Abstract
Glioblastoma (WHO grade IV astrocytoma) is the most common and most aggressive primary brain tumor in adults. Optimal treatment of a patient with glioblastoma requires collaborative care across numerous specialties. The diagnosis of glioblastoma may be suggested by the symptomatic presentation and imaging, but it must be pathologically confirmed via surgery, which can have dual diagnostic and therapeutic roles. Standard of care postsurgical treatment for newly diagnosed patients involves radiation therapy and oral temozolomide chemotherapy. Despite numerous recent trials of novel therapeutic approaches, this standard of care has not changed in over a decade. Treatment options under active investigation include molecularly targeted therapies, immunotherapeutic approaches, and the use of alternating electrical field to disrupt tumor cell division. These trials may be aided by new insights into glioblastoma heterogeneity, allowing for focused evaluation of new treatments in the patient subpopulations most likely to benefit from them. Because glioblastoma is incurable by current therapies, frequent clinical and radiographic assessment is needed after initial treatment to allow for early intervention upon progressive tumor when it occurs.
Keywords: chemotherapy, glioblastoma, radiation, surgery, temozolomide
Clinical Case Presentation
A 73-year-old man presented to his local emergency department after experiencing a generalized seizure. He had moderate left-sided weakness in the initial postictal period which quickly resolved. In retrospect, the patient had noted subjective left-hand “clumsiness” for a month prior to the seizure, but had not reported it to his family or physician. A CT scan was obtained in the emergency room and was followed shortly by an MRI (Fig. 1). The patient was then referred to our institution for further care.
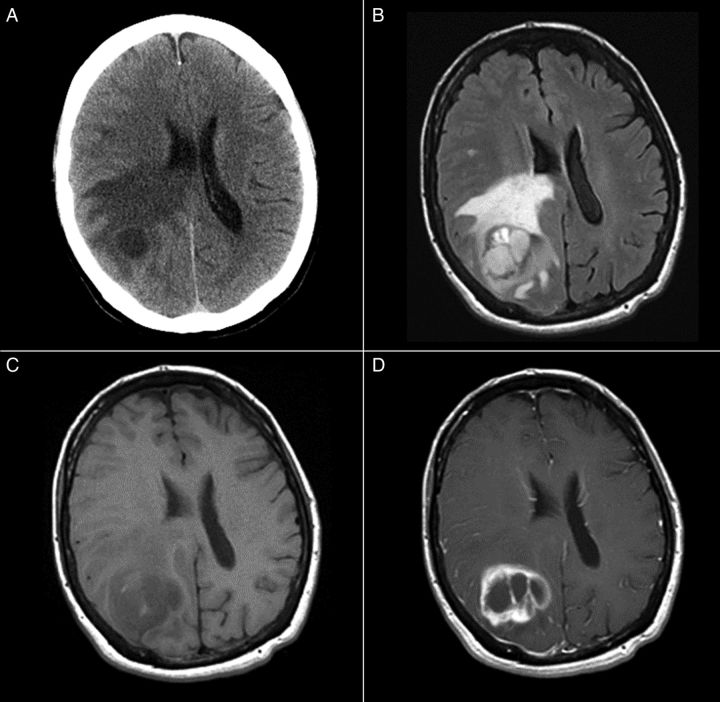
Fig. 1.
(A) Unenhanced CT, (B) T2-weighted FLAIR, (C) gradient echo T1-weighted, and (D) post-gadolinium spin echo T1-weighted images depict a relatively circumscribed mass in the left superior temporal lobe with both solid, enhancing components and some cystic or necrotic areas. Moderate edema signal surrounds a portion of the mass.
Initial Supportive Care
The presentation of high-grade glioma is variable, depending on the location of the lesion within the brain. Headaches, seizures, and subacutely progressive neurological deficits are all common presenting symptoms.
Antiepileptic Therapy
Patients who present with seizure should be treated with antiepileptic drug (AED) therapy. An optimum AED choice would have rapid efficacy, few side effects, and no drug–drug interactions. In clinical practice, levetiracetam is often chosen as the first-line agent in this setting.1 Studies have suggested that some AEDs may have direct antitumor effects. For example, valproic acid is a histone deacetylase (HDAC) inhibitor,2 while levetiracetam is an MGMT inhibitor.3 However, no impact of AED choice on survival has been proven, so AED choice should be based on efficacy and tolerability.
In patients with high-grade glioma who have not had a seizure, there is no proven role for long-term prophylactic AED therapy, and the American Academy of Neurology recommends against the routine use of prophylactic AEDs outside of the immediate perioperative period.4 As previous studies of prophylactic AED therapy evaluated older agents in mixed patient populations, some experts question their applicability to current practice. A large trial of lacosamide vs placebo for seizure prophylaxis in patients with high-grade gliomas is ongoing to address this issue.5
Corticosteroid Therapy
In patients presenting with headaches or focal neurological deficits, symptoms may be due to peritumoral vasogenic edema, which may respond to corticosteroid therapy. Dexamethasone is often started at 16 mg daily in 4 divided doses, and tapered down to the lowest effective dose or discontinued altogether. While this dosing schedule is widely used based on the short pharmacologic half-life of dexamethasone, the biological half-life is in excess of 36 hours, and daily or twice-daily dosing is effective and more convenient for maintenance therapy in most patients. Gastrointestinal prophylaxis and pneumocystis prophylaxis should be considered in patients in whom long-term corticosteroid treatment is anticipated.
Clinical Case Relevance
The patient was started on antiepileptic therapy at the time of his original emergency department visit. He had no further seizures. His exam was pertinent for a Karnofsky Performance Score of 90, and subtle left-sided pronator drift and slowing of rapid hand and foot movements on the left side were his only findings on physical exam. Dexamethasone was not initiated as he did not have symptoms of elevated intracranial pressure, such as headache or papilledema.
Initial Diagnostic Imaging
Because the symptomatic presentation of brain tumors is nonspecific, the presumptive diagnosis of brain tumor is often made only after imaging. Glioblastoma may be initially imaged with CT, particularly in the emergency department setting, but MRI provides more diagnostic information.
The typical CT appearance of glioblastoma is a mass lesion, often iso- to hyperattenuating (bright) in comparison to normal gray matter, with surrounding hypoattenuation due to infiltrating tumor and vasogenic edema. Contrast-enhanced CT classically reveals a centrally necrotic enhancing mass. Given that vascular proliferation is a hallmark of glioblastoma, intratumoral hemorrhage is common and may be visualized on CT, though it is more frequently identified as microhemorrhages on MR susceptibility-weighted imaging (SWI). Calcification is uncommon in glioblastoma, but can occasionally be seen.
On MRI, nearly all glioblastomas enhance with gadolinium contrast, usually showing a thick, irregular rind of tumor surrounding a necrotic cavity. Heterogeneity of signal intensity and contrast enhancement within glioblastomas and irregularity in shape are expected. Vascular hyperpermeability contributes to surrounding vasogenic edema visible as high signal intensity on T2-weighted images. Hemorrhage may complicate the appearance of glioblastoma, with acute and early subacute hemorrhage appearing hypointense on T2-weighted images and iso- to hyperintense on T1-weighted images. This intrinsic T1 hyperintensity of blood is similar in appearance to gadolinium enhancement, so it is crucial to always compare T1-weighted postcontrast images with T1-weighted precontrast images to ensure accurate judgment of enhancement.
The infiltrative nature of glioblastoma is generally more apparent on MRI than on CT. Mass-like signal abnormality infiltrating along white matter tracts is suggestive of glioma as opposed to other entities. However, distinguishing between nonenhancing infiltrative glial tumor with edema and vasogenic edema from any other etiology can be difficult or impossible. Frequently, glial infiltration and thickening of the cerebral cortex can be appreciated on T2-weighted and T2-weighted FLAIR images, which may help to distinguish gliomas from other neoplasms. Multifocality, distant, or diffuse disease may be seen initially in approximately 13% of glioblastoma cases, with some areas sometimes looking less aggressive than the primary mass.6 It is also well established that microscopic glial tumor cell infiltration is expected to extend beyond visualized signal abnormality on MRI.
The differential diagnosis of glioblastoma often includes metastasis and CNS lymphoma. Generally speaking, glioblastoma tends to be more irregularly shaped than metastases because of its predilection for spread along white matter tracts,7 but there is overlap at least in qualitative analysis. Primary CNS lymphoma (PCNSL) in the immunocompetent patient is most often homogeneous in signal intensity and enhancement, though exceptions do occur; while heterogeneity and central necrosis are more common in CNS lymphoma in the immunocompromised.
Advanced MRI techniques including perfusion imaging techniques such as dynamic susceptibility contrast (DSC) imaging, diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI), SWI, and MR spectroscopy may help to distinguish glioblastoma from other tumors. Given the histological hallmark of neovascularity in glioblastoma, increased blood volume (often expressed as rCBV, or relative cerebral blood volume) is expected within at least portions of a glioblastoma.8–10 On MR spectroscopy, glioblastoma typically has the nonspecific findings of elevated choline and decreased N-acetylaspartate (NAA) and may have elevated lipid and lactate resonances. Generally speaking, the choline:NAA ratio increases with astrocytoma grade.11 Due to the infiltrative nature of glioblastoma relative to metastases, one may expect greater CBV12,13 and greater choline:creatine14,15 in the peritumoral areas of glioblastoma relative to metastases. rCBV also tends to be greater in enhancing tumor and peritumoral areas of glioblastoma than in CNS lymphoma.12 Apparent diffusion coefficient tends to be lower in CNS lymphoma than in glioblastoma, given the great hypercellularity of lymphoma.12 Microhemorrhages on SWI are found in most glioblastomas but rarely in CNS lymphoma.16,17 Differentiation of glioblastoma and lymphoma using multiparametric advanced MRI has also been suggested.18 Imaging genomic mapping is a burgeoning area of research that has begun to discover associations between MRI features and glioblastoma genotypes and clinical phenotypes.19
Many published reports using advanced MRI techniques have relied on quantitative analyses, which are currently difficult to standardize across imaging platforms and institutions. For example, with perfusion imaging, there exists great variability in all steps from IV gadolinium bolus injection to scanner platforms used to MRI scan parameters chosen to post-processing software and analysis techniques used.20 Given the technical variabilities of advanced MR techniques and expected glioblastoma heterogeneity, there are limits to the sensitivity and specificity of these techniques. The standardization of advanced MRI is well recognized as a pressing clinical and research need.
