Abstract
Background
Personality changes following brain tumors may be due to disruption of frontal-subcortical networks. The relation between personality changes and tumor parameters such as volumes of the surgical cavity, residual tumor, or nonspecific white matter abnormalities is unknown. In this study we examined the relation between these tumor parameters and abnormal behaviors typically associated with frontal lobe dysfunction.
Methods
Thirty-one patients with intracranial tumors who completed the Frontal Systems Behavior Scale (FrSBe) during clinical neuropsychological assessment and had a solitary, well-delimited brain lesion on MRI within 3 months of that assessment were included. Tumor parameters were manually segmented using OsiriX. Nonparametric statistics were used to determine the relationship between tumor parameters and frontal behavioral dysfunction as measured by FrSBe scores.
Results
Patients reported significantly more behavior problems after tumor diagnosis. Tumor cavity volume was correlated with self-reported Executive Dysfunction (rho = 0.450, P = .047), and there was a trend in the relationship with self-reported Apathy (rho = 0.438, P = .053). Nonspecific white matter abnormality volume was also correlated with self-reported Apathy (rho = 0.810, P = .01). There were no correlations between FrSBe scores and residual tumor volume or summed volumes of tumor-related parameters.
Conclusion
Our results suggest that tumor parameters have differential effects on behaviors associated with frontal-subcortical networks and corroborate the high frequency of behavioral dysfunction in brain tumor patients. Examination of these relationships in a prospective trial is warranted to establish incidence, prevalence, risk factors, and consequences of behavioral disturbances in brain tumor patients.
Keywords: executive function, intracranial tumors, neurocognitive, quality of life
Primary malignant brain tumors account for about 1.4% of all newly diagnosed cancers, and in North America alone over 20 000 new cases are diagnosed every year.1 With advances in technology and more adequate treatments, the average life expectancy has significantly increased in the last few decades. Although most patients with malignant, grade 4 tumors still have a median survival of only 14.6 months,2 other patients with malignant brain tumors such as oligoastrocytomas have overall survival rates beyond 15 years.3–6 Brain tumor survivors have poorer quality of life compared with healthy controls and with cancer patients with a similar prognosis but without CNS involvement.7–11 Both patients and their caregivers report a decline in their quality of life compared with healthy controls in most evaluated domains including overall health-related quality of life, as well as physical, emotional, cognitive, and social functioning.7,8,11 Several factors have been implicated in the functional decline of patients with brain tumors, including impairment in cognitive, visual, and motor functioning.7,8,12
The role of brain tumors as the cause of cognitive decline may be difficult to establish in the absence of premorbid estimates of cognitive abilities.13,14 However, although radiotherapy is associated with neurocognitive sequelae, data from primary brain tumor patients seen prior to radiation suggest that the tumor itself and its progression have the most deleterious effect on cognitive function.15–17 Patients with larger lesions score significantly worse on tasks assessing verbal and visual memory, verbal fluency, shifting, and visuospatial skills.12 In addition, widespread edema has been associated with higher rates of impairment.12
Little is known about the impact of a brain tumor on personality and behavior. Behavioral changes significantly impact social functioning, quality of life, and caregiver burden in other populations with brain injuries affecting the frontal lobes. For example, patients with traumatic brain injury with few overt limitations still experience significantly reduced community integration and goal-directed behavior, diminished motivation, and lack of dynamism compared with population controls.18–20 Since Phineas Gage, it is well known that damage to the frontal lobe leads to change in personality and behavior.21–24 However, it was not until the 1980s that the intricate neuroanatomic substrate of frontal-lobe-related behaviors was delineated in detail25 describing three frontal circuits responsible for human behavior: the dorsolateral prefrontal circuit associated with executive control, the orbitofrontal circuit associated with self-regulation, and the anterior cingulate circuit associated with motivated behavior. Disruption to these regions is associated with frontal behavioral syndromes characterized by executive dysfunction, disinhibition, or apathy.21,22,26 With the advent of novel imaging techniques, in particular resting-state fMRI, support for these original circuits and a better understanding of their functions has become possible.27,28
Several scales have been designed to rate behavioral changes in patients with frontal dysfunction.29–31 The Frontal Systems Behavior Scale (FrSBe) has been used in large numbers of patients with neurological diseases including Alzheimer′s disease, Parkinson′s disease, and schizophrenia.32,33 It is brief, reliable, and valid for assessing behavior related to frontal lobe circuits29,31 and has been shown to predict community integration (home integration, social integration, and productivity) in patients with frontal lobe dysfunction.20
Few studies have investigated the incidence and impact of behavioral syndromes in brain tumor patients.14,30,34 One recent publication reported clinically significant levels of apathy, disinhibition, and executive dysfunction in about 40% to 60% of brain tumor patients in their sample. A significant proportion of them experienced behavioral syndromes regardless of tumor location.14 However, there has been little work done to explore the relationship between behavioral syndromes and other tumor-related features, such as volumes of the postsurgical cavity, residual tumor, or nonspecific white matter abnormalities. In this study we evaluated frontal behavioral syndromes among patients with primary brain tumors using the FrSBe and explored the relationship between these behaviors and tumor-related and treatment-related variables.
