Abstract
Background
Long-term cancer treatment complications become more prevalent as survival improves. Little is known about the psychological complications in long-term survivors of head and neck cancer (HNC). We investigated cognitive functioning and its relation with depression, fatigue, cognitive complaints, and brain lesions on MRI.
Methods
This study is part of a multicentre, prospective cohort study of 65 patients treated for HNC. A comprehensive neuropsychological assessment was combined with validated questionnaires on subjective memory complaints, depression, and fatigue after a median of 7 years follow-up. Results were compared with age- and education-adjusted normative data. Further, we evaluated cerebral white matter hyperintensities (WMH), brain volume, and infarctions on MRI.
Results
HNC patients had worse cognitive performance in two of the five assessed cognitive domains: episodic memory (z = −0.48, P = .003) and speed of information processing (z = −0.47, P < 0.001). Patients with fatigue performed worse than patients without fatigue on verbal fluency (mean difference in z-score 0.52, P = .02) and speed of information processing (0.49, P = .04). Patients with subjective memory complaints had a worse episodic memory performance (mean difference in z-score −0.96; P = .02). Patients with cerebral infarction(s) on MRI performed worse on fluency (mean difference in z-score 0.74, P = .005). A lower cognitive performance was not associated with depression, WMH or brain volume.
Conclusion
Long-term HNC survivors showed worse cognitive functioning 7 years after treatment. Cognitive function was associated with subjective complaints and fatigue, but not with depressive symptoms. Cerebral infarctions on MRI were correlated with cognitive function, whereas WMH, and brain volume were not.
Keywords: cognition, depression, fatigue, head and neck cancer, white matter hyperintensities
An increase in successful treatment for cancer has resulted in a growing population of long-term survivors. A consequence is a parallel increase in long-term, treatment-related complications. A well-known, long-term complication of radiotherapy (RT) of the neck is carotid artery vasculopathy, which ultimately can lead to stroke.1,2 In the current study cohort, we previously showed a significant increase in carotid wall pathology and stroke risk in the first seven years after RT.3 Apart from this known, direct radiation-related damage, limited clinical data exist on long-term cognitive function, mood, fatigue, and quality of life (QoL).
In the general population vascular white matter hyperintensities (WMH) and silent brain infarctions are related to cognitive impairment, depression, and risk of dementia.4–6 Small prospective studies showed evidence of cognitive failure in HNC patients within the first two years after RT.7,8 A high rate of depression with preserved QoL was observed in 5-year survivors of HNC.9 To date, no long-term prospective studies assessing the relation between cognitive functioning, depression and fatigue exist in these patients. The aim of the current study was to evaluate cognitive functioning, depression, fatigue, QoL and their interrelation 7 years after treatment with RT in HNC patients. Furthermore, we assessed WMH, atrophy and infarctions on brain MRI. We hypothesized a multifactorial etiology of cognitive problems in long-term HNC survivors, consisting of RT-induced carotid vasculopathy, fatigue or depression, and vascular changes of the brain, such as cerebral infarction.
Materials and Methods
This study was a part of a multicentre, prospective cohort study designed to investigate the long-term vascular and cerebral complications after RT of the neck. Details of this study and the treatment protocols have been described elsewhere.10
In short, patients were prospectively recruited 7 years ago in two centers of the Netherlands (the Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital (NCI/AvL), Amsterdam and the Radboud University Medical Center, Nijmegen). Inclusion criterion was RT of the neck because of a HNC, which included carcinoma of the larynx, parotid, nasopharynx, oropharynx, hypopharynx, pleiomorphic adenoma of the parotid, and lymphoma (Table 1). Patients were assessed for cerebrovascular risk factors at baseline; patients with a history of cerebrovascular disease were excluded. At follow-up 7 years after RT all patients underwent a standardized neuropsychological assessment and fulfilled self-reported questionnaires about cognitive complaints, depression, fatigue, and quality of life (QoL). The Medical Ethics Review Committee approved the study (NL 41008.091.12). All patients gave written informed consent.
Table 1.