Clinical Case Relevance
The initial imaging obtained for this patient included a CT and contrast-enhanced MRI, shown in Fig. 1. Both of these images, and the MRI in particular, were concerning for glioblastoma, and metastasis and non-neuroplastic entities such as infection or demyelination were thought to be significantly less likely.
Surgery
Surgical resection is the primary treatment for glioblastomas. The goals of surgery are tissue diagnosis, including molecular and genetic tumor analysis, as well as cytoreduction for alleviation of presenting symptoms and improved tumor control. As previously discussed, in the appropriate context imaging can be very suggestive of glioblastoma. However, tissue diagnosis is the standard of care and only in cases of truly inaccessible tumors (such as brainstem lesions) or grave infirmity of the patient, precluding surgical candidacy, should treatment be undertaken without pathological confirmation of disease.
The surgical approach of choice is maximal safe resection. Over the past several years, significant data have accumulated supporting the idea that maximizing the extent of tumor resection positively impacts survival for patients with newly diagnosed glioblastoma.21,22 In a single institution study of 949 patients with high-grade gliomas, more than half of whom were operated on for the first time, the extent of resection was shown to be an independent predictor (gross total resection [GTR] vs near total resection [NTR], NTR vs subtotal resection [STR]) of prolonged survival (median OS 11 months GTR, 9 months NTR, and 5 months STR).23
The association between extent of resection and survival benefit holds true even for tumors that are difficult to resect. In a study of multicentric high-grade glioma, resection of a dominant lesion was strongly predictive of improved overall survival when compared with biopsy only (12 months vs 4 months).24 In the setting of insular high-grade gliomas, one of the most technically difficult eloquent cortical areas to access surgically, extent of resection ≥90% of the tumor provided 2-year overall survival of 91% compared with 75% for volumetric resection <90% of the tumor, in addition to improved progression-free survival.25 The beneficial effect of maximal resection has also been suggested to extend to elderly patients without an increase in mortality or complications.26
Several technical intraoperative adjuncts have been developed in an effort to maximize the extent of safe resection.27,28 Use of frameless stereotactic guidance, which allows for optimal patient positioning, accurate tailoring of the craniotomy, and safe access trajectory to the tumor, has become standard for resection of glioblastomas. Recent advances have made it possible for intraoperative guidance to integrate imaging tools such as tractography, which allows for identification of motor, speech, and visual pathways, as well as MR spectroscopy to facilitate accurate targeting of presumed higher-grade areas within a heterogeneous tumor.29 In cases where only a biopsy is planned, such imaging integration allows targeting of regions likely to optimize diagnostic yield and accuracy.
Direct cortical mapping allows identification of motor pathways and, when combined with awake craniotomy used for mapping of language areas, is an important adjunct to surgical resection of lesions in eloquent cortex.25,30 A systematic review of the literature showed that direct cortical mapping decreases late severe neurological deficits from 8.25% to 3.4% and increases the rate of GTR from 58% to 75%.31
Intraoperative MRI has been used in an attempt to maximize extent of surgical resection and identification of residual resectable tumor.32 In a study including both high-grade and low-grade tumors, use of intraoperative MRI increased the volumetric extent of resection from 76% to 96%.33 However, the high installation cost of an intraoperative magnet as well as the complexities involved in its intraoperative use have led to research into alternate ways to identify residual tumor during surgical resection. The utilization of fluorescence guidance has been recently advocated. The use of fluorophores such as 5-aminolevulenic acid (5-ALA) or fluorescein, which accumulate in areas of blood brain disruption, can be a powerful adjunct that allows for the accurate identification of tumor borders and possible residual disease at the time of resection.34–36 In a systematic review of 10 studies, patients who underwent surgery utilizing 5-ALA for maximizing resection had improved 6-month progression-free survival and overall survival.37 A multicenter, randomized, phase III trial of 5-ALA-guided surgery found higher rates of gross total resection and 6-month progression-free survival in the 5-ALA group without any increase in adverse events.38
Following the initial multicenter, randomized trial of implantable carmustine polymer wafers in the treatment of recurrent high-grade glioma, Attenello et al reported their experience with their use during surgery for newly diagnosed high-grade gliomas and found an overall median survival of 13.5 months without any increased incidence of complications.39,40 Although the use of chemotherapy implants appears to be safe in the setting of primary glioblastoma, the relative lack of improved survival and the fact that much of the data regarding the use of chemotherapy wafer implants predates the use of temozolomide (TMZ), has limited enthusiasm for this approach.
Surgery for high-grade gliomas is in general associated with relatively low rates of major complications. In an analysis of the patients in the Glioma Outcomes Project, an overall complication rate of 24% was reported for surgical treatment of newly diagnosed high-grade gliomas. In decreasing frequency, major complications included: depression (11%), worsened neurological status (8.1%), seizures (7.5%), adverse drug reaction (5.2%), DVT (4.2%), intracranial bleeding (1.6%), and pulmonary embolism and wound infection (0.5% each). Perioperative mortality was reported as 1.5%.41 These results were similar to an earlier study that reported 13% major complications and 1.7% mortality in patient undergoing craniotomy for intraparenchymal tumors.42 In an analysis of the California Inpatient Database, Marcus et al reported a 30-day readmission rate of 13.2% for patients who underwent surgical treatment for a glioma who were originally discharged home. The most common presentations at readmission were seizures (20.9%) and surgical infection (14.5%).43
In summary, surgery remains the first and very important treatment modality for a newly diagnosed glioblastoma. Its effectiveness for optimizing overall survival is related to the extent of resection and its safety is dependent on various intraoperative adjuncts that allow for accurate localization of the tumor as well as eloquent cortical areas.
Clinical Case Relevance
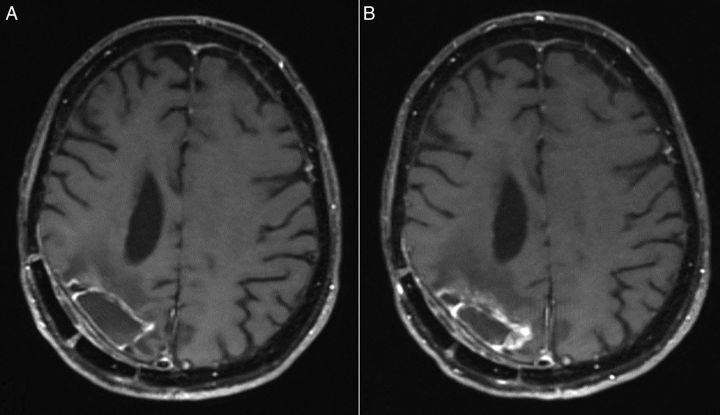
The patient underwent resection of his tumor without use of awake craniotomy or intraoperative MRI. Following surgery, his left-sided weakness was transiently worse but it then improved back to the presurgical baseline. His extensive resection placed him in a more favorable prognostic group than biopsy alone would have. Preoperative and postoperative MRI images are displayed in Fig. 2.
Fig. 2.
Post-gadolinium spin echo T1-weighted images (A) before and (B) after surgery.
Pathology
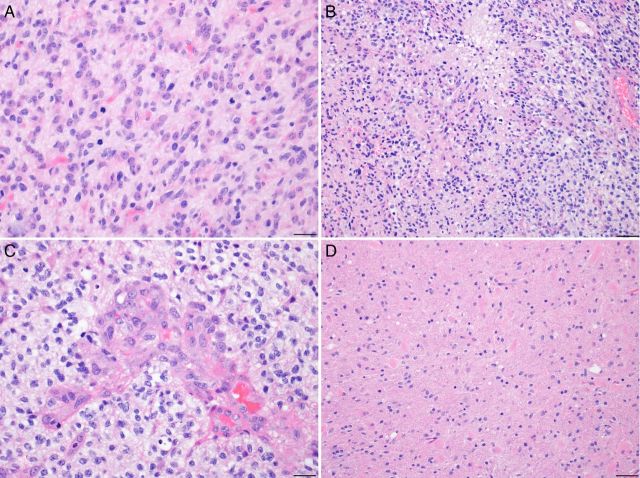
The histological diagnosis in this case was WHO grade IV astrocytoma (glioblastoma). It was an infiltrative astrocytoma showing areas of high cellularity and brisk mitotic activity (Fig. 3A), tumor necrosis (Fig. 3B), and microvascular proliferation (Fig. 3C). The diagnostic criteria from the WHO (2007) include presence of cytological atypia, mitotic activity, microvascular proliferation, and/or tumor necrosis.44 Briefly, an infiltrative astrocytoma exhibiting cytological atypia alone, including elongated, irregular and hyperchromatic nuclei, is considered WHO grade II (diffuse astrocytoma). The presence of increased cellularity, nuclear atypia, and mitotic activity warrant a WHO grade III (anaplastic astrocytoma) designation. Tumors that additionally show microvascular proliferation and/or necrosis are WHO grade IV (glioblastoma). Classic microvascular proliferation has the appearance of “glomeruloid tufts,” consisting of multilayered, mitotically active endothelial cells admixed with smooth muscle cells/pericytes (as represented in Fig. 3C). Although necrosis surrounded by pseudopalisading tumor cells is most characteristic of glioblastoma (Fig. 3B), both geographic and pseudopalisading tumor necrosis can be present and are associated with similarly dismal prognoses. Astrocytoma grading is based on the highest histological grade. Since infiltrative astrocytomas can have considerable regional heterogeneity, especially toward their infiltrative border into surrounding parenchyma, it is important to assess whether a biopsy sample is representative of the entire tumor by correlating histological, clinical, and radiological findings. A biopsy taken at the periphery of a ring-enhancing mass could well show a low to moderately cellular tumor (as seen in Fig. 3D) with/without mitoses, prompting an inaccurate diagnosis of diffuse or anaplastic astrocytoma (WHO grade II or III) rather than glioblastoma (WHO grade IV).