Materials and Methods
We conducted a retrospective chart review of patients with intracranial tumors who completed the FrSBe as part of comprehensive clinical neuropsychological assessments between 2004 and 2012 at the Princess Margaret Cancer Centre. Eighty-six patients were identified with FrSBe scores; 31 of them had a brain MRI scan within 3 months of the neuropsychological evaluation (ie, either before or after), a solitary, well-delimited brain lesion, and a confirmed diagnosis of malignant glioma (n = 28) or meningioma (n = 3).
Frontal behaviors were identified from patient and informant responses to the FrSBe, a 46-item standardized questionnaire that asks respondents to rate behavior problems on a 5-point Likert scale, before diagnosis (retrospective rating; respondents were asked to think about how they were before they started having symptoms of their disease) and at the time of assessment. It provides composite and 3 subscale scores for Apathy, Disinhibition, and Executive Dysfunction, both before and after diagnosis. We also analyzed performance on tests of executive function (Trailmaking Test, Part B; Wisconsin Card Sorting Test, number of errors) obtained during the neuropsychological assessment to compare objective measures of executive functions with subjective concerns reported in the FrSBe.
To explore the relationship between problem behaviors and different features of the tumor and its treatment, we obtained medical information about diagnosis (tumor type, location, grade) and treatment (antiseizure medication, surgery, radiation therapy, elapsed time after radiation therapy) via chart review. Tumor-related parameters (volumes of tumor cavity, residual tumor, and nonspecific white matter abnormalities) were obtained via volumetric analyses of MRI scans. Specifically, we used OsiriX (Pixmeo, Bernex, Switzerland),35 an image navigation and display software package designed for navigation and visualization of multimodal and multidimensional images36 to view and manually segment the brain lesions into tumor-related MRI parameters that included tumor cavity, residual tumor, and nonspecific white matter abnormalities in order to calculate their volumes. Tumor cavity was defined as a T1/FLAIR hypointense image in the area where the surgery was done. Residual tumor was defined as an enhanced or nonenhanced T1 hypointense, T2/FLAIR hyperintense homogeneous or heterogeneous lesion. Residual white matter abnormality was defined as a diffuse, nonenhanced T1 isointense or hypointense, T2/FLAIR hyperintense lesion that spared subcortical U-fibers. This work was conducted in accordance with the guidelines of the University Health Network's research ethics board.
Statistical Analyses
FrSBe raw scores were converted to T-scores (mean 50, standard deviation 10) for each domain according to published normative data.31 T-scores equal or greater than 65 are clinically significant and scores between 60 and 64 are borderline impaired. Performance measures were scored according to published criteria37–41 and scaled scores transformed to Z-scores (mean 0, standard deviation 1), and averaged to create an executive function performance score.
Comparisons between retrospective ratings of behavioral syndromes before diagnosis to those at the time of the assessment were conducted separately for patients and informants using Wilcoxon signed rank tests, and effect sizes were calculated using Cohen's D.
Correlations between self-report and informant ratings on the FrSBe, executive function performance, and MRI tumor-related characteristics were made using Spearman's rho. The Mann-Whitney U test was used to calculate the relation between other tumor and treatment variables (hemisphere [right/left], grade [high/low], radiation treatment [yes/no], antiseizure medications [yes/no]) and FrSBe scores.
