Abstract
Glioblastoma is a highly malignant neoplasm, notorious for its poor prognosis. The median age of diagnosis is 64 years, with an increasing number of patients diagnosed over the age of seventy. Managing elderly patients with this condition is challenging. Management pathways may include surgery, radiotherapy, chemotherapy, and best supportive care. Many clinical trials in oncology exclude elderly patients, including some of those for malignant brain tumors, leaving less evidence to guide treatment in these patients. Recent advances in molecular diagnostics and biomarkers, such as 06-methylguanine-DNA-methyltransferase (MGMT) promoter methylation status, may help guide optimal treatment selection. Focusing on available randomized data, this review provides a practical overview of the evidence for treating newly diagnosed glioblastoma in the elderly, including management recommendations.
Keywords: chemotherapy, elderly, glioblastoma, radiotherapy, surgery
Convention is to define the “elderly” population as exceeding a specified chronological age that varies with temporal, geographical, social, and cultural factors. Managing elderly patients can be challenging; medical comorbidities, multiple concomitant medications, and increasing fragility of health alter drug efficacy and the magnitude and spectrum of adverse effects related to treatment. With age, the natural insidious change of physiology and constitution affects pharmacokinetic processes with regard to absorption, metabolism, distribution, and drug clearance.1 Many clinical trials in oncology exclude elderly patients, including some of those for malignant brain tumors; as such there is less evidence to guide treatment in the elderly cohort. This review provides a practical overview of the evidence for treating newly diagnosed glioblastoma in the elderly.
Epidemiology
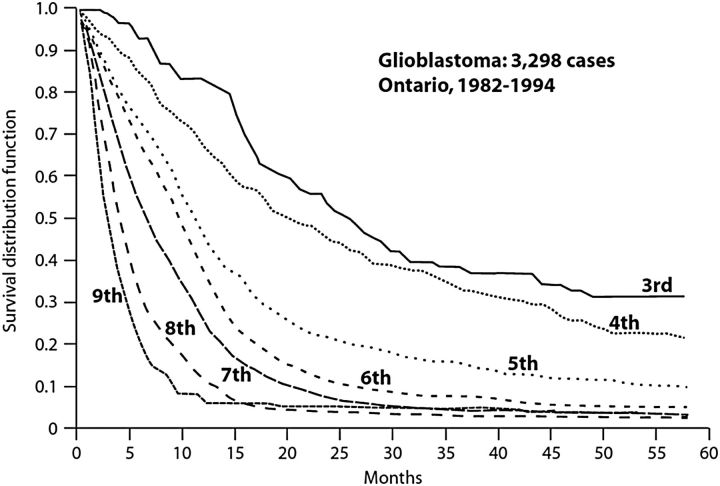
Glioblastoma is a highly malignant neoplasm, notorious for its poor prognosis. The Central Brain Tumour Registry of the United States (CBTRUS) statistical report, which collates epidemiological data from over 50 state cancer registries, identified 112 458 malignant primary brain and central nervous system (CNS) tumors between 2006 and 2010 of which 45.2% were glioblastomas.2 A median age of 64 at diagnosis and an average age-adjusted incidence rate of 3.19 (3.16–3.21) per 100 000 were reported. Stratification by age detected an increase in incidence with age, and the peak rate of 14.93 in the 75 to 84-year age range. Of note is the marked decrease in survival with advancing age (Table 1). The 1-year and 2-year relative survival rates of 40.7% and 14.2% for patients aged 55 to 64 falls to 9.2% and 2.6% for patients aged 75 or older. An Ontario (Canada) population-based cohort study of all patients diagnosed with glioblastoma between 1982 and 1994 found poorer survival with respect to each increasing decade of age (Fig. 1 courtesy of Paszat et al. Unpublished 1999). Whether this poorer survival is a reflection of differing provisions of care based on chronological age or reflects more aggressive tumor biology, or both, is presently unclear.
Table 1.
Average age-adjusted incidence per 100 000 and relative survival for patients with glioblastoma, stratified by age (CBTRUS).2
| Age (years) | Average Age Adjusted Incidence per 100 000 | 1-year Relative Survival | 2-year Relative Survival | 5-year Relative Survival |
|---|---|---|---|---|
| 45–54 | 3.62 | 52.7% | 20.8% | 5.9% |
| 55–64 | 8.08 | 40.7% | 14.2% | 3.8% |
| 65–74 | 13.09 | 23.7% | 7.2% | 1.7% |
| 75–84 | 14.93 | 9.2% (≥75) | 2.6% (≥75) | 0.8% (≥75) |
| ≥85 | 9.24 |
Fig. 1.
Overall survival of glioblastoma patients treated in Ontario, Canada, stratified by decade of age.
