Abstract
Background
Wernicke's encephalopathy is a serious medical condition associated with high morbidity and mortality caused by deficiency of thiamine. This disease is classically associated with alcoholism, but is underappreciated in the nonalcoholic population. There is growing acknowledgement of the development of Wernicke's encephalopathy in patients with malignancies.
Methods
We conducted a literature review in PubMed for cases of Wernicke's encephalopathy occurring in patients with malignancy. We also present the case of a 47-year-old woman with recurrent laryngeal cancer and multiple hospital admissions for malnutrition. Neurological examination was notable for pendular nystagmus, severe gait ataxia, confusion, and poor memory consolidation. MRI of the brain was significant for T2-weighted fluid-attenuated inversion recovery hyperintensities in periaqueductal regions, medial thalami, and the tectal plate, typical for Wernicke's encephalopathy. She was treated with thiamine repletion, and had marked improvement in her mental status and some improvement in her vision problems and ataxia, although some nystagmus and significant short-term memory impairment persisted.
Results
The literature review yielded dozens of case reports of Wernicke's encephalopathy in patients with malignancy, dominated by cases of patients with malignancies of the gastrointestinal system, followed by those with hematologic malignancies.
Conclusions
Malignancy is an important risk factor for the development of Wernicke's encephalopathy. This diagnosis is underappreciated and difficult for the clinician to discern from multifactorial delirium. Clinicians should be aware to treat at-risk patients with thiamine immediately, especially if multiple risk factors are present.
Keywords: cancer, encephalopathy, malignancy, non-alcoholic, Wernicke's
Vitamin B1, also known as thiamine, is utilized by neurons in several essential biochemical pathways including those of carbohydrate metabolism, lipid metabolism, and production of amino acids and neurotransmitters.1 At the cellular level, thiamine is converted into thiamine pyrophosphate, which acts as a co-factor for the enzymes α-ketoglutarate-dehydrogenase and pyruvate-dehydrogenase in the citric acid cycle, as well as the enzyme transketolase in the pentose phosphate pathway of energy metabolism.1 A deficiency of thiamine may lead to oxidative stress, which produces focal accumulation of lactic acid, free radicals, and cytokines, ultimately leading to neuronal death by apoptosis and necrosis.1 This neuronal damage can occur in the periphery, causing a neuropathy commonly known as Beriberi. In the central nervous system, neurons in the periaqueductal gray matter, medial thalami, and mamillary bodies have demonstrated high utilization and turnover of thiamine and glucose, and are particularly prone to damage in instances of thiamine deficiency, producing a syndrome commonly known as Wernicke's encephalopathy.1
Fig. 2.
T2-weighted FLAIR hyperintensities demonstrated in tectal plate, periaqueductal area, and area near mammillary bodies. Some motion artifact.
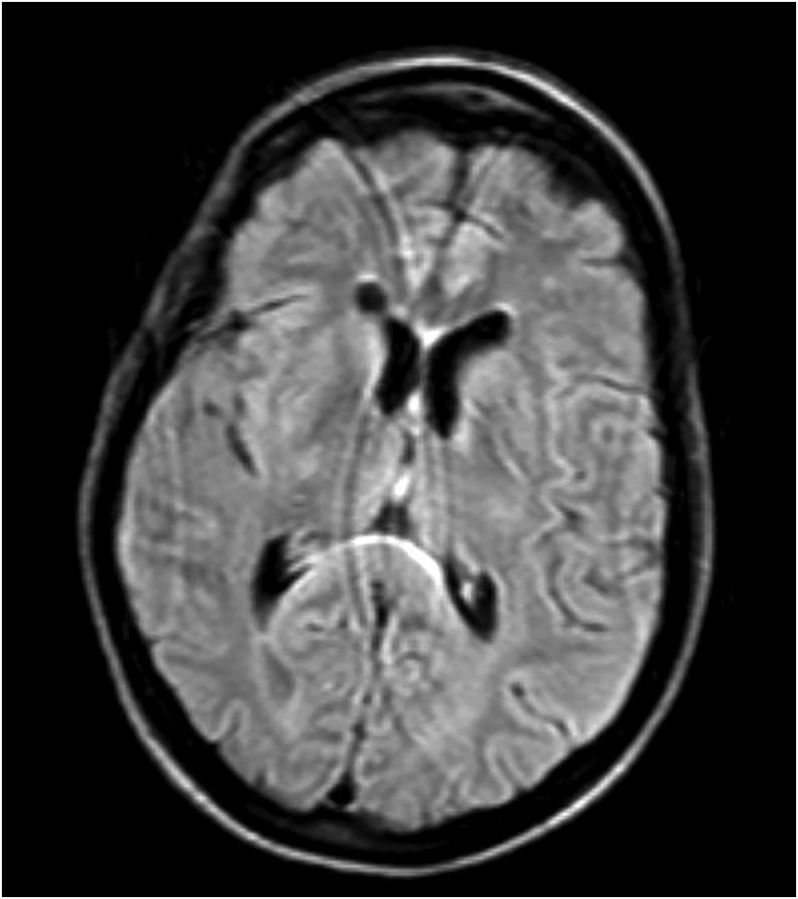
Fig. 3.
T2-weighted FLAIR hyperintensities demonstrated in medial thalami and area around third ventricle. Some motion artifact.