Fig. 3.
The biopsies demonstrated an infiltrating population of atypical astrocytic cells, showing (A) brisk mitotic activity, (B) tumor necrosis, and (C) microvascular proliferation, consistent with a diagnosis of glioblastoma. A biopsy from the periphery of this mass may show (D) a low-to-moderately cellular tumor, with or without mitoses, corresponding to a lower histological grade. In images (A) and (C), photographed at 400× magnification, the scale bars on the bottom right represent 20 µm. In images (B) and (D), photographed at 200× magnification, the scale bars represent 50 µm.
Historically, glioblastomas have been distinguished based on their clinical presentation as primary (de novo) or secondary glioblastomas that develop in progression from a lower grade astrocytoma. Primary glioblastomas, the most common (>90%), develop with a short clinical history without clinical or pathological evidence of a lower grade precursor, and are typically seen in older patients. There are no definite histological features to distinguish primary and secondary glioblastomas. With advancing molecular information, however, it is clear that primary and secondary glioblastoma are two different diseases.
Mutation of isocitrate dehydrogenase (IDH) is frequently seen in low-grade glioma, and is also found in approximately 12% of glioblastomas.45 The presence of IDH mutation within a glioblastoma is suggestive of secondary glioblastoma, regardless of any previous history of low-grade glioma.46–50 In glioblastoma, mutations almost exclusively involve residue 132 (R132) of IDH1 resulting in the substitution of arginine.46,51 The presence of IDH1 mutation in glioblastoma has been associated with younger age and relatively longer survival.52–56 Alterations in receptor tyrosine kinase pathways have also been frequently identified in glioblastomas.57 Epidermal growth factor receptor (EGFR) activation, either by amplification of wild-type EGFR or by deletion of exons 2–7 that encode the extracellular domain (the variant III mutation) resulting in ligand-independent constitutive activation of EGFR,47–49 is more commonly seen among primary glioblastomas. Mutations of the TP53 gene are frequent in, but not exclusive of, secondary glioblastomas.50,58,59 On the other hand, mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene are predominantly found in primary glioblastomas that lack IDH1 mutations, and appear to be associated with worse prognosis.49,60–62
The gene for the DNA-repair enzyme O(6)-methylguanine-DNA methyltransferase (MGMT) has a promoter region that is rich in CG dinucleotide (CpG) sites that are normally unmethylated. However, in glioblastomas, the cytosine in these CpG sites may become methylated, resulting in transcriptional silencing of MGMT and subsequent impairment of the DNA-repair process.63–65 Glioblastomas with MGMT-promoter hypermethylation are unable to repair the DNA damage caused by alkylating agents (such as TMZ), and carry a more favorable prognosis than tumors without methylation of MGMT.66–69 Tumors with IDH mutation frequently have MGMT promoter methylation.70
Integrated genomic analysis has revealed subsets of high-grade astrocytomas based on the differential expression of prognostic markers.71,72 Three to four major subgroups are generally well recognized, and their distinction appears to be relatively robust on meta-analysis.73 At one end of the spectrum is the proneural subgroup, characterized by alterations in markers associated with neurogenesis, strongly associated with IDH1 mutations, and tending to have more favorable outcomes. The mesenchymal subgroup is at the other end of the spectrum, expressing markers usually associated with mesenchymal tissue and increased angiogenesis, associated with loss and/or mutation of the NF1 gene, and tending to have relatively worse outcomes. These expression-based subgroups were also distinguished by their CpG island methylation status.74 Glioblastomas in the proneural subgroup were more frequently found to show widespread CpG island hypermethylation, termed a glioma CpG island methylator phenotype (G-CIMP), which was not usually seen in tumors of the mesenchymal subgroup.
At present, the diagnosis of glioblastomas per the 2007 WHO guidelines is based on well-established histological features. However, testing for MGMT status is becoming accepted as a standard of care for newly diagnosed glioblastomas, as is screening for IDH1 mutations. As newer therapeutic modalities emerge, there is increasing recognition for the need to communicate the status of prognostically and therapeutically relevant genomic markers to help guide clinical decisions for patient management. In a preliminary attempt to address this, consensus guidelines suggested at an international meeting of neuropathologists recommended that pathologists to provide an integrated diagnosis that incorporates the histological diagnosis and relevant molecular information.75 For example, the diagnosis of glioblastoma, WHO grade IV, might also include the results of the IDH1 mutation status, MGMT status, and other relevant markers.
Clinical Case Relevance
Pathology revealed glioblastoma, MGMT methylated. The MGMT methylation status is prognostic of better survival, relative to patients with unmethylated MGMT, and it also may predict response to treatment in some situations, to be discussed later.
Epidemiology
Glioblastoma is the most common primary brain tumor in adults. In the United States, the age-adjusted incidence rate is 3.19 (95% confidence interval 3.16–3.21) per 100 000 persons.76 The lifetime risk of being diagnosed with an invasive cancer of the nervous system, most of which are either glioblastoma or glioblastoma precursors, is approximately 1 in 161, with the risk of dying from an invasive cancer of the nervous system being approximately 1 in 222.77 Thus, while glioblastoma remains a relatively rare tumor, many patients will be aware of one or more acquaintances or relatives with this condition purely by chance, sometimes raising concern of clustering of tumors by geography or within a family.
Glioblastoma is more common in men than women, as are all infiltrating gliomas. Incidence rises with age, peaking in the 75–84 age range. Thus, though the overall age-adjusted incidence rate of glioblastoma does not appear to be rising, the raw number of tumors diagnosed each year is expected to climb in coming decades due to aging of the population at large. In the United States, glioblastoma is more common amongst non-Hispanic whites than in black, Asian/Pacific Islander, or American Indian/Alaskan native groups.
Ionizing radiation remains the only proven exposure risk factor for glioblastoma. Typically, radiation-induced glioblastoma is seen years after therapeutic radiation for another tumor or medical condition. Diagnostic radiation, for example from CT scanning or even dental X-rays may theoretically confer increased risk of glioma, but this has not yet been confirmed in large-scale epidemiological studies. Nonionizing radiation, specifically related to the use of cellular telephones, has not been convincingly linked to glioma incidence, but this is an area of active investigation. Asthma and atopic disease are associated with lower risk of glioblastoma in multiple studies, with one meta-analysis showing a reduction in glioma risk of 40% in patients with allergies.78
Many patients with glioblastoma are concerned about possible genetic risk factors, and ask about the advisability of screening for relatives. A heritable component to glioblastoma risk has long been demonstrated by the association between glioblastoma and Mendelian cancer syndromes including the Lynch and Li-Fraumeni syndromes. Recently, genome-wide association studies have revealed single nucleotide polymorphisms (SNPs) associated with glioblastoma risk. These SNPs have been identified within the candidate genes TERT (chromosome 5p15.33), EGFR (7p11.2), CDKN2B (9p21.3), TP53 (17p13.1), and RTEL1 (20q13.33).79 Other SNP associations such as those within CCDC26 (8q24.21) and PHLDB1 (11q23.3) are mainly associated with IDH-mutant tumors and thus risk of secondary glioblastoma. Most of the currently identified risk SNPs confer only a modest increase in risk of glioma, and thus the absolute risk of glioma remains low even in individuals carrying the risk SNPs.
Clinical Case Relevance
The patient came from a large family with a history of a number of different tumor types, but no first or second degree relatives with primary brain tumors. He was reassured that his family members were at low risk of primary brain tumor, and no screening studies were recommended.
Standard-of-Care Treatment
Glioblastoma is an infiltrative tumor, with residual disease present after surgery, even in cases of radiographic gross total resection. Additional treatment to address this residual tumor is thus necessary as soon as the operative site has healed appropriately. This additional treatment may take the form radiation therapy (RT), chemotherapy, or both, depending on the clinical scenario.