The histologic hallmarks of glioblastoma, as defined by the World Health Organization (WHO), include cellular polymorphism, nuclear atypia, a high mitotic index, microvascular proliferation, and necrosis.3 With the emergence of personalized medicine, molecular diagnostics are increasingly used to improve the treatment and survival associated with glioblastoma. Prognostic biomarkers such as TP53 mutation, 1p deletion, cyclin dependent kinase (CDK) N2A/p16 deletion, and epidermal growth factor receptor (EGFR) amplification vary with age.4 In a histological review of 140 patients, TP53 mutations and EGFR amplification had differing prognostic significance when stratifying by age, with TP53 mutations being positively prognostic for younger patients and negative for older patients (<70 years HR = 0.84; 95% CI, 0.49-1.42 vs >70 years HR = 7.54; 95% CI, 2.38-23.87).4 Conversely EGFR amplification in the context of older patients was positively prognostic yet in younger patients it was negatively prognostic.4
More recently gene expression-based molecular analysis has been used to categorize glioblastoma into the proneural, neural, classical, and mesenchymal subtypes.5 Lee et al performed a meta-analysis that substantiated the presence of these subtypes, as identified by genetic signature, and suggested that the prognostic effect of age may in fact be a reflection of the differing prevalence of specific subtypes at differing ages; for example, the proneural subtype appears to occur more often in younger patients and is associated with longer survival.6 Presently these markers do not have a defined role in clinical practice with regard to daily management decisions and remain under investigation. Of note, positive prognostic biomarkers, like mutations of isocitrate dehydrogenase (IDH) are virtually absent in glioblastoma of the elderly; similarly the general DNA methylation levels in the tumor tissue seem to be low. Despite this the frequency of O6-Methylguanine-DNA methyltransferase (MGMT) promoter methylation, itself an important positive predictive marker, does not vary with age.7
Surgery
In younger patients, maximal safe resection is advocated with the intent of preserving neurological function, providing maximal tissue for molecular profiling, and improving overall survival. Analysis of the extent of surgery in Radiation Therapy Oncology Group (RTOG) randomized trials found significant improvement in survival with partial/total resection vs biopsy alone.8 Review of an unselected population of 345 newly diagnosed glioblastoma patients from the German Glioma Network (GGN) demonstrated gross tumor resection to be associated with superior overall survival (OS) (median 17.1 months) compared with incomplete resection (median 11.7 months) and biopsy alone (median 8.7 months).9 A multivariate analysis of 416 glioblastoma patients treated at a single institution between 1993 and 1999 reported resections of tumor volume in the order of 98% or greater to be associated with significant survival advantage (median survival 13 months [95% CI, 11.4-14.6 months] vs 8.8 months [95% CI, 7.4-10.2 months; P < .0001]).10
There is one randomized trial pertaining to surgical intervention that includes elderly patients with glioblastoma. This small study of 30 patients assessed the role of debulking surgery compared with biopsy alone.11 Patients aged 65 or older with KPS >60 were randomized to open craniotomy and resection (14 patients with a median age of 70 [66–80]) or stereotactic biopsy (16 patients with a median of age 72 [67–79]). Surgical resection resulted in superior overall survival (171 days [95% CI, 146-278 days] vs 85 days [95% CI 55-157 days] P = .0346). More recently, a case-control study with a subgroup analysis of 52 patients aged 70 or over found a median survival of 4.5 months and 3 months for surgical resection and needle biopsy, respectively (P = .03).12 Perhaps the most relevant trial for the topic is the Neuro-oncology Working Group of the German Cancer Society NOA-08, study which found extent of surgery to be an independent prognostic factor for overall survival among glioblastoma patients 65 years and older.13 Furthermore, multivariate analysis of all patients (n = 342) participating in the Nordic trial of standard vs hypofractionated radiotherapy vs chemotherapy alone in newly diagnosed glioblastoma patients 65 years of age or older also demonstrated a survival benefit favoring surgery over biopsy alone (biopsy vs resection HR = 1.50 [95% CI, 1.17–1.92] P = .001).14
Standard postoperative management for newly diagnosed glioblastoma
The European Organisation for Research and Treatment of Cancer (EORTC) 26981–22981/National Cancer Institute of Canada Clinical Trials Group (NCIC) CE.3 randomised phase III trial assessed the addition of temozolomide (TMZ) to radiotherapy in the concomitant and sequential adjuvant setting in glioblastoma patients aged 18 to 70 years.15 Median age was 56 (range 19–71) and the selected population required Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. The addition of TMZ resulted in a median survival benefit of 2.5 months; 14.6 months (95% CI, 13.2–16.8) compared with 12.1 months (95% CI, 11.2–13.0) for radiotherapy alone. The 5-year analysis of this trial confirmed a persisting advantage and this has become the standard of care postsurgical resection for patients <70 years of age and of appropriate performance status.16 In a recent review Laperriere et al noted that subgroup analysis of this trial demonstrated a trend to benefit in the elderly subgroups, without reaching statistical significance. Specifically, this benefit of combined treated appeared to diminish with increasing age (61–65 years: HR = 0.64; P = .096 and 66–70 years: HR = 0.78; P = 0.34) compared with the overall group (HR = 0.6, 95% CI, 0.5-0.7; P < .0001).17 This may reflect less robust effects of the combined approach in the elderly or may be due to lower statistical power in the subgroup analysis.
Radiation for elderly patients
Randomized trials have long demonstrated a survival benefit from postoperative radiotherapy in the management of glioblastoma and more recently several trials have focused on the elderly. The French ANOCEF group found a median survival benefit of 12.2 weeks in favour of RT plus best supportive care vs best supportive care alone.18 Patients aged 70 or older with a KPS >70 and a diagnosis of glioblastoma or anaplastic astrocytoma were randomized postoperatively to receive best supportive care (42 patients [median age 73; range 70–85]) or best supportive care and 50 Gy in 1.8 Gy fractions to a clinical target volume consisting of enhancing tumour with a 2-cm margin (39 patients [median age 75; range 70–84]). Overall survival was 16.9 (95% CI, 13.4–21.4) and 29.1 weeks (95% CI, 25.4–34.9), respectively. No significant difference was detected in quality of life; however, Health Related Quality of Life (HRQoL) assessments were incomplete. Cox proportional hazard modeling revealed that extent of surgery was associated with increased survival.
