Abstract
Background: Norovirus, also known as the winter vomiting bug, is the predominant cause of non-bacterial gastroenteritis worldwide. Disease control is predicated on a robust innate immune response during the early stages of infection. Double-stranded RNA intermediates generated during viral genome replication are recognised by host innate immune sensors in the cytoplasm, activating the strongly antiviral interferon gene programme. Ifit proteins (interferon induced proteins with tetratricopeptide repeats), which are highly expressed during the interferon response, have been shown to directly inhibit viral protein synthesis as well as regulate innate immune signalling pathways. Ifit1 is well-characterised to inhibit viral translation by sequestration of eukaryotic initiation factors or by directly binding to the 5' terminus of foreign RNA, particularly those with non-self cap structures. However, noroviruses have a viral protein, VPg, covalently linked to the 5' end of the genomic RNA, which acts as a cap substitute to recruit the translation initiation machinery.
Methods: Ifit1 knockout RAW264.7 murine macrophage-like cells were generated using CRISPR-Cas9 gene editing. These cells were analysed for their ability to support murine norovirus infection, determined by virus yield, and respond to different immune stimuli, assayed by quantitative PCR. The effect of Ifit proteins on norovirus translation was also tested in vitro.
Results: Here, we show that VPg-dependent translation is completely refractory to Ifit1-mediated translation inhibition in vitro and Ifit1 cannot bind the 5' end of VPg-linked RNA. Nevertheless, knockout of Ifit1 promoted viral replication in murine norovirus infected cells. We then demonstrate that Ifit1 promoted interferon-beta expression following transfection of synthetic double-stranded RNA but had little effect on toll-like receptor 3 and 4 signalling.
Conclusions: Ifit1 is an antiviral factor during norovirus infection but cannot directly inhibit viral translation. Instead, Ifit1 stimulates the antiviral state following cytoplasmic RNA sensing, contributing to restriction of norovirus replication.
Keywords: IFIT, norovirus, innate immunity, interferon
Introduction
The Caliciviridae family of small positive-sense RNA viruses comprises 11 genera, including Norovirus and Sapovirus. Noroviruses are the leading cause of non-bacterial gastroenteritis in humans, accounting for 18% of acute gastroenteric disease worldwide 1. While recent advancements in human intestinal organoids have made it possible to study human noroviruses in culture 2, murine norovirus (MNV) remains a valuable model for dissecting interactions between noroviruses and their host, owing to readily cultivable permissive cell lines and a flexible reverse genetics system 3.
The innate immune response to viral infection is essential for the control of norovirus replication and clearance 4. Sensing of calicivirus infection is predominantly mediated by cytoplasmic double-stranded RNA sensors; both RIG-I and MDA5 have been implicated in controlling the innate immune response at different stages of infection 5– 7. By contrast, TLR3, an endosomal dsRNA sensor, has little effect on norovirus replication 5. RIG-I and MDA5 signalling converge on the activation of the antiviral signalling complex MAVS, which recruits TBK1 to induce the phosphorylation of interferon regulatory factor (IRF)-3. Activated IRF-3 dimerises and translocates into the nucleus where it promotes the transcription of type I interferon (IFN) and early antiviral genes.
During the antiviral response, among the most strongly upregulated IFN-stimulated genes are the IFIT family of RNA-binding proteins 8– 10. In humans, IFIT1 directly inhibits the translation of non-self RNAs at the initiation stage, by binding over the 5′ terminus, occluding the recruitment of eukaryotic translation initiation factor (eIF) 4F 11– 13. IFIT1 binding is highly specific for capped mRNA which lacks methylation at the first or second cap-proximal nucleotides (cap0) 14. Murine Ifit1 similarly binds cap0 RNA and mediates the inhibition of cap0 viruses in vivo 11, 15– 18. It is important to note, however, that murine Ifit1 and human IFIT1, which share 52% sequence identity, have distinct evolutionary origins, with murine Ifit1 being more closely related to another gene family member, IFIT1B 19.
However, IFIT1 may have antiviral activity independent of its RNA-binding capability. IFIT1 was reported to inhibit hepatitis C virus replication 20, 21 by binding to eIF3 to prevent viral translation initiation 22– 24. Additionally, direct binding to the human papilloma virus DNA helicase, E1, was reported to inhibit viral DNA replication 25, 26. IFIT1 also modulates different stages of the host innate immune response during both viral and bacterial infection 27– 29 and may regulate the inflammatory response in human astrocytes 30. MNV can antagonise innate immune sensing and was consequently shown to inhibit the expression of a number of interferon-stimulated genes, including Ifit2 31. However, associations between noroviruses and other members of the Ifit family have not been established.
We investigated whether Ifit1 played a role in the antiviral response to calicivirus infection. We show that Ifit1 knockout promoted MNV replication in a macrophage cell line. However, calicivirus translation was not inhibited by Ifit1. Instead, we show that Ifit1 knockout cells have impaired cytoplasmic double-stranded RNA sensing, resulting in a weaker type I IFN response, which permits increased viral replication.
