Abstract
Objective:
A growth hormone receptor (GHR) gene polymorphism impacts sensitivity to endogenous and exogenous growth hormone (GH) to moderate growth and development. Increased sensitivity may accelerate spinal growth and contribute to scoliosis, particularly in GH-deficient and treated populations such as Prader-Willi syndrome (PWS). Therefore, we examined the relationship between GHR genotype and scoliosis (case and control) in PWS cohorts.
Design:
We utilized a case-control design in a study of 73 subjects (34M; 39F) with genetically confirmed PWS in 32 individuals previously diagnosed with moderate to severe scoliosis (mean age = 16.9 ± 10.2 years; age range of 1 to 41 years) and 41 adults with no evidence of scoliosis (mean age = 30.8 ± 9.7 years; age range of 18 to 56 years). The GHR gene polymorphism was determined using PCR specific primers to capture the two recognized GHR gene fragment sizes [i.e., full length (fl) or exon 3 deletions (d3)].
Results:
Twenty-three (72%) of the 32 case subjects with scoliosis required surgical correction with an approximately equal balance for gender and PWS genetic subtype among cases and 41 control subjects without scoliosis. The GHR d3/d3 genotype was identified in N = 2 of 8 (25%) cases with scoliosis and the d3/fl genotype was identified in N = 11 of 25 (44%) cases with scoliosis but the distribution difference did not statistically differ. The GHR fl/fl genotype was correlated with a significantly faster rate and heavier weight gain among case subjects.
Conclusion:
Our examination of demographic and genetic markers associated with scoliosis and surgical repair in PWS found no evidence to support differences in gender, PWS genetic subtype or GHR d3 allele distributions among the case vs control groups. Those with fl/fl alleles were heavier than those with d3/d3 or d3/fl genotypes and warrant further study with a larger sample size and possibly to include other vulnerable populations requiring growth hormone treatment.
Keywords: Prader-Willi syndrome (PWS), Scoliosis, Growth hormone receptor (GHR), polymorphism, GHR d3 allele, Growth hormone treatment
1. Introduction
Prader-Willi syndrome (PWS) is a rare genetic obesity-related disorder occurring in about 1 in 15,000–30,000 births [1–4]. PWS results from the loss of paternally expressed genes from the genomic imprinted 15q11-q13 region [1–3] usually from a de novo paternally derived 15q11-q13 deletion [5]. PWS is characterized by infantile central hypotonia with failure to thrive, a poor suck and feeding difficulties; growth and other hormone deficiency leading to short stature, small hands and feet and decreased muscle mass; hypogonadism with genital hypoplasia in both males and females and reduced cognition with behavioral problems [1–3,6–8]. Increased feeding and hyperphagia in early childhood leads to precocious obesity without strict oversight and obesity-related morbidity and mortality [1–3,9]. Both hyperphagia and decreased growth in PWS are hormone-based due to dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis [1–3,6–10].
Individuals with PWS respond to treatment with growth hormone (GH) approved by the FDA in 2000 for children with PWS with increased stature, lean body mass and physical activity with reduced obesity status [9–15]. The vast majority of children with PWS in the United States are treated with GH with a positive impact on growth and body composition by as little as two years of treatment [15]. Furthermore, GH exposure for at least 40% of the life span results in near normal height by 18 years of age according to syndrome-specific standardized PWS growth charts [16]. However, increased growth associated with growth hormone treatment combined with lower muscle mass and strength and severe hypotonia, common in PWS at baseline, may increase the risk or propensity for scoliosis defined as a skeletal back curve > 10° using standing X-rays [2,17].
Before the approval of GH therapy in PWS, scoliosis was reported in 44% of individuals [1]. Later, about 30% of individuals treated with GH during childhood typically presented with lumbar or thoracolumbar skeletal curves [18]. Musculoskeletal manifestations following physical, orthopedic and radiological evaluations of the spine, hip joints and lower extremities found that scoliosis and limb malalignment were present in 64% of patients with PWS. Scoliosis correlated with a higher body mass index (BMI) which generally followed a bimodal distribution pattern developing prior to 4 years of age or later at the time of normal puberty which is delayed in PWS [17]. In addition, central hypotonia and effects on development of infantile spinal curvature may be impacted by GH treatment in PWS. GH-mediated effects are influenced by genetic factors that may influence the rate of skeletal growth, muscle response and the development of scoliosis including the growth hormone receptor (GHR). Growth hormone is the endogenous ligand of this transmembrane receptor which is responsible for intra and intercellular signaling pathways which leads to growth.