Scott et al performed a large retrospective review of elderly glioblastoma patients diagnosed between 1993 and 2005.19 The study sample of 2836 patients identified from the Surveillance, Epidemiology, and End Results (SEER) registry database had a median age of 76.9 years (range 71.0–98.0). Kaplan-Meier analysis revealed median cancer-specific survivals of 8 months for patients undergoing both surgery and postoperative radiotherapy, 4 months for radiation alone, 3 months for surgery alone and 2 months for neither surgery nor radiotherapy (log rank P < .001). Multivariate analysis suggested radiotherapy significantly increased cancer-specific survival after adjusting for tumor size, tumor location, surgery, and patient demographics with a hazard ratio of 0.43 (95% CI, 0.38–0.49).19
The biological effect of radiation on tumour and normal tissues is dependent upon the provision of dose over time as well as intrinsic radio-sensitivity (α) and repair capability (β). Glioblastoma has an alpha-beta ratio (α/β) = 8 Gy (range = 5.0–10.8 Gy),20 which is in the range of most tumors, while the α/β ratio is ∼2 for the normal central nervous system. As a result of this difference, hypofractionation reduces overall treatment time and may minimize the potential for tumor cell repopulation and provides a practical convenience for an elderly frail population. Roa et al randomized patients aged 60 or older to radiotherapy given as 60 Gy in 30 fractions (47 patients, mean age = 72.4 years, SD = 5.4) or a hypofractionated regimen of 40 Gy in 15 fractions (48 patients, mean age = 71.0 years, SD = 5.5).21 While this study was not sufficiently powered to conclude equivalence of these two fractionation schedules it suggested no significant differences in overall survival (median 5.1 months for the standard radiotherapy arm vs 5.6 months for the shorter course [log rank P = .57]); survival at 6 months (44.7% standard radiotherapy vs 41.7% hypofractionated radiotherapy); or HRQoL. More patients required an increase of corticosteroid dose following the standard radiotherapy schedule compared with the hypofractionated course (P = .02).
The Nordic trial incorporated a different hypofractionated radiotherapy schedule.14 There were 3 treatment arms including standard radiotherapy of 60 Gy in 30 fractions; hypofractionated radiotherapy of 34 Gy in 10 fractions; or TMZ dose of 200 mg/m2 days 1 to 5 every 28 days for up to 6 cycles. Standard radiotherapy (60 Gy/30 fractions) was not routinely offered to elderly patients in some study sites so only randomization between the hypofractionated radiotherapy and TMZ arms was permitted. Two hundred ninety-one glioblastoma patients (initially aged 60 years or older, then, in view of EORTC 26981-22981/NCIC-CTG CE.3, the age eligibility was adjusted so that patients 60–65 years old fit for combined treatment were excluded) were randomized to standard radiotherapy (n = 100), hypofractionated radiotherapy (n = 98), or TMZ alone (n = 93). A further 51 patients were randomized to either hypofractionated radiotherapy (n = 25) or TMZ (n = 26) by those centers that did not offer 60 Gy in 30 fractions as their standard care.
The median age was 70 for both the hypofractionated and the standard radiotherapy groups. In the 3-arm comparison, median survival in the hypofractionated group was 1.5 months longer than in the standard radiation group. Interestingly, on stratification by age, the advantage of hypofractionated radiation appeared better for patients over the age of 70 (7.0 [5.2–8.8] vs 5.2 [4.0–6.3] months). Treatment completion according to protocol was more frequent with the hypofractionated schedule (95% vs 72%). Salvage treatment was received for a similar proportion of patients in both groups, while reported toxicity was not different between groups.
Temozolomide and O-6-methylguanine-DNA methyltransferase
The alkylating agent TMZ has activity in glioblastoma, and in combination with radiotherapy followed sequentially by a 6-month maintenance course represents the current standard of care for many patients. The mechanism of anti-tumor activity is believed to arise through methylation of DNA at the O-6 position of guanine by monomethyl-triazeno-imidazole-carboxyamide (MTIC), a nonenzymatic chemical degradation product of TMZ.22
MGMT is a DNA repair protein implicated in resistance to alkylating agents.23 Methylation of the MGMT promoter, located at 10q26, leads to suppression of MGMT gene expression and an increased likelihood of clinical benefit.23–25 Hegi et al assessed the MGMT promoter methylation status of patients randomized in the EORTC trial 26981/ NCIC CE.3 trial.25 Regardless of treatment arm, patients with MGMT promoter methylation had greater overall survival; 18.2 months compared with 12.2 months (HR for death = 0.45 [95% CI, 0.32–0.61]). The magnitude of this effect was more substantial for patients receiving TMZ than for those receiving radiation alone (P = .007 vs P = .06, log-rank test). Of note, the majority of patients allocated to radiotherapy alone received alkylating agent chemotherapy as salvage treatment further supporting the use of concomitant therapy upfront in newly diagnosed patients.25 The prognostic significance of MGMT promoter methylation status was prospectively corroborated in the RTOG 0525 randomized study of TMZ dose density in the adjuvant setting. In this study, dose-dense TMZ (n = 422) failed to demonstrate a survival advantage over standard dosing (n = 411).26 The absence of a TMZ-free control arm did not allow distinction between prognostic and predictive properties.
