanopatch™ in the plastic holder is depicted in Fig. 2B. Details on the use of the applicator are described in the Materials in Methods.
Fig. 2.
(A) The Proof-of-Principle 4 (PoP4) applicator, (B) Patch in holder assembly.
3.2. Nanopatch™ cold shipping studies
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jvacx.2019.100030.
Following the coating of Nanopatches™ with antigen, it was important to understand if the HVP VLP coating could withstand cold shipping. Nanopatches™ were coated with unadjuvanted HPV VLPs in Australia and cold shipped to the United States for evaluation/administration to cadaver pig skin (ears) or rhesus macaques (see Materials and Methods for packaging of the Nanopatches™). The storage and transport of vaccine coated Nanopatches™ to the site of Nanopatch™ administration had temperature and humidity ranges that are shown in Supplementary Fig. 1. The temperature was maintained at approximately 0.5 °C, with small excursions occurring during transport. A change in temperature and humidity was observed once the foil bag was opened and the temperature logging devices were exposed to ambient conditions (arrow, A, Supplementary Fig. 1). Additionally, a period of acclimatization to ambient conditions was also observed (arrow, B, Supplementary Fig. 1).
Supplementary Fig. 1.
3.3. Nanopatch™ transfer efficiency studies in pig cadaver skin (Pig Ears)
Following evaluation of the cold shipping profile, the Nanopatches™ were evaluated in cadaver pig skin (ears) at both the site of Nanopatch™ coating, drying, and administration (Australia) under ambient conditions and following cold shipment of coated/dried Nanopatches™ and administration in the United States. These studies were performed to determine if a difference would be observed between Nanopatches (TM) that were coated/dried and administered immediately vs. Nanopatches (TM) that were cold shipped then administered. In addition, because the rhesus Nanopatch™ studies (#1, #2, and #3, see Materials and Methods) would each be performed in one day, it was important to know if the Nanopatches™ would deliver the same amount of antigen at both the beginning and end of the day. This would also determine if any changes occurred to the applicator during this time period.
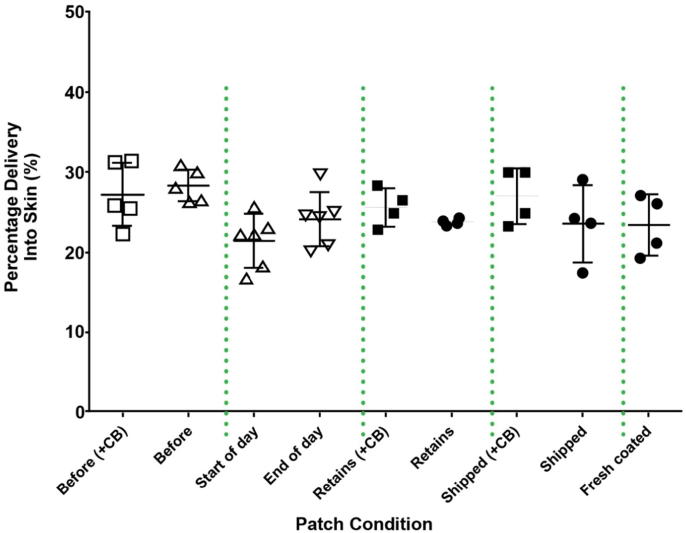
Nanopatches™ that contained 14C-labeled HPV VLPs and some which contained Coomassie blue (see Materials and Methods) were applied to pig ears using the PoP4 applicator. These Nanopatches™ contained a total of 54 μg unadjuvanted HPV VLPs. The results of the pig ear administration studies are depicted in Fig. 3. The 14C delivery results of applications to pig ears are shown as the two “Before” groups with average delivery <30%, approximately 27% and 28% (CV of 14% and 6%) with and without Coomassie blue, respectively.
Fig. 3.
(A) Delivery performance of Nanopatches™ prior to and post transportation, and validation of the Nanopatch™, PoP4 applicator, and force used in rhesus studies. 14C labelled HPV type 11 Nanopatches™ were applied to pig ears. ‘Before’ samples with or without Coomassie blue (CB) were applied in Australia. ‘Start of Day’ indicates Nanopatches™ that were cold shipped and tested in the USA prior to administration for Rhesus study #1. ‘End of Day’ indicates evaluation of the Nanopatches™ at the conclusion of Rhesus study #1. ‘Start’ and ‘End’ of the day contained Coomassie blue. ‘Retains’ are the same batch of Nanopatches™ tested at Vaxxas (no shipping). ‘Shipped’ samples were Nanopatches™ sent to the USA under cold conditions and returned to Australia. The ‘Fresh coated’ group were not part of the study and were coated after the study, using an identical formulation to the study batch as an experimental control. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Immediately before commencement of the Rhesus #1 Study applications, Nanopatches™ from the batch were applied to defrosted pig ears to confirm that Nanopatches™ had not been detrimentally affected by cold shipping from Australia to the United States. These applications are the “Start of Day” group in Fig. 3. Two more defrosted pig ears were patched after the Rhesus #1 Study to confirm the consistency of the PoP4 applicator device throughout the day. These applications are the “End of Day” group in Fig. 3. Both the “Start of Day” and “End of Day” samples contained Coomassie blue. Following the experiments conducted in the United States, additional Nanopatch™ application to defrosted pig ears was conducted in Australia. The “Retains” groups in Fig. 3 are samples from the study batch but retained in Australia and stored at 4 °C. The “Cold Shipped” samples were Nanopatches™ sent to the US but not used in the study and were cold shipped back to Australia for Nanopatch™ studies. The “Fresh coated” group were not a part of the study and were coated after the study, using an identical formulation to the study batch as an experimental control. The results from all groups were consistent, despite the significant variations in environmental conditions experienced between some of the groups. All applications to pig ears were for two minutes. Percentage of delivery was calculated by dividing the amount of 14C delivered to the skin (disintegrations per minute) by the amount of 14C present on control Nanopatches™ (not applied to skin).