Current Standard-of-Care Therapy
The current standard-of-care treatment for newly diagnosed glioblastoma was established by a landmark trial conducted by the European Organization for Research and Treatment of Cancer (EORTC) and the National Cancer Institute of Canada Trials Group (NCIC).80 In this trial patients were randomized to RT alone (the previous standard of care) vs RT with concurrent and adjuvant oral TMZ chemotherapy. In this trial, median survival was 12.1 months in the radiation-only arm and 14.6 months in the TMZ arm. More importantly, combined chemoradiotherapy significantly increased the proportion of relatively long-term survivors from 10.9% to 27.2% at two years and from 1.9% to 9.8% at five years. Patients whose tumors had methylation of the promoter for the MGMT gene (MGMT methylated) had greater benefit from the addition of TMZ (46% 2-year survival vs 27% for the MGMT unmethylated patients), but MGMT unmethylated patients still had incremental benefit from the addition of TMZ (14% 2-year survival vs <2%).69
Numerous studies have indicated a benefit to using RT in the treatment of gliomas.81,82 A dose of 60 Gy in 2 Gy fractions is the recommended dose based on prior studies indicating that doses up to but not exceeding 60 Gy impact survival.83,84 Despite agreement of a standard dose recommended in both clinical trials and practice, there is tremendous variability in the volume of tissue irradiated. A common approach endorsed by the Radiation Therapy Oncology Group and other cooperative groups is to target the tumor edema with a 2.5-cm margin to a dose of 46 Gy followed by a cone down to the resection cavity and contrast-enhancing tumor with similar margin to a dose of 60 Gy.85 Other cooperative groups also recommend a staged approach initially including tumor edema with a cone down to the enhancing tumor, but only add a 1-cm margin. Additional strategies include treating the enhancing tumor and resection cavity with a 2–3-cm margin to a total dose of 60 Gy without a staged volume reduction.80 The volume of brain irradiated in each of these scenarios is substantially different. While studies have demonstrated comparable outcomes comparing conformal radiation to whole brain irradiation, similar comparisons have not been made between these varied radiation approaches.86
Typically, TMZ is given daily during RT at a dose of 75 mg per square meter of surface area each day, followed by a rest period of approximately a month at the end of radiation. TMZ is then resumed at the dose of 150 mg per square meter on days 1–5 of a single 28-day cycle, and subsequent 28-day cycles are dosed at 200 mg per square meter on days 1–5 if the first adjuvant cycle was well tolerated. In the clinical trial that proved the efficacy of this approach, a total of 6 adjuvant cycles were given.80 In practice, some physicians recommend more than 6 cycles, though there is currently no definitive data demonstrating that more prolonged regimens are associated with superior survival.
Side Effect Management
The most common symptomatic side effects of treatment are mild fatigue, nausea, and constipation. All patients receiving TMZ should be provided with antiemetic therapy, both to take prior to each TMZ dose to prevent nausea and also for as-needed use. Antiemetics of the serotonin 5-HT3 receptor antagonist class, such as ondansetron and granisetron, are often used for this purpose. TMZ often causes mild thrombocytopenia, but severe thrombocytopenia, neutropenia, and anemia are much less common. Asymptomatic leukopenia is very common in patients on TMZ. Prophylaxis against Pneumocystis jiroveci pneumonia with trimethoprim/sulfamethoxazole or an alternative agent is recommended for the full duration of TMZ therapy.
Common acute radiation side effects include headaches, nausea, exacerbation of presenting symptoms, hair loss, skin reaction at the site of radiation, and fatigue. Less common acute side effects can include dry mouth or altered taste, hearing impairment or seizures. Possible late side effects of radiation include decreased pituitary hormonal production, cataract formation, secondary cancers, and nerve damage. Radiation necrosis can occur and cause symptoms similar to tumor recurrence or stroke. Necrosis is initially managed with steroids but may require more intensive options such as surgery or bevacizumab therapy.87
Neurocognition can be altered during initial therapy secondary to inflammation, anxiety, and medications, but radiation can also cause cognitive impairment months to years after it is completed, even in the absence of tumor progression. Learning and memory are the most commonly impaired cognitive domains. The extent of impairment may be related to patient-specific factors, but also related to volume of tissue irradiated and location of the radiation field. For example, dose to hippocampal structures has been found to influence extent of neurocognitive dysfunction.88–90
Elderly Patients and Patients with Poor Performance Status
The pivotal trial that demonstrated the efficacy of TMZ for newly diagnosed glioblastoma did not include patients over the age of 70, leaving unanswered the question of whether this regimen is effective and well tolerated in elderly patients.80 Moreover, a number of trials have been conducted that suggest, at least in some circumstances, abbreviated or less intensive treatments may be effective in older patients. In a randomized, phase III trial conducted by the Nordic Clinical Brain Tumor Study Group, patients age 60 or older with glioblastoma were randomized to the typical 6 weeks of RT, 2 weeks of hypofractionated RT, or TMZ without RT.91 The trial demonstrated that both the hypofractionated RT and TMZ monotherapy were superior to 6 weeks of RT with respect to overall survival. Similarly, in the German NOA-08 trial, patients over 65 years of age were randomized to dose-dense TMZ or standard 6-week RT, with similar outcomes in each group.92 Both the Nordic and NOA-08 trials demonstrated that MGMT methylation was predictive of response to TMZ therapy. Patients whose tumors demonstrated methylation of MGMT had better survival when treated with regimens that contained TMZ, whereas patients with unmethylated MGMT did better when treated with RT. Although both these studies were randomized phase III studies, there are significant limitations in interpreting the data. Notably, 6 weeks of RT with concurrent and adjuvant TMZ was not an arm in either study, so direct comparisons of these alternative approaches to the current standard of care are not possible. In addition, the definition of elderly varied across these trials and across other, retrospective analyses.
Currently, as no therapy has been proven superior or equivalent to standard combined chemoradiotherapy in elderly patients, this therapy is a reasonable option for patients of any age with good performance status who are felt likely to tolerate intensive treatment. In patients with poor performance status or those in whom treatment tolerability is a concern, treatment choice should be informed by MGMT methylation testing. In patients with MGMT methylation, TMZ monotherapy is reasonable, whereas RT monotherapy is an option for patients without MGMT methylation. Given the results of the Nordic trial, as well as a previous trial that demonstrated that abbreviated RT was not inferior to 6-week RT, hypofractionated RT is preferable to the standard six-week schedule in this patient group if not combined with chemotherapy.93
Optune (NovoTTF-100A)
Recently released information, in the form of a press release and a presentation at a neuro-oncology specialty meeting, suggests that the addition of therapy with alternating electric fields via the Optune device (NovoCure) may prolong both progression-free and overall survival by several months when combined with standard chemoradiotherapy for newly diagnosed glioblastoma.94 In the trial for which data were presented, Optune therapy was initiated at the time of initiation of adjuvant TMZ therapy and continued until tumor progression. While this result is cause for optimism, the results presented were from an interim data analysis, and publication of the full trial data in a peer-reviewed journal will be necessary before Optune therapy can be critically evaluated for possible inclusion in a new standard of care for glioblastoma.
Recent Clinical Trial Results
After combined chemoradiotherapy became the standard of care, more aggressive dosing of TMZ was tested in hopes that it might provide additional efficacy. RTOG 0525, a large phase III study, compared a dose-dense schedule of TMZ after RT with the standard 5-day schedule. No difference in survival was noted in either MGMT methylated or MGMT unmethylated patients.85 Cilengitide, a targeted drug that inhibits integrins, was added to standard RT and TMZ in two separate clinical trials, a phase III study in MGMT methylated patients95 and a phase II study in MGMT unmethylated patients,96 but did not show improvement in survival in either case. The addition of the antiangiogenic agent bevacizumab, an antibody against VEGF, to standard RT and TMZ was tested in two large phase III trials. While there was some improvement in progression-free survival, no improvement in overall survival was noted in either of these trials.97,98 As such, bevacizumab has not at this time been approved for use in initial treatment of glioblastoma.
Important Ongoing Clinical Trials
There are numerous phase I and II trials around the world testing the addition of other drugs to standard therapy, including targeted agents, cytotoxic agents, and a variety of immune-targeting approaches. In addition, there are trials in progress testing alternative radiation techniques such as proton therapy or imaging-guided radiation dose escalation, and trials testing metabolic approaches against cancer such as variations on the ketogenic diet.
With regard to phase III trials, a randomized, placebo-controlled trial is currently ongoing that tests the addition of an autologous vaccine made from a patient's own tumor and their own dendritic cells (DCVax-L) to initial adjuvant TMZ.99 Another glioblastoma immunotherapy approach, using the EGFRvIII-targeted experimental cancer vaccine rindopepimut (CDX-110), is being evaluated in the ACT IV trial.100 Within the elderly population specifically, a randomized, phase III trial comparing hypofractionated RT alone or in combination with TMZ has completed accrual in Canada, Europe, and Japan101; the results are eagerly awaited to further inform whether combination therapy is effective in this patient population.
Clinical Case Relevance
The patient had excellent performance status and was felt to be a candidate for standard chemoradiotherapy, despite his age of 73. He developed mild nausea and moderate fatigue during chemoradiotherapy, but no life-threatening toxicities. He likewise tolerated adjuvant TMZ well from a symptomatic standpoint, though several cycles had to be briefly delayed due to mild thrombocytopenia. He discontinued TMZ therapy and moved to an observational phase following the sixth adjuvant cycle. Had he not been judged a good candidate for standard therapy, a TMZ-only approach, sparing radiation, would have been an acceptable alternative for an elderly man with an MGMT-methylated tumor.
Follow-up Imaging
Initial Postoperative Imaging
Postoperative imaging is strongly recommended within the first 48 hours following surgical resection in order to establish a postoperative baseline. If MRI is obtained, it is important to compare precontrast T1-weighted images with post-contrast T1-weighted images in order to detect true residual enhancement, as blood products in or around the surgical bed are usually present and may cause T1 shortening on both pre-gadolinium and post-gadolinium images. It is also crucial to perform DWI in order to detect any perioperative infarction, which may subsequently gain enhancement in the subacute phase, lose restricted diffusion, and present as a troubling, new enhancing lesion on subsequent MRI follow-ups.102
Long-term Follow-up
Generally, patients undergo monthly clinical evaluation and blood work during adjuvant TMZ, with MRI every other month. After the completion of TMZ, imaging is recommended every 2 to 3 months until 2 years from the end of treatment. After this time, MRI can be performed less often, provided the patient and physician are comfortable with this approach. Regardless of duration of disease-free survival, clinical evaluation and imaging should take place on at least an annual basis.