A common polymorphism of the GHR gene includes a deletion of exon-3 (d3) which is seen in about 50% of Caucasians [19]. Two recognized isoforms [full length (fl); exon 3 deletion (d3)] exist with the GHR d3 polymorphism associated with increased sensitivity to growth hormone [19,20]. This gene polymorphism leads to an accelerated growth rate including for PWS when treated with GH [19,21]. It is not clear if increased sensitivity towards growth hormone associated with the GHR d3 polymorphism influences the rate of scoliosis and severity. The aims for our study were to assess the relationship between GHR gene allele subtypes and risk for scoliosis in a cohort of individuals with PWS and GH treatment by studying those with moderate to severe scoliosis and those without a history of scoliosis.
2. Methods
2.1. Subjects
Seventy-three subjects (34M; 39F) were studied with genetic confirmation of PWS and available genetic subtypes (15q11-q13 deletion, N = 38; maternal disomy 15 (UPD), N = 30; imprinting defect, N = 5) with medical, skeletal and surgical information including growth hormone therapy data. Case reports and samples were drawn from existing clinical trial data and longitudinal study of PWS clinical course. A case-control study design was undertaken of individuals with PWS from the clinical setting following signed informed consent forms approved by the local Institutional Review Board for research on human subjects. Thirty-two individuals (mean age = 16.9 ± 10.2 years, age range 1 to 41 years) were previously diagnosed with moderate to severe scoliosis with 23 requiring surgical intervention and included as case subjects while 41 adults with PWS (mean age = 30.8 ± 9.7 years, age range 18 to 56 years) were selected with no evidence of scoliosis and included as controls. The average age for control subjects with PWS without scoliosis was older than the case subject group due to our selection and inclusion/exclusion criteria which required control participants with PWS to reach their final height without scoliosis.
2.2. GHR gene polymorphism
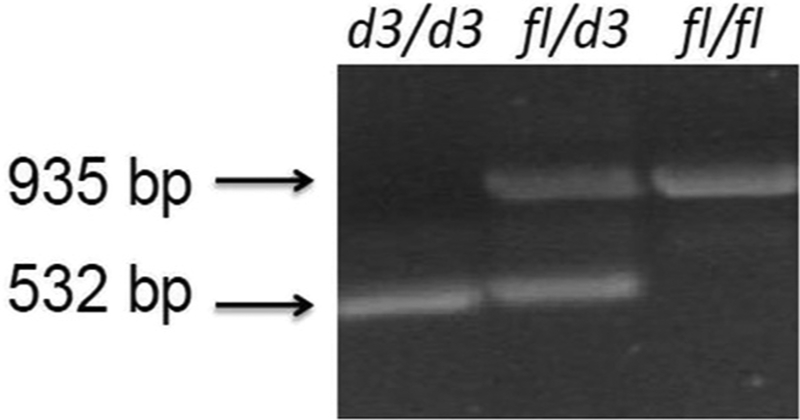
Polymerase chain reaction (PCR) generated fragments using specific primers to capture the two GHR gene fragment sizes [i.e., full length (fl) or exon 3 deletion (d3)] (see Fig. 1) to identify allele subtypes following protocols previously reported [21]. The GHR exon 3 deletion was identified using PCR with primers G1, G2, and G3 obtained from Integrated DNA Technologies (Coralville, Iowa; GenBank accession number AF155912). Genotyping was performed on DNA collected from blood or saliva on each subject to identify the GHRfl and GHRd3 alleles, using two separate PCR procedures with primers G1/G2 and G1/G3 run as separate reactions. The following PCR cycle parameters were used for G1/G2: the initial denaturation for 5 min at 94 °C followed by 35 cycles of 30 s at 94 °C, 60 s at 60 °C; and for 90 s at 72 °C, followed by a final extension period for 7 min at 72 °C. The following PCR cycle parameters were used for G1/G3: the initial denaturation for 5 min at 94 °C followed by 40 cycles of 30 s at 94 °C, 60 s at 60 °C; and for 90 s at 72 °C, followed by a final extension period for 7 min at 72 °C. Electrophoresis of the PCR fragments was performed with 2% agarose. Primers for G1 and G3 generate a PCR fragment of 935 bp indicating the wild type full length (fl) allele while primers G1 and G2 generate a PCR fragment of 532 bp representing the deletion (d3) allele indicating the exon 3 deletion (see Fig. 1).