3.4. Delivery of vaccine into rhesus macaque skin
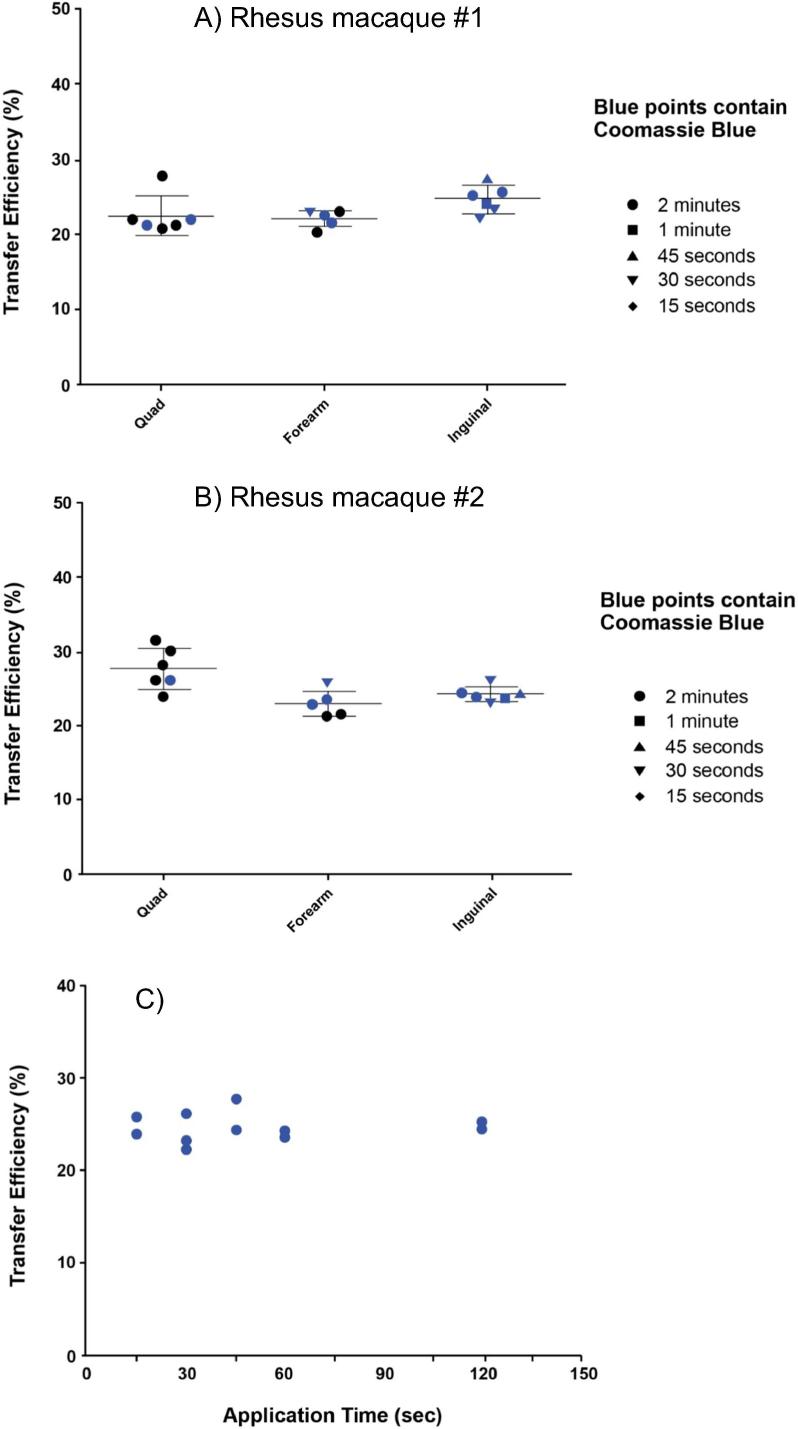
Following the completion of the cold shipping and delivery performance studies with the Nanopatch™ in pig ears, the release times and depth of delivery of material that was coated on the Nanopatch™ using rhesus skin were evaluated (Rhesus Study #1). A study with 54 μg 14C-labeled HPV genotype 11 coated on to Nanopatches™ was conducted to evaluate transfer efficiency with varied times of skin engagement and different locations in rhesus macaques (Fig. 4). A range of different application times, from 15 s to 2 min, was evaluated at different sites (for additional details, see the Materials and Methods). These Nanopatches™ were administered to the thigh (quadriceps), forearm, and inguinal regions of two rhesus macaques using the PoP4 applicator (Rhesus Study #1, Fig. 4A, 4B). It was determined in these studies that approximately 20–25% of 14C-labeled HPV genotype 11 on the Nanopatch™ was delivered to the skin (Fig. 4A, 4B). In another study, the amount of 14C-labeled HPV genotype 11 from Nanopatches™ delivered to the skin site (quadriceps) ranging from 15 s to 2 min was performed (Fig. 4C). These results indicated that the unadjuvanted HPV VLPs were delivered within approximately the first 15 s.
Fig. 4.
Rhesus Study #1. Delivered dose of vaccine and time of administration at different sites on rhesus using Nanopatches™ coated with 14C-labeled HPV Type 11. (A, B) Transfer efficiency on the quadriceps, forearm, and inguinal in two rhesus macaques, (C) Timed patch study and transfer efficiency on the quadriceps.