Wernicke's encephalopathy and its complication, Korsakoff's amnestic syndrome, are well known complications of chronic alcohol abuse.2 Wernicke's encephalopathy is characterized by the triad of confusion, ophthalmoplegia, and gait ataxia. Korsakoff's syndrome is the result of advanced Wernicke's encephalopathy and is characterized by memory impairment and confabulation. Wernicke's encephalopathy has serious sequelae if untreated and is associated with high risk of morbidity and mortality.2 While Wernicke's encephalopathy can be treated and reversed, Korsakoff's syndrome is, unfortunately, a permanent condition. Untreated Wernicke's encephalopathy can also lead to coma and eventually death.2
Alcoholics are typically deficient in thiamine due to a combination of poor dietary intake, gastric malabsorption, and impaired hepatic storage.3 Alcoholism is a major risk factor for the development of Wernicke's encephalopathy, and alcoholics comprise the majority of cases, which is estimated to be anywhere from 60% to 75% of diagnoses.4 In addition, according to one study, 12.5% of noted alcohol abusers were found to have evidence of Wernicke's encephalopathy at autopsy, which suggests that Wernicke's encephalopathy is currently underdiagnosed in this population.2
While Wernicke's encephalopathy is usually a disease associated with alcoholism, it is often underappreciated in nonalcoholics, who may develop thiamine deficiency through other means. Severe malnutrition can cause thiamine deficiency through obvious mechanisms of poor dietary intake.5 HIV infection and other systemic disorders can induce thiamine deficiency by increasing the basal metabolic rate.6–8 It has been suggested that thiamine may be lost in hemodialysis.9–11
There is a growing appreciation of Wernicke's encephalopathy in patients with malignancies. Patients with malignancies, as patients with other systemic disorders, have increased basal metabolic rates, which may induce depletion of thiamine. Malignancies of the gastrointestinal system have been particularly associated with the development of Wernicke's encephalopathy, believed to be due to the added mechanisms of impaired absorption, especially when compounded by intervention such as resection of parts of the gastrointestinal tract, itself a profound risk factor for thiamine deficiency.12 The fluoropyrimidine class of chemotherapeutic agents, which includes the agent 5-fluorouracil, has also been associated with the development of Wernicke's encephalopathy by inducing increased thiamine metabolism, placing those with malignancies getting these treatments at further risk.13,14
Wernicke's encephalopathy is currently underdiagnosed in the population. This is owed in no small part to the clinical manifestations of the disease, which are often hard to recognize and rarely present as a complete triad. Additionally, those at risk for nonalcoholic Wernicke's encephalopathy often have multiple co-morbid conditions, which can make it difficult for the clinician to discern from multifactorial delirium. Caine's diagnostic criteria has been established as a clinical tool to assess for Wernicke's encephalopathy, which improves the sensitivity of recognizing and diagnosing this disease from 22%, with the classic triad, to 85% (Table 1).15
Table 1.
Caine's diagnostic criteria
| Sign | Value |
|---|---|
| Ocular abnormalities | 1 |
| Cerebellar abnormalities | 1 |
| Confusion or memory impairment | 1 |
| History of malnutrition | 1 |
Total values of 2 or higher indicate 85% sensitivity when suspecting Wernicke's encephalopathy.
Case Presentation
We present the case of a 47-year-old woman diagnosed with laryngeal cancer 3 years prior to admission, which had been treated with chemotherapy (1 cycle of docetaxel, cisplatin, and 5-fluorouracil [TPF]), radiation therapy, and resection (transoral robotic surgery [TORS] and bilateral neck dissection), but found to have recurred several months prior to admission. Her past medical history is also significant for hypertension and end-stage renal disease for which she receives hemodialysis. She had been recently discharged from our hospital 1 week prior to admission, when she was noted to have severe malnutrition as she was not tolerating oral intake. The severity of her malnutrition was illustrated by her rapid and profound weight loss of 43 kg over the course of 6 months. At that time, she refused percutaneous endoscopic gastrostomy tube placement, but recovered her ability to eat food after speech and laryngeal rehabilitation. She returned to our hospital complaining only of nausea, but appeared acutely ill with delirium, and also appeared malnourished.
Initial evaluation revealed bacteremia and her mental condition improved with antibiotics and supportive therapy. She required a prolonged hospital stay, during which time she began to complain of double vision. Neurological examination at that time was significant for persistent, pendular nystagmus in primary gaze and in all directions, as well as jerky ophthalmologic pursuit and slow saccades. After several weeks, while still in the hospital, she had deterioration in her mental status and again developed acute confusion. She also had a newly ataxic gait and impaired short-term memory and alertness.
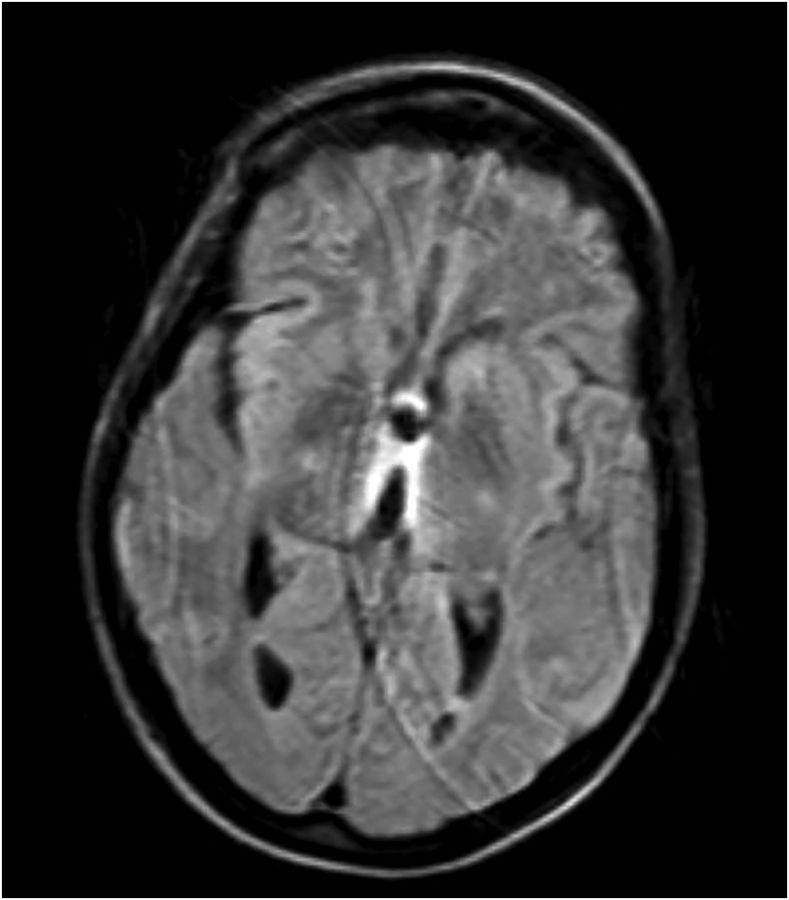
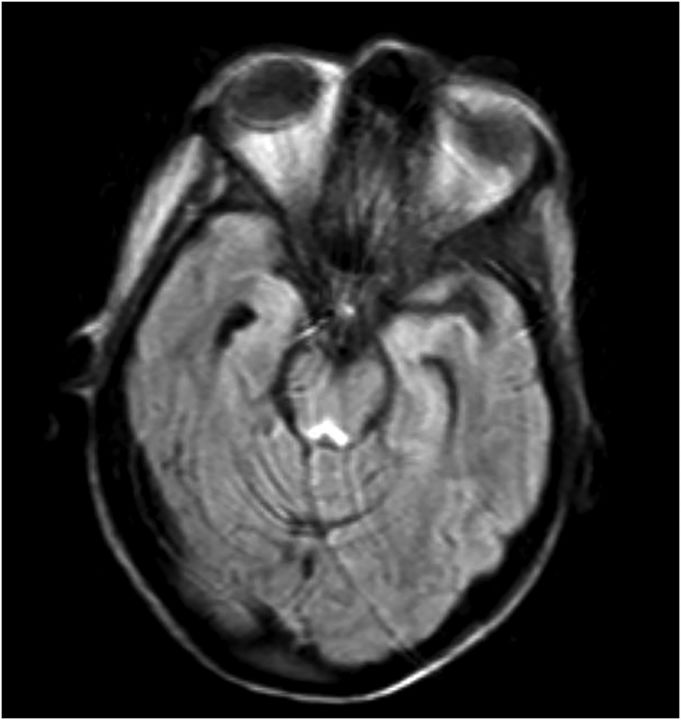
She had a full evaluation for infection, which was negative, and she remained afebrile and with normal white blood cell count. Her serology, including basic metabolic panel and complete blood count, were unremarkable. Given her recurrent laryngeal cancer, there was high suspicion for CNS metastases and leptomeningeal disease, but lumbar puncture was normal, with unremarkable cytology and flow cytometry. MRI of her brain revealed T2-weighted fluid-attenuated inversion recovery (FLAIR) hyperintensities in the periaqueductal area, medial thalami, and tectal plate, which is typical for Wernicke's encephalopathy (Figs. 1–4).16,17
Fig. 1.