Methods
Cells, viruses and plasmids
Murine macrophage RAW264.7, microglial BV2 and Crandell-Rees feline kidney cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% (v/v) foetal calf serum (FCS) and 1% penicillin/streptomycin (P/S). LLC-PK cells, expressing bovine viral diarrhoea virus NPro to render them IFN-deficient, were cultured in Eagle's minimal essential medium (EMEM) supplemented with 200 μM glycochenodeoxycholic acid (GCDCA; Sigma), 2.5% FCS, and 1% P/S 32. MNV-1 strain CW.1 was recovered from the pT7:MNV-G 3'Rz plasmid as described 33. Feline calicivirus (FCV) strain Urbana was recovered from the pQ14 full length infectious clone 34. The porcine sapovirus (PSaV) Cowden tissue culture adapted strain was obtained from K. O. Chang (Kansas State University) and recovered from the full-length infectious clone pCV4A 35. For lentivirus generation, psPAX2 (Addgene plasmid # 12260) and pMD2.G (Addgene plasmid # 12259) were gifts from Didier Trono. For bacterial expression, murine Ifit1 (NM_008331.3), Ifit2 (NM_008332.3) and Ifit3 (NM_010501.2) were cloned between NcoI and XhoI sites in pTriEx1.1, to contain a C-terminal His 8 tag.
Knockout cells
First, five guide RNAs designed against the 5' end of the second exon of Ifit1 ( Table 1), were cloned into lentiCRISPR v2 36. Next, 3 μg guide RNA plasmid was cotranfected into 5 × 10 6 HEK293T cells with 3 μg psPAX2 packaging vector and 1.5 μg pMD2.G VSV-G envelope vector using lipofectamine 2000 (Invitrogen). Supernatants were harvested over 72 hours, pooled and used directly for transduction of subconfluent RAW264.7 cells. After 3 days, transduced cells were selected with puromycin for one week, before single cell clones were generated by dilution in 96-well plates. Knockout was verified by western blotting, as described below, after treatment with murine IFNβ for 12 hours and harvesting in passive lysis buffer (Promega).
Table 1. Guide RNA sequences for CRISPR-Cas9 knockout of Ifit1.
Guide RNAs were generated using crispr.mit.edu and cloned into LentiCRISPRv2 36. The 3' protospacer adjacent motif (PAM) sequences are underlined in bold.
| Guide RNA sequence |
|---|
| GGAGGTTGTGCATCCCCAAT GGG |
| ATTGGGGATGCACAACCTCC TGG |
| CTTGACATCAAGAACCCATT GGG |
| GAAGCAGATTCTCCATGACC TGG |
| AAATAATGACATACCTGATT TGG |
Infections
Cells were infected for 1 hour at 37°C at the multiplicity of infection (MOI) indicated in the legend to Figure 1. Cells were harvested by freezing at the indicated times and titres were determined by 50% tissue culture infectious dose (TCID 50) in BV2 cells, as described 3, performed in technical quadruplicate. Plates were scored by cytopathic effect after 5 days and titres were calculated by the Reed and Muensch method 3.
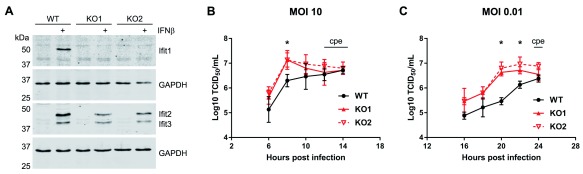
Figure 1. Ifit1 decreases MNV infection in RAW264.7 cells.
( A) Ifit1 knockout RAW264.7 cells were generated by CRISPR-Cas9 gene editing. Cells were stimulated with IFNβ for 12 hours then analysed by western blotting against Ifit1 and Ifit2/Ifit3. GAPDH was included as a loading control for each membrane. ( B, C) Infection of wild-type (WT) and Ifit1 knockout (KO) RAW264.7 cells at ( B) high or ( C) low multiplicity of infection (MOI) with murine norovirus (MNV-1). Viral titres were determined by 50% tissue culture infectious dose (TCID 50) in BV2 cells and expressed as log 10-transformed values. At late time points, indicated, severe cytopathic effect (cpe) was visible. Graphs show the mean and the standard error of three biological replicates. Titres were compared between WT and KO cells for each time point by two-tailed Student’s t-test. Asterisks indicate that a statistically significant difference (p < 0.05) was observed for both KO cell lines.
Stimulation of RAW264.7 cells
Cells were treated with 10 ng/mL LPS (Sigma) or 1 μg/mL polyI:C (Sigma), or transfected with 2 μg polyI:C using lipofectamine 2000 (Invitrogen). Cells were harvested by washing twice in PBS before lysis in passive lysis buffer (Promega) and RNA was extracted using TRIreagent (Sigma).
Reverse transcription-quantitative PCR (RT-qPCR)
For RT-qPCR analysis, cDNA was generated using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega) with random hexamer primers. qPCR was performed on cDNA using primers for murine IFNβ 37, TNFα 38 and GAPDH, using the qPCR core kit for SYBR green I with low ROX passive reference (Eurogentec), using the manufacturer’s recommended parameters: 95°C for 15 seconds then 60°C for 1 minute, for 50 cycles. Data were normalised against GAPDH, expressed as fold change over mock (2 -ΔΔCq).