Patient and treatment-related characteristics at baseline
| Characteristic | n = 65 |
|---|---|
| Demographics | |
| Men, % | 60 |
| Mean age at baseline, years (SD) | 54.3 (13.3) |
| Mean follow-up post RT, years (SD) | 6.7 (1.2) |
| Radiotherapy | |
| Dose on irradiated carotid arteries, Gy (mean, min-max, [SD]) | |
| CCA | 58, 30–70, (12) |
| ICA | 61, 30–70, (12) |
| Bilateral, % | 63 |
| Cancer diagnosis | |
| Carcinoma of larynx, % | 45 |
| Carcinoma of parotid, % | 14 |
| Pleiomorphic adenoma of parotid, % | 15 |
| Carcinoma of nasopharynx, % | 2 |
| Carcinoma of oropharynx, % | 17 |
| Carcinoma of hypopharynx, % | 2 |
| Lymphoma, % | 6 |
| Number CV risk factors at baselineb (%) | |
| 0 | 32 |
| 1 | 43 |
| ≥2 | 25 |
| Education levela at follow-up, mean (SD) | 5 (1.4) |
| Marital status at follow-up | |
| Married, % | 66 |
| Widowed, % | 9 |
| Divorced, % | 8 |
| Never married, % | 17 |
| Employment status at follow-up | |
| Working, % | 42 |
| Full or partially unemployed, % | 6 |
| Retired, % | 44 |
| Other, % | 8 |
aEducation level is classified using seven categories; 1 being less than primary school and 7 reflecting an academic degree. Educational level 5 is comparable to 10–11 years of education.
bCerebrovascular (CV) risk factors at baseline consist of: hypertension, diabetes, hypercholesterolemia, overweight and current smoking.
Abbreviations: RT, radiotherapy; CCA, common carotid artery; ICA, internal carotid artery.
Radiation therapy
Radiotherapy was usually given with a linear accelerator using 4–6 MV photons with the patient immobilized using a thermoplastic mask. The target area included at least the unilateral neck consistent to the tumor side, including part of the carotid artery system (e.g. in cases of parotid tumors or well-lateralized oropharyngeal carcinomas). In other patients, both sides of the neck and the vascular system were irradiated (e.g. in cases of laryngeal or hypopharyngeal carcinomas).
The radiation treatment was delivered with external beam radiotherapy using either standard techniques (parallel opposing beams or wedge-pair techniques) or intensity-modulated radiotherapy, depending on resources.
The radiation dose given was typically 30–36 Gy for lymphoma, 50–60 Gy for parotid tumors (pleiomorphic adenoma or parotid carcinoma), 60–70 Gy for laryngeal carcinoma, and 70 Gy for oropharyngeal and hypopharyngeal carcinoma. In the majority of cases, 2 Gy per fraction was delivered up to the specified total dose. For patients with lymphoma, parotid tumors, or T1N0 oropharyngeal carcinoma, a standard once-daily fractionation schedule was used. Accelerated fractionation was used for patients with T2 or N1 oropharyngeal carcinomas, hypopharyngeal carcinomas, and larynx carcinoma beyond stage T1N0. In patients with T1N0 glottic laryngeal carcinomas the fractionation schedule was 60 Gy in 25 fractions over 5 weeks to the larynx only, using 2 lateral opposing fields. In few cases with large nodal metastases in the upper neck, the base of the posterior cranial fossa received some radiation dose, but always <30 Gy. In the Netherlands standard treatment protocols of early stage HNC patients do not include chemotherapy or targeted therapy.