Results
Demographics
Clinical and demographic details for 31 study patients are presented in Table 1. There were more men than women in our group (χ2 = 4.65, P = .03). There were no differences in tumor grade (high vs low grade; χ2 = 0.03, P = .85) or hemisphere (left vs right; χ2 = 2.13, P = .14) in our sample. Tumors were located in the frontal lobe in 24 (77%) patients and the dorsolateral prefrontal region was affected in all of those cases. Orbitofrontal and anterior cingulate cortices were affected in 4 and 2 of those patients, respectively. Among the 7 patients with nonfrontal tumors, 4 were located in the right temporal lobe, 1 in the left temporal lobe, and 2 in the right cerebellar hemisphere. There were no patients with tumors located in parietal or occipital lobes. In terms of treatment, all patients underwent surgical resection of the tumor, and there was no difference in the proportion of patients who had radiation compared with those who did not (61% vs. 39% respectively; χ2 = 1.6, P = .2). The proportion of patients who were on anti-seizure medication was greater than that of patients who were not (67% vs 33% respectively; χ2 = 3.9, P = .05).
Table 1.
Participant demographic and medical information
| N | % | |
|---|---|---|
| Sex | ||
| Male | 22 | 71 |
| Female | 9 | 29 |
| Tumor type | ||
| Meningioma | 3 | 9.7 |
| Low-grade glioma | 13 | 42 |
| High-grade glioma | 15 | 48.3 |
| Hemisphere | ||
| Left | 19 | 61.3 |
| Right | 11 | 35.5 |
| Both | 1 | 3.2 |
| Tumor location | ||
| Frontal | 24 | 77 |
| Nonfrontal | 7 | 23 |
| Treatment | ||
| Surgery | 31 | 100 |
| Radiation | 19 | 61.3 |
| Chemotherapy | 9 | 29 |
| Antiseizure medication | 21 | 67.7 |
| Median | IQR | |
| Age and time (yrs) | ||
| Age at assessment | 45 | 14 |
| Age at diagnosis | 43 | 12 |
| Time since diagnosis | .92 | 3 |
Abbreviation: IQR, interquartile range.
Frontal Behavioral Syndromes
Mean FrSBe subscale scores are provided in Table 2. Patient and informant retrospective ratings of behavior problems prior to the brain tumor diagnosis were not clinically significant (Table 2). In contrast, 78% (n = 24) of the patients endorsed significant problems with at least one frontal behavioral syndrome after diagnosis. Clinically significant levels of Apathy were reported by 48.8% (n = 15) of patients and 61.1% (n = 11) of informants; Disinhibition was reported by 48.8% (n = 15) of patients and 15.8% (n = 3) of informants, and Executive Dysfunction reported by 61.3% (n = 19) of patients and 53% (n = 10) of informants. It is interesting to note that frontal behavior syndromes were frequently reported by patients and informants in cases with nonfrontal tumors (Table 2).
Table 2.
Self-reported and informant-rated Apathy, Disinhibition, and Executive Dysfunction before diagnosis and at the time of the assessment
| Median | IQR | Frequency impaired |
|||
|---|---|---|---|---|---|
| Frontal (n = 24) | Nonfrontal (n = 7) | ||||
| Apathy | |||||
| Before diagnosis | Self-report | 51 | 17 | 3 (12.5) | 1 (14.3) |
| Informant-rated | 50 | 15 | 2 (8.3) | 0 (0) | |
| At assessment | Self-reporta | 62 | 28 | 11 (45.8) | 4 (57.1) |
| Informant-rateda | 72 | 33 | 8 (33.3) | 3 (42.9) | |
| Disinhibition | |||||
| Before diagnosis | Self-report | 53 | 21 | 2 (8.3) | 3 (42.9) |
| Informant-rated | 48 | 19 | 1 (4.2) | 0 (0) | |
| At assessment | Self-reporta | 64 | 25 | 10 (41.7) | 5 (71.4) |
| Informant-rated | 56 | 16 | 2 (8.3) | 1 (14.3) | |
| Executive Dysfunction | |||||
| Before diagnosis | Self-report | 53 | 24 | 7 (29.1) | 2 (28.6) |
| Informant-rated | 50 | 18 | 3 (12.5) | 1 (14.3) | |
| At assessment | Self-reporta | 68 | 23 | 14 (58.3) | 5 (71.4) |
| Informant-rateda | 66 | 21 | 8 (33.3) | 2 (28.6) | |
Data are median T-scores that are age-adjusted and sex-adjusted according to published norms. Frequency impaired scores are the number of patients (% in parentheses) with frontal or nonfrontal tumors whose scores are clinically significant (ie T > 65).