Western blotting
Cell lysates were separated in 12.5% SDS-PAGE and transferred to 0.45-μm nitrocellulose membrane by semi-dry blotting. Membranes were blocked in 5% milk phosphate buffered saline with 0.1% tween-20 (PBS-T) and primary antibodies were incubated in 5% BSA PBS-T at 4°C overnight. Anti-Ifit1 (Santa Cruz, sc-134949, rabbit polyclonal) was used at 1:500, anti-Ifit2/3 (ProteinTech, 12604-1-AP, rabbit polyclonal) was used at 1:800 and anti-GAPDH (Invitrogen, AM4300, mouse monoclonal) was used at 1:8000. Blots were incubated with IRDye 680LT Goat anti-Mouse IgG (Li-Cor, 926-68020) and IRDye 800CW Donkey anti-Rabbit IgG (Li-Cor, 926-32213) secondary antibodies at 1:10000 in PBS-T, for 1 hour at room temperature, then imaged on an Odyssey CLx Imaging System (Li-Cor).
RNA extraction and in vitro transcription
Preparation of VPg-linked RNA from MNV, FCV 39, 40 and PSaV 32 infected cells was performed as described using the GenElute total RNA extraction kit (Sigma). In vitro transcribed RNAs were generated with T7 polymerase (New England Biosciences) from linearised plasmids and subsequently capped using the ScriptCap Capping System (CellScript).
Recombinant protein purification
Recombinant Ifit1, Ifit2 and Ifit3 were expressed in BL21 (DE3) Star Escherichia coli (Invitrogen). Cells were grown to an OD600 of ~1.0 in 2x TY media at 37°C. Expression was induced with 1 mM isopropyl b-D-1-thogalactopyranoside at 22°C for 16 hours. Cells were harvested in a lysis buffer containing 400 mM KCl, 40 mM Tris pH 7.5, 5% glycerol, 2 mM DTT and 0.5 mM phenylmethylsophonyl fluoride with 1 mg/mL lysozyme. Proteins were purified by affinity chromatography on NiNTA agarose (Qiagen), followed by FPLC on MonoQ (GE Healthcare) as described 41.
In vitro translation
Using the Flexi Rabbit Reticulocyte Lysate system (Promega), 8 nM cap0 or 20 ng/μL VPg-linked RNA was translated in the presence or absence of 1.5 μM Ifit proteins, including 5 uCi EasyTag™ L-[ 35S]-Methionine (Perkin-Elmer). After 90 min at 30°C, reactions were terminated by addition of 50 mM EDTA and 0.5 μg/μL RNaseA. Labelled proteins were separated by 12.5% PAGE and detected by autoradiography using an FLA7000 Typhoon Scanner (GE).
Primer extension inhibition
Primer extension inhibition assays were performed as described 12. Briefly, 1 nM cap0 or VPg-linked RNA were incubated with 1.5 μM Ifit proteins for 10 minutes at 37°C in reactions containing 20 mM Tris pH 7.5, 100 mM KCl, 2.5 mM MgCl 2, 1 mM ATP, 0.2 mM GTP, 1 mM DTT and 0.25 mM spermidine. RT was carried out using 2.5 U avian myeloblastosis virus (AMV) reverse transcriptase (Promega) and a 32P-labelled primer in the presence of 4 mM MgCl 2 and 0.5 mM dNTPs. Primer sequences used for RT were CCTGCTCAGGAGGGGTCATG (MNV-1), GTCATAACTGGCACAAGAAGG (FCV) and GTCGTGGGGTGCCAGAAATC (PSaV). Sequencing reactions were performed using the Sequenase Version 2.0 DNA Sequencing Kit (ThermoFisher) in the presence of 35S-labelled ATP. cDNA products were resolved on 6% denaturing PAGE and detected by autoradiography using an FLA7000 Typhoon Scanner (GE).
Statistical analysis
Log viral titres and RT-qPCR fold changes were analysed by two-tailed Student’s t-test, assuming unequal variance, using Microsoft Excel (Microsoft Office 2013, Version 15.0.5119.1000). Values were compared between wildtype cells and each knockout cell line, for each time point. Where both knockout cell lines were significantly different to wildtype (p < 0.05), this is indicated in the figure with an asterisk. Graphs were generated in GraphPad Prism 7 (Version 7.03). Full statistics are available as Underlying data 42.
An earlier version of this article can be found on bioRxiv (doi: https://doi.org/10.1101/611236).
Results
Ifit1 inhibits MNV in RAW264.7 cells
We examined the effect of Ifit1 on calicivirus replication, using MNV as a model. Ifit1 knockout RAW264.7 cell lines were generated by CRISPR-Cas9 gene editing and complete knockout was verified by western blotting. Images of all uncropped blots are available as Underlying data 43. Ifit1 expression was undetectable in two independent Ifit1 -/- clones after 12 hours treatment with IFNβ, while expression of Ifit2 and Ifit3 was maintained ( Figure 1A). Wild-type and Ifit1 -/- RAW264.7 cells were then infected with MNV-1 at low or high MOI and samples were harvested by freezing at the indicated time points. Viral titres were determined by TCID 50 assay in BV2 cells. Raw viral titres are available as Underlying data 42.
