Abstract
Craniopharyngioma is a rare tumor that is expected to occur in ∼400 patients/year in the United States. While surgical resection is considered to be the primary treatment when a patient presents with a craniopharyngioma, only 30% of such tumors present in locations that permit complete resection. Radiotherapy has been used as both primary and adjuvant therapy in the treatment of craniopharyngiomas for over 50 years. Modern radiotherapeutic techniques, via the use of CT-based treatment planning and MRI fusion, have permitted tighter treatment volumes that allow for better tumor control while limiting complications. Modern radiotherapeutic series have shown high control rates with lower doses than traditionally used in the two-dimensional treatment era. Intracavitary radiotherapy with radio-isotopes and stereotactic radiosurgery may have a role in the treatment of recurrent cystic and solid recurrences, respectively. Recently, due to the exclusive expression of the Beta-catenin clonal mutations and the exclusive expression of BRAF V600E clonal mutations in the overwhelming majority of adamantinomatous and papillary tumors respectively, it is felt that inhibitors of each pathway may play a role in the future treatment of these rare tumors.
Keywords: craniopharyngioma, proton therapy, radiosurgery, review, surgery
Classification and Intracranial Location
Craniopharyngiomas are benign tumors of squamous epithelial origin and are believed to arise from the remnants of Rathke's pouch in and around the suprasellar region. These tumors can adhere to vital structures like the optic chiasm, optic nerves, major vessels of the circle of Willis, and the hypothalamus; consequently, complete surgical resection is often difficult. Craniopharyngiomas can be purely cystic or solid, but they are typically some combination. Histologically, they are classified as adamantinomatous or papillary.1–3
Methods and Materials
For this review of the most recent treatments and outcomes for craniopharyngioma, an updated literature search strategy was conducted using the PubMed.gov database (http://www.ncbi.nlm.nih.gov/pubmed) of the United States National Library of Medicine at the NIH.
For updated information regarding treatment and outcomes of craniopharyngioma with surgical excision, the PubMed/MEDLINE database (1992–2014) was searched using the search strategy (“surgery”) and (“craniopharyngioma”) and (“outcome”) and (“review”). For updated information regarding treatment and outcomes of craniopharyngioma with intracavitary radiation, the PubMed/MEDLINE database (1992–2014) was searched using the search strategy (“intracavitary radiation”) and (“craniopharyngioma”). For updated information regarding treatment and outcomes of craniopharyngioma with radiosurgery, the PubMed/MEDLINE database (1992–2014) was searched using the search strategy (“radiosurgery”) and (“craniopharyngioma”) and (“outcome”). For updated information regarding treatment and outcomes of craniopharyngioma with fractionated radiation, the PubMed/MEDLINE database (1992–2014) was searched using the search strategy (“radiotherapy”) and (“craniopharyngioma”) and (“outcome”) and (“review”).
Table 1 describes the outcomes of patients treated by surgical resection and includes details regarding the percentage receiving salvage therapy, the percentage receiving radiotherapy, postoperative mortality, recurrence rate, degrees of resection (subtotal or complete), and the surgical approach. Series with fewer than 50 participants were excluded. Table 2 describes the outcomes of patients treated by intracavitary radiation and includes details regarding the percentage receiving salvage therapy, isotopes used, recurrence rate (%), response rate (%), and median follow-up period (in years). Series with fewer than 20 participants were excluded. Table 3 describes the outcomes of patients treated by radiosurgery and includes details regarding the percentage receiving salvage therapy, the median follow-up period (in years), and degrees of local control (%). Table 4 describes the outcomes of patients treated by conventional radiotherapy and includes details regarding percentage receiving salvage therapy, median follow-up period (in years), and dose (Gy). In the series reported in Tables 3 and 4, Local control and progression-free survival (PFS) were used as an outcome variable as described in the original reports while recurrence rate was used as an outcome variable in the series reported in Tables 1 and 2 (evaluating surgical resection and intracavitary radiation treatment modalities). Series with fewer than 20 participants were excluded.
Table 1.
Outcomes for patients treated by surgical resection
| Institution | Patient# | Years | Follow-up (Median Years) | % Salvage | % Complete Resection (CR) | % Receiving Radiotherapy (RT) | Post-Op Mortality (%) | Treatment | Recurrence Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| Mayo (Duff et al, 2000)15 | 121 | 1974–1991 | 10 | 0 | 69, S,R | 20.7 | 1.7 | 66 CR 30 SR 3 CR/RT 22 SR/RT |
12/66 (18.2) 15/30 (50) 0 (0) 2/22 (9.1) |
| Erlangen (Fahlbusch et al, 1999)19 | 148 | 1983–1997 | 5.4 | 12.8 | 49.3, S,R | Unknown | 2.7 | 72CR 62 SR* 13 part |
8/72 (11.1) 51.2 9/13 (69.2) |
| Groupe Hospitalier Pitié-Salpêtrière (Van Effenterre and Boch, 2002)20 | 122 | 1975–2000 | 7 | 0 | 60.9 S,R | 0 | 2.4 | 71 CR 46 SR |
9/71 (12.7) 20/46 (43.5) |
| University of Pennsylvania (Stripp et al, 2004)21 | 75 | 1974–2001 | 7.6 | 0 | 64 S &/or R | 24 | 1.33 | 48 CR 9 SR 18 SR/RT |
25/48 (52.1) 7/9 (77.8) 3/18 (16.7) |
| Northwestern (Tomita and Bowman, 2005)22 | 54 | 1984–2003 | Unknown | 0 | 79.6 S 66.6 R |
14.8 | 0 | 33 CR-S,R 10 CR-S 8 SR/RT 3 SR |
9/27 (33.3) 9/10 (90.0) 3/8 (37.5) 3/3 (100) |
| UCSF (Schoenfeld et al, 2012)23 | 122 | 1980–2009 | 4.7 | 0 | 27 S,R | 41.8 | 8.2 | 30 CR 3 CR/RT 41 SR 48 SR/RT |
24.8** NS 63.8 26.7 |
| UCLA, YALE, University of Utah (Chakrabarti et al, 2005)24 |
86 | 1984–1994 | >5 | 0 | TN 90% radio TC 61% radio |
12.8 | 16.7 | TC CR 11 ST 7 ST + RT 6 TN CR 61 ST 7 ST + RT 5 |
TN 10% TC 22% TN/ST 43% TC/ST 43% |
| Brain Science Institute of Beijing (Shi et al, 2008)25 | 309 | 1996–2006 | 2 | 0 | 89.3 S,R | Unknown | 3.9 | 276 CR 20 SR |
13.7 75 |
| Okinaka Memorial Institute for Medical research, Toranomon Hospital (Yamada et al, 2010)26 | 90 | 1990–2008 | 4 0.6 | 0 | 77.8 S,R | 11.1 | 2.2 | 69 CR 8 SR 1CR/RT 9SR/RT |
7.8 total |
| University of Halle, University of Erlangen-Nuremberg, International Neuroscience Institute (Hoffman et al, 2012)27 | 73 | 1997–2012 | 2 | 0 | 83.1 R | Unknown | 0 | 60 CR | 7/60 (11.7) |
| University of Pittsburgh School of Medicine (Koutourousiou et al, 2013)28 | 64 | 1999–2011 | Mean = 2.2 | 0 | 37.5 R | 14 (9/64) | 14 | 24 CR 40 SR 9 ES/RT 55 ES |
25 (6/24) 40 (16/40) 33.3 (3/9) 34.5 (19/55) |
Abbreviations: ES, endoscopic endonasal surgery; S, complete resection noted at surgery; R, complete resection noted radiographically; CR, complete resection; PR, partial resection; ST, subtotal resection; RT, radiation therapy; TN, Transnasal surgical approach; TC, Transcranial surgical approach; SR, anything ≤95% tumor removal as defined within publication.
**Progression rate at two years.
Table 2.
Outcomes for patients treated with intracavitary radiation
| Institution | Number of Patients | Isotope(s) | Salvage (%) | Response Rate (%) | Follow-up (Median Years) | Recurrence Rate (%) |
|---|---|---|---|---|---|---|
| Erasmus (Van den Berge et al, 1992)30 | 31 | Y90 | 100 | N/A | 3.3 | 28.5 |
| Koln (Voges et al, 1997)31 | 78 | Y90, P32, 136RH | N/A | 79.5 | 11.9 | N/A |
| St. John's Hospital (Julow et al, 2007)32 | 73 | Y90 | 100 | 100 | 9.4 | 16.6 |
| Pittsburgh (Hasegawa et al, 2004)33 | 49 | P32 | 49.0 | 80 | 4 | 24% at 5 years 30% at 10 years |
| Indiana University (Barriger et al, 2011)34 | 22 | P32 | 36.3 | 42 | 5.2 | N/A |
| Shaheed Beheshti University of Medical Sciences, Tehran, Iran (Shahzadi et al, 2008)35 | 22 | P32 | N/A | 73 | 10.5 | N/A |
| Fourth Military Medical University, Xi'an, Shaanxi 710032, China (Zhao et al, 2010)36 | 20 | P32 | N/A | 90 | 3.9 | N/A |
Abbreviation: N/A, not available.