Abbreviation: IQR, interquartile range.
aSignificant change in behavior compared with rating before diagnosis (P < .05).
Patients reported having significantly more behavior problems at the time of assessment than before diagnosis (Apathy, Wilcoxon Z = − 2.7, P = .01, Cohen's D = 0.50; Disinhibition, Z = − 2.0, P = .04, D = 0.38; Executive Dysfunction, Z = − 2.4, P = .02, D = 0.45). Similarly, informants reported increased Apathy (Z = − 2.7, P = .01, D = .64) and Executive Dysfunction (−2.7, P = .01, D = 0.64) at the time of assessment compared with before diagnosis, but no change in Disinhibition (Z = − 1.5, P = .1, D = 0.35). There was no difference in the pattern of results when we analyzed our data excluding the 7 patients who did not have frontal lobe tumors (data not shown).
Patient and informant ratings were positively correlated for Disinhibition (before diagnosis: Spearman's rho = 0.61, P = .01; time of assessment: rho = 0.68, P = .002), Executive Dysfunction (before: rho = 0.56, P = .02; time of assessment: rho = 0.62, P = .01), and Apathy (time of assessment: rho = 0.61, P = .01). There was no correlation between patient and informant retrospective ratings of Apathy before diagnosis (P > .1). There was a significant correlation between the executive function performance score and FrSBe symptoms of Apathy (self-rated: rho = − 0.417, P = .02; informant-rated: rho = − 0.507, P = .03) and Executive Dysfunction (self-rated: rho = − 0.391, P = .033).
Tumor and Treatment Effects and Frontal Behavioral Syndromes
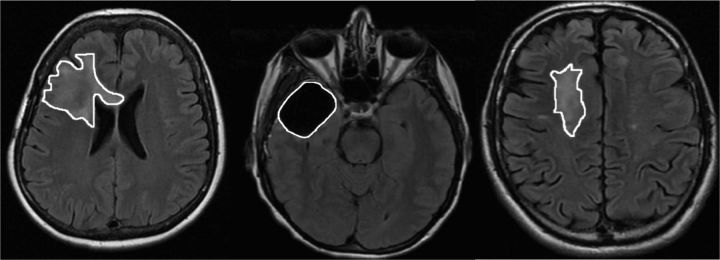
In our sample, tumor cavity and residual tumor were evident in 20 patients. Nonspecific white matter abnormalities were evident in only 8 of our patients. Examples of these tumor parameters are provided in Fig. 1. Tumor cavity volume was positively correlated with self-reported Executive Dysfunction at the time of the assessment (rho = 0.450, P = .047), and there was a trend in the relationship with self-reported Apathy at the time of the assessment (rho = 0.438, P = .053). Nonspecific white matter abnormality volume was also correlated with self-reported Apathy at the time of the assessment (rho = 0.810, P = .01), although this finding should be interpreted with caution because of the small number of patients with these abnormalities. There were no correlations between FrSBe scores and residual tumor volume or summed volumes of tumor-related parameters (tumor cavity, residual tumor, and nonspecific white matter unspecific abnormality volumes).
Fig. 1.
Example of residual tumor (left), tumor cavity (center), and white matter abnormality (right).
We found no significant differences between FrSBe subscale scores and other tumor characteristics (ie, tumor grade or hemisphere; all P values > .1), or treatment variables (ie, whether or not patients received radiation or were on antiseizure medications, all P values > .1; Table 3).
Table 3.