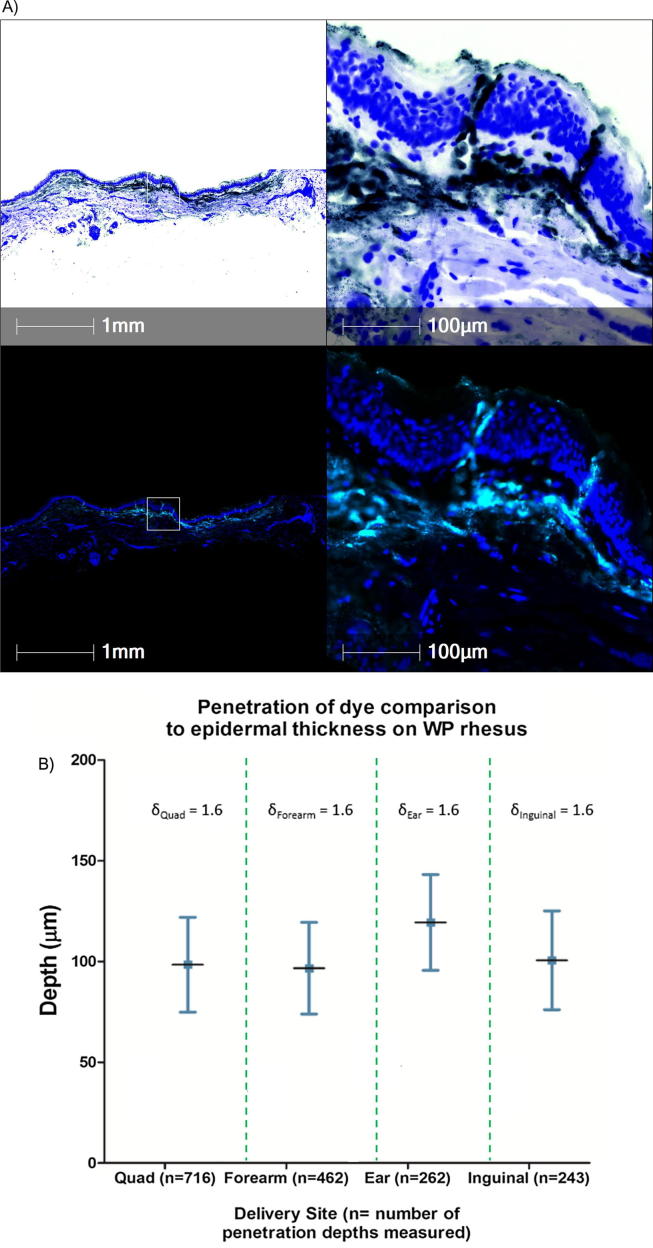
The next experiment was performed to evaluate the depth of delivery of material from the Nanopatch™ to rhesus skin (Rhesus Study #2). These experiments were performed by coating Nanopatches™ with FluoSpheres®. FluoSpheres® are particles that fluoresce following excitation at a wavelength of 488 nm, and histological sections containing the FluoSpheres® can be visualized by fluorescence microscopy (for additional details, see the Materials and Methods). Nanopatches™ coated with FluoSpheres® were delivered to the thigh (quadriceps), forearm, ear, and inguinal areas. The results indicated that FluoSpheres® are delivered to both the epidermis and dermis, and the penetration depth of projections in skin was on the order of ∼100 µm ± ∼20 µm (Fig. 5). Penetration depth into ear skin was slightly deeper (Fig. 5B). Despite the sites having substantially different subcutaneous tissue/muscle composition, there was no significant difference between the penetration depths achieved (Fig. 5B).
Fig. 5.
Rhesus Study #2. (A) Histological sections of delivered vaccine payload into Rhesus skin by Nanopatches™. Top Panel: Pseudo-brightfield images generated from the emittance/absorption spectra of the fluorescent images shown in the bottom panel illustrate fine morphological details of the skin tissue. Black is indicative of the injected FluoSpheres®, and blue demonstrates the cellular nuclei. Bottom Panel: Fluorescent green signal indicates the location of the injected FluoSpheres® and blue represents the cellular nuclei, (B) Depth measurements obtained from four quadriceps applications, three forearm applications, one ear application, and two inguinal applications. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Accelerated stability of nanopatches™
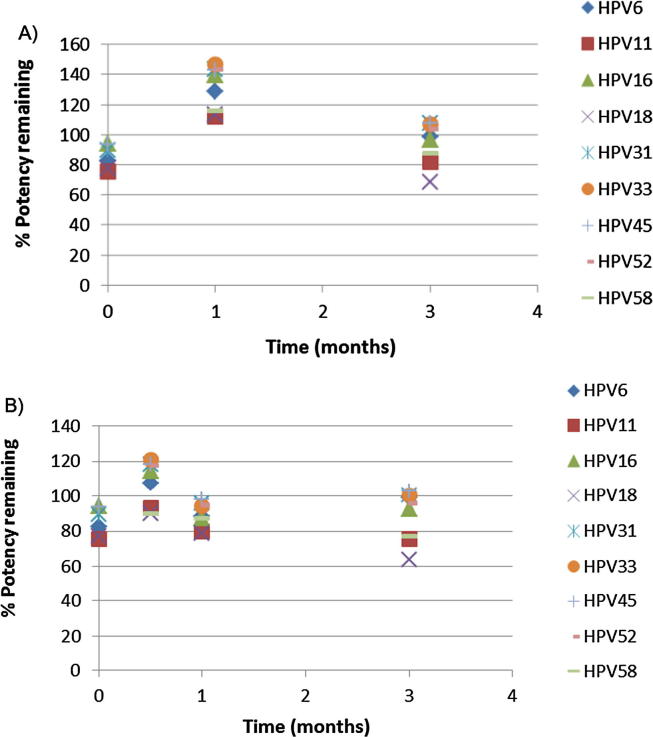
Following the evaluation of vaccine delivery to the skin, accelerated stability of unadjuvanted HPV VLPs on the Nanopatch™ was evaluated over 3 months at 25 °C and 37 °C. A total of 54 μg HPV VLPs, consisting of the 9 different genotypes, was loaded on to each Nanopatch™. Following the incubation of samples over a 3-month period and sampling at Time 0, 0.5 (37 °C sample only), 1, and 3 months, HPV VLPs were released from the Nanopatches™ and evaluated using the Biacore™. The results of this study are shown in Fig. 6, and demonstrated that unadjuvanted HPV VLPs coated on the Nanopatch™ retained their functional stability over a period of 3 months at both 25⁰C and 37 °C.
Fig. 6.
3 Month Stability study performed on Nanopatches™, (A) 25 °C, (B) 37 °C. The dose of each HPV VLP serotype at Time 0 was 70 μg total, consisting of 9 types, in a total volume of 41 mcl.