Fig. 1.
Representative examples of growth hormone receptor gene (GHR) alleles using polymerase chain reaction to identify the wild type fl/fl; heterozygous fl/d3 and homozygous exon-3 deletion (d3/d3) alleles.
2.3. Statistical analysis
The study methodology utilized a case-control study design with two case classifications: those with diagnostically confirmed scoliosis in childhood (standing skeletal back X-ray with curve > 10°) and those with progressing scoliosis that required bracing in childhood or surgical repair if not responsive to bracing. ANOVA was used for age comparisons and Chi Square analysis was employed with categorical variables to determine the gender, PWS genetic subtype and GHR d3 gene polymorphism distribution for case vs control participants under each classification system. A sample size of N = 38 subjects per group (comparable to an N = 35/N = 42 ratio of participants) has 80% power to detect a 30% increase in the frequency of the d3 gene polymorphism in scoliosis patients at a p = 0.05 level of alpha which would be deemed clinically meaningful. A sample size of N = 71 subjects per group would be required to detect a 20% difference. The relationship between GHR receptor allele type and body measures over the lifespan was examined using trend lines, linear and polynomial regression modeling. Q-Q residual plots were examined for model fit and linearity of height and weight distributions for model designation. The impact of GHR allele on growth parameters were determined by the interaction term for the “Age by GHR allele.”
Additionally, the probability of any GH treatment or GH treatment under 12 years of age was calculated using the Chi Square test and logistic regression modeling controlling for age, PWS genetic subtype and GHR gene polymorphism. The average length in years of GH treatment for case vs control subjects was examined using linear regression modeling thereby controlling for age, PWS genetic subtype and GHR gene subtype. All statistical analysis was completed using SAS Software System version 8.02.
3. Results
Thirty-two (44%) of the 73 subjects with genetically confirmed PWS were diagnosed with scoliosis (12 males, 20 females), and of those, 23 (72%) required surgical correction due to severe scoliosis (8 males, 15 females; see Table 1). The number of male and female participants had nearly equal representation in both case and control subject groups. The distribution of PWS genetic subtypes among subjects were also in approximately equal proportions for those with or without scoliosis with a higher, but not statistically significant, percentage of maternal disomy 15 (see Table 2) among scoliosis cases and related surgery representing the degree of scoliosis. The control group consisted of adults, by design, in order to ensure that they were beyond the age of onset for scoliosis and thus the mean age for the controls (30.8 ± 9.7 yrs) was significantly higher than in the case group (16.9 ± 10.2 yrs; F = 35, p < 0.0001).
Table 1.
Characteristics of subjects with Prader-Willi syndrome (PWS) with and without scoliosis and related surgical repair.
| Scoliosis | Surgery for subjects with scoliosis | |||||
|---|---|---|---|---|---|---|
| Variable | N (%) Yes N = 32 |
N (%) No N = 41 |
Test statistics | N (%) Yes N = 23 of 32 |
N (%) No N = 50 |
Test statistics |
| Gender | ||||||
| Male | 12 (35%) | 22 (65%) | 8 (23%) | 26 (76%) | ||
| Female | 20 (51%) | 19 (49%) | χ2 = 1.9, p < 0.17 | 15 (38%) | 24 (61%) | χ2 = 1.9, p < 0.17 |
| PWS subtype | ||||||
| Deletion | 15 (39%) | 23 (60%) | 11 (29%) | 27 (71%) | ||
| UPD | 16 (53%) | 14 (47%) | 12 (40%) | 18 (60%) | ||
| Imprinting | 1 (20%) | 4 (80%) | χ2 = 2.5, p < 0.28 | 0 (0%) | 5 (100%) | χ2 = 3.4, p < 0.18 |
| Mean age | 16.9 ± 10.2 yrs | 30.8 ± 9.7 yrs | F = 35, p < 0.0001* | 19.1 ± 8.7 yrs | 27.2 ± 12.6 yrs | F = 7.8, p < 0.007* |
We chose older patients with PWS as control subjects who achieved adult height without evidence of scoliosis. Table percentages represent row percentages for each variable.