Behavioral syndromes in relation to tumor and treatment factors
| FrSBe Subscale | Tumor grade |
Hemisphere |
Antiepileptics |
Radiotherapy |
||||
|---|---|---|---|---|---|---|---|---|
| High | Low | Right | Left | Yes | No | Yes | No | |
| Apathy | ||||||||
| Self-report | 71 (40) | 62 (26) | 66 (28) | 62 (25) | 62 (35) | 65 (23) | 62 (25) | 76 (37) |
| Informant-rated | 75 (23) | 51 (31) | 77 (45) | 68 (28) | 73 (39) | 68 (33) | 60 (39) | 74 (23) |
| Disinhibition | ||||||||
| Self-report | 53 (19) | 68 (29) | 57 (28) | 66 (21) | 31 (23) | 68 (26) | 63 (21) | 67 (30) |
| Informant-rated | 56 (20) | 56 (23) | 57 (15) | 56 (27) | 55 (16) | 59 (51) | 56 (22) | 54 (13) |
| Executive Dysfunction | ||||||||
| Self-report | 70 (25) | 65 (26) | 68 (24) | 68 (25) | 69 (22) | 61 (23) | 68 (15) | 69 (37) |
| Informant-rated | 62 (17) | 71 (28) | 66 (16) | 67 (28) | 66 (18) | 56 (33) | 62 (26) | 67 (18) |
Data are median FrSBe subscale scores, interquartile range in parentheses.
No effect of tumor or treatment variables on FrSBe scores; all P values > .1
Discussion
We found that patients with primary brain tumors and their caregivers reported significant behavioral problems typically associated with frontal lobe dysfunction after the diagnosis of the tumor. Retrospective ratings of these behaviors prior to diagnosis were within the normal range for most of our sample suggesting that the perceived decline in these behaviors was a consequence of the tumor or its treatment. These results are in keeping with those of Gregg et al, who reported a similar frequency of frontal behavioral syndromes after a brain tumor diagnosis.14 In that study, they found significant behavioral syndromes regardless of tumor location, although these syndromes occurred with a higher frequency among patients with frontal lobe lesions than among patients with tumors in other brain regions. Our data appear to be consistent with those findings, although the small number of patients with nonfrontal lesions in our sample limits our ability to explore this systematically. Patients with nonfrontal tumors may develop these behavioral changes because parietal and temporal lobes are important afferents to frontal behavioral circuits.21 Lesions in those regions may result in blood flow reduction and dysregulation of the monoaminergic system in the frontal cortex.42,43 The right temporal lobe in particular is highly interconnected with other structures involved in behavior regulation, including orbito-frontal cortex, and is associated with emotional processing of auditory and olfactory stimuli and social cognition.44
Interestingly, although most of our patients had lesions involving dorsolateral prefrontal cortex (74%), clinically significant levels of Apathy and Disinhibition were observed in addition to Executive Dysfunction in our sample. This is concordant with evidence of broad afferent and efferent interconnections between the circuits involved in the control of these behaviors both within the frontal lobes and between frontal-subcortical regions including basal ganglia and temporal, parietal, and occipital lobes.44–46 This may explain the widespread cortical dysfunction commonly seen among patients with brain tumors regardless of tumor location.42
Our study revealed that postsurgical tumor cavity volume and white matter abnormalities correlated positively with behavioral problems. Tumor cavity size reflects loss of both cortex and axons, encompassing both focal cortical loss and the disruption of interconnected networks, resulting in more widespread disturbance and more extensive cognitive and behavioral deficits than what might be expected from a focal lesion.47,48 Similarly, T2/FLAIR residual, nonspecific white matter abnormalities may correspond to late-delayed leucoencephalopathy, microangiopathic changes, or gliosis that have been associated with cognitive or behavioral dysfunction.49–51 These lesions are related to demyelination and/or axonal loss with a subsequent dysfunction of the involved network.15 White matter integrity is critical for cognitive functioning, and changes to white matter microstructure seen with more sophisticated imaging such as diffusion tensor imaging, but not evident on routine clinical scans, are associated with cognitive deficits in people with traumatic brain injuries52 and brain tumors.53,54 Tumor location, size, and edema have been reported to have a significant impact on cognitive functioning12,13 and so it is not unexpected to find that some of these factors could also impact behavior. In contrast, some tumors may infiltrate brain parenchyma without causing death of the neurons and axons55,56 with some functions remaining relatively spared.57,58