3.6. Immunogenicity study in rhesus macaques
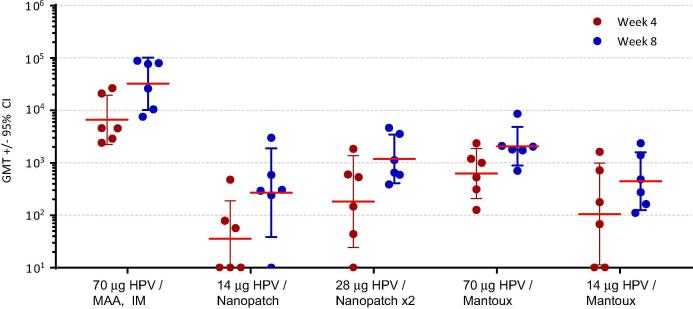
Immunogenicity from unadjuvanted HPV VLPs delivered with the Nanopatch™ was next assessed (Rhesus Study #3). Table 1 summarizes the groups that were evaluated in the study. Nanopatches™ coated with a mixture of HPV VLPs (genotypes 6, 11, 16, 18, 31, 33, 45, 52, 58) were administered at Time 0 and 4 weeks on the thigh (quadriceps) (see Materials and Methods for details). The first group was administered an IM injection of 70 μg of HPV vaccine with aluminum adjuvant (MAA). This formulation and administration route (IM) most closely represents the current approved adjuvanted HPV vaccine (GARDASIL™9). The dose of 70 μg HPV VLPs in rhesus macaques is the target dose to mount a similar immune response that is equivalent the human dose of 270 μg HPV VLPs. The second group was evaluated with the Nanopatch™ loaded with the same dose, 70 μg, of unadjuvanted HPV VLPs, with approximately 14 μg (20%) delivered to the skin. The third group was administered two Nanopatches™, 70 μg loaded on each, with approximately 28 μg unadjuvanted HPV VLPs delivered. The fourth and fifth groups were evaluated with 70 μg and 14 μg of unadjuvanted HPV vaccine (no aluminum adjuvant) in a liquid formulation delivered ID with the Mantoux technique (Fig. 7). Blood was drawn to evaluate HPV-specific antibody responses by ELISA to a mixture of VLP genotypes 6, 11, 16 and 18 at 4 weeks post-dose 1 (Week 4) and 4 weeks post-dose 2 (Week 8), as depicted in Fig. 7. The immune response to the benchmark IM dose of 70 μg HPV vaccine containing aluminum adjuvant was the strongest, as expected, and was statistically significant with a p value >0.001 when compared to the Nanopatch™ containing 70 μg unadjuvanted HPV VLPs. This was the only group that contained an adjuvant. The single Nanopatch™ loaded with 70 μg (approximately 14 μg delivered) had antibody titers similar to those induced by the Mantoux injection with 14 μg. These results suggest that the Nanopatch™ induces a comparable magnitude of HPV-specific antibodies using the same effective dose (14 μg) when compared to the traditional Mantoux technique, and statistical differences between these groups were not observed using Tukey’s multiple comparison’s test (Fig. 7 and Table 2). Increasing the unadjuvanted HPV VLP dose by using two Nanopatches™ resulted in a higher trend in the IgG titer, however, it was similar to administration with a single Nanopatch™ and were statistically the same when comparing one Nanopatch™ with 70 μg unadjuvanted HPV VLPs to two Nanopatches™ each with 70 μg unadjuvanted HPV VLPs (Fig. 7 and Table 2). The immune response of two Nanopatches™ delivering 28 μg was similar to the response of the 70 μg dose delivered via the Mantoux technique, and were statistically equivalent (Fig. 7 and Table 2). There were no statistically significant differences in the immune response with unadjuvanted HPV VLPs either on the Nanopatch™ or Mantoux injection following post-prime or post-boost (data not shown).
Fig. 7.
Rhesus Study #3. ELISA of HPV-specific IgG titers induced by vaccination of rhesus macaques using Nanopatches™ compared to intramuscular injection (IM) or intradermal injection (Mantoux method), at doses delivered shown on the x axis. Doses for Nanopatch™ groups indicate the amount of HPV VLPs (total of 9 genotypes) that were delivered. The individual animal endpoint titers and the group geometric mean titers with 95% confidence intervals are plotted for each group at study week 4 (4 weeks post dose 1, red symbols) and study week 8 (4 weeks post dose 2, blue symbols). Titers of HPV-specific IgG antibodies that bind to a mixture of VLP genotypes 6, 11, 16 and 18 are defined as the reciprocal of the highest serum dilution that was detected at a level of two times the assay background. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Comparison of group, route, method, and dose delivered, 8-weeks post-time 0 for rhesus immunogenicity studies.
| Group | Route | Method | Dose Delivered |
vs. | Group | Route | Method | Dose Delivered |
Significant | Adjusted P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Intradermal | Nanopatch™ | 14 μg | 5 | Intradermal | Mantoux Needle/Syringe |
14 μg | No | 0.9542 | |
| 2 | Intradermal | Nanopatch™ | 14 μg | 3 | Intradermal | 2X Nanopatch™ | 28 μg | No | 0.2702 | |
| 3 | Intradermal | 2X Nanopatch™ | 28 μg | 4 | Intradermal | Mantoux Needle/Syringe |
70 μg | No | 0.9339 | |
| 2 | Intradermal | Nanopatch™ | 14 μg | 1 | Intramuscular | Needle/ Syringe with aluminium adjuvant |
70 μg | Yes | <0.0001 |
Note: all μg values refer to HPV VLPs administered.