Neuropsychological assessment
Neuropsychological assessments were administered between November 2012 and October 2013 by a trained neuropsychologist. Education level was classified using 7 categories based on the Dutch educational system; 1 being less than primary school and 7 reflecting an academic degree.11 The Mini Mental State Examination (MMSE)12 and the Frontal Assessment Battery (FAB)13 were used as descriptive tools of overall cognitive function and orientation. An extensive, objective test battery covered 5 cognitive domains: (i) Episodic memory was assessed using the 5-trial Dutch version of the Rey Auditory Verbal Learning Test (RAVLT), in which the ability to acquire and retain new verbal information was measured, immediately and after a delay.14 The total number of correctly recalled words over trials 1 to 5 was used as measure for immediate memory; total number of words recalled after a delay was used as delayed recall measure. (ii) Working memory was assessed by using the Digit Span test,15 both forwards and backwards. (iii) Executive functioning was assessed using the Dutch version of the Stroop color-word task. This task consists of three cards: Card I – the word card– which contains color words in black ink that have to be read, Card II – the color card – which consists of colored patches, the colors have to be named, and Card III – the color-word card – which contains color names printed in incongruent colors that have to be named (i.e. the word “yellow” printed in red). The Stroop interference score was used here as a measure of response inhibition (i.e. number of seconds needed to complete Card III/ mean number of seconds needed to complete Cards I and II)16; and the Brixton Spatial Anticipation Test that measures rule detection and set shifting.17,18 (iv) Verbal fluency was assessed using two tasks. First, a semantic fluency task19 in which participants had to name as many animals as possible within 60 s followed by as many professions within 60 s. Second, a letter fluency task20 in which as many words beginning with a given initial letter (D-A-T) had to be generated within 60 s. (v) Speed of information processing was evaluated by using three tasks; the Stroop test (mean score Parts I and II) and the Symbol Digit Substitution Task,21 which is a modified version of the Symbol Digital Modalities Task.
For each individual, performance on the neuropsychological tests was adjusted for age and education level using available neuropsychological normative data sets and converted into standardized z-scores. The compound domain scores were constructed by averaging the z-scores of the used subtests. For a test performance to be classified as clinically impaired, the age- and education-adjusted individual z-score had to be lower than −1.65, corresponding with the 5th percentile.22
Subjective memory complaints, depression, fatigue and QoL
Subjective memory complaints were assessed by a 15-item semi-structured interview based on the Cognitive Failure Questionnaire.6,23 The Center for Epidemiologic Studies Depression Scale (CES-D)24 was used to diagnose a probable depression (score ≥16). Fatigue was assessed by using the Checklist on Individual Strength (CIS-20R).25 A score > 27 indicated a heightened experience of fatigue.25 QoL was assessed with the Short Form 36 Health Survey (SF-36).26 For each individual, performance on the SF-36 subdomains was adjusted for age using available Dutch normative data.
MRI
The MRI studies were performed on a 3 Tesla MR-scanner (Skyra, Siemens Erlangen). The scanning protocol included T1 TSE, T2 TSE and T2 fluid attenuated inversion recovery (FLAIR) transversal sequences of the brain. Parameters for the imaging sequences were as follows: (i) T1 (Repetition Time (TR)/Time to Echo (TE) 500/9.2 ms, slice thickness 5 mm, flip angle 70 degree, voxel size 0.7 × 0.7 × 5 mm); (ii)T2 TR/TE 3500/92 ms, slice thickness 5 mm, flip angle 120 degree, voxel size 0.4 × 0.4 × 5 mm; and (iii) T2 FLAIR TR/TE 9000/ 87 ms, slice thickness 5 mm, flip angle 150 degree, voxel size 0.6 × 0.6 × 5 mm. The complete scanning protocol took ∼10 min. Total WMH were manually segmented on FLAIR images and the volume of WMH was calculated by summing the segmented areas multiplied by slice thickness. Total white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) volume were calculated by brain volumetry using a 6-class segmentation tool, Statistical Parametric Mapping Software (http://www.fil.ion.ucl.ac.uk/spm) (SPM 8), on the T1 images. The volumes were calculated by summing all the voxel volumes belonging to the tissue class. We used the formula: (WM volume + GM volume)/(WM + GM + CSF volume) as a covariate to adjust for total brain volume.27 Brain infarctions were rated separately by two experienced neuro-radiologists. In case of inconsistency, a consensus meeting was held. The MRI protocol included a 3-dimensional time-of-flight angiography of the carotid arteries to measure grade of stenosis.
Statistical analysis
Mean composed z-scores were calculated for each cognitive domain by averaging the z-scores of the individual tasks in that domain. These mean z-scores of the HNC group were statistically compared to the normative standardized mean (z = 0) using one-sample t tests. Also, the frequency of patients classified as ‘cognitively impaired’ at an individual level was quantified for each neuropsychological test. The results of the SF-36 were also analyzed by calculating a mean z-score, testing against z = 0 by using a one-sample t test, and defining an abnormal score as z-score ≤−1.65. The relation between subjective memory complaints, depression or fatigue and cognitive functioning was tested by comparing mean composed z-scores per cognitive domain with an independent t test. ANCOVA was used to test the relation between volume of WMH (with correction for brain volume) or brain volume (with correction for age) and composed z-scores on the cognitive domains. Total volume of WMH was log transformed. The relation between the presence of brain infarctions on MRI and cognitive functioning was determined by comparing mean composed z-scores per cognitive domain with an independent t test. Effect sizes were calculated for all comparisons (Cohen's d).