For elderly patients not suitable for the combined modality approach, recent evidence supports consideration of TMZ alone particularly for tumors harbouring MGMT promoter methylation.27 TMZ alone was assessed in the Nordic study,14 which found longer survival for both TMZ alone and hypofractionated radiotherapy over standard radiotherapy for patients older than 65 years of age. Comparison of TMZ and hypofractionated radiotherapy revealed no significant difference in overall survival (7.4 vs 8.4 months; HR = 0.82 [95% CI, 0.63-1.06]). In the head-to-head comparison of TMZ vs hypofractionated radiation, 36% of the TMZ recipients had subsequent radiation and 29% of the hypofractionated group had salvage chemotherapy. MGMT promoter methylation status was available in 258 (75%) of 342 patients. Patients with MGMT promoter-methylated tumors receiving TMZ survived 2.9 months longer than those with unmethylated tumors (HR = 0.56 [95% CI, 0.34-0.93]; P = .02). No survival advantage was identified based on MGMT promoter methylation status within the cohort receiving radiation (HR = 0.97 [95% CI, 0.69-1.38]; P = .81). Although the intent for the TMZ group was to complete 6 cycles, at least 2 cycles were administered to 86% of patients, and only 34% completed all 6 cycles. Hematological toxicity as well as nausea and vomiting were more frequent, as would be expected in the TMZ cohort. In addition, a treatment-related death involving thrombocytopenia highlights that prescribing chemotherapy is not without the potential for serious toxicity.
In the NOA-08 study,13 192 patients received TMZ (1 week on, 1 week off schedule of 100 mg/m2 days 1–7) and 178 patients received 60 Gy radiotherapy alone over 6 to 7 weeks to the gross tumour volume with a 2-cm margin. Median overall survival was similar for the 2 treatment arms: 8.6 months in the TMZ group and 9.6 months in the radiotherapy group (HR = 1.09 [95% CI, 0.84-1.42]; P noninferiority = .033). MGMT promoter methylation status was available in 55% of patients receiving TMZ and 57% of patients receiving radiation with a predictive benefit seen for patients receiving the alkylating agent in the context of MGMT promoter methylated tumors. Hematological toxicity, abnormal liver function tests, infections and thromboembolic events were more prevalent in the TMZ group.
These trials found that MGMT promoter methylation is a predictive biomarker of benefit from TMZ, but not radiotherapy. The randomized international NCIC/EORTC/TROG study, which completed accrual in September 2013 (James R Perry M.D., personal communication), aims to address the potential benefit of combining short course radiotherapy (40 Gy in 15 fractions) with concurrent and adjuvant TMZ for patients over 65 years who have had prior surgery/biopsy at diagnosis and are not deemed suitable for the standard radiotherapy regimen of 60 Gy.28MGMT status will be assessed in this study.
A phase 2 ANOCEF study suggests that older age and poor KPS should not preclude the use of TMZ alone.29 This was a nonrandomized study that recruited 70 patients with a median age of 77 years (range 70–87) and a median KPS of 60 (range 30–60). Intriguingly this study found an improvement of KPS in excess of 10 for 23 (33%) of treated patients with 18 (26%) having a rise to 70 or more. A maximum of 12 cycles of TMZ was planned; however, the median number of cycles received was only 2 with 20% and 24% of patients having dose delays and dose reductions for hematological toxicity, respectively. Grade 3 or 4 hematological toxicities were not insignificant with 13% experiencing grade 3 or 4 neutropenia and 14% grade 3 or 4 thrombocytopenia. No deaths were attributed to treatment. Although only 44% of patients were able to have tumor material assessed for MGMT promoter methylation, this study again demonstrated its predictive role with a hazard ratio for death of 2.307 (95% CI, 1.073-4.962) for patients with unmethylated MGMT promoter status (P = .03). This phase 2 study introduces the questions: Should more elderly patients with poor performance status be primarily treated with TMZ monotherapy? Or should TMZ monotherapy be employed only in those whose tumor harbors a methylated MGMT promoter?
Although MGMT promoter methylation status is increasingly available it still not used in all centers. In the future increasing evidence favoring MGMT testing is likely to demand more widespread availability; for example, the European Association for Neuro-Oncology guideline for the diagnosis and treatment of malignant gliomas has already declared that MGMT promoter methylation status testing is standard of care.30 There have been some controversies regarding the methodology of MGMT testing, with some centers preferring pyrosequencing and others utilizing PCR. Immunohistochemistical assessment of MGMT does not appear to correlate with overall survival.31
Bevacizumab
Three uncontrolled studies indicate that the vascular endothelial growth factor (VEGF) antibody bevacizumab may have increased activity in elderly patients with glioblastoma.32–34 In 2014, the efficacy of bevacizumab in newly diagnosed glioblastoma patients had been reported by 2 large, placebo-controlled, randomized trials.35,36 The Avastin in Glioblastoma (AVAglio) phase 3 study evaluated the effect of the addition of bevacizumab to focal radiotherapy with concurrent TMZ, to the adjuvant component and then beyond the adjuvant component until progression.34 Although improved progression-free survival (HR = 0.65 [95% CI, 0.56–0.75]), preservation of baseline quality of life, and performance status were reported, there was no improvement in overall survival. Stratified by age over 70 years, the statistical significance with regards to PFS was lost (HR = 0.78 [95% CI, 0.46–1.33]); however, this may reflect an issue of statistical power and small subgroups rather than a lack of clinical efficacy. The RTOG 0825 trial, sharing a similar design, also failed to demonstrate an overall survival benefit,35 but in contrast to the AVAglio study, greater clinical deterioration (assessed by patient-reported outcome questionnaires) was evident in the bevacizumab group. There were differences in the design of these 2 studies that may influence determination of progression and patient-reported outcomes. Radiological assessment in the RTOG 0825 study was by serial measurement of cross-sectional diameter and use of the international criteria proposed by the Response Evaluation Criteria in Solid Tumors (RECIST)37 committee, whereas the AVAglio study used an adaptation of the Macdonald criteria, similar to the newer RANO criteria, which take into account the issues of pseudoprogression, pseudoresponse, and changes in the nonenhancing disease.38 Ongoing discussion and analyses may further clarify the apparent discordant results with regard to progression-free survival in these 2 pivotal trials.