Table 3.
Outcomes for patients treated with radiosurgery
| Institution | Patients (n) | Salvage (%) | Follow-up (Median Years) | Local Control |
|---|---|---|---|---|
| Tapei (Chung et al, 2000)37 | 31 | 80.6 | 3.0 | 87% |
| University of Miami (Amendola et al, 2003)38 | 14 | 85.7 | 3.25 (mean) | 85.7% |
| Karolinska (Ulfarsson et al, 2002)39 | 21 | 70 | 3.5 | 33.3% |
| Komaki City Hospital (Kobayashi et al, 2005)40 | 98 | – | 5.25 | 60.8% at 5 years 53.8% at 10 years |
| University of Pittsburgh (Niranjan et al, 2010)41 | 46 | 93.5 | 5.18 | 91% at 1 year 81% at 2 years 68% at 3 years |
| UVA (Xu et al, 2011)42 | 37 | – | 4.16 | 84.8% (3-year PFS) 67% (5-year PFS) |
| Nagoya Kyoritsu Hospital, Japan (Kobayashi et al, 2009)43 | 98 | – | 5.5 (mean) | 79.6% |
Table 4.
Outcomes for patients treated with fractionated radiotherapy
| Institution | Number of Patients | Years | Follow-up (Median Years) | % Receiving Surgery | Salvage (%) | Dose (Gy) | Progression-free Survival |
|---|---|---|---|---|---|---|---|
| Harvard (Tarbell et al, 1996)46 | 21 | 1992–1995 | 1.3 | 100% | – | 50–54 | 100% |
| Royal Marsden (Minniti et al, 2007)47 | 39 | 1994–2003 | 3.33 | 100% | 35.9 | 50 | 97% at 3 years 92% at 5 years |
| University of Heidelberg (Combs et al, 2007)48 | 40 | 1989–2006 | 8.2 | 100% | 70 | Median 52.2 (50.4–56) |
100% at 5 years 100% at 10 years |
| St. Jude Children's Research Hospital* Merchant et al, 201349 | 88 | 1998–2009 | 5.0 | 55.6% | – | 54 Gy | 88.1–98.2%* |
Abbreviations: OS, Overall Survival.
*Results reported for clinical target volume margins >5 mm (88.1%) and <5 mm (98.2%) at 5 years.
For each table described above, only full English publications describing patients treated for craniopharyngioma were included.
Epidemiology of Craniopharyngiomas
Craniopharyngiomas have a bimodal age distribution. They account for 5% of intracranial tumors in the pediatric age group, with a peak incidence between 5 and 14 years. A second peak in incidence has been found in persons between 50 and 74 years. The adamantinomatous subtype is more common in the younger group, whereas the papillary subtype is more common in the older group.1 Although the adamantinomatous subtype may cause a florid gliotic reaction in adjacent brain parenchymal tissue, resulting in difficulties with identification and manipulation of surgical planes, histologic subtype is generally not used for treatment selection as this is not prognostic or predictive of treatment outcome.4,5 The overall incidence is 0.13 per 100 000 persons, and does not vary by gender or race. Higher rates have been demonstrated in Asia and Africa than in Western countries. Approximately 383 cases are expected to occur annually in the United States.2
Presentation
Most craniopharyngiomas become symptomatic because of mass effects of the tumor and cyst on the optic nerves, optic chiasm, and/or the hypothalamus. Patients frequently present with headaches, visual complaints, nausea, vomiting, and intellectual dysfunction (especially memory loss).
Diagnosis
Diagnosis is made after careful physical examination, laboratory tests, neuroimaging, and histopathology. Physical examination should include evaluation of visual fields for symptoms of bitemporal hemianopia and evaluation of growth and sexual development, including Tanner staging in children and adolescents. Patients can also have optic atrophy, papilledema, diplopia, and menstrual cycle irregularities. Diabetes insipidus is uncommon at presentation. Laboratory tests should include serum electrolytes and measurements of anterior pituitary hormone function (thyroid-stimulating hormone [TSH], free thyroxin, cortisol, growth hormone, insulin-like growth factor 1, follicle-stimulating hormone, lutenizing hormone, prolactin, and sex hormones [testosterone, estradiol]). Differential diagnosis includes Rathke's cleft cyst, pituitary adenoma, dermoid/epidermoid cysts, pituicytoma, and rarely, malignant craniopharyngioma.4,6
Neuroimaging
Craniopharyngiomas present as a mass in the sellar or suprasellar location. MRI is the most common form of neuroimaging used for diagnosis and evaluation of tumor location, but a CT scan is helpful in examination of bony structures and tumor calcification. Craniopharyngiomas show various degrees of solid or cystic architecture; some lesions are completely cystic, and ∼10% present as purely solid lesions. The solid portions and cystic capsule typically demonstrate avid enhancement. CT scans demonstrate calcifications in 30% to 50% of cases.3,7 There is conflicting evidence for the reliability of imaging for the differentiation of the two histologic subtypes.1,8 However, it is agreed that calcification is typically seen in younger individuals with the adamantinomatous subtype.
Histopathology
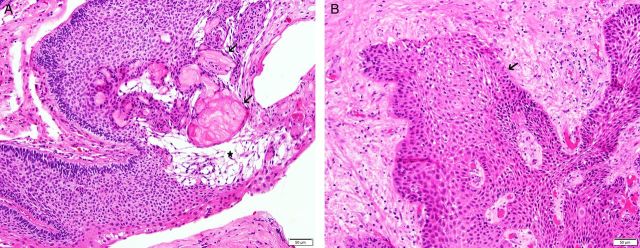
Despite their sellar location, craniopharyngiomas are epithelial neoplasms and are not derived from glial or neuronal cells. Craniopharyngioma is a WHO Grade I neoplasm.9,10 The adamantinomatous type is most common in children, but examples have been reported in adults of any age. This tumor contains cords or lobules of bland squamous epithelium that are bordered by epithelial palisades. Characteristic diagnostic features include foci of loose-spindled squamous cells (“stellate reticulum”) and devitalized keratin nodules (“wet keratin”) (Fig. 1A). Cysts and calcification often develop as degenerative changes, and adjacent brain tissue may be intensely gliotic. The cystic fluid contains desquamated epithelial cells, phospholipids, and keratin; this fluid resembles machinery oil.11 The papillary craniopharyngioma is more common in adults and consists of bland squamous epithelial lobules (Fig. 1B). Papillary craniopharyngioma lacks peripheral palisading, stellate reticulum, and wet keratin.
Fig. 1.
(A) Craniopharyngioma, adamantinomatous type. This lesion is composed of squamous epithelium with peripheral palisading. Wet keratin (arrows) and stellate reticulum (star) are characteristic. Hematoxylin and eosin (H&E) stain, 200x. (B) Craniopharyngioma, papillary type. This lesion is composed of squamous epithelium (arrow) that lacks peripheral palisading, stellate reticulum or wet keratin. H&E stain, 200x.
Recently, mutually exclusive and clonal mutations have been identified in the two histologic subtypes of craniopharyngioma. Beta-catenin expression with nuclear accumulation of beta-catenin (a downstream element of the Wnt transduction pathway) has been demonstrated in the adamantinomatous tumors.12,13 Recently, a multi-institutional investigative group confirmed the mutations in Beta-catenin exclusively in the overwhelming majority of adamantinomatous tumors (11/12 tumors in the initial cohort and 51/53 in the validation cohort), while further demonstrating that BRAFV600E was found exclusively in the overwhelming majority of papillary neoplasms (3/3 tumors in the initial cohort and 34/36 in the validation cohort).14
Treatment
General guidelines
Because of their rarity, proximal location to critical structures, and various presenting symptoms, craniopharyngiomas remain a challenging disease entity in terms of treatment. Although past retrospective chart reviews have been supportive of aggressive resection alone as well as limited resection and radiotherapy (RT), due to the lack of prospective randomized trials, it cannot be stated with certainty which treatment is optimal. Therefore, treatment plans must be individualized. By considering the tumor location adjacent or adherent to critical intracranial structures, adverse outcomes (recurrence or treatment complication) may be predicted prior to initiation of treatment. Because no differences in outcomes or recurrence rates have been noted due to the age of the patient (child or adult) or tumor histology, these factors are not usually used as treatment selection factors.15 Future prospective studies should perform formal neuropsychological, endocrine, and visual testing prior to and following any intervention.