T2-weighted FLAIR hyperintensity demonstrated in periaqueductal area. Some motion artifact.
Fig. 4.
T2-weighted FLAIR hyperintensity demonstrated in area around third ventricle. Some motion artifact.
Given clinical findings and imaging results, she was diagnosed with acute Wernicke's encephalopathy, and she was treated with aggressive thiamine repletion at a dosage of 500 mg intravenously every 8 hours for 5 days. She had immediate improvement in her mental status, became attentive, and was no longer confused. She had persistence, however, of her memory impairment and had poor scoring on recall tasks. There was partial improvement in her nystagmus and ataxia.
She agreed to percutaneous endoscopic gastrostomy tube placement, and was also instructed to take regular, lifetime thiamine supplementation at 100 mg daily via gastric tube. She was discharged to a sub-acute rehab facility, where she regained much of her strength, and was able to ambulate independently for short distances 4 weeks after arrival. She was last seen in our hospital 2 months after discharge, when she was found to have gained 2 kg in body weight.
Literature Review
A literature review was conducted in PubMed. The terms “Wernicke,” “Encephalopathy,” “Malignancy,” “Cancer,” and all combinations of those terms were used in our literature search. The review yielded 40 relevant papers and abstracts, largely in the form of case reports, representing 46 total cases of Wernicke's encephalopathy diagnosed in patients with active malignancy. Malignancies of the gastrointestinal system represented 17 of the 46 total case reports (Table 2). Hematologic malignancies also represented 17 of the 46 total case reports (Table 3). The remainder of the case reports included 5 incidences of head and neck malignancies, 2 incidences of primary central nervous system malignancies, and 1 incidence each of gynecological, pulmonary, skeletal, genitourinary, and endocrinological malignancies (Table 4).
Table 2.
Results of a literature review in PubMed
| Malignancy | Age | Sex | Barriers to Nutrition | Chemotherapeutics | Surgical Interventions | Imaging | Clinical Findings | Outcome | Author, Year, and Citation |
|---|---|---|---|---|---|---|---|---|---|
| Colon cancer | 59 | Female | Unknown | 5-FU, otherwise unknown | Unknown | MRI | Confusion, ataxia (NOS), otherwise unknown | Unknown | Guilloton L. 2005.19 |
| Colon cancer | 54 | Male | Persistent nausea, TPN | Unknown | Colectomy (subtotal) | MRI | Lethargy, inattentiveness, memory impairment, ophthalmoplegia (lateral movements), nystagmus (pendular), ataxia (NOS), muscle stiffness, hyporeflexia | Slight resolution; persistence of all symptoms | Nolli M. 2005.20 |
| Colon cancer | 56 | Female | Ileus, TPN | Unknown | Hemicolectomy (right) | MRI | Lethargy, nystagmus (horizontal), ataxia (limbs), hyporeflexia | Nearly full resolution; persistence of trace nystagmus and ataxia | Pagnan L. 1998.21 |
| Colon cancer | 56 | Female | Unknown | Calcium folinate, 5-FU | Unknown | MRI | Alteration of consciousness, seizures, ophthalmoplegia (NOS), nystagmus (horizontal), hyperreflexia | Nearly full resolution; persistence of trace ataxia | Papila B. 2010.22 |
| Gastric cancer | 66 | Female | Persistent nausea and vomiting, TPN | NOS | Unknown | Unknown | Disorientation, memory impairment | Full resolution | Onishi H. 2004.23 |
| Gastric cancer | 68 | Male | Unknown | Unknown | Gastrectomy (subtotal) | MRI | Memory disturbance, diplopia, ophthalmoplegia (total), ataxia (gait), hyporeflexia | No improvement | Kikuchi A. 2000.24 |
| Gastric cancer | 48 | Male | Alcoholism | Unknown | Gastrectomy (total) | MRI negative | Memory impairment, ophthalmoplegia (bilateral lateral recti), nystagmus (horizonta), ataxia (truncal and gait), areflexia | Improved, NOS | Arai M. 1997.25 |
| Gastric cancer | 71 | Female | Unknown | Unknown | Unknown | MRI | Confusion, memory impairment, nystagmus (horizontal), ataxia (limbs), hyporeflexia | Slight improvement; persistence of all symptoms | Weidauer S. 2004.26 |
| Gastric cancer, adenocarcinoma | 28 | Female | Esophageal stenosis, poor oral intake | 5-FU | Unknown | Unknown | Confusion, dizziness, deafness, nystagmus (NOS), deafness, ataxia (gait) | Full resolution | Kondo K. 1996.13 |
| Gastric cancer, adenocarcinoma | 35 | Male | Partial gastric outlet obstruction, vomiting, poor oral intake | Unknown | Unknown | MRI | Confusion, dysarthria, ataxia (gait) | Full resolution | Kudru CU. 2014.27 |
| Gastric cancer, signet ring cell | 48 | Female | Esophagogastric junction obstruction, nausea and vomiting, primarily liquid diet, intermittent parenteral nutrition | Paclitaxel, S-1 | Esophagogastric junction stent placement | Unknown | Nystagmus (NOS), ataxia (gait) | Nearly full resolution; persistence of mild nystagmus | Jung ES. 2010.28 |
| Gastric cancer, signet ring cell | 58 | Female | Nausea and vomiting | Oxaliplatin, 5-FU, leucovorin | MRI | Confusion, disorientation, ophthalmoplegia (NOS), ataxia (NOS) | Died from neurological complications | Jung ES. 2010.28 | |
| Gastric cancer, submucosal | 56 | Male | Alcoholism | Unknown | Gastrectomy (total) | Unknown | Ophthalmoplegia (bilateral lateral recti), severe ataxia (gait), areflexia | Improved, NOS | Arai M. 1997.25 |
| Hepatocellular carcinoma | 72 | Male | None | None | Hepatic lobectomy | None | Confusion, inattention, memory impairment, insomnia | Full resolution | Onishi H. 2005.29 |
| Pancreatic cancer, adenocarcinoma | 52 | Male | Alcoholism | Unknown | Whipple procedure | MRI | Confusion, memory impairment, ataxia (trunk and gait) | Full resolution | Karayiannakis AJ. 2011.30 |