Results
A total of 103 patients fulfilled the inclusion and exclusion criteria for our study. The number of patients lost to follow-up is reported in Fig. 1. No significant differences were found between patients with or without exclusion criteria for neuropsychological assessment or MRI for the variables of baseline age (mean [SD]) 52 [14] years and 55 [13] years, respectively, Mann Whitney U test, P = .5); sex distribution (9:4 and 30:22 ratio male:female, respectively, Pearson Chi-Square, P = .4); and education level (mean [SD] 4.6 [1.9] and 4.9 [1.3], respectively, Mann Whitney U test, P = .9). A total of 65 patients completed the questionnaires. Fifty-one patients had a follow-up protocol between November 2012 and November 2013. In total, a neuropsychological assessment was administered to 44 patients, 38 of whom also underwent MRI of the brain. Characteristics of our sample at baseline are reported in Table 1. Mean follow-up time after RT was 6.7 (range 4.5–9.6) years and mean age at follow-up was 54 years.
Fig. 1.
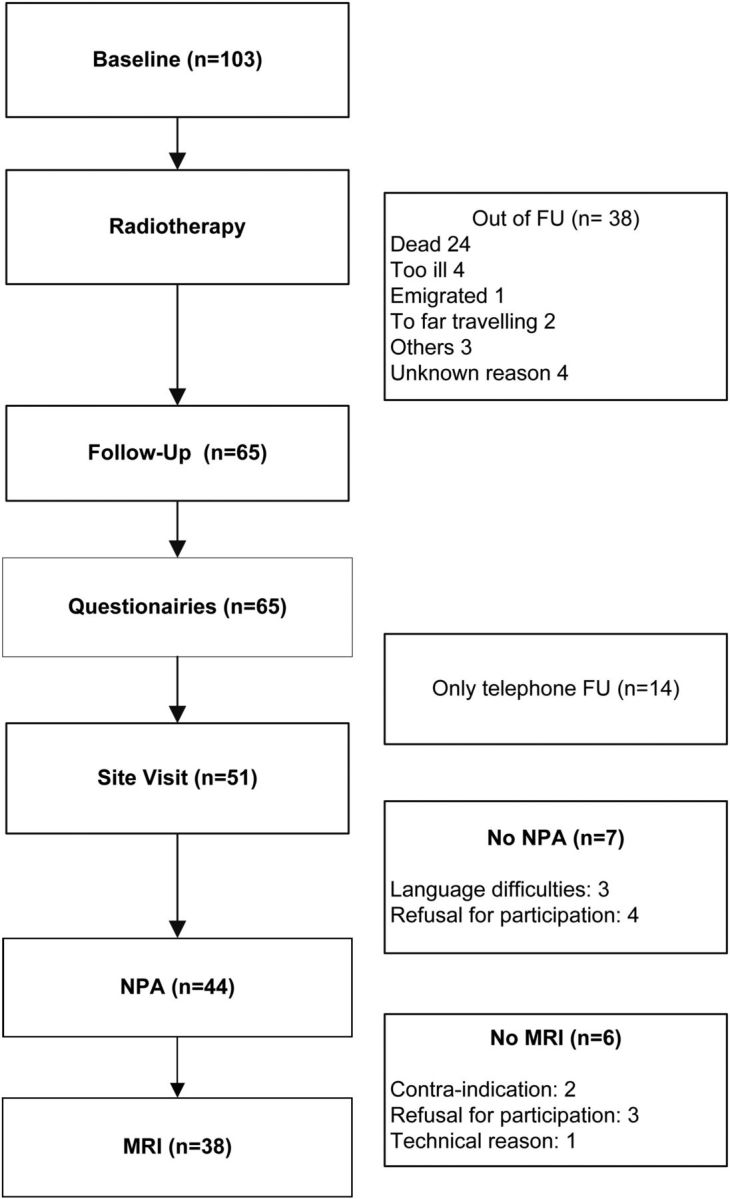
Flow Chart. FU = Follow-up. NPA = Neuropsychological assessment.