Table 2.
Scoliosis and surgical repair by growth hormone receptor (GHR) genotype compared with controls without scoliosis.
| GHR genotype | Scoliosis | Scoliosis surgery | ||||
|---|---|---|---|---|---|---|
| N (%) Yes N = 32 |
N (%) No N = 41 |
χ2, p-value | N (%) Yes N = 23 |
N (%) No N = 50 |
χ2, p-value | |
| d3/d3 | 2 (25%) | 6 (75%) | 1 (12%) | 7 (87%) | ||
| fl/d3 | 11 (44%) | 14 (56%) | 8 (32%) | 17 (68%) | ||
| fl/fl | 19 (47%) | 21 (52%) | χ2 = 1.4, p < 0.5 | 14 (35%) | 26 (65%) | χ2 = 1.6, p < 0.4 |
Table percentages for each variable represent row percentages.
Table 2 shows the distribution and row percentages of GHR d3 and fl alleles of 32 individuals with scoliosis in which DNA was available for GHR genotyping. Forty-one percent of those with scoliosis and 49% of those without scoliosis had at least one GHR d3 allele, while 39% of those with scoliosis and surgery had at least one GHR d3 allele. Fig. 1 shows representative PCR generated GHR gene polymorphisms. The low number of individuals with the d3/d3 genotype reduced statistical power and limited our interpretation of results related to independent effects for that genotype. Two of 8 (25%) of individuals homozygous for the d3 allele had scoliosis compared to thirty of 65 (46%) of the combined d3/fl and fl/fl subject groups which was not statistically significant (χ2 = 1.3, p = 0.2). Similarly, one of 8 (12%) participants with the d3/d3 genotype required surgical correction for scoliosis compared to 22 of 65 (34%) of those with an fl allele (χ2 = 1.5, p = 0.2). A larger sample of individuals with the d3/d3 genotype are needed for conclusive assessment of these relationships.
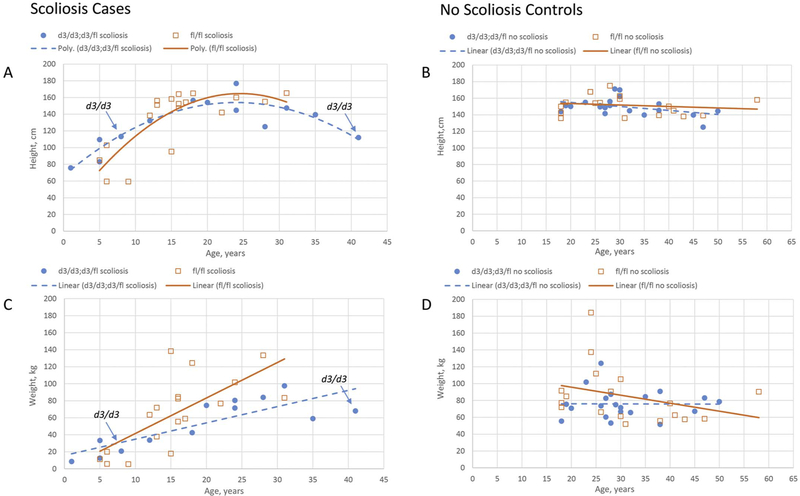
Secondary analyses considered the effects of GHR subtype on body measures over the lifespan which were modeled and presented in scatter plots for height and weight by age for individual carriers of the GHR d3 allele relative to carriers of the GHR fl/fl allele (see Fig. 2). A curvilinear growth profile was observed for height by age in individuals with scoliosis (Fig. 2A) with carriers of the GHR fl/fl allele showing a sharper increase in slope than individuals with the GHR d3 allele. The model for this relationship was statistically significant (F = 11.5, df = 4, p < 0.0001) but the age X GHR allele interaction term did not meet statistical significance (F = 1.56, p = 0.22). Weight for scoliosis cases increased in a linear fashion for both allele types; however, individual cases who carried the GHR fl/fl allele showed a significantly faster rate of increase in weight than those with the GHR d3 allele and apparently greater terminal weight (F = 6.24, df = 1, p < 0.02, Fig. 2C). No difference in height or weight by GHR allele type was found among adult controls (Fig. 2B, D).