In Ifit1 -/- cells infected at a high multiplicity of infection, MNV-1 titres were slightly higher than wild-type cells at 6–8 hours post infection ( Figure 1B). By 12–14 hours post infection, viral titres from wild-type and knockout cells were similar. At these times, a high degree of cytopathic effect was observed, hence infection did not progress any further. When infected at low multiplicity, the differences between wild-type and Ifit1 -/- cells were more apparent ( Figure 1C). Infection of Ifit1 -/- cells resulted in up to 20x higher MNV-1 yields compared to wild-type cells over the course of the infection, suggesting that Ifit1 has antiviral activity during norovirus infection.
Ifit1 cannot inhibit VPg-dependent translation
Ifit1 primarily mediates its antiviral activity by binding to the 5′ cap of non-self RNA, to occlude translation factor recruitment and prevent viral translation. However, members of the Caliciviridae family possess a viral protein, VPg, covalently linked to the 5′ end of the genome which promotes viral translation, in place of a 5′ cap 32, 39, 44– 46. Since knockout of Ifit1 promoted MNV replication in vitro, this suggests that Ifit1 may restrict MNV replication directly, by inhibiting viral translation, or indirectly, by creating a cellular environment which is less permissive to infection. To differentiate these possibilities, we first examined whether Ifit1 could inhibit calicivirus translation in vitro. A similar in vitro translation approach was originally used by Guo et al. to describe the activity of IFIT proteins 22, and since has been successfully used to investigate IFIT1 translation inhibition on human parainfluenza virus 47 and Zika virus model RNAs 41.
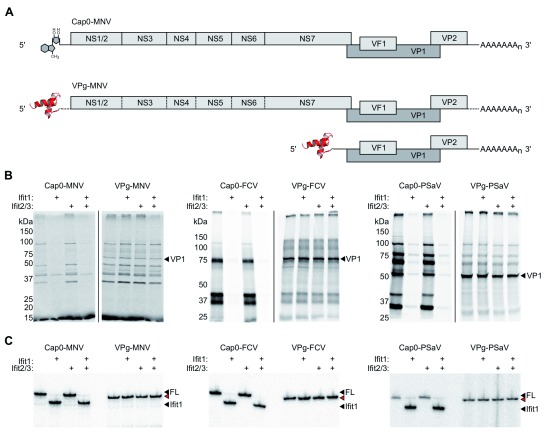
To generate VPg-linked RNA for examination, total RNA was extracted from cells infected with MNV, PSaV or FCV. For PSaV and FCV, translation from VPg-linked RNA prepared in this way predominantly consists of VP1, the major viral capsid protein, which is translated from a highly abundant subgenomic RNA ( Figure 2A) 32, 40. VPg-linked RNAs were translated in rabbit reticulocyte lysate in the presence or absence of recombinantly expressed and purified murine Ifit1, Ifit2 and Ifit3. 35S-Met-labelled translation products were separated by SDS-PAGE and detected by autoradiography. Full-length in vitro transcribed cap0 RNA was included as a positive control for Ifit1 activity ( Figure 2A). As expected, Ifit1 strongly inhibited the translation of artificial cap0 viral RNA 12, but had no effect on the translation of VPg-linked RNAs ( Figure 2B). Addition of Ifit2 and Ifit3 did not enhance translation inhibition on any RNA tested.
Figure 2. Calicivirus translation is resistant to Ifit1 inhibition.
( A) Schematic representations of in vitro transcribed cap0 genomic RNA or VPg-linked genomic and subgenomic RNAs, purified from infected cells, used for in vitro translation and toeprint assays. ( B) In vitro translation of cap0 or VPg-linked RNA from murine norovirus (MNV), porcine sapovirus (PSaV) and feline calicivirus (FCV). VP1, the dominant protein product produced from the VPg-linked subgenomic RNA, is indicated. ( C). Toeprint analysis of MNV, PSaV and FCV VPg-linked and cap0 RNA. Ifit1 binding is indicated by a cDNA product 6-7 nt shorter than the full-length signal (FL), indicated by black arrowheads. Red arrowheads indicate a 1-2 nt shorter full-length signal on VPg-linked RNAs.
Consistently, we observed no evidence of direct Ifit1 binding to VPg-linked RNA when examined in a primer extension inhibition assay, an approach we have used previously to quantify IFIT binding to different RNAs 12, 41. Ifit1, alone or with Ifit2 and Ifit3, was incubated with viral RNA before reverse transcription from a radiolabelled primer specific for the full-length genomic RNA of each virus. cDNA products were resolved by denaturing polyacrylamide gel electrophoresis. Ifit1 was capable of forming a toeprint 6-7 nt downstream of the full-length cDNA product on artificial cap0 RNAs, consistent with binding to the 5′ end ( Figure 2C). However, VPg-linked RNAs derived from infected cells were not bound by Ifit1. Addition of Ifit2 and Ifit3 did not affect Ifit1 binding.