| Pancreatic cancer, neuro-endocrine | 45 | Female | Recurrent hypoglycemia, persistent nausea and vomiting | Cisplatin, etoposide, streptozotocin, doxorubicin, interferon, somatostatin analogues | Unknown | MRI negative | Confusion, nonreactive pupils, ophthalmoplegia (bilateral lateral recti), ataxia (trunk and gait) | Full resolution | Grunenwald S. 2009.31 |
| Rectal cancer | 73 | Male | Severe nausea, poor oral intake primarily liquid | Unknown | Hemicolectomy (right) | MRI | Diplopia, ophthalmoplegia (left lateral rectus), gait impairment, ataxia (limbs) | Partial resolution; persistence of mild confabulation | Chu K. 2002.32 |
This table summarizes the clinical history, features, and outcomes of case reports of Wernicke's encephalopathy in patients with gastrointestinal system malignancies.
Abbreviations: 5-FU, 5-fluorouracil; NOS, not otherwise specified; TPN, total parenteral nutrition.
Table 3.
Results of a literature review in PubMed
| Malignancy | Age | Sex | Barriers to nutrition | Chemotherapeutics | Surgical interventions | Imaging | Clinical findings | Outcome | Author, Year, and Citation |
|---|---|---|---|---|---|---|---|---|---|
| Acute leukemia, NOS | 15 | Female | Unknown | NOS | Unknown | MRI | Lethargy, amnesia, ataxia, occulomotor deficits (NOS) | Full resolution | Vanhulle C. 1997.33 |
| ALL | 9 | Male | Limited oral intake (carbohydrates including soft drinks) | Methotrexate (intrathecal), vincristine, prednisone, cyclophosphamide, adriamycin | None | None | Diffuse weakness, double vision, unsteadiness, alteration of consciousness | Died before complete neurological improvement | Miyajami Y. 1993.34 |
| Acute mixed lineage leukemia | 12 | Male | Nausea, persistent diarrhea, TPN | Cytosine arabinoside, aclarabucin, granulocyte colony stimulating factor | None | MRI | Occulomotor palsy (NOS), nystagmus | Full resolution | Onodera N. 1998.35 |
| AML | 42 | Female | TPN | Cyclosporin A, methotrexate | Allogeneic peripheral blood stem cell transplantation | MRI | Confusion, nystagmus (horizontal), generalized weakness, ataxia (truncal and lower limbs) | Full resolution | Baek JH. 2005.36 |
| ALL | 16 | Male | Acute pancreatitis, vomiting, TPN | Prednisone, L-asparaginase, daunorubicin, vincristine, low-dose cytarabine, 6-mercaptopurine, high-dose methotrexate | None | MRI | Confusion, disorientation, dizziness, vertigo, diplopia. | Full resolution | Muwakkit S. 2009.37 |
| ALL | 3 | Female | Diarrhea, parenteral nutrition | NOS | None | Unknown | Abnormal eye movements | Wernicke's encephalopathy diagnosed at autopsy | Brück W. 1991.38 |
| ALL, T-cell | 20 | Male | Poor oral intake (limited to soft drinks)/6 weeks | None | None | MRI | Confusion | Full resolution | Lacasse L. 2004.39 |
| AML | 13 | Female | Persistent nausea and vomiting | Idarubicin, etoposide, cytarabine | None | MRI | Confusion, nystagmus (NOS), ophthalmoplegia (NOS) | Full resolution | D'Aprile P. 2000.40 |
| B-cell lymphoma, diffuse large cell | 37 | Female | Alcoholism, nausea (with good oral intake) | R-CHOP | Unknown | MRI | Obtunded, disconjugate gaze | Full resolution | Turner JE. 2004.41 |
| B-cell lymphoma, high-grade | 51 | Male | Unknown | Methotrexate, NOS | Unknown | Unknown | Confusion, ophthalmoplegia (bilateral, NOS), nonreactive pupils, ptosis, visual hallucinations | Full resolution. Also treated for new-onset seizures (hallucinations). | Gregory J. 2012.42 |
| B-cell lymphoma, Hodgkin's | 60 | Female | Anorexia, nausea | NOS | Unknown | MRI negative | Disorientation, depression, nystagmus (horizontal), psychomotor retardation proximal weakness, hyporeflexia | Partial resolution; died 2 months later before full recovery | Macleod AD. 2000.43 |
| B-cell lymphoma, Non-Hodgkin's | 56 | Male | Persistent nausea and vomiting | R-CHOP | Gastric biopsy | MRI negative | Vertical nystagmus, ataxia (gait) | Full resolution | Boniol S. 2007.44 |
| B-cell lymphona, diffuse large cell | 47 | Male | Nausea, TPN | R-CHOP | Hemicolectomy (right) | MRI | Confusion, lethargy, hallucinations (visual and auditory) | Slight improvement; died shortly after diagnosis | Lee SM. 2012.45 |
| CNS lymphoma, primary | 62 | Female | Nausea, poor oral intake primarily carbohydrates | High-dose methotrexate, cytarabine | Unknown | MRI | Confusion, memory impairment, ataxia (mild, NOS) | Slight improvement; persistence of confusion and memory impairment | Richardson S. 2010.46 |
| Multiple myeloma | 50 | Female | Nausea and vomiting, poor oral intake | Unknown | Autologous stem cell transplantation | MRI | Confusion, lethargy, inattention, ophthalmoplegia (left lateral rectus), nystagmus (horizontal) | Partial resolution; persistence of memory impairment | Morcos Z. 2004.47 |
| Promyelocytic leukemia | 32 | Female | Parenteral nutrition | Daunorubicin, cytosine arabinoside, 6-mercaptopurine | Unknown | Unknown | Memory impairment | Died; Wernicke's encephalopathy diagnosed at autopsy | Pittella JE. 1990.48 |
| T-cell lymphoma, diffuse high-grade large cell (histiocytic) | 54 | Male | Mucositis causing anorexia, persistent nausea and vomiting | Cyclophosphamide, vincristine, prednisone, bleomycin, doxorubicin, procarbazine | Radiotherapy | None | Confusion, lethargy, memory impairment, nystagmus (horizontal) | Partial resolution; persistence of memory impairment | Engel PA. 1991.49 |
This table summarizes the clinical history, features, and outcomes of case reports of Wernicke's encephalopathy in patients with hematologic malignancies.