No clinical or tissue-based biomarkers have yet been prospectively shown to be associated with benefit from bevacizumab, although patients with glioblastomas harboring a proneural subtype may derive the most benefit.39 At present bevacizumab has no role in the standard upfront treatment of glioblastoma; however, future clinical trials may attempt to target specific groups of patients defined by sets of biomarkers. The randomized Avastin plus Radiotherapy in Elderly Patients with Glioblastoma (ARTE) study, a phase 2 trial, will explore whether the addition of bevacizumab to radiotherapy improves outcome in elderly patients with newly diagnosed glioblastoma without MGMT promoter methylation (Michael Weller M.D., personal communication).
Symptomatic management
Glioblastoma can cause many difficult symptoms ranging from fatigue to those associated with raised intracranial pressure. Seizures may often occur as well as cognitive, motor or sensory deficits occurring in a location-dependent manner. Corticosteroids are often required to control symptoms of cerebral edema and their utility over time must be balanced with potential side effects such as proximal myopathy, steroid-induced diabetes, and osteoporotic fractures, which can be debilitating. Furthermore, corticosteroids may reduce the benefit from TMZ in the most promising MGMT promoter methylated subgroup.40 Antiseizure medications are also often warranted. There is no randomized evidence pertaining to palliative care in the glioblastoma setting. However, based on a randomized study in non-small lung cancer, which demonstrated the addition of palliative care not only improved quality of life but also increased overall survival, many would advocate the early incorporation of palliative care support.41
Population-based retrospective studies
For glioblastoma, like many other cancers, results from randomized clinical trials may not reflect real-world outcomes as described in population-based studies. Several large population-based studies have shown that many elderly patients do not receive the gold standard treatment. For example, despite the increasing body of evidence regarding the important benefit of resection rather than biopsy, numerous international pattern of care studies42–46 demonstrate a much higher rate of biopsy alone rather than attempted resection in the elderly population.
The SEER database study published by Scott et al reported that among 2836 patients, only 46% of those over the age of 70 received both surgery and radiotherapy, with omission of treatment associated with poorer survival.19 A similar SEER study of 4137 patients with glioblastoma, aged 65 or older, reported a median overall survival of 4 months and described age to be associated with lower odds of resection and provision of radiotherapy or chemotherapy.19 The Princess Margaret Cancer Centre published outcomes of 131 patients aged >70 treated between 2004 and 2008.47 Elderly patients were more likely to receive best supportive care or palliative doses of radiotherapy with only 1 patient receiving 60 Gy in 30 fractions in combination with TMZ. Only 6 patients (5%) received TMZ following radiation, with only a median of 2.5 cycles administered. A retrospective review of 235 patients aged 65 or over treated between 2006 and 2013 at the Odette Cancer Center in Toronto provides a more contemporary overview regarding provision of care in the elderly setting.48 With a median survival of ∼2 months, 19% of patients were deemed not suitable for active treatment.
There is likely another subgroup of elderly patients not reflected in statistics who might be presumptively diagnosed on radiological investigations (eg, imaging for suspected stroke) but for various reasons (eg, comorbidities, patient and family preference) do not proceed even to a biopsy. Of course, in certain scenarios (eg, bedbound patient with dementia) it may be inappropriate to pursue active management.
Survivorship in the real world would appear shorter than in randomized clinical trials and may be a reflection of both physician preference to not administer treatment in a group previously not studied, as well as patient choice. The definition of “elderly” varies across clinical trials and may appear to limit the ability to cross-compare data from these studies. However, the NOA-08 trial had a median age of 72 (66–84) years in the TMZ arm and 72 (66–82) years in the radiotherapy arm. Age as a continuous variable or dichotomized at age 70 was not an independent prognostic factor for either overall survival or event-free survival13; thus the association with age may not be as important among patients older than 70 years. Patients from population-based studies are clearly different that those included in the randomized trials. A patient-centered approach is important, as in all aspects of medicine, and treatment decisions need to involve a patient's own preferences and goals of care should be a focus early in the discussion regarding management.
Practical aspects
Practical considerations such as performance status and even the ability of the patient to get to appointments can also come into play, as many of these patients are no longer driving. For example, a mobile elderly patient with a poor short-term memory, but with a strong family network advocating for active treatment, is far more likely to be treated than a socially isolated patient. If a cognitively intact patient with poor mobility is keen for treatment, again the presence of supportive family will often make a difference in decision making.
Often rehabilitation is not offered for glioblastoma patients postoperatively. However, there is evidence that postoperative rehabilitation in this setting is just as useful as in the stroke setting49,50 and should be considered where possible. There are observational studies which show improvement in patients' functional status during the course of rehabilitation therapy, including the functional independence measure51,52 and referral for rehabilitation is advocated.53
Elderly patients and their caregivers may have numerous symptoms or challenges ahead. Challenges include treatment and tumor-related symptoms and deficits, seizures, headaches, communication difficulties (eg, expressive or receptive aphasia), personality and behavior changes (eg, frontal syndrome with disinhibition and emotional lability), poor concentration, poor memory, fatigue, weakness, mobility, hemiparesis, impaired judgement/insight, and depression (reactive vs major). These challenges can be even more difficult to manage in the setting of comorbidities and polypharmacy often faced by elderly patients.