Fig. 2.
Scatter plot representations of height (A, B) and weight (C, D) for scoliosis cases (A, C) and control (B, D) subjects with Prader-Willi syndrome. Open squares with solid trendlines represent individuals with the fl/fl genotype. Closed circles with dotted trendlines indicate individuals with a d3/d3 or d3/fl genotype. Scoliosis case subjects homozygous for the d3/d3 allele are highlighted with arrows.
Seventy-one percent of those with scoliosis were treated with GH in the past or currently compared to 36% for those without scoliosis (χ2 = 9.6, p < 0.002) although those without scoliosis were generally older due to our inclusion/exclusion criteria to allow age maturation and absence of scoliosis. Growth hormone was not as widely available to adult PWS cohorts. Sixty-two percent of those with scoliosis were treated with GH under 12 years of age compared to 15% for controls (x2 = 18.8, p < 0.0001). The average length of GH treatment was for 6.2 years for those with scoliosis and 1.3 years for those without scoliosis. Exposure to GH therapy in PWS is often related to cohort differences in subject age since GH therapy has become widely available as standard treatment in PWS since the early 2000s and many adults with PWS did not have access to GH treatment in childhood prior to that time. There was no difference in the frequency of growth hormone treatment for d3 (N = 8, 61%) fl/fl (N = 13, 68%) allele types for the scoliosis cases (χ2 = 0.16, p = 0.69) and exposure to growth hormone did not significantly impact the growth parameters.
4. Discussion
Our examination of the distribution of demographic and genetic markers associated with scoliosis and surgical scoliosis repair in PWS did not find evidence to support differences in gender, PWS genetic subtype or GHR d3 allele distribution among case vs control groups. Individuals with scoliosis and scoliosis related surgery were significantly more likely to have prior GH treatment particularly in childhood (60–70% vs 20–40%) and for a significantly longer period of treatment time (6.2 years vs 1.3 years for scoliosis; 7.2 years vs 1.6 years for surgical correction). This relationship is impacted by the age disparity between case and control subjects, which would differentially increase access to GH treatment in the younger case subjects.
A relatively small number of individuals with the GHR d3/d3 genotype presented with scoliosis and surgical repair compared to those with a GHR fl allele although our number of subjects was low (< 10 subjects) and insufficient for statistical analysis. The increased sensitivity to growth hormone associated with the homozygous GHR d3/d3 genotype and enhanced early musculoskeletal growth and strength could possibly increase spinal stability and thereby reducing the later risk for scoliosis. Conversely, the accelerated growth response to standard GH treatment may lead to closer supervision and monitoring by care providers to examine for growth rate, skeletal maturation and scoliosis. In this respect, the GHR d3/d3 genotype may indeed serve as a protective genetic status against the development of scoliosis particularly in combination with early GH therapy and surveillance. These relationships did not achieve statistical significance but the data trends and potential clinical implications of the association do warrant further study.
Secondary analyses of GHR d3 allele type and growth parameters indicated accelerated weight gain and higher maximal weight among individuals with scoliosis who carried the fl/fl genotype. Conversely, individuals who carried a d3 allele showed a more gradual growth in height and weight with age which again may indicate a protective impact of increased GH sensitivity in scoliosis. It is possible that increased growth hormone sensitivity throughout life improves physiological parameters such as bone density and muscle mass leading to improved growth parameters. This may be particularly beneficial in a context of low endogenous growth hormone in early development. This association was not found in control subjects or a similar PWS cohort with normal spinal development published previously [21].
Scoliosis is an understudied feature in PWS with and without GH treatment as noted in a report by Shim et al. [22] who found that 23 (64%) of their 36 patients with musculoskeletal manifestations had scoliosis and significantly associated limb malalignment. Higher BMI values in PWS were also associated with kyphotic deformities and a higher rate of surgical intervention [17]. As noted, we found increased weight in individuals in our PWS cohort with scoliosis and having the GHR fl/fl allele which may influence risk of skeletal abnormalities [21].