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; NOS, not otherwise specified; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; TPN, total parenteral nutrition.
Table 4.
Results of a literature review in PubMed
| Malignancy | Age | Sex | Barriers to nutrition | Chemotherapeutics | Surgical interventions | Imaging | Clinical findings | Outcome | Author, Year, and Citation |
|---|---|---|---|---|---|---|---|---|---|
| Adrenal gland cortical carcinoma | 70 | Female | None | Mitotane, cisplatin, doxorubicin, etoposide | Unknown | None | Disorientation, inattention | Full resolution | Yae S. 2005.50 |
| Endometrial adenocarcinoma | 57 | Female | Unknown | Unknown | Total abdominal hysterectomy and bilateral salpingo-oophorectomy | MRI | Lethargy, memory impairment, nystagmus (all directions) | Partial resolution; persistence of memory impairment | Flint AC. 2006.51 |
| Lung cancer | 71 | Male | Loss of appetite | Unknown | Unknown | Unknown | Confusion, inattention, ataxia (gait) | Full resolution | Onishi H. 2004.23 |
| Maxillary cancer | 77 | Female | Dysphagia | NOS | Radiotherapy | Unknown | Confusion, memory disturbance, nystagmus (horizontal), ataxia (gait) | Full resolution | Onishi H. 2004.23 |
| Nasopharyngeal carcinoma | 46 | Female | Unknown | Cisplatin, 5-FU | Radiotherapy | MRI | Confusion, lethargy, diplopia, headaches | Full resolution | Cho IJ. 2009.52 |
| Nasopharyngeal carcinoma | 62 | Male | Ischemic colitis, diarrhea | Docetaxel | Unknown | MRI | Confusion, nystagmus (NOS), ataxia (NOS) | Died | Kosta P. 2005.53 |
| Neuroectodermal tumor, Frontal lobe | 12 | Male | Mucositis causing anorexia, TPN | Methotrexate, etoposide, cyclophosphamide, carboplatinum | Tumor resection, radiotherapy, autologous peripheral hematopoietic stem cell rescue | MRI | Confusion, hallucinations, tremors (diffuse) | Slight improvement, NOS | Cefalo MG. 2014.54 |
| Osteosarcoma | 17 | Female | Frequent vomiting, parenteral nutrition | Doxorubicin, methotrexate, ifosfamide, cisplatin | Unknown | Unknown | Somnolence, blurry vision, nystagmus (horizontal and vertical), ataxia (trunk and limbs), hyporeflexia | Died; Wernicke's encephalopathy diagnosed at autopsy | Kálmánchey R. 1994.55 |
| Peritoneal carcinoma, primary | 76 | Female | Persistent nausea, TPN | Paclitaxel, carboplatin | Unknown | MRI | Confusion, memory impairment, diplopia, ataxia (NOS) | Partial resolution; peristent confusion and memory impairment | Kim KH. 2013.56 |
| Pontine glioma, diffuse intrinsic | 6 | Male | None | Vinorelbine, nimotuzumab | Unknown | MRI | Restlessness, dysarthria, blurry vision, opthalmoplegia (bilateral lateral recti), ataxia (truncal) | Partial resolution; persistence of occulmotor deficits and ataxia | Cefalo MG. 2014.54 |
| Squamous cell carcinoma, tongue | 28 | Female | Persistent nausea and vomiting | NOS | Partial glossectomy with lateral neck dissection, radiotherapy to neck | Unknown | Confusion, inattention, memory impairment, nystagmus (horizontal gaze-evoked), diffuse weakness, ataxia (NOS), sensory impairment (distal) | Nearly full resolution | Fikhman G. 2011.57 |
| Squamous cell carcinoma, tonsil | 63 | Female | Persistent nausea and vomiting | NOS | Cholecystectomy, radiotherapy to neck | Unknown | Confusion, diplopia, vertigo, ataxia (NOS) | Nearly full resolution; persistence of mild ataxia | Fikhman G. 2011.57 |
This table summarizes the clinical history, features, and outcomes of case reports of Wernicke's encephalopathy in patients with various malignancies.
Abbreviations: 5-FU, 5-fluorouracil; NOS, not otherwise specified; TPN, total parenteral nutrition.
Discussion
There is a growing literature base suggesting association between malignancies and the development of Wernicke's encephalopathy. Our literature review chiefly associates gastrointestinal and hematologic malignancies with the development of this condition. Gastrointestinal malignancies are particularly well represented in our literature review because of the many means by which they induce inadequate supply of the vitamin thiamine. This particular patient population is particularly prone to poor nutritional intake, poor nutritional absorption, and increased thiamine utilization through the very nature of their disease. They are also prone to complications such as persistent nausea and vomiting as well as gastrointestinal tract obstruction, which further impair adequate intake of thiamine. These factors may be additionally exacerbated by iatrogenic intervention, such as surgical resection of the gastrointestinal tract, which can expose these patients to further risk of thiamine deficiency.