Surgery
Gross total resection via craniotomy or transsphenoidal approach has historically been recommended as the initial management strategy. Historically, neurosurgeons advocated for aggressive local resection because it was felt that the dense gliosis characteristically intervening between these epithelial tumors and normal brain constitutes a margin of safety for the surgeon.16 However, the location of the tumor, in the suprasellar space with direct apposition to the optic apparatus, carotid arteries and their branches, and hypothalamic-infundibular structures, may create a situation in which aggressive resection cannot be achieved without unacceptably high postsurgical morbidity. Additionally, although craniopharyngiomas are not histologically malignant, their capsule is often densely adherent to the neighboring critical structures, placing these structures at risk with attempted total resection. Tumors that are prechiasmatic in location are generally more accessible and often less adherent to vital structures, but only 30% have this favorable positioning.17 The remaining 70% are retro- and subchiasmatic and are much more difficult to resect because these tumors can extend superiorly into the third ventricle and along the medial surfaces of the hypothalami.17 Surgical management is most beneficial and most practical if the tumors are small, located beneath the diaphragm (the rare “purely intrasellar” craniopharyngioma), and without hypothalamic symptoms. The recommended approach to patients with other tumors where curative surgery is less practical is to undergo excisional biopsy, cyst decompression, and tumor debulking within safe limits, and then receive radiotherapy. For patients with recurrent craniopharyngiomas, surgical resection is rarely curative, so tempered resection and debulking is clearly the goal.
Hoffman et al classified tumor location as intrasellar (subdiaphragmatic), subchiasmatic, prechiasmatic, retro-chiasmatic, and purely ventricular.18 Although there is general consensus on the surgical approach to sellar (transsphenoidal) and purely intraventricular lesions (transcallosal or transcortical), the surgical technique used for the other lesions is debatable.4 Some advocate for endoscopic endonasal surgery because this approach may allow for comparable surgical resections while achieving better endocrine function preservation and visual improvement.4
Modern surgical series are listed below in Table 1.15,19–28 It should be noted that the rates of complete resection greatly vary because the surgical policies can differ by institution and over time even within the same institution. Most of the patients in these series were treated with surgery at the time of initial diagnosis (ie, a low rate of salvage procedures, 0% to 12.8%). In some, there was no mention of radiation therapy,19,25,27 and in others,24 it is impossible to assess the impact of radiation in addition to surgical resection.
Rates of complete resection range from 27.0% to 100%. Even with complete resection, crude recurrence rates are noted in 11.1% to 90.0% of patients with higher rates defined either by surgical or radiographic assessment21,22 instead of both methods of evaluation. Chakrabarti et al24 reported higher recurrence rates with a transcranial approach (22%) than with a transnasal approach (10%), but this may reflect factors related to the individual patient or tumor location. The overall crude rate of recurrence in patients undergoing complete resection in all series was 21.6% (76 recurrences in 351 complete resections). Two-year actuarial PFS was 24.8% in the complete resection group from UCSF.23 The lowest recurrence rate after complete surgical resection was noted by Fahlsbuch,19 but there was no mention as to whether any patients received postoperative radiotherapy. Postoperative imaging has revealed residual calcifications in 15% to 50% of cases that were thought to be completely resected at the time of surgery.18 In the series of Tomita and Bowman,22 rate of recurrence based on surgical impression and postoperative imaging of complete resection was noted to be 33.3% (9/27), but rose to 90% (9/10) based solely upon the surgeon's operative assessment of complete resection. Only two groups reported recurrences after complete resection and radiotherapy15,22; in these two groups 0/3 and 9/27 of tumors recurred, respectively.
The recurrence rates from subtotal resection alone ranged from 40.0% to 100%. The crude recurrence rate from subtotal resection was 52.5% (117 recurrences in 223 patients). Two-year actuarial PFS was 63.8% in the subtotal resection group from UCSF.23 Using radiotherapy after subtotal resection appears to result in lower recurrence rates than subtotal resection alone and appears similar to those after complete resection, with rates ranging from 9.1% to 37.5%, and a mean crude rate of 18.8% (9 of 48 patients). Two-year actuarial PFS was 26.7% in the subtotal resection and postoperative radiotherapy group from UCSF.23 It has been long-recognized that recurrences from may occur many years after initial treatment,17 so it is likely that actual recurrence rates are higher than those reported in the literature, even in series with significant follow-up.
Several groups have shown no significant difference in recurrence rates among patients who received subtotal resection followed by radiotherapy versus patients who underwent gross total resection. This approach has also been shown to reduce the neurologic, endocrine, and ophthalmic side effects frequently associated with gross total resection. The combination of fewer long-term sequelae and the increase in both PFS and overall survival seen with subtotal resection followed by radiotherapy suggest that this may be a superior treatment approach than an overly aggressive gross total resection.21,29
Intracavitary Radiation
Cystic craniopharyngiomas can be treated with implantation of radioisotopes via stereotactic or endoscopic techniques. A variety of radioisotopes are used, all of which emit beta particles (RH-136 emits both alpha and beta particles) that have short path lengths, thereby treating the epithelial cells lining the cyst without significant dose to neighboring structures. High rates of response (ie, reduction of cyst size) have been reported (between 73%–100%31–33,35–36) with variable rates of long-term control, if reported (Table 2). The lower response rate at one institution (40.0%) suggests that this technique should be performed in experienced centers.34 This technique should be limited to solitary, cystic lesions only. Additionally, the nonuniformity of cystic doses and the lack of known dose responses for toxicity and tumor control make this technique particularly worrisome. For example, in one series, the solid components of lesions responded to intracavitary radiation, but blindness resulted, despite the limited half value tissue penetrance of P-32 of only 1.1 mm.33 Nevertheless, this technique may be a reasonable salvage option for patients with solitary cystic recurrences that cannot be salvaged with external beam radiotherapy and/or surgical resection.
Stereotactic Radiosurgery
More recently, stereotactic radiosurgery has been used to treat craniopharyngiomas. Control ranges from 33.3% to 87.0% (Table 3). This technique is generally limited to solid, small tumors that are at least 3 to 5 mm away from the optic chiasm. Due to the proximity of the optic apparatus and its known sensitivity to single-fraction doses as low as 8 Gy, some clinicians have given low peripheral doses,39 prescribed to low isodose lines (eg, 30%–35%) with lower margin doses (10 Gy),40 or excluded a small rim of tumor outside of the prescription isodose line with the use of an effective dose for benign tumor control (median peripheral dose = 13 Gy).41 Ulfarsson et al39 suggested that the peripheral dose needed for local control of craniopharyngiomas may be relatively low. With an observation time period ranging from 0.5 to 29 years (mean 7.5 years, median 3.5 years) 11/13 tumors progressed after receiving a marginal dose of <6 Gy, but only 3/9 tumors were not controlled when a peripheral dose of 6 Gy was administered. However, investigators at the University of Virginia demonstrated that marginal doses of >14.5 Gy were associated with a longer PFS.42 The report from Niranjan et al41 demonstrated that 5-year local control rates for solid tumors (n = 22) was 77.5%, but dropped to 64.3% for tumors with both solid and cystic components (n = 14). The 5-year rate of local control for solid tumors (excluding cystic enlargement) was 91.6%. However, with a median tumor size of only 1 cc, it appears that patient selection may have played a role in the relatively high long-term control rates. Likewise, Kobayashi43 demonstrated that cystic/mixed tumors were an unfavorable prognostic factor. Due to the long natural history of this tumor, further investigations with longer periods of follow-up are needed. However, due to the invasiveness of this tumor and the known sensitivity of the nearby optic structures to single-dose radiation, proper patient selection is necessary to achieve success with stereotactic radiosurgery.