The clinical journey is a complex one and can involve interaction with many health professionals—such as neurosurgeons, radiation oncologists (and radiation therapists), medical oncologists, palliative care physicians, occupational therapists, physiotherapists, neurologists, endocrinologists (for steroid-induced diabetes) or diabetic educator, social worker, pharmacist, psychologist, and speech pathologists—and ideally a cancer care coordinator should be available, where possible, to help the patient navigate through this difficult pathway.
Caregiver burnout is also very important for clinicians to be aware of. Recent studies have demonstrated that global quality of life is often poorer in caregivers than in the patients themselves.54,55 Often, in the elderly setting, a spouse (if there is one) has their own comorbidities to deal with and struggles to manage both physically and emotionally with the complexities involved with caring for a partner with glioblastoma.
Conclusion and recommendations
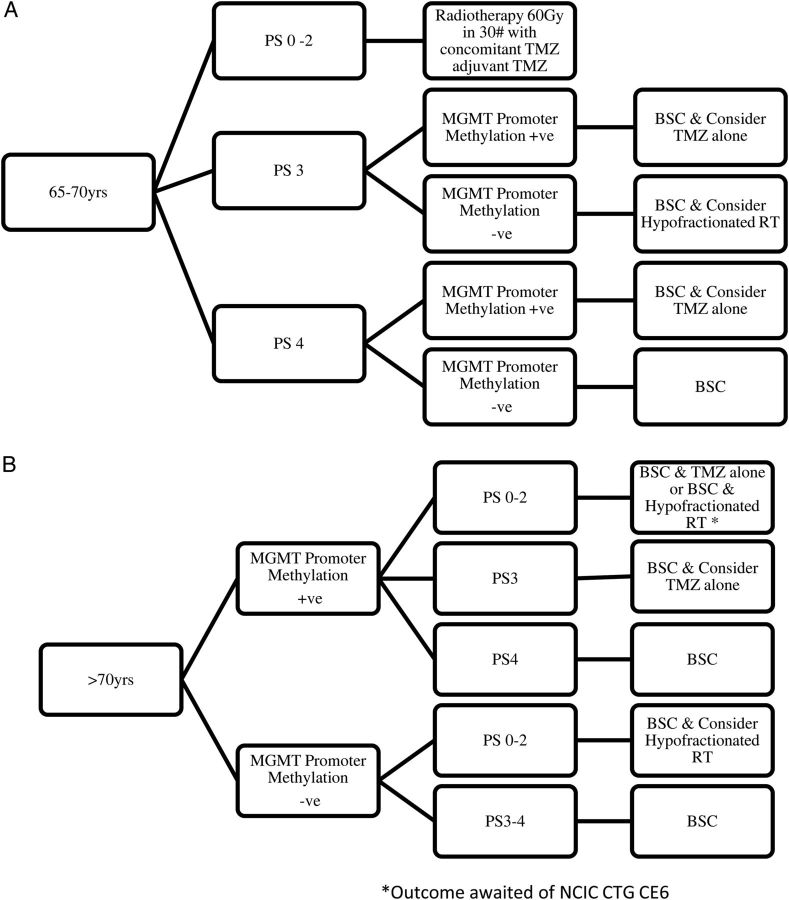
Selecting the appropriate treatment for an elderly patient with a newly diagnosed glioblastoma is challenging and a patient-centered approach is essential. Randomized evidence to guide treatment decisions is emerging (Table 2) and there is less reason for nihilism. Initial consideration should include the appropriateness and extent of surgical intervention. With frailty and potential comorbidities there may be increased risk of perioperative complications and prolonged recovery; however, maximal safe surgical resection should be considered. Subsequent management should incorporate initial symptomatic management including titration of corticosteroids and suitable antiseizure medication if required. Early introduction of palliative care may have a role for many patients. Management should be based on the fitness of the patient, performance status, and MGMT promoter methylation status (Fig. 2).56 Standard radiotherapy of 60 Gy in 30 fractions with concurrent and adjuvant TMZ can be used for most patients under the age of 70 and of appropriate fitness. For patients over the age of 70 there is evidence of efficacy for both radiotherapy alone and TMZ monotherapy; the results of the NCIC-CTG/EORTC/TROG clinical trial will assess the benefit of hypofractionated radiotherapy with concurrent TMZ compared with radiotherapy alone. Most patients over 70 years of age appear not to benefit from conventional radiation schedules such as 60 Gy in 30 fractions and a hypofractionated schedule is recommended. We acknowledge that some practitioners continue to recommend radical treatment (60 Gy in 30 fractions with TMZ) for fit patients over the age of 70; however there are no randomized data to support this practice. MGMT may turn out to be even more important in the setting of elderly patients than in younger patients in terms of guiding management decisions. Ideally MGMT promoter methylation status should be determined for all patients 65 years and older. Patients lacking MGMT promoter methylation should be considered for a course of hypofractionated radiation therapy alone while those with methylated tumors may be offered TMZ alone. Selection of these treatments requires an interdisciplinary discussion of the risks and benefits of radiotherapy vs TMZ, incorporation of the patient's own goals of care, and patient preference.
Table 2.