This phenomenon is not unique to gastrointestinal malignancies, as Wernicke's encephalopathy is also well represented in hematologic malignancies and observed in other malignancies. The many factors described in our discussion of gastrointestinal malignancies are also likely working in tandem to induce thiamine deficiencies in these cases.
The vast majority of case reports describe significant barriers to adequate nutritional intake including persistent nausea and vomiting, anorexia, mucositis, obstruction, and total parenteral nutrition, which patients with malignancies are predisposed to as direct sequelae of their disease processes and their treatments. The clinician should be extra cautious and have high suspicion for thiamine deficiency in cases of significantly poor nutritional intake. There are several case reports of patients with malignancies who do not have any barriers to nutrition or any other known risk factors for developing Wernicke's encephalopathy, which suggests that malignancies are an independent risk factor for developing this disorder.
An overwhelming number of the diagnoses of Wernicke's encephalopathy in our literature review were made at the time of MRI. Given the low sensitivity of MRI in diagnosing Wernicke's encephalopathy (53% by one study16), this disease is likely being underdiagnosed and undertreated. Additionally, our literature review reveals that significant time often passes between the initial manifestation of Wernicke's encephalopathy and its diagnosis. Unfortunately, these factors ultimately lead to worse patient outcomes; some patients in our review died from this acute neurological condition, while many others did not achieve full recovery from this eminently treatable disease. These facts also illustrate the difficulty of diagnosing this particular condition in cancer patients, who often have complex medical histories and disease presentations.
A high clinical suspicion remains the best diagnostic tool for identifying Wernicke's encephalopathy. As above, MRI remains the most prevalent and valuable imaging modality for making a real-time diagnosis of Wernicke's encephalopathy, but while highly specific, this test has a low sensitivity for detection of this disease and is not reliable for making this diagnosis.16 Thiamine levels can be normal in patients with florid Wernicke's encephalopathy, and there are no other available serological markers to assist the clinician with this diagnosis.18 Caine's criteria is a useful diagnostic tool for the clinician who suspects a diagnosis of Wernicke's encephalopathy, and its employment vastly increases the sensitivity in detecting this disorder. If Caine's criteria is employed to case presentations in our literature review retrospectively, an early diagnosis of Wernicke's encephalopathy can be made in all but one case. A good understanding of the pathophysiology and epidemiology of Wernicke's encephalopathy, when coupled with the use of Caine's criteria, can further increase the sensitivity of detecting this disease.
Our literature review illustrates the growing association between malignancy and Wernicke's encephalopathy. Consistent with recent literature, Caine's criteria concerns itself only with history of malnutrition, and does not otherwise delineate between alcoholic and nonalcoholic causes.
Once suspected, treatment should be initiated as soon as possible. A recent review reveals that there is currently insufficient evidence from randomized, controlled trials to suggest an optimal dose, route, and duration of thiamine repletion in the treatment of Wernicke's encephalopathy.1 Existing literature, however, supports the use of thiamine 500 mg intravenously (administered over 30 minutes) or intramuscularly, three times per day for 2 to 3 days; the intravenous and intramuscular routes are used to ensure adequate absorption.1 Where an effective response is observed, the dosage can be reduced to 250 mg intravenously or intramuscularly daily for 3 to 5 more days, or until no further clinical improvement is seen.1 The existing literature shows that dosages of 100 to 250 mg daily may be inadequate to improve clinical signs or prevent death.1 Care should also be taken if patients have a history of malnutrition without any evidence of Wernicke's encephalopathy, and thiamine supplementation should be offered to these patients especially if parenteral administration of nutrition or glucose is being considered.
The patient in our case report illustrates the diagnostic difficulty in a patient with malignancy. She was not an alcoholic, but had several other known risk factors for Wernicke's encephalopathy including malignancy, severe malnutrition, and hemodialysis treatments. She was noted to have alteration of mental status on admission, which was thought to be due to her underlying sepsis as well as her severe nutritional deficiencies; indeed, her mental status was noted to have improved after administration of antibiotics and nutritional support, but slowly deteriorated during her hospital course as she developed acute confusion and inattentiveness, as well as ataxia and nystagmus. There was large concern for recurrent sepsis as well as metastatic or leptomeningeal spread of her malignancy as the cause of her symptomatology. Utilizing Caine's criteria, however, we would have high suspicion for this condition given her nutritional deficits, occulomotor abnormalities, acute confusion, and ataxia (Caine's criteria suggests Wernicke's encephalopathy if satisfying 2 out of the 4 criteria). We would also have had higher suspicion for this disorder in the face of mounting evidence associating this condition with malignancy.
Based on our literature search, our case report may be the first describing Wernicke's encephalopathy in a patient with laryngeal cancer.
Funding
This case report and literature review was not supported by any funding.
Conflict of interest statement. None declared.