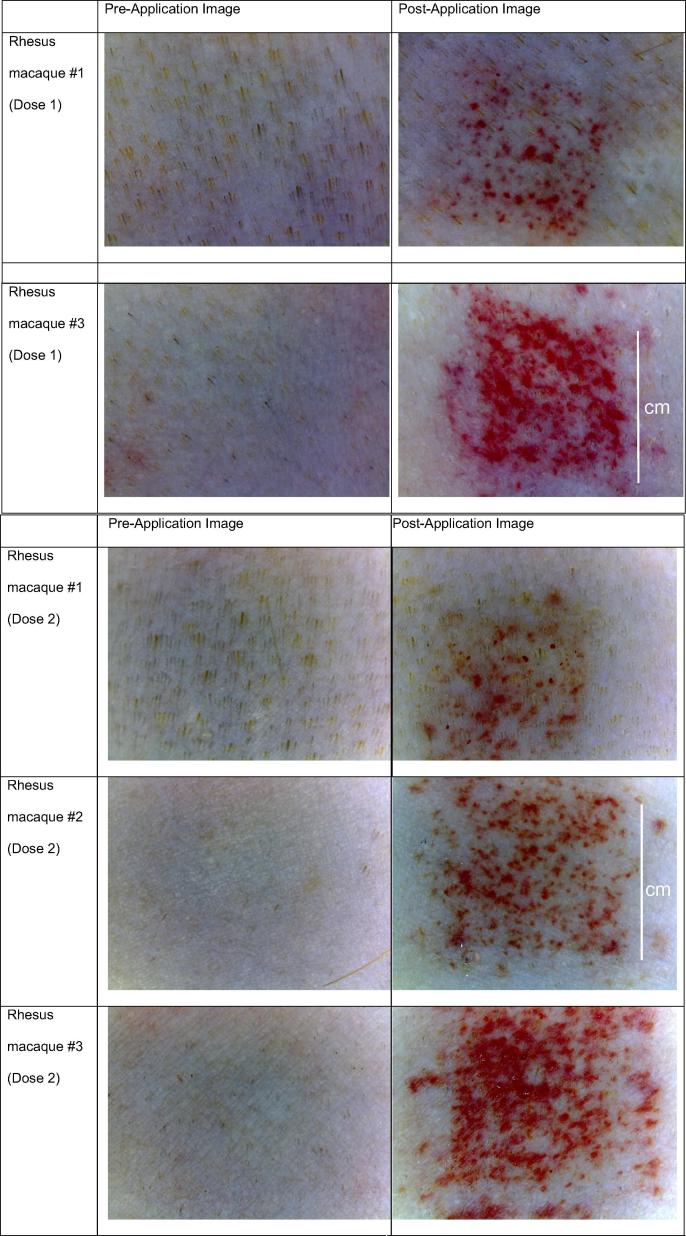
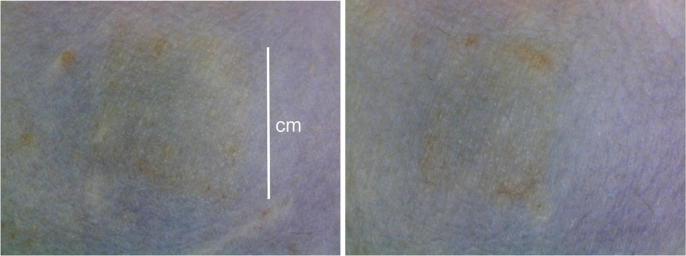
The local skin reactogenicity of the Nanopatch™ was also evaluated following the dosing at Time 0 (Dose 1) and at 4 weeks (Dose 2), as shown in Fig. 8. Close-up photographs of the skin from three rhesus macaques are depicted. Erythema and petechiae could be seen on the surface of the skin immediately following administration (Fig. 8). Fig. 9 shows application sites from a rhesus 28 days after dose 1 which were representative. The monkeys were observed daily following application of the Nanopatch™ and no signs of pain or distress were observed.
Fig. 8.
Photographs of rhesus macaque skin pre- and post- Nanopatch™ application for doses 1 and 2. Scale bar indicates 1 cm.
Fig. 9.
Application sites of two Nanopatches™ 28 days following Dose 1 (Week 4). Scale bar indicates 1 cm.
4. Discussion
GARDASIL™ and GARDASIL™9 for the prevention of HPV infection are administered intramuscularly. These vaccines are provided as liquids in pre-filled syringes (PFS) for easy administration to the patient as well as individual single-dose vials which are used with a needle/syringe for delivery, and are stored at 2–8 °C. In this report, an unadjuvanted HPV vaccine (in the absence of aluminum adjuvant) in a dried form on a patch (Nanopatch™) for intradermal delivery, was investigated. Potential advantages of a vaccine patch compared to a liquid vaccine include the following: potential improved thermostability (dried vs. liquid), beneficial for needle-phobic patients, removal/reduction of needles/sharps, and smaller footprint and weight.
The Nanopatch™ was coated using a gas-drying approach, in which 41 μL of the vaccine formulation was placed on the Nanopatch™ and dried using a stream of gas, as previously described [38]. One challenge with this method is that the vaccine formulation coats both the projections and base of the Nanopatch™. Fig. 1 showed that areas of dark contrast, which correspond to the vaccine formulation, was present on the middle and lower portion of the projections, as well as the base. Areas of lighter contrast, corresponding to no vaccine formulation, was seen at the distal tips. This was one challenge with the gas-jet drying approach and may have resulted in the approximate 20% vaccine transfer efficiency to the skin, as any material on the base of the Nanopatch™ or bottom portions of the projections would likely not transfer vaccine to the skin.
It was demonstrated that the Nanopatch™ could be shipped cold internationally without impacting the amount of vaccine that was delivered from the Nanopatch™ to the skin, as shown from the Time 0 and shipped samples. Additionally, to ensure that the unadjuvanted HPV VLPs were structurally intact on the Nanopatches™, accelerated thermostability experiments were performed at 25 °C and 37 °C. It was demonstrated that the dried HPV Nanopatch™ retained its potency at least 3 months at 37 °C. When compared to the accelerated stability standards used for Vaccine Vial Monitors (VVM), 1 month at 37 °C is equivalent to >4 years at 5 °C.
Delivery studies of the Nanopatch™ in pig ears and rhesus macaque skin were performed with either 14C-labeled HPV VLPs or FluoSpheres®. In both pig ears and rhesus skin, the delivery efficiency using 14C-labeled HPV VLPs was approximately 20–25%, with the remainder not being delivered (remained on the Nanopatch™). This was a limitation of the Nanopatch™ coating approach at the time of the study which was based on earlier work in the mouse model, as mentioned [30]. Using 14C-labeled HPV VLPs on the Nanopatch™, it was demonstrated that the unadjuvanted HPV vaccine could be delivered within 15 s. Other Nanopatch™ vaccine coating methods (e.g. dip coating), increase the transfer efficiency to >85% [44].
Data from the transfer studies for example, 15 s for antigen transfer, would be beneficial as it would reduce the possibility of the Nanopatch™ being removed or dislodged in the clinic when compared to an application time of 2 min. In pediatric populations, it would therefore be more advantageous to reduce the possibility of Nanopatch™ removal within the first 2 min. The evaluation of the site for Nanopatch™ administration is also a factor to consider when administering to specific populations (i.e. skin over the deltoid may be more accessible for young adult populations as compared with the thigh). These rapid release kinetics of the vaccine from the microprojections into the skin are broadly consistent with the fast release times observed when Nanopatches™ delivered conventional influenza vaccine into the skin of live mice [14]. It is believed the rapid dissolution is due to the fact that the high density array has a thin coating of vaccine (approximately one micron, data not shown) which allows rapid release into the skin.
Delivery of FluoSpheres® with the Nanopatch™ in rhesus skin demonstrated that the average depth of delivery was 100 µm and reached both the epidermis and dermis, thereby targeting the same strata as Nanopatch™ prototypes deployed to vaccinate mice and rats [30], [31], [32].