Objective cognitive functioning
One patient obtained an MMSE score below 24. No patients obtained a below cut-off score on the FAB. The composed mean age- and education-adjusted z-scores were significantly worse at group level on two cognitive domains: episodic memory z = −0.48 (P = .003) and speed of information processing z = − 0.47 (P < 0.001). No group differences were found on working memory, executive functioning, or verbal fluency. Examining the percentages of patients classified as being cognitively impaired (compared to the expected 5% impaired individuals in the normative sample using a cut-off of z = 1.65), 11.4% of the patients were classified as being clinically impaired on the domain episodic memory and 6.8% were impaired on the domain speed of information processing (note that in the domain working memory, the percentage of impaired patients (4.5%) was within the expected rate of impairment). (Table 2)
Table 2.
Neuropsychological domain scores
| Cognitive Domain | Composed z-score (SEM) | P-value | Impaired (%) |
|---|---|---|---|
| Episodic memory | −0.48 (0.15) | 0.003 | 11.4 |
| Working memory | 0.36 (0.16) | 0.025 | 4.5 |
| Executive functioning | 0.10 (0.097) | 0.297 | 0 |
| Verbal fluency | −0.05 (0.11) | 0.630 | 0 |
| Speed of information processing | −0.47 (0.12) | <0.001 | 6.8 |
Data of composed z-scores are expressed as means (SEM) based on normative data with a mean z-score of zero. Impaired composed z-score is defined as Z ≤ −1.65 (performance worse than 95% of the norm population).
Subjective cognitive complaints, depression, fatigue and QoL
The proportions of patients having subjective memory complaints, depressive symptoms (CES-D), or abnormal fatigue were 80%, 21%, and 36%, respectively (Table 4). Domain-specific QoL scores were not significantly different from norm scores of the general population, except for a trend towards a lower z-score on the subdomain og general health (z = −0.26, P = .05) (Table 3). Patients with subjective memory complaints performed significantly worse than patients without subjective memory complaints on episodic memory (mean difference in z score 0.96, P = .02, d = −1.0). Patients with fatigue scored significantly worse than patients without fatigue on verbal fluency (mean difference in z-score 0.52, P = .02, d = −0.7) and speed of information processing (mean difference in z-score 0.49, P = .04, d = −0.7). Mean z-score per cognitive domain for patients with or without depressive symptoms was not significantly different (Table 4).
Table 4.
Association between subjective memory complaints, depressive symptoms, fatigue and objective cognitive failure
| SMC |
Depressiona |
Fatigueb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (80%) | No (20%) | P | d | Yes (21%) | No (79%) | P | d | Yes (36%) | No (64%) | P | d | |
| EM | −0.65 | 0.31 | .02 | −1.0 | −0.63 | −0.41 | .57 | −0.2 | −0.80 | −0.30 | .12 | −0.5 |
| WM | 0.26 | 0.66 | .33 | −0.4 | 0.29 | 0.42 | .74 | −0.1 | 0.18 | 0.46 | .40 | −0.3 |
| EF | 0.01 | 0.46 | .08 | −0.6 | 0.02 | 0.13 | .65 | −0.2 | −0.07 | 0.20 | .19 | −0.4 |
| VF | −0.19 | 0.25 | .11 | −0.7 | −0.14 | −0.00 | .64 | −0.2 | −0.39 | 0.13 | .02 | −0.7 |
| SP | −0.53 | −0.24 | .36 | −0.4 | −0.74 | −0.40 | .24 | −0.4 | −0.78 | −0.29 | .04 | −0.7 |
All data are expressed as mean composed z-scores.
Abbreviations: EM, episodic memory; WM, working memory; EF, executive functioning; VF, verbal fluency; SP, speed of information processing; SMC, subjective memory complaints.
aDepressive symptoms, based on CESD ≥16.
bFatigue, based on CIS20R >27.
d = Cohen's d effect size.
Table 3.