References
- 1. Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442–455. [DOI] [PubMed] [Google Scholar]
- 2. Harper C. The incidence of Wernicke's encephalopathy in Australia - a neuropathological study of 131 cases. J Neurol Neurosurg Psychiatry. 1983;46(7):593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomson AD, Ryle PR, Shaw GK. Ethanol, thiamine, and brain damage. Alcohol Alcohol. 1983;18(1):27–43. [Google Scholar]
- 4. Lindboe CF, Loberg EM. Wernicke's encephalopathy in non-alcoholics. An autopsy study. J Neurol Sci. 1989;90(2):125. [DOI] [PubMed] [Google Scholar]
- 5. Newman ME, Adityanjee, Sobolewski E, Jampala VC. Wernicke-Korsakoff amnestic syndrome secondary to malnutrition in a patient with schizoaffective disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11(4):241–244. [PubMed] [Google Scholar]
- 6. Davtyan DG, Vinters HV. Wernicke's encephalopathy in AIDS patient treated with zidovudine. Lancet. 1987;1(8538):919. [DOI] [PubMed] [Google Scholar]
- 7. Schwenk J, Gosztonyi G, Thierauf P, Iglesias J, Langer E. Wernicke's encephalopathy in two patients with acquired immunodeficiency syndrome. J Neurol. 1990;237(7):445. [DOI] [PubMed] [Google Scholar]
- 8. Soffer D, Zirkin H, Alkan M, Berginer VM. Wernicke's encephalopathy in acquired immune deficiency syndrome (AIDS): a case report. Clin Neuropathol. 1989;8(4):192. [PubMed] [Google Scholar]
- 9. Hung SC, Hung SH, Tarng DC, Yang WC, Chen TW, Huang TP. Thiamine deficiency and unexplained encephalopathy in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2001;38(5):941. [DOI] [PubMed] [Google Scholar]
- 10. Jagadha V, Deck JH, Halliday WC, Smyth HS. Wernicke's encephalopathy in patients on peritoneal dialysis or hemodialysis. Ann Neurol. 1987;21(1):78. [DOI] [PubMed] [Google Scholar]
- 11. Descombes E, Dessibourg CA, Fellay G. Acute encephalopathy due to thiamine deficiency (Wernicke's encephalopathy) in a chronic hemodialyzed patient: a case report. Clin Nephrol. 1991;35(4):171. [PubMed] [Google Scholar]
- 12. Aasheim ET. Wernicke encephalopathy after bariatric surgery: a systematic review. Ann Surg. 2008;248(5):714. [DOI] [PubMed] [Google Scholar]
- 13. Kondo K, Fujiwara M, Murase M et al. Severe acute metabolic acidosis and Wernicke's encephalopathy following chemotherapy with 5-fluorouracil and cisplatin: case report and review of the literature. Jpn J Clin Oncol. 1996;26(4):234–236. [DOI] [PubMed] [Google Scholar]
- 14. Heier MS, Dornish JM. Effect of the fluoropyrimidines 5-fluorouracil and doxifluridine on cellular uptake of thiamin. Anticancer Res. 1989;9(4):1073–1077. [PubMed] [Google Scholar]
- 15. Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antunez E, Estruch R, Cardenal C, Nicolas JM, Fernandez-Sola J, Urbano-Marquez A. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke's encephalopathy. AJR Am J Roentgenol. 1998;171(4):1131. [DOI] [PubMed] [Google Scholar]
- 17. Zhong C, Jin L, Fei G. MR Imaging of Nonalcoholic Wernicke Encephalopathy: A Follow-Up Study. AJNR. 2005;26:2301–2305. [PMC free article] [PubMed] [Google Scholar]
- 18. Lough ME. Wernicke's encephalopathy: expanding the diagnostic toolbox. Neuropsychol Rev. 2012;22(2):181–194. [DOI] [PubMed] [Google Scholar]
- 19. Guilloton L, Michaud A, Potier V, Le Berre J, Drouet A, Felten D. Ataxia and confusion after treatment with 5 Fluorouracile. Rev Med Interne. 2005;26(12):986–987. [DOI] [PubMed] [Google Scholar]
- 20. Nolli M, Barbieri A, Pinna C, Pasetto A, Nicosia F. Wernicke's encephalopathy in a malnourished surgical patient: clinical features and magnetic resonance imaging. Acta Anaesthesiol Scand. 2005;49(10):1566–1570. [DOI] [PubMed] [Google Scholar]
- 21. Pagnan L, Berlot G, Pozzi-Mucelli RS. Magnetic resonance imaging in a case of Wernicke's encephalopathy. Eur Radiol. 1998;8(6):977–980. [DOI] [PubMed] [Google Scholar]
- 22. Papila B, Yildiz O, Tural D et al. Wernicke's Encephalopathy in Colon Cancer. Case Rep Oncol. 2010;3(3):362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Onishi H, Kawanishi C, Onose M. Successful treatment of Wernicke encephalopathy in terminally ill cancer patients: report of 3 cases and review of the literature. Support Care Cancer. 2004;12(8):604–608. [DOI] [PubMed] [Google Scholar]
- 24. Kikuchi A, Chida K, Misu T et al. A case of Wernicke-Korsakoff syndrome with dramatic improvement in consciousness immediately after intravenous infusion of thiamine. No To Shinkei. 2000;52(1):59–63. [PubMed] [Google Scholar]
- 25. Arai M, Nara K, Awazu N. Wernicke's encephalopathy developed several years after total gastrectomy. Report of 2 cases. Rinsho Shinkeigaku. 1997;37(11):1027–1029. [PubMed] [Google Scholar]
- 26. Weidauer S, Rösler A, Zanella FE, Lanfermann H. Diffusion-weighted imaging in Wernicke encephalopathy associated with stomach cancer: case report and review of the literature. Eur Neurol. 2004;51(1):55–57. [DOI] [PubMed] [Google Scholar]
- 27. Kudru CU, Nagiri SK, Rao S. Wernicke's encephalopathy in a patient with gastric carcinoma: a diagnosis not to miss. BMJ Case Rep. 20142014:bcr2013203511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jung ES, Kwon O, Lee SH et al. Wernicke's Encephalopathy in Advanced Gastric Cancer. Cancer Res Treat. 2010;42(2):77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Onishi H, Sugimasa Y, Kawanishi C, Onose M. Wernicke encephalopathy presented in the form of postoperative delirium in a patient with hepatocellular carcinoma and liver cirrhosis: a case report and review of the literature. Palliat Support Care. 2005;3(4):337–340. [DOI] [PubMed] [Google Scholar]
- 30. Karayiannakis AJ, Souftas VD, Bolanaki H, Prassopoulos P, Simopoulos C. Wernicke encephalopathy after pancreaticoduodenectomy for pancreatic cancer. Pancreas. 2011;40(7):1157–1159. [DOI] [PubMed] [Google Scholar]