We found no relationship between frontal behavioral syndromes, as measured by the FrSBe, and other variables associated with the tumor and its treatment including tumor laterality, type, WHO grade, antiseizure medication, surgery, radiation therapy, and elapsed time after radiation therapy. The heterogeneity of our sample, small sample size, and retrospective design may have precluded a proper assessment of these factors. The retrospective component of the FrSBe may not provide an accurate measure of the patient's behavior prior to diagnosis due to issues related to recall bias. Nonetheless, premorbid ratings are sensitive to behavior change when compared with current behavior in patients with focal frontal lesions, and as such provide a useful indicator of the impact of the tumor on current functioning.30,59 Another limitation of this study was the challenge of accurately distinguishing residual, nonenhancing tumor from nonspecific white matter abnormality. Notwithstanding these limitations, to our knowledge, the relation between behavioral syndromes and tumor-related MRI characteristics in adult brain tumor patients has not been previously explored. Our results suggest that a prospective study with a larger sample is warranted to clarify the relations between tumor parameters and behavioral changes. Those relations could have implications for treatment, particularly for patients with low-grade tumors, for whom it is considered acceptable to defer surgery until evidence of clinical or significant radiological progression.60–63 Understanding the repercussions of surgery on cognition and behavior will provide much-needed education for physicians, patients, and families to inform treatment decision making.
In summary, in addition to focal neurologic deficits and cognitive comorbidities that have been reported among patients with brain tumors,7,13,16 our study corroborates the high frequency of frontal behavioral dysfunction in this population and suggests that certain factors may play a more important role in their development. These findings must be corroborated in prospective studies with a larger sample to definitively establish the incidence and prevalence of frontal behavioral syndromes in brain tumor patients, as well as the factors associated with frontal dysfunction. Better appreciation of behavioral changes in brain tumor patients will allow us to understand the role they play in caregiver burden, patient community integration, and patient quality of life. The FrSBe is a relatively brief scale with good factor structure and construct validity hence it is a useful instrument that may be used in clinical research to assess changes in behavior and the three frontal behavioral syndromes separately30,32,64 so that individualized interventions based on abnormal behavior profiles might be implemented. Finally, understanding the relationship between behavioral symptoms and tumor-related MRI parameters may have implications for treatment. Behavioral symptoms are important deficits in brain tumor patients so they need to be considered as end points in clinical trials, in addition to enriching the information gathered in routine neuropsychological assessment.
Funding
This work was supported in part by the Kirchmann Family Chair in Neuro-Oncology and the Ontario Ministry of Health and Long-Term Care (OMOHLTC).
Acknowledgments
This work was supported in part by the Kirchmann Family Chair in Neuro-Oncology and the Ontario Ministry of Health and Long-Term Care. The views expressed do not necessarily reflect those of the OMOHLTC.
Conflict of interest statement. None declared.