It is expected that the imaging appearance of a treated glioblastoma will evolve over time. One hopes for tumor regression after chemoradiation, but a temporary worsening of imaging findings, including increased contrast enhancement and edema signal with mass effect, is very common as a reaction to treated and dying tumor. This phenomenon is known as pseudoprogression, named so because the MRI appearance may be identical to tumor progression when, in fact, subsequent follow-up examinations with no change in therapy show regression of these imaging findings.103 There is, unfortunately, often no basis on which to discriminate post-treatment related enhancement from enhancement related to viable tumor using standard morphological MRI.
Pseudoprogression may be more common with the combined use of TMZ with RT, it is more common in those with MGMT promoter methylation, and it has been associated with an improved clinical outcome in some cohorts.104,105 The incidence of pseudoprogression is on the order of 30%,106 depending on how it is judged, and most pseudoprogression occurs within 3 months of the end of RT. However, pseudoprogression occurring after this 3-month period but within the first year is not uncommon, particularly in those with MGMT promoter methylation.107
The Macdonald criteria has been used as a framework for judging glioblastoma progression or regression, relying mainly upon 2-dimensional maximal diameters of contrast-enhancing lesions.108 In 2010, the Response Assessment in Neurooncology (RANO) criteria were published, updating the Macdonald criteria in several important ways, while maintaining a reliance upon the product of perpendicular diameters of contrast-enhancing lesions as an indicator of tumor size and status.109 One very important caveat in the RANO criteria is that, because of the common occurrence of pseudoprogression with modern chemoradiation therapy, a radiographic diagnosis of tumor progression cannot be made within the first 3 months of the end of chemoradiation if an enlarging, enhancing lesion is within the high-dose field of RT. Any apparent tumor progression within the first 3 months after radiation must be closely followed to differentiate early tumor progression from pseudoprogression. Because pseudoprogression occasionally occurs beyond 3 months from the end of radiotherapy, some would advocate early follow-up scans to confirm or deny pseudoprogression if there is apparent radiographic progression occurring even beyond the 3-month window after radiotherapy. RANO also allows for the determination of progression when there is nonenhancing tumoral progression, as nonenhancing tumor progression is not uncommon.6 New, discrete, masslike abnormalities or new cortical expansion with T2 lengthening suggest nonenhancing tumor progression per RANO criteria. Unfortunately, distinguishing nonenhancing tumor from other treatment-related effects and edema is often difficult or impossible.
Given the complexity of MRI interpretation, other means of glioblastoma treatment response assessment are needed. Estimates of tumor cell proliferation rates and invasion have been made through the analysis of MRI coupled with computational modeling as a means to monitor treated glioblastomas over time.110,111 Physiologic or mechanistic imaging techniques such as dynamic susceptibility contrast (DSC) perfusion imaging, dynamic contrast enhanced perfusion imaging (DCE), DWI, spectroscopy, and PET with various tracers also may aid in distinguishing progressing tumor from treatment-related changes such as pseudoprogression and radiation necrosis, but their implementation has to date been so variable site-to-site that these techniques have not been included in the RANO criteria.
Generally speaking, it is expected that CBV will be elevated with viable high-grade glioma but not radiation necrosis, and possibly not elevated with pseudoprogression, though data for pseudoprogression are as yet less clear.112–119 However, the variability in perfusion imaging has already been mentioned, there is often overlap between CBV in viable glioblastoma and radiation-induced changes, there is frequently an admixture of viable tumor and radiation-induced changes, and leakage of gadolinium into the interstitium breaks tracer-kinetic modeling assumptions and presents a challenge to accurate determination of CBV. In order to optimize CBV measurement, the use of a leakage-correction option is advised during image postprocessing. Many experts also recommend the use of a gadolinium preload prior to the acquisition of DSC images. Histogram and voxel-wise analyses of perfusion data hold promise for improving the differentiation between viable tumor and treatment-related changes, but they are postprocessing intensive.120,121 Given the frequent admixture of residual glioma and radiation-related changes, determination of a residual/recurrent tumor fraction would be desirable and such a metric using CBV has been shown to correlate with overall survival.122 Importantly, the relative change in lesion CBV over time may be crucial in judging tumoral stability or progression,123 though it bears stating explicitly that uniformity in perfusion technique between time points is mandatory for the best chance at fair comparisons.
DCE perfusion imaging, which is generally more technically demanding than DSC perfusion imaging, also holds promise for differentiating recurrent glioblastoma from treatment-related changes, using metrics such as the volume transfer coefficient ktrans and initial area under the curve.118,124–126 MR spectroscopy can be technically challenging to perform well and interpret, but after therapy choline and lipid and lactate levels have been shown to correlate with glioma outcomes.119,127 More data on the value of MR spectroscopy in the glioblastoma post-treatment setting is needed. ADC values from DWI imaging, particularly when using advanced analysis techniques such as histogram analysis and functional diffusion maps (fDMs),128–133 may also help to differentiate recurrent tumor from treatment-related changes. Amino acid PET may also aid in this discrimination better than with FDG PET but these techniques need further evaluation.134–138 Finally, it is likely that multiparametric approaches with advanced MRI techniques will add to assessment of glioblastoma treatment response.119
Clinical Case Relevance
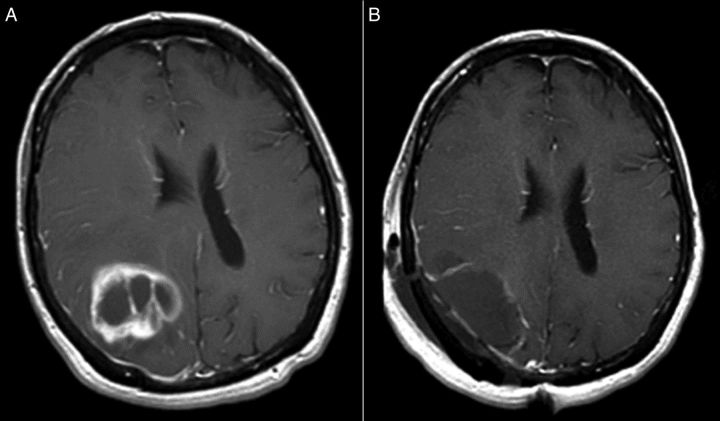
The first MRI following the completion of chemoradiotherapy, shown in Fig. 4A, demonstrated a rim of contrast enhancement around the resection cavity that had not been visible on the initial postoperative imaging (Fig. 2B). The patient was asymptomatic, the changes were suspected to be treatment-related, and no change of plan was recommended. He remained clinically and radiographically stable for nearly 2 years until he had tumor progression along the medial margin of his resection cavity, as shown in Fig. 4B. His KPS at the time of progression was 80, due to increased left-sided weakness, but he was still able to live independently with his wife. After discussing options including continuing to focus on aggressive tumor treatment, potentially at the cost of short-term quality of life, vs prioritizing quality of life and supportive care with hospice, he chose to pursue salvage chemotherapy, the details of which are beyond the scope of this review.
Fig. 4.
(A) The first MRI following chemoradiotherapy and (B) MRI evidence of tumor progression approximately 2 years later. Both are post-gadolinium spin echo T1-weighted images.
Prognosis and Survivorship
Overall Survival
Survival after diagnosis of glioblastoma has been steadily improving over the course of the last decade, for reasons both known and unknown. The widespread adoption of TMZ for newly diagnosed glioblastoma in and after 2005 was associated with in an increase in population-level survival.139 Likewise, though a survival benefit has yet to be demonstrated in a randomized prospective trial, population-based data suggest that survival also improved after the FDA approval of bevacizumab for recurrent glioblastoma.140 Additional improvements in the survival of patients with glioblastoma may be due to incremental improvement in surgery, radiation, and supportive care.
The median survival figure of 14.6 months from the pivotal TMZ trial is often shared with patients with newly diagnosed glioblastoma, but clinical trial median survival numbers have little relevance when predicting the specific prognosis of an individual patient. Many factors can significantly impact survival, including but not limited to age at diagnosis, performance status, extent of resection, MGMT methylation status, and IDH mutation status. Median survival by MGMT methylation status in recent phase III trials is shown in Table 1. A number of survival prognostication systems have been published, but again these are more relevant to cohorts than patients as individuals. In the long-term follow-up of the pivotal phase III TMZ trial, among patients treated with radiation and TMZ, 27.2% were still alive at 2 years, 16.0% at 3 years, 12.1% at 4 years, and 9.8% at 5 years.141 Survival to 10 years or longer is very rare.
Table 1.
Survival by MGMT methylation status in recent phase III trials for newly diagnosed glioblastoma
| Trial | MGMT Status | OS Median (95% CI) | PFS Median (95% CI) |
|---|---|---|---|
| EORTC 26981/22981 and NCIC trial CE.3a, 69,141 | Unmethylated | 12.6 (11.6–14.4) | 5.3 (not reported) |
| Methylated | 23.4 (18.6–32.8) | 10.9 (not reported) | |
| RTOG 052585 | Unmethylated | 14.0 (12.9–14.7) | 5.7 (5.1–6.1) |
| Methylated | 21.2 (17.9–24.8) | 8.7 (6.6–11.2) | |
| RTOG 082598 | Unmethylated | 14.3 (13.6–15.3) | 8.2 (7.5–9.2) |
| Methylated | 23.2 (20.1–28.3) | 14.1 (10.5–16.1) |
aOS statistics from Stupp Lancet Oncology 2009; PFS statistics from Hegi NEJM 2005. All statistics regard the RT + TMZ trial arm.