Self reported quality of life
| SF-domain | Mean z-score (SEM) | P-value | Impaired (%) |
|---|---|---|---|
| Physical functioning | 0.15 (0.13) | .233 | 6.2 |
| Social functioning | −0.17 (0.15) | .280 | 12.5 |
| Role physical | 0.10 (0.11) | .348 | 6.2 |
| Role emotional | 0.03 (0.12) | .779 | 6.3 |
| Mental health | 0.04 (0.14) | .788 | 7.8 |
| Vitality | 0.04 (0.14) | .794 | 6.3 |
| Pain | 0.08 (0.11) | .483 | 1.6 |
| General health | −0.26 (0.13) | .049 | 9.4 |
| Change in health | −0.06 (0.13) | .659 | 6.2 |
Data of composed z-scores are expressed as means (SEM) based on normative data with a mean z-score of zero. Impaired composed z-score is defined as Z ≤ −1.65 (performance worse than 95% of the norm population).
White matter hyperintensities, brain volume, infarctions on MRI and carotid stenosis
The 38 MRIs showed 14 infarctions in 9 patients (5 patients had 2 infarctions). Only one patient had a microbleed. From the 14 infarctions, 7 were (sub)cortical, 4 watershed, and 3 lacunar. Seven infarctions were located in the anterior circulation (all in irradiated vascular territory) and seven were located in the posterior circulation.
Mean (SD) volume of gray matter, white matter and cerebral spinal fluid was 610.0 (57.8), 512.2 (52.8) and 268.3 (32.3) ml, respectively. Mean (SD) volume of WMH on MRI was 18.9 ml. No correlations were found between volume of WMH (with correction for brain volume) or brain volume (with correction for age) and the cognitive domain z-scores. Patients with one or more brain infarctions on MRI performed worse than patients without brain infarctions on verbal fluency (mean difference in z-score 0.74, P = .005, d = −1.1) Cerebral infarctions were not correlated with the other cognitive domains. Only one patient had a carotid artery stenosis of more than 50%, which was in the high-dose RT portal. Unfortunately, no subgroup analysis was possible on carotid pathology.
Discussion
The current study showed worse functioning in some cognitive domains in long-term HNC survivors on average 7 years after treatment compared to the general population. A lower cognitive performance in this cohort was associated with subjective memory complaints and fatigue, but not with depression. Brain infarctions on MRI were correlated with worse cognitive function (verbal fluency); whereas WMH and brain volume were not. Carotid artery vasculopathy on MRI was mild. QoL was relatively unaffected in these long term HNC survivors.
Although attention for long-term physical complications in HNC survivors is growing, limited clinical data exist on the long-term neuropsychological sequelae. The current study is the first long-term follow-up study combining cognitive functioning, depression, fatigue, QoL, and brain imaging. The cognitive test battery consisted of sensitive and widely used neuropsychological tests for which good normative data are available. We showed that on average 7 years after treatment HNC patients had worse cognitive functioning on episodic memory and speed of information processing. Previous prospective studies in small samples (n = 10–30) and relatively short follow-up durations (12–20 months) also showed impaired cognitive functioning after RT of the neck, especially in the memory domain.7,8 Larger studies with longer follow-up time are to date lacking. Since cognitive function can be influenced by several conditions, it is important to find the underlying mechanisms. In our cohort of long-term survivors, a worse cognitive function was related to subjective memory complaints and fatigue, but not to depression. This is a clinically useful finding, because it demonstrates the importance of screening for subjective memory complaints and fatigue in long-term survivors after RT of the neck. Furthermore, brain infarctions on MRI were associated with impaired cognitive function. Therefore, counseling of patients on the importance of preventing cardiovascular risk factors is a potential prevention strategy that could be studied in future. However, a trial on screening and prevention of cardiovascular risk factors, infarctions and cognition is probably not feasible, because follow-up duration has to be several years. Cancer-related fatigue affects 70%–100% of the cancer-patient population,28 even months to years after treatment,29 which results in substantial adverse physical, psychosocial, and economic consequences for both patients and caregivers.30 Studies assessing cancer-related fatigue specific for HNC patients are scarce. Short-term follow-up studies reported increased levels of fatigue during the treatment