References
- 1. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. National Cancer Institute; 2014. http://seer.cancer.gov/statfacts/html/brain.html Accessed April 2014. [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Li J, Wang M, Won M, et al. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(3):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curran WJ, Jr., Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704–710. [DOI] [PubMed] [Google Scholar]
- 5. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000;54(7):1442–1448. [DOI] [PubMed] [Google Scholar]
- 7. Klein M, Taphoorn MJ, Heimans JJ, et al. Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J Clin Oncol. 2001;19(20):4037–4047. [DOI] [PubMed] [Google Scholar]
- 8. Boele FW, Heimans JJ, Aaronson NK, et al. Health-related quality of life of significant others of patients with malignant CNS versus non-CNS tumors: a comparative study. J Neurooncol. 2013;115(1):87–94. [DOI] [PubMed] [Google Scholar]
- 9. Perez-Campdepadros M, Castellano-Tejedor C, Sabado-Alvarez C, Gros-Subias L, Capdevila L, Blasco-Blasco T. Type of tumour, gender and time since diagnosis affect differently health-related quality of life in adolescent survivors. Eur J Cancer Care. 2015;24(5):635–641. [DOI] [PubMed] [Google Scholar]
- 10. Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade glioma. J Neurol Neurosurg Psychiatry. 2005;76(4):562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taphoorn MJ, Stupp R, Coens C, et al. Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol. 2005;6(12):937–944. [DOI] [PubMed] [Google Scholar]
- 12. Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324–333; discussion 333–324. [DOI] [PubMed] [Google Scholar]
- 13. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 14. Gregg N, Arber A, Ashkan K, et al. Neurobehavioural changes in patients following brain tumour: patients and relatives perspective. Support Care Cancer. 2014;22(11):2965–2972. [DOI] [PubMed] [Google Scholar]
- 15. Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. [DOI] [PubMed] [Google Scholar]
- 16. Brown PD, Jensen AW, Felten SJ, et al. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24(34):5427–5433. [DOI] [PubMed] [Google Scholar]
- 17. Noll KR, Sullaway C, Ziu M, Weinberg JS, Wefel JS. Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro Oncol. 2015;17(4):580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35(12):1731–1741. [DOI] [PubMed] [Google Scholar]
- 19. Cattelani R, Roberti R, Lombardi F. Adverse effects of apathy and neurobehavioral deficits on the community integration of traumatic brain injury subjects. Eur J Phys Rehabil Med. 2008;44(3):245–251. [PubMed] [Google Scholar]
- 20. Reid-Arndt SA, Nehl C, Hinkebein J. The Frontal Systems Behaviour Scale (FrSBe) as a predictor of community integration following a traumatic brain injury. Brain Inj. 2007;21(13–14):1361–1369. [DOI] [PubMed] [Google Scholar]
- 21. Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci. 1994;6(4):358–370. [DOI] [PubMed] [Google Scholar]
- 22. Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50(8):873–880. [DOI] [PubMed] [Google Scholar]
- 23. Harlow JM. Passage of an iron rod through the head. Boston Med Surg J. 1848;39(20):389–393. [Google Scholar]
- 24. Harlow JM. Recovery from the passage of an iron bar through the head. Bull Mass Med Soc. 1868;2:327–347. [Google Scholar]
- 25. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 26. Masterman DL, Cummings JL. Frontal-subcortical circuits: the anatomic basis of executive, social and motivated behaviors. J Psychopharmacol. 1997;11(2):107–114. [DOI] [PubMed] [Google Scholar]
- 27. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. [DOI] [PubMed] [Google Scholar]
- 28. Sporns O. From simple graphs to the connectome: networks in neuroimaging. Neuroimage. 2012;62(2):881–886. [DOI] [PubMed] [Google Scholar]
- 29. Malloy P, Grace J. A review of rating scales for measuring behavior change due to frontal systems damage. Cogn Behav Neurol. 2005;18(1):18–27. [DOI] [PubMed] [Google Scholar]
- 30. Grace J, Stout JC, Malloy PF. Assessing frontal lobe behavioral syndromes with the frontal lobe personality scale. Assessment. 1999;6(3):269–284. [DOI] [PubMed] [Google Scholar]
- 31. Grace J, Malloy P. The Frontal Systems Behavior Scale: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc.; 2001. [Google Scholar]
- 32. Stout JC, Ready RE, Grace J, Malloy PF, Paulsen JS. Factor analysis of the frontal systems behavior scale (FrSBe). Assessment. 2003;10(1):79–85. [DOI] [PubMed] [Google Scholar]
- 33. Velligan DI, Ritch JL, Sui D, DiCocco M, Huntzinger CD. Frontal Systems Behavior Scale in schizophrenia: relationships with psychiatric symptomatology, cognition and adaptive function. Psychiatry Res. 2002;113(3):227–236. [DOI] [PubMed] [Google Scholar]
- 34. Andrewes DG, Kaye A, Murphy M, et al. Emotional and social dysfunction in patients following surgical treatment for brain tumour. J Clin Neurosci. 2003;10(4):428–433. [DOI] [PubMed] [Google Scholar]
- 35. About OsiriX. OsiriX Web site. http://www.osirix-viewer.com/AboutOsiriX.html. Accessed October 15, 2015.