Conventional Radiotherapy
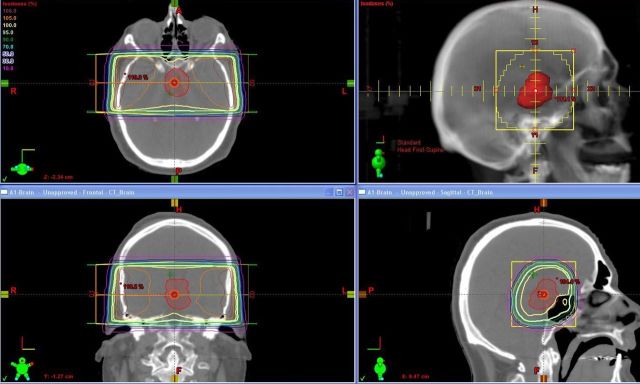
Prior to the availability of modern imaging and linear accelerators, radiation was aimed at the sella turcica for uncalcified tumors, or at calcifications beyond the sella turcica with parallel opposed portals and low-energy radiotherapy. This technique treated the tumor at the expense of giving a high dose to the temporal lobes (Fig. 2). Because tumor doses ranged from 50 to 60 Gy, the hot spots in the temporal lobes exceeded doses associated with necrosis of brain tissue (50 Gy).44 In one such series,45 impaired functional outcome (partial dependence or total dependence) increased from 14% to 34% of 35 patients, and visual and neurologic functions were affected in 34% and 40%, respectively.
Fig. 2.
Treatment of a patient with a craniopharyngioma with traditional opposed lateral fields and low-energy photon beam (6 MV). The tumor area was treated with a 2 cm margin. A large volume of neural tissue was treated to the 105% isodose line (orange areas) or higher. Axial, sagittal, and coronal views of the isodoses as well as a portal image view are demonstrated.
Modern Radiotherapy Techniques
The results of the various series of fractionated radiotherapy in the era of CT-based treatment planning with or without MRI fusion show high control rates of 92.0% to 100.0%, albeit with short follow-up (1.3–8.2 years) (Table 4). It should be noted that high control rates were noted in the series reported by Merchant et al despite 40 of 88 patients treated (45.5%) receiving surgery described as “minimal to none” and that the extent of resection was not related to PFS.49
Modern imaging, utilizing CT simulation with MRI fusion, has allowed for better delineation of intracranial tumor volume. This has allowed for expansion of the gross tumor volume by 5 mm to cover microscopic disease (ie, clinical target volume) with an additional expansion margin of 3 mm to account for daily setup (planning tumor volume). This technique has been found to yield very high rates of local control with short-term follow-up.50
Special care must be given to patients whose tumor has a cystic component. In one recent series, it was revealed that 6 of 17 patients who underwent repeat imaging during radiotherapy required a change in radiation therapy volume due to changes in cystic dimensions.51 Therefore, we recommend the treatment of these patients on linear accelerators with On-Board cone-beam CT-imaging systems. Cone-beam CT scans should be obtained at least weekly to assess changes in cystic volume.
We also recommend fusion of the CT-based treatment planning scans with gadolinium-contrast enhanced T1-weighted and T2-weighted MRI FLAIR images in order to help define the solid and cystic components of the tumor, respectively. Treatment should be given via conformal radiation approaches using protons or specialized photon techniques. Multiple nonopposed fields are recommended if intensity-modulated radiotherapy (IMRT) or volumetric arc therapy (VMAT) are not used in order to concentrate the high dose of radiation to the tumor while sparing normal structures. Examples of static field IMRT and VMAT are noted in Fig. 3A and B.
Fig. 3.
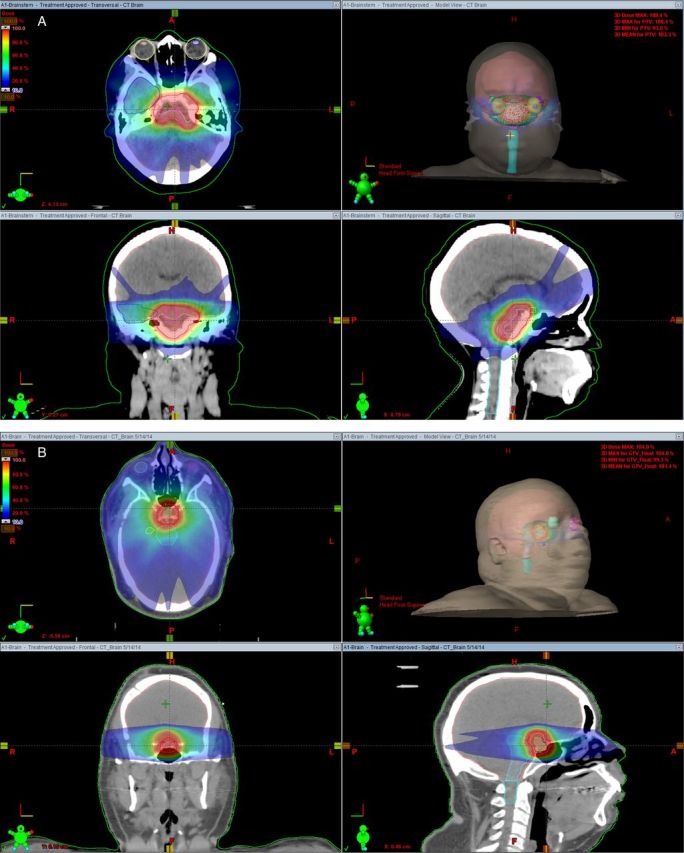
(A) Treatment of a patient with a craniopharyngioma using intensity-modulated radiation therapy with multiple non-opposed fields. Axial, sagittal, and coronal views of the isodoses as well as a room's eye view are demonstrated. (B) Treatment of a patient with a craniopharyngioma with volumetric arc therapy. Axial, sagittal, and coronal views of the isodoses as well as a room's eye view are demonstrated.
Proton Therapy
Proton therapy allows the clinician to conform the dose better to the tumor volume and reduce the integral dose that the patient receives. As a result, proton therapy is becoming a more commonly used treatment option, especially in younger patients who are most susceptible to the long-term functional effects of radiation to the brain.52 Boehling et al53 compared IMRT, 3D-conformal proton therapy (3DCPT) and intensity-modulated proton therapy (IMPT) plans for 10 pediatric craniopharyngioma patients. IMRT and IMPT proved to be more conformal than 3DCPT, though target coverage was adequate for all modalities. The proton therapy techniques yielded significantly lower doses to the hippocampus, dentate gyrus, subventricular zones, brainstem and both infra- and supratentorial brain. Merchant et al54 modeled the potential benefit in cognitive function from the reduced integral dose using proton therapy. Their models suggest that IMPT leads to a smaller cognitive decline than IMRT, mainly due to a reduction of the low-dose region to the supratentorial brain.
However, the use of proton therapy can be a challenge because these tumors often contain both solid and cystic components (low electron–density tissue). Furthermore, these tumors are prone to size, shape, and volume changes throughout treatment, which affects proton therapy plans generally more than IMRT plans. One approach to this issue is to repeat MRI examinations throughout the course of treatment and change treatment plans accordingly. Beltran et al55 have reported that 3DCPT is relatively insensitive to changes in tumor target volume, whereas IMPT is the most conformal and spares the greatest volume of normal tissue, but is highly sensitive to changes in target volume, therefore requiring closer monitoring. These investigators determined that 3DCPT may be the best proton method in absence of high-frequency (more than once per week) and high resolution imaging during treatment. Additionally, there remains uncertainty regarding how the proton beam traverses low electron–density tissue such as a cyst, which may result in dose uncertainty within the tumor and a degradation of the sharp distal fall-off.56
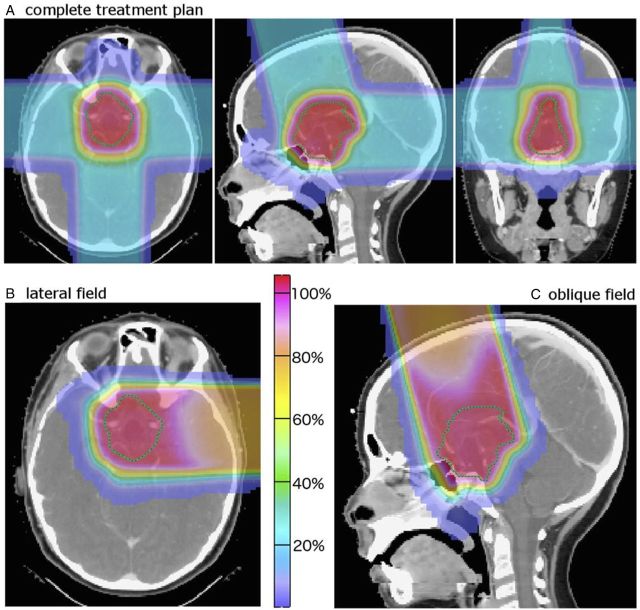
Clinical outcome data for proton therapy in craniopharyngioma are very limited. A study of 16 patients treated with proton therapy reports 1 case of local failure, 12 long-term survivors, and little acute toxicity after a mean follow-up time of 60.2 months.57 Fitzek et al58 report long-term outcomes of a cohort of 15 patients treated with a combination of photons and protons. Five-year local control, 10-year local control, and 10–year overall survival rates were 93%, 85%, and 72%, respectively. Fig. 4 shows an example of 3D-conformal proton therapy plan using lateral, posterior, and superior oblique beams.