Abbreviations: MGMT, O(6)-methylguanine-DNA methyltransferase; OS, overall survival; PFS, progression-free survival; EORTC, European Organisation for Research and Treatment of Cancer; NCIC, National Cancer Institute of Canada; RTOG, Radiation Therapy Oncology Group.
Because glioblastoma is an intrinsically fatal diagnosis, and treatment can cause significant side effects, most tumor treatment plans require the sacrifice of some quality of life in the short term in the hope of gaining duration of life in the longer term. Early in the course of disease, this is a trade-off that most patients readily accept, though some choose to forgo aggressive therapy. With each successive treatment, the expected duration of benefit tends to decrease and toxicity may increase, so the merits of focusing purely on quality of life should be readdressed regularly, for example at the time of clinical or radiographic progression. When the service is available, patients may benefit from speaking with a palliative care physician early in the course of their disease, and maintaining the relationship until the end of their lives. While death may occur precipitously as the result of a pulmonary embolism or intracerebral hemorrhage, most patients die after weeks or rarely months of progressive decline following the decision to discontinue aggressive tumor therapy, and hospice services can be extremely valuable to patients and their families in this situation.
Supportive Care (Long-term)
Antiepileptic Therapy
Most patients with glioblastoma who have experienced a seizure require lifelong AED therapy, particularly if seizures occur during or after initial tumor therapy. There is variability in practice regarding patients who experienced seizure at initial presentation and are seizure-free on anti-epileptic therapy following tumor treatment. Many neuro-oncologists recommend lifelong AED therapy in this situation as well, whereas others will consider tapering patients off of antiepileptic therapy after 1 to 2 years if he patient is interested in doing so and electroencephalogram (EEG) at that time does not demonstrate epileptiform discharges. Of course, freedom from seizures cannot be guaranteed, with or without continuation of antiepileptic therapy. Patients electing to attempt to discontinue AEDs should be counseled about seizure safety and avoiding high-risk activities.
Anticoagulation
Patients with glioblastoma are at significant risk of venous thromboembolism and related complications. There is no proven role for prophylactic anticoagulant therapy to prevent deep venous thrombosis (DVT).142 Instead, patients should be educated about the symptoms of DVT and pulmonary embolism (PE), and physicians should have a low threshold for obtaining confirmatory testing if these symptoms occur. The diagnosis of glioblastoma is not a contraindication for treatment with systemic anticoagulation if a DVT/PE occurs, even in the setting of antiangiogenic therapy.143 Treatment with low molecular weight heparin products has been shown to be more effective that oral anticoagulation with warfarin in patients with cancer.144
Psychological and Emotional Well-being
Symptoms of depression and anxiety are common and undertreated in patients with glioblastoma. Many factors may contribute to depression in patients with glioblastoma, including loss of independence and function, the adjustment to the idea of a significantly shortened life, and possibly a direct biological effect of the tumor on neurotransmitter signaling.145 Many patients and their families find cancer support groups, or ideally brain-tumor-specific support groups, very beneficial both for practical advice and the knowledge that they are not alone in facing this diagnosis. Antidepressant therapy and/or referral to a mental health professional should be considered for patients with symptoms that extend beyond the range of normal adjustment and negatively impact the quality of their lives.
Conclusion
Despite recent progress, glioblastoma remains an incurable tumor with survival under a year and a half for most patients. Multidisciplinary care is necessary to maximize survival time and preserve quality of life. In appropriately selected patients, aggressive surgery may relieve symptoms and prolong survival. Medical oncologists, radiation oncologists, and neurologists work as a team to design and deliver the initial treatment plan, typically involving the combination of RT and TMZ chemotherapy. Radiologists with expertise in the complexities of glioblastoma imaging help make the initial diagnosis and monitor response to therapy.
For glioblastoma survival to continue to improve, advances in each of these specialties and collaboration between specialties will be required. Advances in neurosurgical technique aided by advanced imaging modalities will allow for more extensive safe tumor resection at time of diagnosis and at time of recurrence. Ongoing clinical trials may help refine the long-recognized role of RT for the treatment of newly diagnosed glioblastoma in general and also define its role relative to that of TMZ in elderly patients. Novel therapeutic strategies, recently with an emphasis on molecularly targeted therapies and immunotherapeutic approaches, also hold the potential to significantly change the care of glioblastoma.
Funding
None.
Conflict of interest statement. No conflicts of interest are reported for any author.
References
- 1. Rosati A, Buttolo L, Stefini R et al. . Efficacy and safety of levetiracetam in patients with glioma: a clinical prospective study. Arch Neurol. 2010;67(3):343–346. [DOI] [PubMed] [Google Scholar]
- 2. Barker CA, Bishop AJ, Chang M et al. . Valproic acid use during radiation therapy for glioblastoma associated with improved survival. Int J Radiat Oncol Biol Phys. 2013;86(3):504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bobustuc GC, Baker CH, Limaye A et al. . Levetiracetam enhances p53-mediated MGMT inhibition and sensitizes glioblastoma cells to temozolomide. Neuro Oncol. 2010;12(9):917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glantz MJ, Cole BF, Forsyth PA et al. . Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(10):1886–1893. [DOI] [PubMed] [Google Scholar]
- 5. Health USNIo. Lacosamide for Seizure Prophylaxis in High-Grade Gliomas. https://clinicaltrials.gov/ct2/show/NCT01432171 Accessed February 1, 2015.
- 6. Chamberlain MC. Radiographic patterns of relapse in glioblastoma. J Neurooncol. 2011;101(2):319–323. [DOI] [PubMed] [Google Scholar]
- 7. Blanchet L, Krooshof PW, Postma GJ et al. . Discrimination between metastasis and glioblastoma multiforme based on morphometric analysis of MR images. AJNR Am J Neuroradiol. 2011;32(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackson A, Kassner A, Annesley-Williams D et al. . Abnormalities in the recirculation phase of contrast agent bolus passage in cerebral gliomas: comparison with relative blood volume and tumor grade. AJNR Am J Neuroradiol. 2002;23(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- 9. Law M, Yang S, Babb JS et al. . Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2004;25(5):746–755. [PMC free article] [PubMed] [Google Scholar]
- 10. Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27(4):859–867. [PMC free article] [PubMed] [Google Scholar]
- 11. Liu ZL, Zhou Q, Zeng QS et al. . Noninvasive evaluation of cerebral glioma grade by using diffusion-weighted imaging-guided single-voxel proton magnetic resonance spectroscopy. J Int Med Res. 2012;40(1):76–84. [DOI] [PubMed] [Google Scholar]
- 12. Wang S, Kim S, Chawla S et al. . Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2011;32(3):507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cha S, Lupo JM, Chen MH et al. . Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol. 2007;28(6):1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chawla S, Zhang Y, Wang S et al. . Proton magnetic resonance spectroscopy in differentiating glioblastomas from primary cerebral lymphomas and brain metastases. J Comput Assist Tomogr. 2010;34(6):836–841. [DOI] [PubMed] [Google Scholar]
- 15. Server A, Josefsen R, Kulle B et al. . Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors. Acta Radiol. 2010;51(3):316–325. [DOI] [PubMed] [Google Scholar]
- 16. Radbruch A, Wiestler B, Kramp L et al. . Differentiation of glioblastoma and primary CNS lymphomas using susceptibility weighted imaging. Eur J Radiol. 2013;82(3):552–556. [DOI] [PubMed] [Google Scholar]
- 17. Deistung A, Schweser F, Wiestler B et al. . Quantitative susceptibility mapping differentiates between blood depositions and calcifications in patients with glioblastoma. PloS One. 2013;8(3):e57924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kickingereder P, Wiestler B, Sahm F et al. . Primary central nervous system lymphoma and atypical glioblastoma: multiparametric differentiation by using diffusion-, perfusion-, and susceptibility-weighted MR imaging. Radiology. 2014;272(3):843–850. [DOI] [PubMed] [Google Scholar]
- 19. Pope WB. Genomics of brain tumor imaging. Neuroimaging Clin N Am. 2015;25(1):105–119. [DOI] [PubMed] [Google Scholar]
- 20. Willats L, Calamante F. The 39 steps: evading error and deciphering the secrets for accurate dynamic susceptibility contrast MRI. NMR Biomed. 2013;26(8):913–931. [DOI] [PubMed] [Google Scholar]
- 21. Laws ER, Parney IF, Huang W et al. . Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467–473. [DOI] [PubMed] [Google Scholar]
- 22. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764; discussion 264–756. [DOI] [PubMed] [Google Scholar]
- 23. McGirt MJ, Chaichana KL, Gathinji M et al. . Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. [DOI] [PubMed] [Google Scholar]
- 24. di Russo P, Perrini P, Pasqualetti F et al. . Management and outcome of high-grade multicentric gliomas: a contemporary single-institution series and review of the literature. Acta Neurochir (Wien). 2013;155(12):2245–2251. [DOI] [PubMed] [Google Scholar]
- 25. Sanai N, Polley MY, Berger MS. Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg. 2010;112(1):1–9. [DOI] [PubMed] [Google Scholar]
- 26. Holdhoff M, Rosner GL, Alcorn S et al. . ‘Elderly’ patients with newly diagnosed glioblastoma deserve optimal care. J Neurooncol. 2013;113(2):343–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang SM, Nelson S, Vandenberg S et al. . Integration of preoperative anatomic and metabolic physiologic imaging of newly diagnosed glioma. J Neurooncol. 2009;92(3):401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Farshidfar Z, Faeghi F, Mohseni M et al. . Diffusion tensor tractography in the presurgical assessment of cerebral gliomas. Neuroradiol J. 2014;27(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gempt J, Soehngen E, Forster S et al. . Multimodal imaging in cerebral gliomas and its neuropathological correlation. Eur J Radiol. 2014;83(5):829–834. [DOI] [PubMed] [Google Scholar]