- 36. Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17(3):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. [DOI] [PubMed] [Google Scholar]
- 38. Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14(2):167–177. [PubMed] [Google Scholar]
- 39. Wechsler D. Wechsler Adult Intelligence Scale - Third Edition (WAIS-III) Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 40. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 41. Heaton R, Chelune G, Talley J, Kay G, Curtiss G. Wisconsin Card Sorting Test Manual—Revised and Expanded. Odessa, FL: Psychological Assessment Resources, Inc.; 1993. [Google Scholar]
- 42. Lilja Å, Skagerberg G, Salford LG. Frontal lobe dysfunction in patients with non-frontal malignant gliomas: a monoaminergic dysregulation? Med Hypotheses. 1999;53(3):190–193. [DOI] [PubMed] [Google Scholar]
- 43. Lilja Å, Hagstadius S, Risberg J, Salford LG, Smith GJW, Ohman R. Frontal lobe dynamics in brain tumor patients: a study of regional cerebral blood flow and affective changes before and after surgery. Neuropsychiatry Neuropsychol Behav Neurol. 1992;5(4):294–300. [Google Scholar]
- 44. Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(Pt 7):1718–1731. [DOI] [PubMed] [Google Scholar]
- 45. Best JR, Miller PH, Jones LL. Executive functions after age 5: changes and correlates. Dev Rev. 2009;29(3):180–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann N Y Acad Sci. 1995;769:1–13. [DOI] [PubMed] [Google Scholar]
- 47. Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. [DOI] [PubMed] [Google Scholar]
- 48. Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128(Pt 11):2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11(3):157–165. [DOI] [PubMed] [Google Scholar]
- 50. Postma TJ, Klein M, Verstappen CC, et al. Radiotherapy-induced cerebral abnormalities in patients with low-grade glioma. Neurology. 2002;59(1):121–123. [DOI] [PubMed] [Google Scholar]
- 51. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 52. Astafiev SV, Shulman GL, Metcalf NV, et al. Abnormal white matter BOLD signals in chronic mild traumatic brain injury. J Neurotrauma. 2015;32(16):1254–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brinkman TM, Reddick WE, Luxton J, et al. Cerebral white matter integrity and executive function in adult survivors of childhood medulloblastoma. Neuro Oncol. 2012;14(suppl 4):iv25–iv36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mabbott DJ, Noseworthy MD, Bouffet E, Rockel C, Laughlin S. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro Oncol. 2006;8(3):244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Duffau H, Capelle L, Denvil D, et al. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J Neurol Neurosurg Psychiatry. 2003;74(7):901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duffau H, Denvil D, Capelle L. Long term reshaping of language, sensory, and motor maps after glioma resection: a new parameter to integrate in the surgical strategy. J Neurol Neurosurg Psychiatry. 2002;72(4):511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ojemann JG, Miller JW, Silbergeld DL. Preserved function in brain invaded by tumor. Neurosurgery. 1996;39(2):253–258; discussion 258–259. [DOI] [PubMed] [Google Scholar]
- 58. Skirboll SS, Ojemann GA, Berger MS, Lettich E, Winn HR. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38(4):678–684; discussion 684–675. [PubMed] [Google Scholar]
- 59. Malloy P, Tremont G, Grace J, Frakey L. The Frontal Systems Behavior Scale discriminates frontotemporal dementia from Alzheimer's disease. Alzheimers Dement. 2007;3(3):200–203. [DOI] [PubMed] [Google Scholar]
- 60. Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95(5):735–745. [DOI] [PubMed] [Google Scholar]
- 61. Recht LD, Lew R, Smith TW. Suspected low-grade glioma: is deferring treatment safe? Ann Neurol. 1992;31(4):431–436. [DOI] [PubMed] [Google Scholar]
- 62. van Veelen ML, Avezaat CJ, Kros JM, van Putten W, Vecht C. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery. J Neurol Neurosurg Psychiatry. 1998;64(5):581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 64. Carvalho JO, Ready RE, Malloy P, Grace J. Confirmatory factor analysis of the Frontal Systems Behavior Scale (FrSBe). Assessment. 2013;20(5):632–641. [DOI] [PubMed] [Google Scholar]