Ifit1 knockout cells have defective innate immune sensing
Ifit1 was previously shown to regulate different stages during innate immune signalling, including signalling downstream of MAVS and TLR3 27, 29, 30. Therefore, we hypothesised that Ifit1 may mediate its antiviral activity during norovirus infection by promoting the innate immune response to infection. We therefore tested our knockout cell lines for their ability to respond to different stimuli. Wild-type and Ifit1 -/- RAW264.7 cells were incubated with LPS or polyI:C, or transfected with polyI:C, to stimulate TLR4, TLR3 or cytoplasmic RNA sensing pathways, respectively. Samples were taken up to 24 hours post infection and RNA or protein was extracted for analysis.
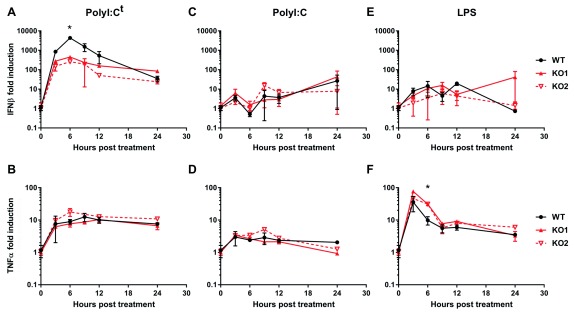
When polyI:C was transfected, to stimulate cytoplasmic RNA sensing, IFNβ expression was strongly upregulated 3 to 9 hours post transfection, decreasing by 12 to 24 hours ( Figure 3A). Expression was 4- to 10-fold higher in wild-type cells during the peak of expression, compared to Ifit1 -/- cells. TNFα was induced to a much lesser extent and expression was comparable between all cell lines ( Figure 3B). We observed weak induction of both IFNβ and TNFα when polyI:C was added to the cell culture medium, rather than transfected, and expression levels were comparable between all cell lines tested ( Figure 3C–D). This indicates that the differential response in Ifit1 -/- cells is specific to cytoplasmic, rather than endosomal, RNA sensing. Cells treated with LPS showed little upregulation of IFNβ mRNA expression when analysed by RT-qPCR ( Figure 3E). However, TNFα was strongly upregulated 3 to 6 hours post LPS treatment in all cell lines, returning to near baseline expression by 9 hours post treatment ( Figure 3F). At 6 hours, TNFα expression was 2- to 3-fold higher in IFIT1 -/- cells compared to wild type, consistent with a recent report 29. Raw gene expression data are available as Underlying data 42.
Figure 3. Ifit1 promotes type I IFN expression following cytoplasmic RNA sensing.
( A– F) Wild-type (WT) and Ifit1 knockout (KO) RAW264.7 cells were stimulated with ( A, B) 2 μg transfected polyI:C (polyI:C t), or ( C, D) 1 μg/mL polyI:C or ( E, F) 10 ng/mL lipopolysaccharide (LPS) in the cell culture medium. RNA was extracted and analysed by RT-qPCR for IFNβ ( A, C, E) and TNFα ( B, D, F) mRNA, expressed as fold induction over untreated cells, normalised against GAPDH (2 -ΔΔCq). Graphs show the mean and the standard error of three biological replicates. Fold induction was compared between WT and KO cells for each time point by two-tailed Student’s t-test. Asterisks indicate that a statistically significant difference (p < 0.05) was observed for both KO cell lines.
Discussion
Noroviruses replicate in the cytoplasm, where they establish membrane-associated replication complexes in which the viral genome is replicated via a dsRNA intermediate. Cytoplasmic double-stranded RNA sensors, RIG-I and MDA5, are principally responsible for detecting replicating calicivirus RNA, activating the type I IFN response 5– 7. This rapid and robust antiviral programme is necessary for viral clearance 4. Here, we demonstrated that the antiviral protein Ifit1 promotes type I IFN responses in RAW264.7 cells and as such contributes to the host antiviral response to restrict murine norovirus infection.
We observed that Ifit1 -/- RAW264.7 cells were more susceptible to MNV infection. In most cells, Ifit1 is not expressed to detectable levels under basal conditions, but expression is induced within a few hours of IFN treatment or viral infection 8, 48, 49. As such, we noticed a more pronounced difference between wild-type and Ifit1-deficient cells following a low multiplicity infection, since type I IFN from infected cells will induce naïve cells to establish an antiviral state, including the upregulation of Ifit1 expression.
IFIT proteins have been implicated in regulating different stages of the antiviral and inflammatory responses (reviewed by Mears and Sweeney 50). In humans, IFIT1 was shown to promote type I IFN expression during alphavirus infection 28. Consistently, a recent study in human and murine macrophages has shown that IFIT1 stimulates type I IFN expression, but represses the inflammatory gene programme, in the acute response following a number of different stimuli 29. The authors suggest that a small population of nuclear IFIT1 can modulate the activity of transcription regulatory complex Sin3A-HDAC2, which is responsible for downregulating both type I IFN and inflammatory gene expression.
Another study has suggested that cytoplasmic IFIT1 downregulates IFN expression by disrupting the MAVS-TBK1-STING signalling axis 27. Together, these studies present a model by which a low level of nuclear IFIT1 promotes type I IFN responses by modulating transcriptional activity. Later in infection, when IFIT1 is highly expressed in the cytoplasm, IFIT1 prevents induction of type I IFN by interfering with MAVS signalling. Consistent with this hypothesis, we observed strong IFNβ expression 3 to 9 hours post stimulation in wild-type cells, which sharply decreased from 9 to 24 hours. In Ifit1 -/- cells, IFNβ expression was induced to a lesser extent, but remained constant up to 24 hours post stimulation, indicating that Ifit1 may be necessary both to switch on and switch off IFN induction at different stages of the immune response.