period, reaching a peak on the sixth week of RT.31 To date, long-term studies on fatigue in HNC patients are lacking. Because cancer-related fatigue has physical, psychosocial, and economic consequences, the identification and treatment of this symptom is important, even in long-term HNC survivors, since evidence-based clinical practice guidelines are available.28 Because only one patient had a > 50% carotid artery stenosis, we could not assess a relation between RT-induced carotid vasculopathy and cognitive decline. QoL was not strongly affected in these long term HNC survivors. These results are in line with previous HNC studies with follow-up durations of 2 years after RT that reported deterioration in QoL, which improved over time.32 In a recently published cross-sectional analyses of 50 HNC survivors (at least 5 years after RT), 80% of the patients indicated to be satisfied with their QoL.9
There were some limitations of the current study. First, no baseline (i.e. pre-RT) assessments of cognition, subjective memory complaints, depression, fatigue or QoL were available, which prevents us from studying changes over time and hence from making causal inferences about the effect of RT. To study the RT effect on cognitive performance, the optimal study design would be a randomized controlled trial with a control group consisting of HNC patients who had not been treated with RT. However, since RT is the treatment of choice in early stage HNC, such a design is neither realistic nor ethical. We used age- and education-adjusted, externally validated normative data for the cognitive assessments and the QoL questionnaire to compare the cognitive performance of HNC survivors with the general population. Second, because the current cohort is not a random sample of HNC survivors, there is a potential selection bias of relatively healthy study subjects that could have resulted in an underestimation of the cognitive decline and vascular changes. A substantial part of our study population had a contraindication for either the neuropsychological assessment or the MRI. However, we do not think that the exclusion criteria for MRI or neuropsychological assessment, like claustrophobia or not having Dutch as the primary language, have influenced the relationship under study, as baseline characteristics did not differ between the two groups. Third, because we had no data about the cardiovascular risk factors of the general population, we could not adjust for lifestyle factors. Finally, information bias is possible because the psychological assessments in our cohort were not performed blindly. However, we do not expect a direct influence on the evaluation of the assessments.
In conclusion, this is the first study assessing the psychological complications in long-term HNC survivors. We showed worse cognitive functioning and more cognitively impaired patients in the domains of episodic memory and speed of information processing. A lower cognitive performance was associated with subjective memory complaints and fatigue, but not with depression. Furthermore, cerebral infarctions on MRI were also related to worse cognitive functioning; WMH and brain atrophy were not. There is a need for more awareness of cognitive dysfunction, fatigue and cerebral infarctions in long-term HNC survivors. Screening and prevention guidelines regarding these psychological complications have to be implemented in the standard of care for long-term HNC survivors.
Funding
None declared.
Acknowledgments
We thank C. Frentz for designing the MRI protocol.
Conflict of interest statement. None declared.
References
- 1. Dorresteijn LD, Kappelle AC, Scholz NM, et al. Increased carotid wall thickening after radiotherapy on the neck. Eur J Cancer. 2005;41(7):1026–1030. [DOI] [PubMed] [Google Scholar]
- 2. Dorresteijn LD, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20(1):282–288. [DOI] [PubMed] [Google Scholar]
- 3. Wilbers J, Dorresteijn LD, Haast R, et al. Progression of carotid intima media thickness after radiotherapy: A long-term prospective cohort study. Radiother Oncol. 2014;113(3):359–363. [DOI] [PubMed] [Google Scholar]
- 4. Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. [DOI] [PubMed] [Google Scholar]
- 5. Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61(10):1531–1534. [DOI] [PubMed] [Google Scholar]
- 6. van Norden AG, Fick WF, de Laat KF, et al. Subjective cognitive failures and hippocampal volume in elderly with white matter lesions. Neurology. 2008;71(15):1152–1159. [DOI] [PubMed] [Google Scholar]