Fig. 4.
(A–C) Example of 3D-conformal proton therapy plan (A) using lateral (B), posterior and superior oblique (C) beams.
Timing of Radiotherapy
Although a past patient series demonstrated a potentially worse outcome for patients treated for recurrence than those patients undergoing immediate adjuvant treatment,59 the patients in this series were treated prior to the era of follow-up with modern imaging, and later series did not demonstrate an adverse outcome associated with the treatment of recurrences. Because most subtotally resected craniopharyngiomas recur, it is probably best to treat these tumors with radiotherapy as soon as they have been resected, when their volumes have been maximally reduced. One series noted that control rates did not depend upon the timing of radiation (ie, adjuvant vs salvage therapy), but there were higher rates of diabetes insipidus as well as loss of visual field and acuity in the delayed radiotherapy group.60 Despite our general recommendation for immediate adjuvant treatment for most patients undergoing subtotal resection, it may be best to treat some children at the time of recurrence because of the known greater susceptibility of young children to the carcinogenic61 and adverse neurocognitive effects of radiotherapy.62
Dose Response
Prior to the CT-based treatment planning era, dose responses for high rates of local control were demonstrated to be >55 Gy,45 >54 Gy,59 and 60 Gy or greater.17 However, it should be noted that the three series in Table 4 that used CT-based treatment planning yielded high rates of local control (>90%) despite the use of slightly lower doses. Due to the relatively short follow-up and limited patient numbers, further studies are needed in order to determine if these lower doses are effective for long-term tumor control.
Chemotherapy/Systemic Therapy
Intracavitary bleomycin has been reported in small patient series.63–65 Although the majority of cystic lesions will decrease in size with therapy, drug leakage into surrounding tissues has been associated with serious events and fatal effects such as hypothalamic damage, hearing loss, cerebrovascular events, blindness, and brain edema. Interferon alpha has also been used for intracystic treatment with low rates of morbidity. Although high response rates have been noted, only short-term follow-up is available.66,67
Jakacki et al reported on the use of a 48-week course of interferon alpha 2a for 15 patients with progressive or recurrent craniopharyngioma.68 Twelve patients were available for follow-up. With a median time to progression of 25 months, radiation could be successfully delayed in 6 patients from 18 to 35 months. Lippens et al treated 4 children with recurrent tumors after previous radiotherapy with doxorubicin and lomustine with tumor control noted at 3 to 12 years in 3 patients.69
Treatment Sequelae
Optic neuropathies can occur from radiotherapy and are dose-related. However, no evidence of optic neuritis or optic necrosis has been reported when these structures are treated with 54 to 55.8 Gy in conventional fraction sizes of 1.8 Gy.70 Hypothalamic-pituitary dysfunction can result from radiation doses needed to control craniopharyngiomas. This complication can take months to years to develop. Consultation and follow-up with an endocrinologist is recommended for any patient undergoing radiotherapeutic treatment. It should be noted that diabetes insipidus is generally not related to radiotherapy, but is considered to be a complication associated primarily with surgical management.71 Aggressive surgical management is also associated with a higher incidence of hypothalamic obesity, anterior pituitary dysfunction, and visual field deficits. One of the largest studies to date on this topic suggests that if gross total resection is not possible, most if not all patients with residual tumor will progress within a year without immediate radiotherapy postoperatively.25
Using modern radiation techniques, neurocognitive tests have revealed that the IQ of children remains stable through 5 years of follow-up. One report has revealed that worse neurocognitive outcomes were associated with ages <7 years, hydrocephalus, large cystic tumors, extensive surgery, and diabetes insipidus.50
Vascular complications are generally rare after radiotherapy. However, one small past report indicated that radiologically apparent vasculopathies could be as high as 30% (6/30 patients).72 Another investigation estimated that the risk of Moyamoya disease increased by 7% for every 100 cGy increase in radiation dose above 5000 cGy. Patients with neurofibromatosis 1 may be particularly susceptible to Moyamoya disease.73
Radiation-induced malignancies after the treatment of craniopharyngiomas are exceedingly rare following treatment with external beam radiotherapy. A recent review demonstrated 6 cases of second cancers in adults and 5 cases of second cancers in children.74 After malignant transformation, survival was short and ranged from 2 to 10 months, but one patient was still alive 5 years after malignant transformation. Second malignancies were associated with multiple local recurrences treated with resection followed by at least 1 course of radiotherapy.
Patient Examples – Supplemental
Following are two cases scenarios of an adult and child at presentation, after surgical resection, and after treatment with radiotherapy. These are included to demonstrate the different presentations and sequelae of craniopharyngioma and/or its treatment in the adult and pediatric age groups. We feel that these case scenarios depict the typical difficulties encountered by patients in their respective age groups. Because of the many different symptoms upon presentation, it is often difficult to discern side effects of treatment from symptoms associated with the initial tumor growth. Fig. 1S shows MRI scans of a 58-year-old man at presentation, 1 month after surgery, and 1 year after radiotherapy. Fig. 2S shows MRI scans of a patient who presented at 8 years of age.
Fig. 1Sa. A 58-year-old man developed memory loss, visual difficulties, and headaches over several years. He started to have progressively worsening fatigue, and had trouble tending to his finances, job, and other duties. Physical exam was normal. His Mini Mental State Examination (MMSE) score was 30/30. Brain MRI demonstrated a 4.8 × 3.0 × 2.7 cm mass in the suprasellar cistern that did not involve the sella turcica and was extra-axial, consistent with a craniopharyngioma.
Fig. 1Sb. One-month post-resection brain MRI, sagittal view. During excision, a cyst was noted within the foramen of Monroe/third ventricle. A small nodule on the left anterior wall of the third ventricle was noted. The tumor was removed in piecemeal fashion. Pathology confirmed craniopharyngioma, papillary type. MRI revealed prominent lateral ventricle with dilated third ventricle and hyperdense foci within the occipital horns of the lateral ventricles representing minimal interventricular bleed. After undergoing surgery he continued to experience generalized fatigue and memory issues.
Fig. 1Sc. Due to the subtotal nature of his resection, the patient started external beam radiotherapy ∼3 months after his surgical resection. The patient was treated with an IMRT using five non-opposed, static radiation therapy beams with 6MV photons. One year after therapy, the patient continued to have employment and memory issues, and his MMSE was 27/30. Sagittal MRI depicted no evidence of recurrence 1 year after radiation therapy.
Fig. 2Sa. Brain MRI of an 8-year-old child at presentation. She presented with worsening headaches and periodic vomiting for several months. The MRI demonstrated a large, lobulated, extra-axial, suprasellar, cystic-appearing lesion with rim enhancement that filled the suprasellar and prepontine cisterns. Subtotal resection of a adamantinomatous craniopharyngioma was performed.
Fig. 2Sb. Brain MRI performed 12 months postoperatively. She was complaining of intermittent blurred vision in the left eye and was prescribed Keppra for complex partial seizures. On physical exam, patient had some mild diplopia and slight ptosis on upward gaze, which, with cover/uncover test, was suggestive of a left superior rectus weakness. Gliotic changes in the left anterior temporal lobe were noted following left frontal and temporal craniotomy. Residual craniopharyngioma was noted in the left cerebellopontine angle cistern and measured 16.2 × 11.7 mm. Thin-rim enhancement of residual tumor with mild compression of the pons was seen. The left trigeminal nerve was stretched around the tumor. T1 hyperintense signals of the posterior pituitary gland were absent. Features of partial empty sella were noted. The optic chiasm appeared thin.
Fig. 2Sc. Brain MRI 19 months after the second surgery, 31 months after the first surgery, and preceding her third surgery. The patient was placed on sumatriptan (Imitrex) for headaches. She also began to experience some left eye pain, burning, and watering. Her left pupil was significantly dilated with respect to the right. No other sensory, motor, or visual changes were observed. On physical exam she had mild anisocoria with the left pupil noted to be 4 mm and the right pupil measured at 2 mm. Both pupils were reactive. She had some diplopia on inferior and left inferior gaze. MRI shows a 1.8 × 1.5 × 1.2 cm cystic mass in the left prepontine cistern, which demonstrated a fluid-fluid level, concerning for a recurrent tumor.