In caliciviruses, VPg acts as a substitute for the mRNA 5′ cap, by interacting directly with components of the eIF4F complex, to promote ribosome recruitment via eIF3 32, 39, 45, 46. Additionally, the VPg of MNV and Norwalk virus, the prototypic strain of human norovirus, may also interact with eIF3 to promote efficient translation initiation 51, 52. IFIT proteins have been reported to interact with eIF3 and inhibit translation initiation on certain mRNA transcripts 22– 24. Human IFIT1 binds to the e subunit of eIF3 22 and can inhibit translation from the hepatitis C virus internal ribosome entry site (IRES) 21. However, IFIT1 cannot inhibit translation from the eIF3-dependent encephalomyocarditis virus IRES 22. Murine Ifit1 and Ifit2 have both been shown to bind to different domains of the eIF3c subunit, causing translation inhibition on luciferase reporter mRNA at micromolar concentrations 48. However, while Ifit3 was also reported to bind to eIF3c, it has no impact on translation 9.
We have demonstrated that 5' VPg renders calicivirus genomic RNA resistant to Ifit1-mediated translation inhibition. We saw no effect on translation of either capped or VPg-linked RNA when Ifit2 and Ifit3 were added to in vitro translation lysates, indicating that neither of these proteins can inhibit translation, despite their potential to interact with eIF3. Therefore, it remains to be determined how IFIT-eIF3 interactions can inhibit translation initiation on some transcripts but not on others.
In summary, despite calicivirus RNA being refractory to translation inhibition by Ifit proteins, we have shown that Ifit1 knockout cells support a higher degree of MNV infection compared to wild-type cells. We observed that Ifit1 promoted type I IFN expression downstream of cytoplasmic dsRNA sensing, suggesting it may play a role in potentiating the host antiviral state. This work contributes to a growing body of evidence that IFIT proteins can modulate innate immune signalling, complementing their role in translation inhibition.
Data availability
Underlying data
Figshare: Original gels used for making figures. https://doi.org/10.6084/m9.figshare.7998521.v1 43.
Figshare: Raw and processed data used to generate viral titre and host gene expression figures. https://doi.org/10.6084/m9.figshare.7998563.v1 42.
This project contains the following underlying data:
FIG1BC_TCID50_RAW_MNV.xlsx (containing raw data on viral titre).
FIG3_qPCR_RAW_LPSpolyIC.xlsx (containing raw qPCR data for gene expression).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Funding Statement
This research was funded by a Wellcome Trust/Royal Society Sir Henry Dale Fellowship to T.S. (202471) and a Wellcome Trust Senior Fellowship to I.G. (207498). H.M. is supported by a Department of Pathology PhD studentship.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 1 approved, 2 approved with reservations]
References
- 1. Ahmed SM, Hall AJ, Robinson AE, et al. : Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(8):725–730. 10.1016/S1473-3099(14)70767-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ettayebi K, Crawford SE, Murakami K, et al. : Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016;353(6306):1387–1393. 10.1126/science.aaf5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hwang S, Alhatlani B, Arias A, et al. : Murine norovirus: propagation, quantification, and genetic manipulation. Curr Protoc Microbiol. 2014;33(1):15K.2.1–61. 10.1002/9780471729259.mc15k02s33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nice TJ, Robinson BA, Van Winkle JA: The Role of Interferon in Persistent Viral Infection: Insights from Murine Norovirus. Trends Microbiol. 2018;26(6):510–524. 10.1016/j.tim.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCartney SA, Thackray LB, Gitlin L, et al. : MDA-5 recognition of a murine norovirus. PLoS Pathog. 2008;4(7):e1000108. 10.1371/journal.ppat.1000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chhabra P, Ranjan P, Cromeans T, et al. : Critical role of RIG-I and MDA5 in early and late stages of Tulane virus infection. J Gen Virol. 2017;98(5):1016–1026. 10.1099/jgv.0.000769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dang W, Xu L, Yin Y, et al. : IRF-1, RIG-I and MDA5 display potent antiviral activities against norovirus coordinately induced by different types of interferons. Antiviral Res. 2018;155:48–59. 10.1016/j.antiviral.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 8. Terenzi F, Hui DJ, Merrick WC, et al. : Distinct induction patterns and functions of two closely related interferon-inducible human genes, ISG54 and ISG56. J Biol Chem. 2006;281(45):34064–34071. 10.1074/jbc.M605771200 [DOI] [PubMed] [Google Scholar]
- 9. Fensterl V, White CL, Yamashita M, et al. : Novel characteristics of the function and induction of murine p56 family proteins. J Virol. 2008;82(22):11045–11053. 10.1128/JVI.01593-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pichlmair A, Lassnig C, Eberle CA, et al. : IFIT1 is an antiviral protein that recognizes 5'-triphosphate RNA. Nat Immunol. 2011;12(7):624–630. 10.1038/ni.2048 [DOI] [PubMed] [Google Scholar]