- 30. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18–27. [DOI] [PubMed] [Google Scholar]
- 31. De Witt Hamer PC, Robles SG, Zwinderman AH et al. . Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–2565. [DOI] [PubMed] [Google Scholar]
- 32. Maldaun MV, Khawja SN, Levine NB et al. . Awake craniotomy for gliomas in a high-field intraoperative magnetic resonance imaging suite: analysis of 42 cases. J Neurosurg. 2014;121(4):810–817. [DOI] [PubMed] [Google Scholar]
- 33. Hatiboglu MA, Weinberg JS, Suki D et al. . Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis. Neurosurgery. 2009;64(6):1073–1081; discussion 1081. [DOI] [PubMed] [Google Scholar]
- 34. Acerbi F, Broggi M, Eoli M et al. . Fluorescein-guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: preliminary results in 12 cases. Acta Neurochir (Wien). 2013;155(7):1277–1286. [DOI] [PubMed] [Google Scholar]
- 35. Bi WL, Laws ER Jr. Searching for the light: fluorescence guidance in glioma resection. World Neurosurg. 2014;82(1–2):54–55. [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Rey-Dios R, Roberts DW et al. . Intraoperative fluorescence-guided resection of high-grade gliomas: a comparison of the present techniques and evolution of future strategies. World Neurosurg. 2014;82(1–2):175–185. [DOI] [PubMed] [Google Scholar]
- 37. Zhao S, Wu J, Wang C et al. . Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies. PloS One. 2013;8(5):e63682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stummer W, Pichlmeier U, Meinel T et al. . Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 39. Attenello FJ, Mukherjee D, Datoo G et al. . Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15(10):2887–2893. [DOI] [PubMed] [Google Scholar]
- 40. Brem H, Piantadosi S, Burger PC et al. . Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–1012. [DOI] [PubMed] [Google Scholar]
- 41. Chang SM, Parney IF, McDermott M et al. . Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98(6):1175–1181. [DOI] [PubMed] [Google Scholar]
- 42. Sawaya R, Hammoud M, Schoppa D et al. . Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42(5):1044–1055; discussion 1055–1046. [DOI] [PubMed] [Google Scholar]
- 43. Marcus LP, McCutcheon BA, Noorbakhsh A et al. . Incidence and predictors of 30-day readmission for patients discharged home after craniotomy for malignant supratentorial tumors in California (1995–2010). J Neurosurg. 2014;120(5):1201–1211. [DOI] [PubMed] [Google Scholar]
- 44. Louis DN, Ohgaki H, Wiestler OD et al. . The 2007 WHO classification of tumours of the central nervous system. Vol 114 2007/07/10 ed2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parsons DW, Jones S, Zhang X et al. . An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hartmann C, Meyer J, Balss J et al. . Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–474. [DOI] [PubMed] [Google Scholar]
- 47. Sugawa N, Ekstrand AJ, James CD et al. . Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci USA. 1990;87(21):8602–8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ohgaki H, Dessen P, Jourde B et al. . Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64(19):6892–6899. [DOI] [PubMed] [Google Scholar]
- 49. Brennan CW, Verhaak RG, McKenna A et al. . The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Louis DN, von Deimling A, Chung RY et al. . Comparative study of p53 gene and protein alterations in human astrocytic tumors. J Neuropathol Exp Neurol. 1993;52(1):31–38. [DOI] [PubMed] [Google Scholar]
- 51. Yan H, Parsons DW, Jin G et al. . IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. SongTao Q, Lei Y, Si G et al. . IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. 2012;103(2):269–273. [DOI] [PubMed] [Google Scholar]
- 53. Hartmann C, Hentschel B, Wick W et al. . Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–718. [DOI] [PubMed] [Google Scholar]
- 54. Bujko M, Kober P, Matyja E et al. . Prognostic value of IDH1 mutations identified with PCR-RFLP assay in glioblastoma patients. Mol Diagn Ther. 2010;14(3):163–169. [DOI] [PubMed] [Google Scholar]
- 55. Yan W, Zhang W, You G et al. . Correlation of IDH1 mutation with clinicopathologic factors and prognosis in primary glioblastoma: a report of 118 patients from China. PloS One. 2012;7(1):e30339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Metellus P, Coulibaly B, Colin C et al. . Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010;120(6):719–729. [DOI] [PubMed] [Google Scholar]
- 57. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. von Deimling A, Eibl RH, Ohgaki H et al. . p53 mutations are associated with 17p allelic loss in grade II and grade III astrocytoma. Cancer Res. 1992;52(10):2987–2990. [PubMed] [Google Scholar]
- 59. Newcomb EW, Cohen H, Lee SR et al. . Survival of patients with glioblastoma multiforme is not influenced by altered expression of p16, p53, EGFR, MDM2 or Bcl-2 genes. Brain Pathol. 1998;8(4):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Simon M, Hosen I, Gousias K et al. . TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015;17(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nonoguchi N, Ohta T, Oh JE et al. . TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013;126(6):931–937. [DOI] [PubMed] [Google Scholar]
- 62. Labussiere M, Boisselier B, Mokhtari K et al. . Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83(13):1200–1206. [DOI] [PubMed] [Google Scholar]
- 63. Bello MJ, Alonso ME, Aminoso C et al. . Hypermethylation of the DNA repair gene MGMT: association with TP53 G:C to A:T transitions in a series of 469 nervous system tumors. Mutat Res. 2004;554(1–2):23–32. [DOI] [PubMed] [Google Scholar]
- 64. Nakagawachi T, Soejima H, Urano T et al. . Silencing effect of CpG island hypermethylation and histone modifications on O6-methylguanine-DNA methyltransferase (MGMT) gene expression in human cancer. Oncogene. 2003;22(55):8835–8844. [DOI] [PubMed] [Google Scholar]
- 65. Esteller M, Garcia-Foncillas J, Andion E et al. . Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350–1354. [DOI] [PubMed] [Google Scholar]
- 66. Thon N, Eigenbrod S, Grasbon-Frodl EM et al. . Predominant influence of MGMT methylation in non-resectable glioblastoma after radiotherapy plus temozolomide. J Neurol Neurosurg Psychiatry. 2011;82(4):441–446. [DOI] [PubMed] [Google Scholar]
- 67. Rivera AL, Pelloski CE, Gilbert MR et al. . MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010;12(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ishii D, Natsume A, Wakabayashi T et al. . Efficacy of temozolomide is correlated with 1p loss and methylation of the deoxyribonucleic acid repair gene MGMT in malignant gliomas. Neurol Med Chir (Tokyo). 2007;47(8):341–349; discussion 350. [DOI] [PubMed] [Google Scholar]
- 69. Hegi ME, Diserens AC, Gorlia T et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 70. Mulholland S, Pearson DM, Hamoudi RA et al. . MGMT CpG island is invariably methylated in adult astrocytic and oligodendroglial tumors with IDH1 or IDH2 mutations. Int J Cancer. 2012;1315:1104–1113. [DOI] [PubMed] [Google Scholar]
- 71. Phillips HS, Kharbanda S, Chen R et al. . Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 72. Verhaak RG, Hoadley KA, Purdom E et al. . Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Huse JT, Phillips HS, Brennan CW. Molecular subclassification of diffuse gliomas: seeing order in the chaos. Glia. 2011;59(8):1190–1199. [DOI] [PubMed] [Google Scholar]
- 74. Noushmehr H, Weisenberger DJ, Diefes K et al. . Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Louis DN, Perry A, Burger P et al. . International Society Of Neuropathology–Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(5):429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ostrom QT, Gittleman H, Farah P et al. . CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Howlader N, Noone AM, Krapcho M et al. (eds). SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. [Google Scholar]
- 78. Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99(20):1544–1550. [DOI] [PubMed] [Google Scholar]
- 79. Ostrom QT, Bauchet L, Davis FG et al. . The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stupp RR, Mason WPWP, van den Bent MJMJ et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 81. Shapiro WR, Young DF. Treatment of malignant glioma. A controlled study of chemotherapy and irradiation. Arch Neurol. 1976;33(7):494–450. [DOI] [PubMed] [Google Scholar]
- 82. Walker MD, Alexander E Jr, Hunt WE et al. . Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49(3):333–343. [DOI] [PubMed] [Google Scholar]
- 83. Salazar OM, Rubin P, Feldstein ML et al. . High dose radiation therapy in the treatment of malignant gliomas: final report. Int J Radiat Oncol Biol Phys. 1979;5(10):1733–1740. [DOI] [PubMed] [Google Scholar]
- 84. Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5(10):1725–1731. [DOI] [PubMed] [Google Scholar]
- 85. Gilbert MR, Wang M, Aldape KD et al. . Dose-Dense Temozolomide for Newly Diagnosed Glioblastoma: A Randomized Phase III Clinical Trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kita M, Okawa T, Tanaka M et al. . [Radiotherapy of malignant glioma--prospective randomized clinical study of whole brain vs local irradiation]. Gan No Rinsho. 1989;35(11):1289–1294. [PubMed] [Google Scholar]
- 87. Levin VA, Bidaut L, Hou P et al. . Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Crossen JR, Garwood D, Glatstein E et al. . Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12(3):627–642. [DOI] [PubMed] [Google Scholar]
- 89. Gondi V, Hermann BP, Mehta MP et al. . Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85(2):348–354. [DOI] [PubMed] [Google Scholar]
- 90. Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31(4):983–998. [DOI] [PubMed] [Google Scholar]
- 91. Malmström A, Grønberg BH, Marosi C et al. . Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 92. Wick W, Platten M, Meisner C et al. . Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 93. Ferguson M, Rodrigues G, Cao J et al. . Management of high-grade gliomas in the elderly. Semin Radiat Oncol. 2014;24(4):279–288. [DOI] [PubMed] [Google Scholar]
- 94. Novocure. Novocure Announces the EF-14 Phase III Clinical Trial of Tumor Treating Fields in Patients with Newly Diagnosed Glioblastoma has been Terminated at the Interim Analysis due to Early Success. http://www.novocure.com/~/media/Files/N/Novocure/press-release/2014/201408-EF14-Trial-Results-Press-Release.pdf Accessed February 1, 2015.