- 31. Grunenwald S, Broussaud S, Vezzosi D, Bennet A, Larrue V, Caron P. Well-differentiated endocrine pancreatic tumour and Wernicke's encephalopathy. Clin Endocrinol (Oxf). 2009;70(1):170–171. [DOI] [PubMed] [Google Scholar]
- 32. Chu K, Kang DW, Kim HJ, Lee YS, Park SH. Diffusion-weighted imaging abnormalities in wernicke encephalopathy: reversible cytotoxic edema? Arch Neurol. 2002;59(1):123–127. [DOI] [PubMed] [Google Scholar]
- 33. Vanhulle C, Dacher JN, Delangre T, Garraud V, Vannier JP, Tron P. Antineoplastic chemotherapy and Wernicke's encephalopathy. Arch Pediatr. 1997;4(3):243–246. [DOI] [PubMed] [Google Scholar]
- 34. Miyajima Y, Fukuda M, Kojima S, Matsuyama T, Shylaja N, Aso K. Wernicke's encephalopathy in a child with acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1993;15(3):331–334. [PubMed] [Google Scholar]
- 35. Onodera N, Nakahata T, Tanaka H, Ito R, Honda T. Successful treatment of Wernicke's encephalopathy in a boy with acute mixed lineage leukemia. Acta Paediatr Jpn. 1998;40(3):271–274. [DOI] [PubMed] [Google Scholar]
- 36. Baek JH, Sohn SK, Kim DH et al. Wernicke's encephalopathy after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;35(8):829–830. [DOI] [PubMed] [Google Scholar]
- 37. Muwakkit S, Al-Aridi C, Saab R, Hourani R, Yazbeck N, Abboud M. Wernicke's encephalopathy during total parenteral nutrition in a child with acute lymphoblastic leukemia and acute pancreatitis. Neuropediatrics. 2009;40(5):249–251. [DOI] [PubMed] [Google Scholar]
- 38. Brück W, Christen HJ, Lakomek H, Hanefeld F, Friede RL. Wernicke's encephalopathy in a child with acute lymphoblastic leukemia treated with polychemotherapy. Clin Neuropathol. 1991;10(3):134–136. [PubMed] [Google Scholar]
- 39. Lacasse L, Lum C. Wernicke encephalopathy in a patient with T-cell leukemia and severe malnutrition. Can J Neurol Sci. 2004;31(1):97–98. [DOI] [PubMed] [Google Scholar]
- 40. D'Aprile P, Tarantino A, Santoro N, Carella A. Wernicke's encephalopathy induced by total parenteral nutrition in patient with acute leukaemia: unusual involvement of caudate nuclei and cerebral cortex on MRI. Neuroradiology. 2000;42(10):781–783. [DOI] [PubMed] [Google Scholar]
- 41. Turner JE, Alley JG, Sharpless NE. Medical problems in patients with malignancy: case 2. Wernicke's encephalopathy: an unusual acute neurologic complication of lymphoma and its therapy. J Clin Oncol. 2004;22(19):4020–4022. [DOI] [PubMed] [Google Scholar]
- 42. Gregory J, Philbrick K, Chopra A. Wernicke encephalopathy in a non-alcoholic patient with metastatic CNS lymphoma and new-onset occipital lobe seizures. J Neuropsychiatry Clin Neurosci. 2012Fall;24(4):E53. [DOI] [PubMed] [Google Scholar]
- 43. Macleod AD. Wernicke's encephalopathy and terminal cancer: case report. Palliat Med. 2000;14(3):217–218. [DOI] [PubMed] [Google Scholar]
- 44. Boniol S, Boyd M, Koreth R, Burton GV. Wernicke encephalopathy complicating lymphoma therapy: case report and literature review. South Med J. 2007;100(7):717–719. [DOI] [PubMed] [Google Scholar]
- 45. Lee SM, Kang WS, Cho AR, Park JK. Wernicke's encephalopathy confirmed via brain MRI in cancer patient. Aust N Z J Psychiatry. 2012;46(1):70–71. [DOI] [PubMed] [Google Scholar]
- 46. Richardson S, Malhotra A, Cwynarski K, Hughes D, Prentice A, McNamara C. Patients undergoing high dose chemotherapy for primary CNS lymphoma should receive prophylactic thiamine to prevent Wernike's encephalopathy. Br J Haematol. 2010;149(6):899–901. [DOI] [PubMed] [Google Scholar]
- 47. Morcos Z, Kerns SC, Shapiro BE. Wernicke encephalopathy. Arch Neurol. 2004;61(5):775–776. [DOI] [PubMed] [Google Scholar]
- 48. Pittella JE, de Castro LP. Wernicke's encephalopathy manifested as Korsakoff's syndrome in a patient with promyelocytic leukemia. South Med J. 1990;83(5):570–573. [DOI] [PubMed] [Google Scholar]
- 49. Engel PA, Grunnet M, Jacobs B. Wernicke-Korsakoff syndrome complicating T-cell lymphoma: unusual or unrecognized? South Med J. 1991;84(2):253–256. [DOI] [PubMed] [Google Scholar]
- 50. Yae S, Okuno S, Onishi H, Kawanishi C. Development of Wernicke encephalopathy in a terminally ill cancer patient consuming an adequate diet: a case report and review of the literature. Palliat Support Care. 2005;3(4):333–335. [DOI] [PubMed] [Google Scholar]
- 51. Flint AC, Anziska Y, Rausch ME, Herzog TJ, Williams O. A clinical and radiographic variant of Wernicke-Korsakoff syndrome in a nonalcoholic patient. Neurology. 2006;67(11):2015. [DOI] [PubMed] [Google Scholar]
- 52. Cho IJ, Chang HJ, Lee KE et al. A case of Wernicke's encephalopathy following fluorouracil-based chemotherapy. J Korean Med Sci. 2009;24(4):747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kosta P, Margariti P, Tolis C, Tsimichodimos V, Konitsiotis S, Argyropoulou M. Wernicke's encephalopathy in a patient with rhinopharyngeal carcinoma. J Neurol. 2005;252(12):1539–1540. [DOI] [PubMed] [Google Scholar]
- 54. Cefalo MG, De Ioris MA, Cacchione A et al. Wernicke encephalopathy in pediatric neuro-oncology: presentation of 2 cases and review of literature. J Child Neurol. 2014;29(12):NP181–5. [DOI] [PubMed] [Google Scholar]
- 55. Kálmánchey R, Koós R, Majtényi K, Borsi J. Wernicke-encephalopathy in children with cancer. Med Pediatr Oncol. 1994;22(2):133–136. [DOI] [PubMed] [Google Scholar]
- 56. Kim KH. Wernicke-Korsakoff Syndrome in Primary Peritoneal Cancer. Case Rep Oncol. 2013;6(3):593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fikhman G, Berger JR, Gal TJ. Wernicke's encephalopathy in the course of chemoradiotherapy for head and neck cancer. Am J Otolaryngol. 2011;32(3):250–252. [DOI] [PubMed] [Google Scholar]