Fig. 2Sd-e. Her preradiation axial (d) and sagittal (e) MRI, performed 18 months after her third surgery and 49 months after the first surgery, demonstrated re-growth of rim-enhancing tumor in the right pre-pontine cistern. Before radiation therapy, the patient was noted to have anterior pituitary insufficiency (growth hormone, TSH, adrenocorticotropic hormone), diabetes insipidus, severe headaches, complex partial seizures, BMI = 30, weight at the 97th percentile, height <10th percentile, decreased right temporal vision, and reading and math levels at 1 to 2 years less than her current grade level (5th grade). After radiation, she was placed on Premarin and found to have slight optic nerve atrophy on opthalmalogic exam.
Quality of Life
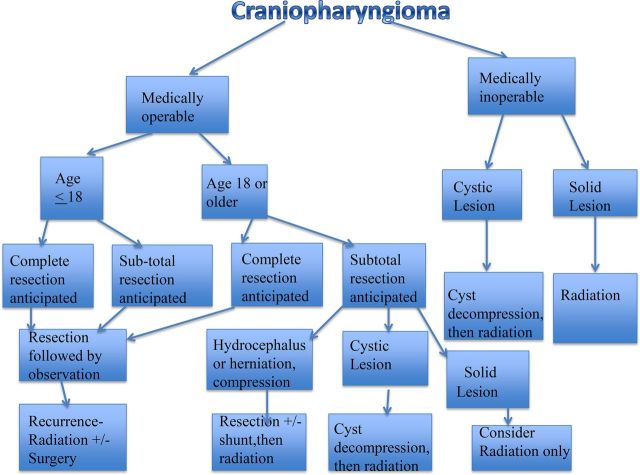
Long-term morbidity caused by craniopharyngiomas can be seen in visual, endocrine, hypothalamic, neurobehavioral and cognitive outcomes. It is difficult to determine whether quality of life is affected by the tumor, treatment, or a combination of both in patients who usually present with several different complaints and because various treatment options can cause harm in different ways. The typical presentation, treatment, and outcome of an adult and child can be seen in the supplemental text and figures. These cases illustrate the difficulty of distinguishing between symptoms due to initial tumor presentation versus side effects from radiation therapy or surgery. Because children are developing in terms of sexual and skeletal growth, we feel that their treatment should differ from that of adults. We have outlined our flow diagram (Fig. 5) at our institutions for the treatment of patients with craniopharyngioma. We feel that surgery should be the main management strategy for all craniopharyngiomas that are likely to be completely resected in both adults and children. In adults, we feel that subtotally resected tumors should be followed by immediate radiation to prevent recurrences.
Fig. 5.
Flow diagram of treatment.
In children, because postsurgical radiation can be associated with a higher risk of endocrine deficiency resulting in precocious puberty and growth deficiency75 and because radiation can affect the normal brain myelination,76 we feel that radiation is best reserved for recurrence after subtotal resection. Indeed, one small patient series demonstrates that hypothalamic obesity was associated with mortality and was increased in patients receiving postoperative radiotherapy.77 Additionally, females in the pediatric age range may be more susceptible to the neurocognitive late effects associated with radiation because follow-up MRIs reveal less normal appearing white matter volume, and this volume is more strongly correlated with working memory difficulties.76 However, it must be emphasized that these patients who are selected for surveillance after subtotal resection should be followed with serial imaging with the intent of administering radiotherapy at the first sign of growth prior to being clinically symptomatic. Because past series have suggested that the number and extent of surgical resections have been associated with poorer neurocognitive outcomes in children,78 that previous surgery has been associated with residual tumor after surgery,79 and that radiation alone with minimal or no surgery has been found to show control rates of >90%49; we feel that judicious selection of surgery is needed. The supplemental pediatric case in Fig. 2Sa-e illustrate the difficulties associated with repeat resection, and the likelihood of recurrence once an initial sub-total resection is performed. Furthermore, the difficulties associated with overly aggressive surgical resection were suggested in a recent systematic review demonstrating that the overall rate of new endocrinopathies for patients undergoing gross total surgical resection alone was 52% as compared with rates of 19% and 20% for patients receiving subtotal resection and subtotal resection with postoperative radiotherapy, respectively.80 Nevertheless, even when gross resection is not possible, we feel that cystic decompression, reduction of hydrocephalus, and prevention of herniation via surgery are important because these surgical procedures will result in less normal brain being radiated, and the total brain volume, supratentorial brain, and left temporal lobe receiving doses in excess of 45 Gy have been associated with decline in longitudinal IQ in pediatric patients.50
Because craniopharyngiomas are invasive and often located near the optic chiasm, and the optic structures are highly sensitive to high-dose-fraction radiosurgery regimens,81 we feel that radiosurgery cases should be highly selected and that this modality may be best reserved for salvage situations after the failure of surgery and fractionated radiation. Likewise, because of the limited experience with intracavitary radiation and the unresolved issues with nonuniform dose distribution and unknown dose responses for toxicity, we feel that this modality is best reserved after surgical and fractionated radiation options have been exhausted.
Because of the rarity of this tumor, and the many different symptoms from presentation or treatment, we feel that large multi-institutional databases are needed. We would encourage the prospective use of the craniopharyngioma clinical classification status81 to see if this predictive test of neurological examination, visual status, pituitary function, hypothalamic dysfunction, and educational/occupational status can be predictive of outcomes in a prospective manner across multiple institutions in both children and adults.
Conclusions
Although craniopharyngiomas are histologically benign, they are usually infiltrative neoplasms and are intricately associated with the optic structures, hypothalamus and the circle of Willis. These tumors have a bimodal age distribution and occur most commonly among patients who are 5 to 14 and 50 to 74 years old. Because of the many different presenting symptoms and potential complications associated with the various treatment options, we advocate for prospective trials that take into consideration not only control rates, but quality of life issues. Until such studies are performed, we recommend surgical resection of all tumors when a complete resection is anticipated. If subtotal resection is performed, we feel that adult patients should be treated with radiation as soon as they recover from surgery, but pediatric patients should be followed and treated with radiation therapy only upon recurrence. Recently, due to the exclusive expression of the beta-catenin clonal mutations and the exclusive expression of BRAFV600E clonal mutations in the overwhelming majority of adamantinomatous and papillary tumors respectively, it is felt that inhibitors of each pathway (WNT/B-catenin or BRAF) may play a role in the future treatment of these rare tumors and may, in combination with radiation, allow for lower effective doses to be given. Because of the rarity of these tumors, large international efforts will be needed prior to any radical changes in radiation treatment doses or techniques. Recently, radiation recall dermatitis was seen in two patients treated with vemurafenib shortly after receiving radiation82 and suggests that future studies involving any molecularly targeted agents must be based on vigorous preclinical models, especially when treatment involves sensitive intracranial structures. Current investigations involving craniopharyngioma can be found on clinicaltrials.gov. However, because the younger age group is largely associated with the adamantinomatous tumors, beta-catenin clonal mutations, and different treatment sequelae than adult patients with craniopharyngioma, we feel that it is best to consider adult and pediatric craniopharyngiomas as two different diseases under the same categorical classification.
Conflict of interest statement
None declared.