- 11. Habjan M, Hubel P, Lacerda L, et al. : Sequestration by IFIT1 impairs translation of 2'O-unmethylated capped RNA. PLoS Pathog. 2013;9(10):e1003663. 10.1371/journal.ppat.1003663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar P, Sweeney TR, Skabkin MA, et al. : Inhibition of translation by IFIT family members is determined by their ability to interact selectively with the 5'-terminal regions of cap0-, cap1- and 5'ppp- mRNAs. Nucleic Acids Res. 2014;42(5):3228–3245. 10.1093/nar/gkt1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abbas YM, Laudenbach BT, Martínez-Montero S, et al. : Structure of human IFIT1 with capped RNA reveals adaptable mRNA binding and mechanisms for sensing N1 and N2 ribose 2'-O methylations. Proc Natl Acad Sci U S A. 2017;114(11):E2106–E2115. 10.1073/pnas.1612444114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson B, VanBlargan LA, Xu W, et al. : Human IFIT3 Modulates IFIT1 RNA Binding Specificity and Protein Stability. Immunity. 2018;48(3):487–499.e5. 10.1016/j.immuni.2018.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daffis S, Szretter KJ, Schriewer J, et al. : 2'- O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468(7322):452–456. 10.1038/nature09489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Züst R, Cervantes-Barragan L, Habjan M, et al. : Ribose 2'- O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat Immunol. 2011;12(2):137–143. 10.1038/ni.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szretter KJ, Daniels BP, Cho H, et al. : 2'- O methylation of the viral mRNA cap by West Nile virus evades ifit1-dependent and -independent mechanisms of host restriction in vivo. PLoS Pathog. 2012;8(5):e1002698. 10.1371/journal.ppat.1002698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hyde JL, Gardner CL, Kimura T, et al. : A viral RNA structural element alters host recognition of nonself RNA. Science. 2014;343(6172):783–7. 10.1126/science.1248465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daugherty MD, Schaller AM, Geballe AP, et al. : Evolution-guided functional analyses reveal diverse antiviral specificities encoded by IFIT1 genes in mammals. eLife. 2016;5: pii: e14228. 10.7554/eLife.14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raychoudhuri A, Shrivastava S, Steele R, et al. : ISG56 and IFITM1 proteins inhibit hepatitis C virus replication. J Virol. 2011;85(24):12881–12889. 10.1128/JVI.05633-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishida Y, Kakuni M, Bang BR, et al. : Hepatic IFN-Induced Protein with Tetratricopeptide Repeats Regulation of HCV Infection. J Interf Cytokine Res. 2019;39(3):133–146. 10.1089/jir.2018.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo J, Hui D, Merrick WC, et al. : A new pathway of translational regulation mediated by eukaryotic initiation factor 3. EMBO J. 2000;19(24):6891–6899. 10.1093/emboj/19.24.6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hui DJ, Bhasker CR, Merrick WC, et al. : Viral stress-inducible protein p56 inhibits translation by blocking the interaction of eIF3 with the ternary complex eIF2.GTP.Met-tRNAi. J Biol Chem. 2003;278(41):39477–39482. 10.1074/jbc.M305038200 [DOI] [PubMed] [Google Scholar]
- 24. Hui DJ, Terenzi F, Merrick WC, et al. : Mouse p56 blocks a distinct function of eukaryotic initiation factor 3 in translation initiation. J Biol Chem. 2005;280(5):3433–3440. 10.1074/jbc.M406700200 [DOI] [PubMed] [Google Scholar]
- 25. Terenzi F, Saikia P, Sen GC: Interferon-inducible protein, P56, inhibits HPV DNA replication by binding to the viral protein E1. EMBO J. 2008;27(24):3311–3321. 10.1038/emboj.2008.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saikia P, Fensterl V, Sen GC: The inhibitory action of P56 on select functions of E1 mediates interferon's effect on human papillomavirus DNA replication. J Virol. 2010;84(24):13036–13039. 10.1128/JVI.01194-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Li C, Xue P, et al. : ISG56 is a negative-feedback regulator of virus-triggered signaling and cellular antiviral response. Proc Natl Acad Sci U S A. 2009;106(19):7945–7950. 10.1073/pnas.0900818106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reynaud JM, Kim DY, Atasheva S, et al. : IFIT1 Differentially Interferes with Translation and Replication of Alphavirus Genomes and Promotes Induction of Type I Interferon. PLoS Pathog. 2015;11(4):e1004863. 10.1371/journal.ppat.1004863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. John SP, Sun J, Carlson RJ, et al. : IFIT1 Exerts Opposing Regulatory Effects on the Inflammatory and Interferon Gene Programs in LPS-Activated Human Macrophages. Cell Rep. 2018;25(1):95–106.e6. 10.1016/j.celrep.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imaizumi T, Yoshida H, Hayakari R, et al. : Interferon-stimulated gene (ISG) 60, as well as ISG56 and ISG54, positively regulates TLR3/IFN-β/STAT1 axis in U373MG human astrocytoma cells. Neurosci Res. 2016;105:35–41. 10.1016/j.neures.