Randomized clinical evidence for elderly patients with glioblastoma
| Title | Treatment Arm | Number of Patients | Age, Median (Range) | Outcome |
|---|---|---|---|---|
| Debulking or biopsy of malignant glioma in elderly people – a randomized study11 | Stereotactic biopsy Open craniotomy/ Resection |
16 14 |
72 (67–79) 70 (66–80) |
85 days (95% CI, 55–157) 171 days (95% CI, 146–278) (P = .035) |
| Radiotherapy for glioblastoma in the elderly18 | Best supportive care Radiotherapy 50 Gy in 28 fractions with best supportive care |
42 39 |
73 (70–85) 75 (70–84) |
16.9 weeks (95% CI, 13.4–21.4) 29.1 weeks (95% CI, 25.4–34.9) HR for death in RT Group = 0.47 (95% CI, 0.29–0.76; P = .002 by the log-rank test) |
| Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: A prospective randomized clinical trial21 | Standard radiotherapy (60 Gy in 2 Gy fractions over 6 weeks) Short-course regimen (40 Gy in 15 fractions over 3 weeks) |
47 48 |
Mean 72.4 (SD 5.4) Mean 71 (SD 5.5) |
Median survival 5.1 months Median survival 5.6 months (HR = 0.89; 95% CI, 0.59–1.36; P = .57) |
| Temozolomide chemotherapy alone vs radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 Randomised, phase 3 trial13 |
Temozolomide alone 100 mg/m2 given on days 1–7 of 1 week every 14 days Radiotherapy 60 Gy, administered over 6-7 weeks in 30 fractions of 1.8–2.0 Gy |
195 178 |
72 (66–84) 71 (66–82) |
8.6 months (95% CI, 7.3–10.2) 9.6 months (95% CI, 8.2–10.8) (HR = 1.09, 95% CI, 0.84–1.42; Pnon inferiority = .033) |
| Temozolomide vs standard 6-week radiotherapy vs hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial14 | Standard radiotherapy (60 Gy in 2 Gy fractions over 6 weeks) Hypofractionated radiotherapy (34.0 Gy in 3·4 Gy fractions over 2 weeks Temozolomide (200 mg/m2 on days 1–5 of every 28 days for up to 6 cycles) |
100 98 93 |
70 years (60–80) 70 (60–83) 70 (60–88) |
6.0 months (95% CI, 5.1–6.8) 7.5 months (95% CI, 6.5–8.6) 8.3 months (95% CI, 7.1–9.5) |
Fig. 2.
Flow diagram of treatment considerations for elderly patients with glioblastoma (A) 65–70 years and (B) >70 years.
Some of the current algorithms for elderly glioblastoma patients are based on extrapolations from small and underpowered studies, but hopefully over the next 5 years higher levels of evidence from larger maturing phase 3 studies will ensure future recommendations are more robust.
Funding
JRP is supported by the Crolla Family Endowed Chair in Neuro-Oncology at the University of Toronto.
Conflicts of interest statement. MM, none declared; NL, Speaker fees with Merck and Roche; WW, Consulting and Trial Steering Committee for Roche (compensated), Consulting and Steering Committee from Apogenix (uncompensated), Lecture fees from Prime Oncology, Research Funding from Apogenix, Boehringer Ingelheim, Eli Lilly, MSD and Roche; DAR, Speakers' Bureau (compensated): Merck and Roche/Genentech; Advisory Board member (compensated): Roche/Genentech; Cavion; Novartis; Midatech; Stemline Therapeutics; Momenta Pharmaceuticals; Research Support: Celldex Therapeutics; Incyte; AM, none declared; EH, Glioma Advisory Board for Roche Australia, MSD Australia; MW, has received research grants from Acceleron, Actelion, Alpinia Institute, Bayer, Isarna, MSD, Merck & Co, PIQUR and Roche and honoraria for lectures or advisory board participation or consulting from Celldex, Immunocellular, Isarna, Magforce, MSD, Merck & Co, Northwest Biotherapeutics, Pfizer, Roche and Teva; JRP, Consulting and Advisory Board fees from Roche Canada, lecture fees from Prime Oncology, Advisory Boards: Merck, Midatech, Roche Canada, Delmar Pharmaceuticals.
References
- 1. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76. [DOI] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batchelor TT, Betensky RA, Esposito JM, et al. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res. 2004;10(1 Pt 1):228–233. [DOI] [PubMed] [Google Scholar]
- 5. Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee Y, Scheck AC, Cloughesy TF, et al. Gene expression analysis of glioblastomas identifies the major molecular basis for the prognostic benefit of younger age. BMC Med Genomics. 2008;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiestler B, Claus R, Hartlieb SA, et al. Malignant astrocytomas of elderly patients lack favorable molecular markers: an analysis of the NOA-08 study collective. Neuro Oncol. 2013;15(8):1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simpson JR, Horton J, Scott C, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26(2):239–244. [DOI] [PubMed] [Google Scholar]
- 9. Kreth FW, Thon N, Simon M, et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol. 2013;24(12):3117–3123. [DOI] [PubMed] [Google Scholar]
- 10. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 11. Vuorinen V, Hinkka S, Farkkila M, Jaaskelainen J. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien). 2003;145(1):5–10. [DOI] [PubMed] [Google Scholar]
- 12. Chaichana KL, Garzon-Muvdi T, Parker S, et al. Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann Surg Oncol. 2011;18(1):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 14. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 15. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 16. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 17. Laperriere N, Weller M, Stupp R, et al. Optimal management of elderly patients with glioblastoma. Cancer Treat Rev. 2013;39(4):350–357. [DOI] [PubMed] [Google Scholar]
- 18. Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. [DOI] [PubMed] [Google Scholar]
- 19. Scott J, Tsai YY, Chinnaiyan P, Yu HH. Effectiveness of radiotherapy for elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(1):206–210. [DOI] [PubMed] [Google Scholar]