Supplementary Material
References
- 1. Sartoretti-Schefer S, Wichmann W, Aguzzi A et al. . MR differentiation of adamantinous and squamous-papillary craniopharyngiomas. Am J of Neuroradiol. 1997;18(1):77–87. [PMC free article] [PubMed] [Google Scholar]
- 2. Bunin GR, Surawicz TS, Witman PA et al. . The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998;89(4):547–551. [DOI] [PubMed] [Google Scholar]
- 3. Jane JA, Laws ER. Craniopharyngioma. Pituitary. 2006;9(4):323–326. [DOI] [PubMed] [Google Scholar]
- 4. Fernandez-Miranda JC, Gardner PA, Snyderman CH et al. . Craniopharyngioma: a pathologic, clinical, and surgical review. Head Neck. 2012;34(7):1036–1044. [DOI] [PubMed] [Google Scholar]
- 5. Mortini P, Gagliardi F, Boari N et al. . Surgical strategies and modern therapeutic options in the treatment of craniopharyngiomas. Crit Rev Oncol Hematol. 2013;88(3):514–529. [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez FJ, Scheithauer BW, Tsundo S et al. . The spectrum of malignancy in craniopharyngioma. Am J Surg Pathol. 2007;31(7):1020–1028. [DOI] [PubMed] [Google Scholar]
- 7. Larijani B, Bastanhagh MH, Pajouhi M et al. . Presentation and outcome of 93 cases of craniopharyngioma. Eur J Cancer Care. 2004;13(1):11–15. [DOI] [PubMed] [Google Scholar]
- 8. Eldevik OP, Blaivas M, Gabrielsen TO et al. . Craniopharyngioma: radiologic and histologic findings and recurrence. Am J Neuroradiol. 1996;17(8):1427–1439. [PMC free article] [PubMed] [Google Scholar]
- 9. Rushing EJ, Giangaspero F, Paulus W et al. . Craniopharyngioma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK et al., eds. WHO Classification of Tumours of the Central Nervous System. 4th ed Lyon, France: IARC; 2007; 238–240. [Google Scholar]
- 10. Zada G, Lin N, Ojerholm E et al. . Craniopharyngioma and other cystic epithelial lesions of the sellar region: a review of clinical, imaging, and histopathological relationships. Neurosurg Focus. 2010;28(4):E4. [DOI] [PubMed] [Google Scholar]
- 11. Karavitaki N, Cudlip S, Adams CB et al. . Craniopharyngiomas. Endocrine Reviews. 2006;27(4):371–397. [DOI] [PubMed] [Google Scholar]
- 12. Oikonomou E, Barreto DC, Soares B et al. . Beta-catenin mutations in craniopharyngiomas and pituitary adenomas. J Neurooncol. 2005;73(3):205–209. [DOI] [PubMed] [Google Scholar]
- 13. Buslei R, Nolde M, Hofmann B et al. . Common mutations of beta-catenin in adamantinomatous craniopharyngiomas but not in other tumors originating from the sellar region. Acta Neuropathol. 2005;109(6):589–597. [DOI] [PubMed] [Google Scholar]
- 14. Brastianos PK, Taylor-Weiner A, Manley PE et al. . Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet. 2014;46(2):161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duff J, Meyer FB, Ilstrup DM et al. . Long-term outcomes for surgically resected craniopharyngiomas. Neurosurgery. 2000;46(2):291–302. [DOI] [PubMed] [Google Scholar]
- 16. Sweet WH. History of surgery for craniopharyngiomas. Pediatr Neurosurg. 1994;21(suppl. 1):28–38. [DOI] [PubMed] [Google Scholar]
- 17. Varlotto JM, Flickinger JC, Kondziolka D et al. . External beam irradiation of craniopharyngiomas: long-term analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys. 2002;54(2):492–499. [DOI] [PubMed] [Google Scholar]
- 18. Hoffman HJ, De Silva M, Humphreys RP et al. . Aggressive surgical management of craniopharyngiomas in children. J Neurosurg. 1992;76(1):47–52. [DOI] [PubMed] [Google Scholar]
- 19. Fahlbusch R, Honegger J, Paulus W et al. . Surgical treatment of craniopharyngiomas: Experience with 168 patients. J Neurosurg. 1999;90(2):237–250. [DOI] [PubMed] [Google Scholar]
- 20. Van Effenterre R, Boch AL. Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg. 2002;97(1):3–11. [DOI] [PubMed] [Google Scholar]
- 21. Stripp DC, Maity A, Janss AJ et al. . Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys. 2004;58(3):714–720. [DOI] [PubMed] [Google Scholar]
- 22. Tomita T, Bowman RM. Craniopharyngiomas in children: surgical experience at Children's Memorial Hospital. Childs Nerv Syst. 2005;21(8–9):729–746. [DOI] [PubMed] [Google Scholar]
- 23. Schoenfeld A, Pekmezci M, Barnes MJ et al. . The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. J Neurooncol. 2012;108(1):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chakrabarti I, Amar AP, Couldwell W et al. . Long-term neurological, visual, and endocrine outcomes following transnasal resection of craniopharyngioma. J Neurosurg. 2005;102(4):650–657. [DOI] [PubMed] [Google Scholar]
- 25. Shi XE, Wu B, Fan T et al. . Craniopharyngioma: surgical experience of 309 cases in China. Clin Neurol Neurosurg. 2008;110(2):151–159. [DOI] [PubMed] [Google Scholar]
- 26. Yamada S, Fukuhara N, Oyama K et al. . Surgical outcome in 90 patients with craniopharyngioma: an evaluation of transsphenoidal surgery. World Neurosurg. 2010;74(2–3):320–330. [DOI] [PubMed] [Google Scholar]
- 27. Hofmann BM, Hollig A, Strauss C et al. . Results after treatment of craniopharyngiomas: further experiences with 73 patients since 1997. J Neurosurg. 2012;116(2):373–384. [DOI] [PubMed] [Google Scholar]
- 28. Koutourousiou M, Gardner PA, Fernandez-Miranda JC et al. . Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J Neurosurg. 2013;119(5):1194–1207. [DOI] [PubMed] [Google Scholar]
- 29. Yang I, Sughrue ME, Rutkowski MJ et al. . Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus. 2010;28(4):E5. [DOI] [PubMed] [Google Scholar]
- 30. Van den Berge JH, Blaauw G, Breeman WA et al. . Intracavitary brachytherapy of cystic craniopharyngiomas. J Neurosurg. 1992;77(4):545–550. [DOI] [PubMed] [Google Scholar]
- 31. Voges J, Strum V, Lehrke R et al. . Cystic craniopharyngioma: long-term results after intracavitary irradiation with stereotactically applied colloidal beta-emitting radioactive sources. Neurosurgery. 1997;40(2):269–270. [DOI] [PubMed] [Google Scholar]
- 32. Julow J, Backlund EO, Lanyi F et al. . Long-term results and late complications after intracavitary yttrium-90 colloid irradiation of recurrent cystic craniopharyngiomas. Neurosurgery. 2007;61(2):288–296. [DOI] [PubMed] [Google Scholar]
- 33. Hasegawa T, Kondziolka D, Hadjipanayis CG et al. . Management of cystic craniopharyngiomas with phosphorus-32 intracavitary irradiation. Neurosurgery. 2004;54(4):813–820; discussion 820–822. [DOI] [PubMed] [Google Scholar]
- 34. Barriger RB, Chang A, Lo SS et al. . Phosophorus-32 therapy for cystic craniopharyngiomas. Radiother Oncol. 2011;98(2):207–212. [DOI] [PubMed] [Google Scholar]
- 35. Shahzadi S, Sharifi G, Andalibi R et al. . Management of cystic craniopharyngiomas with intracavitary irradiation with 32P. Arch Iran Med. 2008;11(1):30–34. [PubMed] [Google Scholar]
- 36. Zhao R, Deng J, Liang X et al. . Treatment of cystic craniopharyngioma with phosphorus-32 intracavitary irradiation. Childs Nerv Syst. 2010;26(5):669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chung WY, Pan DH, Shiau CY et al. . Gamma knife radiosurgery for craniopharyngiomas. J Neurosurg. 2000;93(suppl. 3):47–56. [DOI] [PubMed] [Google Scholar]
- 38. Amendola BE, Wolf A, Coy SR et al. . Role of radiosurgery in craniopharyngiomas: a preliminary report. Med Pediatr Oncol. 2003;41(2):123–127. [DOI] [PubMed] [Google Scholar]
- 39. Ulfarsson E, Lindquist C, Roberts M et al. . Gamma knife radiosurgery for craniopharyngiomas: long-term results in the first Swedish patients. J Neurosurg. 2002;97(suppl. 5):613–622. [DOI] [PubMed] [Google Scholar]
- 40. Kobayashi T, Kida Y, Mori Y et al. . Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg. 2005;103(suppl. 6):482–488. [DOI] [PubMed] [Google Scholar]
- 41. Niranjan A, Kano H, Mathieu D et al. . Radiosurgery for craniopharyngioma. Int J Radiat Oncol Biol Phys. 2010;78(1):64–71. [DOI] [PubMed] [Google Scholar]
- 42. Xu Z, Yen CP, Schlesinger D et al. . Outcomes of gamma knife surgery for craniopharyngiomas. J Neurooncol. 2011;104(1):305–313. [DOI] [PubMed] [Google Scholar]