- 7. Hsiao KY, Yeh SA, Chang CC, et al. Cognitive function before and after intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma: a prospective study. Int J Radiat Oncol Biol Phys. 2010;77(3):722–726. [DOI] [PubMed] [Google Scholar]
- 8. Gan HK, Bernstein LJ, Brown J, et al. Cognitive functioning after radiotherapy or chemoradiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):126–134. [DOI] [PubMed] [Google Scholar]
- 9. Chen AM, Daly ME, Farwell DG, et al. Quality of life among long-term survivors of head and neck cancer treated by intensity-modulated radiotherapy. JAMA Otolaryngol Head Neck Surg 2014;140(2):129–133. [DOI] [PubMed] [Google Scholar]
- 10. Wilbers J, Kappelle AC, Kessels RP, et al. Long term cerebral and vascular complications after irradiation of the neck in head and neck cancer patients: a prospective cohort study: study rationale and protocol. BMC Neurol. 2014;14(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hochstenbach J, Mulder T, van Limbeek J, et al. Cognitive decline following stroke: a comprehensive study of cognitive decline following stroke. J Clin Exp Neuropsychol. 1998;20(4):503–517. [DOI] [PubMed] [Google Scholar]
- 12. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 13. Dubois B, Slachevsky A, Litvan I, et al. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55(11):1621–1626. [DOI] [PubMed] [Google Scholar]
- 14. Van der Elst W, van Boxtel MP, van Breukelen GJ, et al. Rey's verbal learning test: normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc. 2005;11(3):290–302. [DOI] [PubMed] [Google Scholar]
- 15. Wechsler D. Wechsler Adult Intelligence Scale—3rd Edition (WAIS-III). San Antonio: TX: Harcourt Assessment; 1997. [Google Scholar]
- 16. Van der Elst W, Van Boxtel MP, Van Breukelen GJ, et al. The Stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment. 2006;13(1):62–79. [DOI] [PubMed] [Google Scholar]
- 17. van den Berg E, Nys GM, Brands AM, et al. The Brixton Spatial Anticipation Test as a test for executive function: validity in patient groups and norms for older adults. J Int Neuropsychol Soc. 2009;15(5):695–703. [DOI] [PubMed] [Google Scholar]
- 18. Houx PJ, Jolles J, Vreeling FW. Stroop interference: aging effects assessed with the Stroop Color-Word Test. Exp Aging Res. 1993;19(3):209–224. [DOI] [PubMed] [Google Scholar]
- 19. Van der Elst W, Van Boxtel MP, Van Breukelen GJ, et al. Normative data for the Animal, Profession and Letter M Naming verbal fluency tests for Dutch speaking participants and the effects of age, education, and sex. J Int Neuropsychol Soc. 2006;12(1):80–89. [DOI] [PubMed] [Google Scholar]
- 20. Schmand B, Groenink SC, van den Dungen M. [Letter fluency: psychometric properties and Dutch normative data]. Tijdschr Gerontol Geriatr. 2008;39(2):64–76. [DOI] [PubMed] [Google Scholar]
- 21. Schaapsmeerders P, Maaijwee NA, van Dijk EJ, et al. Long-term cognitive impairment after first-ever ischemic stroke in young adults. Stroke. 2013;44(6):1621–1628. [DOI] [PubMed] [Google Scholar]
- 22. Lezak MD. Neuropsychological assessment. 5th ed.Oxford; New York: Oxford University Press; 2012. [Google Scholar]
- 23. Broadbent DE, Cooper PF, FitzGerald P, et al. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(Pt 1):1–16. [DOI] [PubMed] [Google Scholar]
- 24. Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 25. Vercoulen JH, Swanink CM, Fennis JF, et al. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38(5):383–392. [DOI] [PubMed] [Google Scholar]
- 26. Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 27. van der Holst HM, Tuladhar AM, van Norden AG, et al. Microstructural integrity of the cingulum is related to verbal memory performance in elderly with cerebral small vessel disease: the RUN DMC study. Neuroimage. 2013;65:416–423. [DOI] [PubMed] [Google Scholar]
- 28. Mock V. Fatigue management: evidence and guidelines for practice. Cancer. 2001;92:(6 Suppl):1699–1707. [DOI] [PubMed] [Google Scholar]
- 29. Ahlberg K, Ekman T, Gaston-Johansson F, et al. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362(9384):640–650. [DOI] [PubMed] [Google Scholar]
- 30. Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–360. [DOI] [PubMed] [Google Scholar]
- 31. Jereczek-Fossa BA, Santoro L, Alterio D, et al. Fatigue during head-and-neck radiotherapy: prospective study on 117 consecutive patients. Int J Radiat Oncol Biol Phys. 2007;68(2):403–415. [DOI] [PubMed] [Google Scholar]
- 32. Kohda R, Otsubo T, Kuwakado Y, et al. Prospective studies on mental status and quality of life in patients with head and neck cancer treated by radiation. Psychooncology. 2005;14(4):331–336. [DOI] [PubMed] [Google Scholar]