- 95. Stupp R, Hegi ME, Gorlia T et al. . Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 96. Nabors LB, Fink KL, Mikkelsen T et al. . Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro Oncol. 2015;175:708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chinot OL, Wick W, Mason W et al. . Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 98. Gilbert MR, Dignam JJ, Armstrong TS et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Health USNIo. Study of a Drug [DCVax®-L] to Treat Newly Diagnosed GBM Brain Cancer. https://clinicaltrials.gov/ct2/show/NCT00045968 Accessed February 1, 2015.
- 100. Swartz AM, Li QJ, Sampson JH. Rindopepimut: a promising immunotherapeutic for the treatment of glioblastoma multiforme. Immunotherapy. 2014;6(6):679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Health USNIo. Radiation Therapy With or Without Temozolomide in Treating Older Patients With Newly Diagnosed Glioblastoma Multiforme. https://www.clinicaltrials.gov/ct2/show/NCT00045968. Accessed February 1, 2015.
- 102. Smith JS, Cha S, Mayo MC et al. . Serial diffusion-weighted magnetic resonance imaging in cases of glioma: distinguishing tumor recurrence from postresection injury. J Neurosurg. 2005;103(3):428–438. [DOI] [PubMed] [Google Scholar]
- 103. Hygino da Cruz LC Jr, Rodriguez I, Domingues RC et al. . Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32(11):1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Brandes AA, Franceschi E, Tosoni A et al. . MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 105. Gerstner ER, McNamara MB, Norden AD et al. . Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol. 2009;94(1):97–101. [DOI] [PubMed] [Google Scholar]
- 106. Brandsma D, Stalpers L, Taal W et al. . Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 107. Stuplich M, Hadizadeh DR, Kuchelmeister K et al. . Late and prolonged pseudoprogression in glioblastoma after treatment with lomustine and temozolomide. J Clin Oncol. 2012;30(21):e180–e183. [DOI] [PubMed] [Google Scholar]
- 108. Macdonald DR, Cascino TL, Schold SC Jr et al. . Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 109. Wen PY, Macdonald DR, Reardon DA et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 110. Zaw TM, Pope WB, Cloughesy TF, Lai A et al. . Short-interval estimation of proliferation rate using serial diffusion MRI predicts progression-free survival in newly diagnosed glioblastoma treated with radiochemotherapy. J Neurooncol. 2014;116(3):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Neal ML, Trister AD, Cloke T et al. . Discriminating survival outcomes in patients with glioblastoma using a simulation-based, patient-specific response metric. PloS One. 2013;8(1):e51951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Barajas RF Jr, Chang JS, Segal MR et al. . Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009;253(2):486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kim YH, Oh SW, Lim YJ et al. . Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg. 2010;112(9):758–765. [DOI] [PubMed] [Google Scholar]
- 114. Sugahara T, Korogi Y, Tomiguchi S et al. . Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol. 2000;21(5):901–909. [PMC free article] [PubMed] [Google Scholar]
- 115. Hu LS, Baxter LC, Smith KA et al. . Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009;30(3):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mangla R, Singh G, Ziegelitz D et al. . Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology. 2010;256(2):575–584. [DOI] [PubMed] [Google Scholar]
- 117. Gahramanov S, Raslan AM, Muldoon LL et al. . Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys. 2011;79(2):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kim HS, Goh MJ, Kim N et al. . Which combination of MR imaging modalities is best for predicting recurrent glioblastoma? Study of diagnostic accuracy and reproducibility. Radiology. 2014;273(3):831–843. [DOI] [PubMed] [Google Scholar]
- 119. Seeger A, Braun C, Skardelly M et al. . Comparison of three different MR perfusion techniques and MR spectroscopy for multiparametric assessment in distinguishing recurrent high-grade gliomas from stable disease. Acad Radiol. 2013;20(12):1557–1565. [DOI] [PubMed] [Google Scholar]
- 120. Kim HS, Kim JH, Kim SH et al. . Posttreatment high-grade glioma: usefulness of peak height position with semiquantitative MR perfusion histogram analysis in an entire contrast-enhanced lesion for predicting volume fraction of recurrence. Radiology. 2010;256(3):906–915. [DOI] [PubMed] [Google Scholar]
- 121. Tsien C, Galban CJ, Chenevert TL et al. . Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J Clin Oncol. 2010;28(13):2293–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hu LS, Eschbacher JM, Heiserman JE et al. . Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro Oncol. 2012;14(7):919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Boxerman JL, Ellingson BM, Jeyapalan S et al. . Longitudinal DSC-MRI for Distinguishing Tumor Recurrence From Pseudoprogression in Patients With a High-grade Glioma. Am J Clin Oncol. 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 124. Suh CH, Kim HS, Choi YJ et al. . Prediction of pseudoprogression in patients with glioblastomas using the initial and final area under the curves ratio derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. AJNR Am J Neuroradiol. 2013;34(12):2278–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yun TJ, Park CK, Kim TM et al. . Glioblastoma Treated with Concurrent Radiation Therapy and Temozolomide Chemotherapy: Differentiation of True Progression from Pseudoprogression with Quantitative Dynamic Contrast-enhanced MR Imaging. Radiology. 2015;2743:830–840. [DOI] [PubMed] [Google Scholar]
- 126. Shin KE, Ahn KJ, Choi HS et al. . DCE and DSC MR perfusion imaging in the differentiation of recurrent tumour from treatment-related changes in patients with glioma. Clin Radiol. 2014;69(6):e264–e272. [DOI] [PubMed] [Google Scholar]
- 127. Li Y, Lupo JM, Parvataneni R et al. . Survival analysis in patients with newly diagnosed glioblastoma using pre- and postradiotherapy MR spectroscopic imaging. Neuro Oncol. 2013;15(5):607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ellingson BM, Malkin MG, Rand SD et al. . Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging. 2010;31(3):538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ellingson BM, Malkin MG, Rand SD et al. . Volumetric analysis of functional diffusion maps is a predictive imaging biomarker for cytotoxic and anti-angiogenic treatments in malignant gliomas. J Neurooncol. 2011;102(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Moffat BA, Chenevert TL, Lawrence TS et al. . Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci USA 2005;102(15):5524–5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ellingson BM, Cloughesy TF, Lai A et al. . Quantitative probabilistic functional diffusion mapping in newly diagnosed glioblastoma treated with radiochemotherapy. Neuro Oncol. 2013;15(3):382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pope WB, Qiao XJ, Kim HJ et al. . Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol. 2012;108(3):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lutz K, Wiestler B, Graf M et al. . Infiltrative patterns of glioblastoma: Identification of tumor progress using apparent diffusion coefficient histograms. J Magn Reson Imaging. 2014;39(5):1096–1103. [DOI] [PubMed] [Google Scholar]
- 134. Terakawa Y, Tsuyuguchi N, Iwai Y et al. . Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49(5):694–699. [DOI] [PubMed] [Google Scholar]
- 135. Rachinger W, Goetz C, Popperl G et al. . Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57(3):505–511; discussion 505–511. [DOI] [PubMed] [Google Scholar]
- 136. Mehrkens JH, Popperl G, Rachinger W et al. . The positive predictive value of O-(2-[18F]fluoroethyl)-L-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment. J Neurooncol. 2008;88(1):27–35. [DOI] [PubMed] [Google Scholar]
- 137. Chen W, Silverman DH, Delaloye S et al. . 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47(6):904–911. [PubMed] [Google Scholar]
- 138. Ledezma CJ, Chen W, Sai V et al. . 18F-FDOPA PET/MRI fusion in patients with primary/recurrent gliomas: initial experience. Eur J Radiol. 2009;71(2):242–248. [DOI] [PubMed] [Google Scholar]
- 139. Darefsky AS, King JT Jr, Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer. 2012;118(8):2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Johnson DR, Leeper HE, Uhm JH. Glioblastoma survival in the United States improved after Food and Drug Administration approval of bevacizumab: a population-based analysis. Cancer. 2013;119(19):3489–3495. [DOI] [PubMed] [Google Scholar]
- 141. Stupp R, Hegi ME, Mason WP et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 142. Jo JT, Schiff D, Perry JR. Thrombosis in brain tumors. Semin Thromb Hemost. 2014;40(3):325–331. [DOI] [PubMed] [Google Scholar]
- 143. Norden AD, Bartolomeo J, Tanaka S et al. . Safety of concurrent bevacizumab therapy and anticoagulation in glioma patients. J Neurooncol. 2012;106(1):121–125. [DOI] [PubMed] [Google Scholar]
- 144. Lee AY, Levine MN, Baker RI et al. . Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153. [DOI] [PubMed] [Google Scholar]
- 145. Liu R, Page M, Solheim K et al. . Quality of life in adults with brain tumors: current knowledge and future directions. Neuro Oncol. 2009;11(3):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]