- 43. Kobayashi T. Long-term results of gamma knife radiosurgery for 100 consecutive cases of craniopharyngioma and a treatment strategy. Prog Neurol Surg. 2009;22:63–76. [DOI] [PubMed] [Google Scholar]
- 44. Shaw E, Arusell R, Scheithauer B et al. . Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20(9):2267–2276. [DOI] [PubMed] [Google Scholar]
- 45. Habrand JL, Ganry O, Couanet D et al. . The role of radiation therapy in the management of craniopharyngioma: a 25-year experience and review of the literature. Int J Radiat Oncol Biol Phys. 1999;44(2):255–263. [DOI] [PubMed] [Google Scholar]
- 46. Tarbell N, Scott R, Goumnerova L. Craniopharyngioma: Preliminary results of stereotactic radiation therapy. In: Kondziolka D, ed. Radiosurgery. Vol.1 Basel: Karger; 1996:75–82. [Google Scholar]
- 47. Minniti G, Saran F, Traish D et al. . Fractionated stereotactic conformal radiotherapy following conservative surgery in the control of craniopharyngiomas. Radiother Oncol. 2007;82(1):90–95. [DOI] [PubMed] [Google Scholar]
- 48. Combs SE, Thilmann C, Huber PE et al. . Achievement of long-term local control in patients with craniopharyngiomas using high precision stereotactic radiotherapy. Cancer. 2007;109(11):2308–2314. [DOI] [PubMed] [Google Scholar]
- 49. Merchant TE, Kun LE, Hua CH et al. . Disease control after reduced volume conformal and intensity modulated radiation therapy for childhood craniopharyngioma. Int J Radiat Oncol Biol Phys. 2013;85(4):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Merchant TE, Kiehna EN, Kun LE et al. . Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg. 2006;104(suppl. 2):92–104. [DOI] [PubMed] [Google Scholar]
- 51. Winkfield KM, Linsenmeier C, Yock TI et al. . Surveillance of craniopharyngioma cyst growth in children treated with proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73(3):716–721. [DOI] [PubMed] [Google Scholar]
- 52. Merchant TE. Clinical controversies: proton therapy for pediatric tumors. Semin Radiat Oncol. 2013;23(2):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boehling NS, Grosshans DR, Bluett JB et al. . Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int J Radiat Oncol Biol Phys. 2012;82(2):643–652. [DOI] [PubMed] [Google Scholar]
- 54. Merchant TE, Hua CH, Shukla H et al. . Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51(1):110–117. [DOI] [PubMed] [Google Scholar]
- 55. Beltran C, Roca M, Merchant TE. On the benefits and risks of proton therapy in pediatric craniopharyngioma. Int J Radiat Oncol Biol Phys. 2012;82(2):e281–e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hwang UJ, Shin DH, Kim TH et al. . The effect of a contrast agent on proton beam range in radiotherapy planning using computed tomography for patients with locoregionally advanced lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):e317–e324. [DOI] [PubMed] [Google Scholar]
- 57. Luu QT, Loredo LN, Archambeau JO et al. . Fractionated proton radiation treatment for pediatric craniopharyngioma: preliminary report. Cancer J. 2006;12(2):155–159. [PubMed] [Google Scholar]
- 58. Fitzek MM, Linggood RM, Adams J et al. . Combined proton and photon irradiation for craniopharyngioma: long-term results of the early cohort of patients treated at Harvard Cyclotron Laboratory and Massachusetts General Hospital. Int J Radiat Oncol Biol Phys. 2006;64(5):1348–1354. [DOI] [PubMed] [Google Scholar]
- 59. Regine WF, Mohiuddin M, Kramer S. Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol. 1993;27(1):13–21. [DOI] [PubMed] [Google Scholar]
- 60. Moon SH, Kim IH, Park SW et al. . Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngiomas–a study in single institute. Childs Nerv Syst. 2005;21(8–9):799–807. [DOI] [PubMed] [Google Scholar]
- 61. Pui CH. Childhood leukemias. N Engl J Med. 1995;332(34):1618–1630. [DOI] [PubMed] [Google Scholar]
- 62. Eisenstat DD. Craniopharyngioma. Curr Treat Options Neurol. 2001;3(1):77–87. [DOI] [PubMed] [Google Scholar]
- 63. Hader WJ, Steinbok P, Hukin J et al. . Intratumoral therapy with bleomycin for cystic craniopharyngiomas in children. Pediatr Neurosurg. 2000;33(4):211–218. [DOI] [PubMed] [Google Scholar]
- 64. Jiang R, Liu Z, Zhu C. Preliminary exploration of the critical effect of bleomycin on cranipharyngiomas. Stereotact Funct Neurosurg. 2002;78(2):84–94. [DOI] [PubMed] [Google Scholar]
- 65. Takahashi H, Yamaguchi F, Teramoto A. Long-term outcome and reconsideration of intracystic chemotherapy with bleomycin for craniopharyngioma in children. Childs Nerv Syst. 2005;21(8–9):701–704. [DOI] [PubMed] [Google Scholar]
- 66. Dastoli PA, Nicacio JM, Silva NS et al. . Cystic craniopharyngioma: intratumoral chemotherapy with alpha interferon. Arq Neuropsiquiatr. 2011;69(1):50–55. [DOI] [PubMed] [Google Scholar]
- 67. Cavalheiro S, Di Rocco C, Valenzuela S et al. . Craniopharyngiomas: intratumoral chemotherapy with interferon-alpha: a multicenter preliminary study with 60 cases. Neurosurg Focus. 2010;28(4):e12. [DOI] [PubMed] [Google Scholar]
- 68. Jakacki RI, Cohen BH, Jamison C et al. . Phase II evaluation of interferon-alpha-2a for progressive or recurrent craniopharyngiomas. J Neurosurg. 2000;92(2):255–260. [DOI] [PubMed] [Google Scholar]
- 69. Lippens RJ, Rotteveel JJ, Otten BJ et al. . Chemotherapy with Adriamycin (doxorubicin) and CCNU (lomustine) in four children with recurrent craniopharyngioma. Eur J Paediatr Neurol. 1998;2(5):263–268. [DOI] [PubMed] [Google Scholar]
- 70. Kiehna EN, Merchant TE. Radiation therapy for pediatric craniopharyngioma. Neurosurg Focus. 2010;28(4):e10. [DOI] [PubMed] [Google Scholar]
- 71. Honegger J, Buchfelder M, Fahlbusch R. Surgical treatment of craniopharyngiomas: endocrinological results. J Neurosurg. 1999;90(2):251–257. [DOI] [PubMed] [Google Scholar]
- 72. Liu AK, Bagrosky B, Fenton LZ et al. . Vascular abnormalities in pediatric craniopharyngioma patients treated with radiation therapy. Pediatr Blood Cancer. 2009;52(2):227–230. [DOI] [PubMed] [Google Scholar]
- 73. Ullrich NJ, Robertson R, Kinnamon DD et al. . Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007;68(12):932–938. [DOI] [PubMed] [Google Scholar]
- 74. Aquilina K, Merchant TE, Rodriguez-Galindo C et al. . Malignant transformation of irradiated craniopharyngioma in children: report of 2 cases. J Neurosurg Pediatr. 2010;5(2):155–161. [DOI] [PubMed] [Google Scholar]
- 75. Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;10(6):293–310. [DOI] [PubMed] [Google Scholar]
- 76. Jacola LM, Ashford JM, Reddick WE et al. . The relationship between working memory and cerebral white matter volume in survivors of childhood brain tumors treated with conformal radiation therapy. J Neurooncol. 2014;119(1):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rosenfeld A, Arrington D, Miller J et al. . A review of childhood and adolescent craniopharyngiomas with a particular attention to hypothalamic obesity. Pediatr Neurol. 2014;50(1):4–10. [DOI] [PubMed] [Google Scholar]
- 78. Di Pinto M, Conklin HM, Li C et al. . Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys. 2012;84(3):217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mortini P, Losa M, Pozzobon G et al. . Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series. J Neurosurg. 2011;114(5):1350–1359. [DOI] [PubMed] [Google Scholar]
- 80. Sughrue ME, Yang I, Kane AJ et al. . Endocrinologic, neurologic, and visual morbidity after treatment of craniopharyngioma. J Neurooncol. 2010;101(3):463–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Balagamwala EH, Chao ST, Suh JH. Principles of radiobiology of stereotactic radiosurgery and clinical applications in the central nervous system. Technol Cancer Res Treat. 2012;11(1):3–13. [DOI] [PubMed] [Google Scholar]
- 82. Elliott RE, Sands SA, Strom RG et al. . Craniopharyngioma clinical status scale: a standardized metric of preoperative function and posttreatment outcome. Neurosurg Focus. 2010;28(4):e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.