- 20. Pedicini P, Fiorentino A, Simeon V, et al. Clinical radiobiology of glioblastoma multiforme: Estimation of tumor control probability from various radiotherapy fractionation schemes. Strahlenther Onkol. 2014;190(10):925–932. [DOI] [PubMed] [Google Scholar]
- 21. Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 22. Baker SD, Wirth M, Statkevich P, et al. Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin Cancer Res. 1999;5(2):309–317. [PubMed] [Google Scholar]
- 23. Weller M, Stupp R, Reifenberger G, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6(1):39–51. [DOI] [PubMed] [Google Scholar]
- 24. Gerstner ER, Yip S, Wang DL, Louis DN, Iafrate AJ, Batchelor TT. Mgmt methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology. 2009;73(18):1509–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 26. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reifenberger G, Hentschel B, Felsberg J, et al. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer. 2012;131(6):1342–1350. [DOI] [PubMed] [Google Scholar]
- 28. Perry JR, Callaghan CO, Ding K, et al. A phase III randomized controlled trial of short-course radiotherapy with or without concomitant and adjuvant temozolomide in elderly patients with glioblastoma (NCIC CTG CE.6, EORTC 26062–22061, TROG 08.02). J Clin Oncol. 2012;30(suppl; abstr TPS2104). [Google Scholar]
- 29. Gallego Perez-Larraya J, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29(22):3050–3055. [DOI] [PubMed] [Google Scholar]
- 30. Weller M, Van den Bent M, Hopkins K, et al. for the European Association for Neuro-Oncology (EANO) Task Force on Malignant Glioma. EANO Guideline on the Diagnosis and Treatment of Malignant Glioma. Lancet Oncol 2014;15:e395–e403. [DOI] [PubMed] [Google Scholar]
- 31. Karayan-Tapon L, Quillien V, Guilhot J, et al. Prognostic value of O6-methylguanine-DNA methyltransferase status in glioblastoma patients, assessed by five different methods. J Neurooncol. 2010;97(3):311–322. [DOI] [PubMed] [Google Scholar]
- 32. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nghiemphu PL, Liu W, Lee Y, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72(14):1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 36. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 38. Chinot OL, Macdonald DR, Abrey LE, Zahlmann G, Kerloeguen Y, Cloughesy TF. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep .2013;13(5):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sandmann T, Bourgon R, Garcia J, et al. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: Retrospective analysis of the AVAglio trial [published online ahead of print June 29, 2015]. J Clin Oncol. 10.1200/JCO.2015.61.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiler M, Blaes J, Pusch S, et al. The mTOR target NDRG1 confers MGMT-dependent resistance to alkylating chemotherapy. Proc Natl Acad Sci USA. 2014;111(1):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dahlin CM, Kelley JM, Jackson VA, Temel JS. Early palliative care for lung cancer: improving quality of life and increasing survival. Int J Palliat Nurs. 2010;16(9):420–423. [DOI] [PubMed] [Google Scholar]
- 42. Paszat L, Laperriere N, Groome P, Schulze K, Mackillop W, Holowaty E. A population-based study of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51(1):100–107. [DOI] [PubMed] [Google Scholar]
- 43. Rosenthal MA, Drummond KJ, Dally M, et al. Management of glioma in Victoria (1998–2000): retrospective cohort study. Med J Aust. 2006;184(6):270–273. [DOI] [PubMed] [Google Scholar]
- 44. Chang SM, Parney IF, Huang W, et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293(5):557–564. [DOI] [PubMed] [Google Scholar]
- 45. Kita D, Ciernik IF, Vaccarella S, et al. Age as a predictive factor in glioblastomas: population-based study. Neuroepidemiology. 2009;33(1):17–22. [DOI] [PubMed] [Google Scholar]
- 46. Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64(6):628–634. [DOI] [PubMed] [Google Scholar]
- 47. Coate L, McNamara MG, Lwin Z, et al. Glioblastoma treatment in the elderly in the temozolomide therapy era. Can J Neurol Sci. 2014;41(3):357–362. [DOI] [PubMed] [Google Scholar]
- 48. Tsang DS, Khan L, Perry JR, et al. Survival outcomes in elderly patients with glioblastoma. Clin Oncol (R Coll Radiol). 2015;27(3):176–183. [DOI] [PubMed] [Google Scholar]
- 49. Geler-Kulcu D, Gulsen G, Buyukbaba E, Ozkan D. Functional recovery of patients with brain tumor or acute stroke after rehabilitation: a comparative study. J Clin Neurosci. 2009;16(1):74–78. [DOI] [PubMed] [Google Scholar]
- 50. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386–1390. [DOI] [PubMed] [Google Scholar]
- 51. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327–335. [DOI] [PubMed] [Google Scholar]
- 52. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530–1534. [DOI] [PubMed] [Google Scholar]
- 53. Australian Cancer Network Adult Brain Tumour Guidelines Working Party. Clinical Practice Guidelines for the Management of Adult Gliomas: Astrocytomas and Oligodendrogliomas. Sydney, Australia: Cancer Council Australia, Australian Cancer Network and Clinical Oncological Society of Australia Inc.; 2009. [Google Scholar]
- 54. Schubart JR, Kinzie MB, Farace E. Caring for the brain tumor patient: family caregiver burden and unmet needs. Neuro Oncol. 2008;10(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Janda M, Steginga S, Langbecker D, Dunn J, Walker D, Eakin E. Quality of life among patients with a brain tumor and their carers. J Psychosom Res. 2007;63(6):617–623. [DOI] [PubMed] [Google Scholar]
- 56. Wick W, Weller M, van den Bent M, et al. MGMT testing in neurooncology - A paradigm for prospects and challenges of biomarker-based treatment decisions. Nat Rev Neurol. 2014;10:372–385. [DOI] [PubMed] [Google Scholar]