The immunogenicity study to evaluate the HPV-coated Nanopatch™ in rhesus macaques demonstrated that antibody titers induced by the Nanopatch™ were at least comparable to ID injection using the Mantoux technique. With the exception of the 70 μg IM injection (needle/syringe with adjuvant) and Mantoux injections (needle/syringe without adjuvant), the doses delivered with the Nanopatch™ were not equivalent. This was a limitation of the study in that a number of Nanopatches™ would have needed to be applied to reach the 70 μg dose level used for IM and Mantoux injections. It did demonstrate that an immune response was mounted, however, the dose delivered was not considered dose-sparing compared to IM delivery with an aluminum adjuvant.
Other, lower density microneedle patches using pyramidal, dissolvable microneedles 600 µm in height and containing either measles or polio vaccine were also able to generate an immune response in rhesus macaques [18], [19], [20]. In the mouse model, administration of the Nanopatch™, with a high density array and coated with HPV VLPs to the ear resulted in an enhanced immune response (statistically non-inferior to IM doses) [30]. The same response was not seen in the rhesus model, when tested for the potential for dose sparing at 14 and 28 μg unadjuvanted HPV VLPs. There are a number of potential reasons why the Nanopatches™ coated with unadjuvanted HPV VLPs did not demonstrate a clear enhancement of the immune response:
-
(1)
The amount of HPV delivered to the skin was lower than that administered IM (70 μg IM vs. 14 μg and 28 μg delivered with the Nanopatch™) and the IM dose contained an adjuvant, whereas the Nanopatch™ did not.
-
(2)
Rhesus skin has key physiological differences compared to the mouse or human. For example, the individual thickness of rhesus hair are greater than the thickness of human hair (follicles can be >150 µm in diameter), thus rhesus skin requires extensive preparation to remove coarse, dense hair from all skin sites. In this study, a high amount of stubble remained, and dye studies indicated that the stubble may have reduced Nanopatch™/skin engagement. Also, the interaction between Nanopatches™ and the stubble potentially led to higher localized stresses in skin, thereby increasing petechiae and other skin damage. Skin strata are different in rhesus when compared to humans and the type and location of immune cells within rhesus skin is poorly understood.
-
(3)
The applicator used to apply the Nanopatch™ was a first generation design that likely utilized an excess of force for Nanopatch™ application.
-
(4)
The potential physical immune enhancer effect of Nanopatches™, which was observed broadly in vaccination studies in both mice and rats, and a mode of action studied in mice, may not have been optimised in this first study scaling from small rodents to primates [28], [45], [31], [32], [33].
Although anti-HPV IgG levels were substantial with both with the Nanopatch™ and Mantoux technique for intradermal administration, they were lower compared to intramuscular administration with aluminum adjuvant. Because the anti-HPV titer required to confer protection has not been determined, the clinical significance of the lower titers obtained with intradermal delivery is unknown [46].
During the immunogenicity study, skin site reactions induced by HPV Nanopatch™ vaccination were evaluated. It was observed that the rhesus skin response at the quadriceps was quite marked due to the force of the application used in this study with the PoP4 applicator with a varied distribution in erythema (due to hair stubble) and clear petechiae. This was the first study undertaken with Nanopatches™ in an animal larger than a rodent or ferret. The PoP4 was the first applicator developed for this purpose and used a combination of speed and mass (plunger) to apply to the Nanopatch™ to the skin, extending a principle of Nanopatch™ application first developed in mice [44]. However, subsequent to the study reported here, a novel ‘flying patch’ approach has been pioneered that achieves Nanopatch™ engagement in the skin, but with reductions of energy of application by 10–100 fold [39]. Furthermore, because the Nanopatch™ alone makes contact with the skin (i.e. there is no plunger attached to it during skin contact), the energy of application is rapidly dissipated in the outer skin strata. This advanced, lower energy application approach has been applied to humans in clinical studies of Nanopatches™ [34], [37].
In this study, it was expected that the comparatively high energy of application was the cause of capillary damage and bruising at the application site in rhesus macaques immediately following application. Contrastingly, clinical studies with a similar Nanopatch™ design (density, area), but with the described ‘flying patch’ application condition (higher speed, lower overall force) showed a much-improved skin response with good patch-to-skin engagement, and a significant decrease in petechiae and coloration. This improvement from the PoP4 applicator to the newer applicator design therefore shows a lower amount of capillary damage and bruising and therefore has the potential for being more acceptable in a clinical setting. Additionally, this clinical application condition was preferred by subjects to intramuscular injection with a needle and syringe [34], [37]. As mentioned, a vaccine patch may be more advantageous for adolescents and teens who are not as routinely given vaccinations when compared to infants/toddlers and have less exposure to the needle/syringe. Therefore, the absence of the visibility of a needle has the potential to reduce anxiety for needle-phobic populations, or individuals that are not routinely given other vaccinations. Additionally, the potential for a more convenient, needle-free form of the HPV vaccine could be a patch image, such as the Nanopatch™. Regions where uptake could be improved include rural areas of the United States and in Asia, Africa, and other regions [8], [9].
Because the capillary damage and bruising was demonstrated to be minimal when compared to the PoP4 applicator, this may be more acceptable than the use of a needle/syringe. Additionally, it was shown that any capillary damage/bruising is no longer visible after 28 days following administration. While it is believed that the mode of action for the Nanopatch™ results in localized cellular damage to enhance the immune response, it is known from work in rodents that excessive cell death can move the systemic immune response away from the potential for a physical immune enhancer effect [31].
There were a limited number of photographs taken post application due to the movement of the animals in their enclosures and the location of the application site. In most cases, at dose 2 (Day 28) in the majority of cases the previous application site (dose 1) was not visible. The animals were observed daily after application and again, there was no evidence that the application sites were causing the animals discomfort and there seemed to be no signs of scratching or itching. Photographs of IM injections to evaluate for erythema/edema were not taken as these sites were covered with hair. Mantoux injection sites were also not photographed.
In summary, it was demonstrated for the first time that a first-generation version of a high-density vaccine patch, the Nanopatch™, when coated with an unadjuvanted HPV vaccine, could be maintained in a stable condition during cold shipping, retained potency under accelerated stability, could be delivered effectively, and could generate an immune response in non-human primates at least as well as ID delivery by the Mantoux method.