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 31. McFadden N, Bailey D, Carrara G, et al. : Norovirus regulation of the innate immune response and apoptosis occurs via the product of the alternative open reading frame 4. PLoS Pathog. 2011;7(12):e1002413. 10.1371/journal.ppat.1002413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hosmillo M, Chaudhry Y, Kim DS, et al. : Sapovirus translation requires an interaction between VPg and the cap binding protein eIF4E. J Virol. 2014;88(21):12213–12221. 10.1128/JVI.01650-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chaudhry Y, Skinner MA, Goodfellow IG: Recovery of genetically defined murine norovirus in tissue culture by using a fowlpox virus expressing T7 RNA polymerase. J Gen Virol. 2007;88(Pt 8):2091–2100. 10.1099/vir.0.82940-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sosnovtsev S, Green KY: RNA transcripts derived from a cloned full-length copy of the feline calicivirus genome do not require VpG for infectivity. Virology. 1995;210(2):383–90. 10.1006/viro.1995.1354 [DOI] [PubMed] [Google Scholar]
- 35. Chang KO, Sosnovtsev SV, Belliot G, et al. : Reverse genetics system for porcine enteric calicivirus, a prototype sapovirus in the Caliciviridae. J Virol. 2005;79(3):1409–1416. 10.1128/JVI.79.3.1409-1416.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanjana NE, Shalem O, Zhang F: Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11(8):783–784. 10.1038/nmeth.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petro TM: ERK-MAP-kinases differentially regulate expression of IL-23 p19 compared with p40 and IFN-beta in Theiler's virus-infected RAW264.7 cells. Immunol Lett. 2005;97(1):47–53. 10.1016/j.imlet.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 38. Liu G, Friggeri A, Yang Y, et al. : miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 2009;106(37):15819–24. 10.1073/pnas.0901216106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goodfellow I, Chaudhry Y, Gioldasi I, et al. : Calicivirus translation initiation requires an interaction between VPg and eIF 4 E. EMBO Rep. 2005;6(10):968–972. 10.1038/sj.embor.7400510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chaudhry Y, Nayak A, Bordeleau ME, et al. : Caliciviruses differ in their functional requirements for eIF4F components. J Biol Chem. 2006;281(35):25315–25325. 10.1074/jbc.M602230200 [DOI] [PubMed] [Google Scholar]
- 41. Fleith RC, Mears HV, Leong XY, et al. : IFIT3 and IFIT2/3 promote IFIT1-mediated translation inhibition by enhancing binding to non-self RNA. Nucleic Acids Res. 2018;46(10):5269–5285. 10.1093/nar/gky191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mears H, Emmott E, Chaudhry Y, et al. : Raw and processed data used to generate viral titre and host gene expression figures. figshare.Dataset.2019. [Google Scholar]
- 43. Mears H, Emmott E, Chaudhry Y, et al. : Original gels used for making figures. figshare.Dataset.2019. [Google Scholar]
- 44. Olspert A, Hosmillo M, Chaudhry Y, et al. : Protein-RNA linkage and posttranslational modifications of feline calicivirus and murine norovirus VPg proteins. PeerJ. 2016;4:e2134. 10.7717/peerj.2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chung L, Bailey D, Leen EN, et al. : Norovirus translation requires an interaction between the C Terminus of the genome-linked viral protein VPg and eukaryotic translation initiation factor 4G. J Biol Chem. 2014;289(31):21738–50. 10.1074/jbc.M114.550657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leen EN, Sorgeloos F, Correia S, et al. : A Conserved Interaction between a C-Terminal Motif in Norovirus VPg and the HEAT-1 Domain of eIF4G Is Essential for Translation Initiation. PLoS Pathog. 2016;12(1):e1005379. 10.1371/journal.ppat.1005379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Young DF, Andrejeva J, Li X, et al. : Human IFIT1 Inhibits mRNA Translation of Rubulaviruses but Not Other Members of the Paramyxoviridae Family. J Virol. 2016;90(20):9446–9456. 10.1128/JVI.01056-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Terenzi F, Pal S, Sen GC: Induction and mode of action of the viral stress-inducible murine proteins, P56 and P54. Virology. 2005;340(1):116–124. 10.1016/j.virol.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 49. Terenzi F, White C, Pal S, et al. : Tissue-specific and inducer-specific differential induction of ISG56 and ISG54 in mice. J Virol. 2007;81(16):8656–8665. 10.1128/JVI.00322-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mears HV, Sweeney TR: Better together: the role of IFIT protein-protein interactions in the antiviral response. J Gen Virol. 2018;99(11):1463–1477. 10.1099/jgv.0.001149 [DOI] [PubMed] [Google Scholar]
- 51. Daughenbaugh KF, Fraser CS, Hershey JW, et al. : The genome-linked protein VPg of the Norwalk virus binds eIF3, suggesting its role in translation initiation complex recruitment. EMBO J. 2003;22(11):2852–9. 10.1093/emboj/cdg251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Daughenbaugh KF, Wobus CE, Hardy ME: VPg of murine norovirus binds translation initiation factors in infected cells. Virol J. 2006;3:33. 10.1186/1743-422X-3-33 [DOI] [PMC free article] [PubMed] [Google Scholar]