Acknowledgments
Acknowledgements
The authors would like to acknowledge Ken Lodge, Suzie Jendrowski, Kim Michel, and Michael Citron, and Pedro Cejas from Merck & Co., Inc., Kenilworth, NJ, USA for their support of this work. This work was performed in part at the Queensland and Melbourne nodes of the Australian National Fabrication Facility, a company established under the National Collaborative Research Infrastructure Strategy to provide nano and microfabrication facilities for Australia’s Researchers. The authors acknowledge the facilities, and the scientific and technical assistance, of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland. Other team members of the Delivery of Drugs and Genes Group (D2G2) are acknowledged for assisting this study and for scientific insights. Financial support for this work was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Declaration of Competing Interest
Authors Brian K. Meyer, Donna M. Williams, Andrew J. Bett, Sheri Dubey, Renee C. Gentzel, and Akhilesh Bhambhani are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Declaration of Interest by Mark A. F. Kendall:
The Nanopatch™ was developed in Mark A.F. Kendall’s laboratory at the University of Queensland, and Dr. Kendall is an inventor on several Nanopatch™ patents. Dr. Kendall was one of the founders of Vaxxas and served as the Chief Technology Officer at Vaxxas and as Professor at the University of Queensland during the course of most of the work described in this study. He is currently a Vice-Chancellor’s Entrepreneurial Professor at the Australian National University (ANU).
References
- 1.Zhai L., Tubman E. Gardasil-9: a global survey of projected efficacy. Antiviral Res. 2016;130:101–109. doi: 10.1016/j.antiviral.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Sangar V.C., Ghongane B., Mathur G. Development of human papillomavirus (HPV) vaccines: a review of literature and clinical update. Rev Recent Clin Trials. 2016;11(4):284–289. doi: 10.2174/1574887111666160502160748. [DOI] [PubMed] [Google Scholar]
- 3.Haghshenas M.R., Mousavi T., Kheradmand M., Afshari M., Moosazadeh M. Efficacy of human papillomavirus L1 protein vaccines (cervarix and gardasil) in reducing the risk of cervical intraepithelial neoplasia: a meta-analysis. Int J Prev Med. 2017;8:44. doi: 10.4103/ijpvm.IJPVM_413_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Supplement Approval, U.S. Food and Drug Administration; 07-Oct-2016.
- 5.Supplement Approval, U.S. Food and Drug Administration; 05-Oct-2018.
- 6.Walker T.Y., Elam-Evans L.D., Singleton J.A., Yankey D., Markowitz L.E., Fredua B. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874–882. doi: 10.15585/mmwr.mm6633a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorell C.G., Stokley S., Yankey D., Markowitz L.E. Compliance with recommended dosing intervals for HPV vaccination among females, 13–17 years, National Immunization Survey-Teen, 2008–2009. Vaccine. 2012;30(3):503–505. doi: 10.1016/j.vaccine.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Stokley S., Jeyarajah J., Yankey D., Cano M., Gee J., Roark J., Curtis C.R., Markowitz L. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014 — United States. MMWR. 2014;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 9.Bruni L., Diaz M., Barrionuevo-Rosas L., Herrero R., Bray F., Bosch F.X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;2016(4):e453–e463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 10.Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc (Bayl Univ Med Cent) 2005;18(1):21–25. doi: 10.1080/08998280.2005.11928028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen D., Bowersock T., Weeratna R., Yeoh T. Current opportunities and challenges in intradermal vaccination. Ther Deliv. 2015;6(9):1101–1108. doi: 10.4155/tde.15.65. [DOI] [PubMed] [Google Scholar]
- 12.Engelke L., Winter G., Hook S., Engert J. Recent insights into cutaneous immunization: how to vaccinate via the skin. Vaccine. 2015;33(37):4663–4674. doi: 10.1016/j.vaccine.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Filippelli M., Lionetti E., Gennaro A., Lanzafame A., Arrigo T., Salpietro C. Hepatitis B vaccine by intradermal route in non responder patients: anupdate. World J Gastroenterol. 2014;20(30):10383–10394. doi: 10.3748/wjg.v20.i30.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X., Fernando G.J.P., Raphael A.P., Yukiko S.R., Fairmaid E.J., Primiero C.A. Rapid kinetics to peak serum antibodies is achieved following influenza vaccination by dry-coated densely packed microprojections to skin. J Control Release. 2012;158(1):78–84. doi: 10.1016/j.jconrel.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Bui Y., Sow M., Cambron-Goulet E., Levac E., Milford F. Immunogenicity and feasibility of intradermal vaccination against rabies in Quebec. Can Commun Dis Rep. 2015;41(3):55–62. doi: 10.14745/ccdr.v41i03a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beals C.R., Railkar R.A., Schaeffer A.K., Levin Y., Kochba E., Meyer B.K. Immune response and reactogenicity of intradermal administration versus subcutaneous administration of varicella-zoster virus vaccine: an exploratory, randomised, partly blinded trial. Lancet Infect Dis. 2016;16(8):915–922. doi: 10.1016/S1473-3099(16)00133-X. [DOI] [PubMed] [Google Scholar]
- 17.Hung I.F.N., Yuen K.Y. Immunogenicity, safety and tolerability of intradermal Influenza vaccines. Hum Vaccin Immunother. 2018;14(3):565–570. doi: 10.1080/21645515.2017.1328332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edens C., Collins M.L., Ayers J., Rota P.A., Prausnitz M.R. Measles vaccination using a microneedle patch. Vaccine. 2013;31(34):3403–3409. doi: 10.1016/j.vaccine.2012.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edens C., Collins M.L., Goodson J.L., Rota P.A., Prausnitz M.R. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine. 2015;33(37):4712–4718. doi: 10.1016/j.vaccine.2015.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edens C., Dybdahl-Sissoko N.C., Weldon W.C., Oberste M.S., Prausnitz M.R. Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus Macaque. Vaccine. 2015;3(37):4683–4690. doi: 10.1016/j.vaccine.2015.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Z., Van Riet E., Romeijn S., Kersten G.F.A., Jiskoot W., Bouwstra J.A. Immune modulation by adjuvants combined with diphtheria toxoid administered topically in BALB/c mice after microneedle array pretreatment. Pharm Res. 2009;26(7):1635–2164. doi: 10.1007/s11095-009-9874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quan F.S., Kim Y.C., Vunnava A., Yoo D.G., Song J.M., Prausnitz M.R. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84(15):7760–7769. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koutsonanos D.G., Vassilieva E.V., Stavropoulou A., Zarnitsyn V.G., Esser E.S., Taherbhai M.T. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep. 2012;2:357. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuo K., Hirobe S., Yokota Y., Ayabe Y., Seto M., Quan Y.S., Kamiyama F., Tougan T., Horii T., Mukai Y., Okada N., Nakagawa S. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J Control Release. 2012;160(3):495–501. doi: 10.1016/j.jconrel.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Pattani A., McKay P.F., Garland M.J., Curran R.M., Migalska K., Cassidy C.M. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J Control Release. 2012;162(3):529–537. doi: 10.1016/j.jconrel.2012.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeMuth P.C., Min Y., Huang B., Kramer J.A., Miller A.D., Barouch D.H. Polymer multilayer tattooing for enhanced DNA vaccination. Nat Mater. 2013;12(4):367–376. doi: 10.1038/nmat3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kommareddy S., Baudner B.C., Bonificio A., Gallorini S., Palladino G., Determan A.S. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine. 2013;31:3435–3441. doi: 10.1016/j.vaccine.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 28.Fernando G.J., Chen X., Prow T.W., Crichton M.L., Fairmaid E.J., Roberts M.S. Potent immunity to low doses of Influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS One. 2010;5(4):e10266. doi: 10.1371/journal.pone.0010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X., Fernando G.J.P., Crichton M.L., Flaim C., Yukiko S.R., Fairmaid E.J. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Control Release. 2011;152(3):349–355. doi: 10.1016/j.jconrel.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 30.Corbett H.J., Fernando G.J., Chen X., Frazer I.H., Kendall M.A. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PLoS One. 2010;5(10):e13460. doi: 10.1371/journal.pone.0013460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller D.A., Pearson F.E., Fernando G.P., Agyei-Yeboah C., Owens N.S., Corrie S.R. Inactivated poliovirus type 2 vaccine delivered to rat skin via high density microprojection array elicits potent neutralising antibody responses. Sci Rep. 2016;6:22094. doi: 10.1038/srep22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller D.A., Fernando G.J.P., Owens N.S., Agyei-Yeboah C., Wei J.C.J., Depelsenaire A.C.I. High-density microprojection array delivery to rat skin of low doses of trivalent inactivated poliovirus vaccine elicits potent neutralising antibody responses. Sci Rep. 2017;7(1):12644. doi: 10.1038/s41598-017-13011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depelsenaire A.C.I., Meliga S., McNeilly C.L., Pearson F.E., Coffey J.W., Haigh O.L. Colocalization of cell death with antigen deposition in skin enhances vaccine immunogenicity. J Invest Dermatol. 2014;134(9):2361–2370. doi: 10.1038/jid.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernando G.J.P., Hickling J., Jayashi Flores C.M., Griffin P., Anderson C.D., Skinner S.R. Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch. Vaccine. 2018;36(26):3779–3788. doi: 10.1016/j.vaccine.2018.05.053. [DOI] [PubMed] [Google Scholar]
- 35.Hirobe S., Azulizawa H., Hanafusa T., Matsuo K., Quan Y.S., Kamiyama F. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolvable microneedle patch. Biomaterials. 2015;57:50–58. doi: 10.1016/j.biomaterials.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Rouphael N., Paine M., Mosley R., Henry H., McAllister D.V., Kalluri H. The safety, immunogenicity and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded placebo-controlled, phase 1 trial. The Lancet. 2017;390(10095):649–658. doi: 10.1016/S0140-6736(17)30575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin P., Elliot S., Krauer K., Davies C., Skinner R.S., Anderson C.D. Safety, acceptability and tolerability of uncoated and excipient-coated high density silicon micro-projection array patches in human subjects. Vaccine. 2017;35(48B):6676–6684. doi: 10.1016/j.vaccine.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Chen X., Prow T.W., Jenkins D.W.K., Roberts M.S., Frazer I.H., Fernando G.J.P., Kendall M.A.F. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J Control Release. 2009;139(3):212–220. doi: 10.1016/j.jconrel.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins D., Corrie S., Flaim C., Kendall M.A.F. High density and high aspect ratio solid micro-nanoprojection arrays for targeted skin vaccine delivery and specific antibody extraction. RSC Adv. 2012;2(8):3490–3495. [Google Scholar]
- 40.Crichton M.L., Ansaldo A., Chen X., Prow T.W., Fernando G.J., Kendall M.A.F. The effect of strain rate on the precision of penetration of short densely-packed microprojection array patches coated with vaccine. Biomaterials. 2010;31(16):4562–4572. doi: 10.1016/j.biomaterials.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 41.Meliga S.C., Coffey W., Crichton M.L., Flaim C., MartinVeidt M., Kendall M.A.F. The hyperelastic and failure behaviors of skin in relation to the dynamic application of microscopic penetrators in a murine model. Acta Biomater. 2017;48:341–356. doi: 10.1016/j.actbio.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Pearson F.E., McNeilly C.L., Crichton M.L., Primiero C.A., Yukiko S.R., Fernando G.J.P. Dry-coated live viral vector vaccines delivered by Nanopatch™ microprojections retain long-term thermostability and induce transgene-specific T cell responses in mice. PLoS One. 2013;8(7):1–10. doi: 10.1371/journal.pone.0067888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Q., Modis Y., High K., Towne V., Meng Y., Wang Y. Disassembly and reassembly of human papillomavirus virus-like particles produces more virion-like antibody reactivity. Virol J. 2012;9(52):1–13. doi: 10.1186/1743-422X-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X., Corbett H.J., Yukiko S.R., Raphael A.P., Fairmaid E.J., Prow T.W. Site-selectively coated, densely-packed microprojection array patches for targeted delivery of vaccines to skin. Adv Funct Mater. 2011;21:464–473. [Google Scholar]
- 45.Prow T.W., Chen X., Prow N.A., Fernando G.J., Tan C.S., Raphael A.P. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small. 2010;6(16):1776–1784. doi: 10.1002/smll.201000331. [DOI] [PubMed] [Google Scholar]
- 46.Stanley M., Pinto L.A., Trimble C. Human papillomavirus vaccines-immune responses. Vaccine. 2012;30(Suppl 5):F83–F87. doi: 10.1016/j.vaccine.2012.04.106. [DOI] [PubMed] [Google Scholar]