Coupaye et al. [23] compared PWS genetic subtypes and frequency of scoliosis and found no difference between those with the 15q11-q13 deletion subtype compared with maternal disomy 15 (UPD). However, the UPD genotype had a higher frequency of severe or operated scoliosis in their study than the deletion subtype in those exposed to GH treatment during childhood or adolescence. UPD subtypes also appeared to be overrepresented in our sample of moderate to severe scoliosis cases but did not achieve statistical significance.
Our examination of the disturbance of demographic and genetic markers associated with scoliosis and surgical repair in PWS found no evidence to support differences in gender, PWS genetic subtype or GHR d3 allele distributions among the case vs control group. Those with fl/fl alleles were heavier than those with d3/d3 or d3/fl genotypes and warrant further study with a larger sample size and possibly to include other vulnerable populations requiring growth hormone treatment such as Turner syndrome.
Acknowledgements
We thank the participating families and Prayer Will Support PWS Organization (Family & Friends of Kyleigh Ellington) and acknowledge support from the Heartland Genetics Collaborative and NICHD grant HD02528.
Footnotes
Conflict of interest
The submitted manuscript contains original material not submitted or under consideration elsewhere and addresses an important issue in the treatment and care of individuals with Prader-Willi syndrome. We have no conflict of interests in the conduct and reporting of this research to declare.
References
- [1].Butler MG, Prader-Willi syndrome: current understanding of cause and diagnosis, Am. J. Med. Genet 35 (1990) 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Butler MG, Lee PDK, Whitman BY, Management of Prader-Willi Syndrome, Springer, New York, 2006. [Google Scholar]
- [3].Cassidy SB, Schwartz S, Miller JL, Driscoll DJ, Prader-Willi syndrome, Genet. Med 14 (2012) 10–26. [DOI] [PubMed] [Google Scholar]
- [4].Whittington JE, Butler JV, Holland AJ, Changing rates of genetic subtypes of Prader-Willi syndrome in the UK, Eur. J. Hum. Genet 15 (2007) 127–130. [DOI] [PubMed] [Google Scholar]
- [5].Butler MG, Palmer CG, Parental origin of chromosome 15 deletion in Prader-Willi syndrome, Lancet 1 (1983) 1285–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Angulo MA, Butler MG, Cataletto ME, Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings, J. Endocrinol. Investig 38 (2015) 1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Butler MG, Single gene and syndromic causes of obesity: illustrative examples, Prog. Mol. Biol. Transl. Sci 140 (2016) 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Elena G, Bruna C, Benedetta M, Stefania DC, Giuseppe C, Prader-Willi syndrome: clinical aspects, J. Obes 473941 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tauber M, Barbeau C, Jouret B, et al. , Auxological and endocrine evolution of 28 children with Prader-Willi syndrome: effect of GH therapy in 14 children, Horm. Res 53 (2000) 279–287. [DOI] [PubMed] [Google Scholar]
- [10].Oto Y, Obata K, Matsubara K, et al. , Growth hormone secretion and its effect on height in pediatric patients with different genotypes of Prader-Willi syndrome, Am. J. Med. Genet. A 158A (2012) 1477–1480. [DOI] [PubMed] [Google Scholar]
- [11].Lindgren AC, Ritzen EM, Five years of growth hormone treatment in children with Prader-Willi syndrome, Acta Paediatr. Suppl. 433 (1999) 109–111. [DOI] [PubMed] [Google Scholar]
- [12].Eiholzer U, l’Allemand D, van der Sluis I, Steinhert H, Gasser T, Ellis K, Body composition abnormalities in children with Prader-Willi syndrome and long-term effects of growth hormone therapy, Horm. Res 53 (2000) 200–287. [DOI] [PubMed] [Google Scholar]
- [13].Carrel AL, Myers SE, Whitman BY, Eickhoff J, Allen DB, Long-term growth hormone therapy changes the natural history of body composition and motor function in children with Prader-Willi syndrome, J. Clin. Endocrinol. Metab 95 (2010) 1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Butler MG, Smith BK, Lee J, Gibson C, Schmoll C, Moore WV, Donnelly JE, Effects of growth hormone treatment in adults with Prader-Willi syndrome, Growth Hormon. IGF Res 23 (2013) 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Myers SE, Carrel AL, Whitman BY, Allen DB, Sustained benefit after 2 years of growth hormone on body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome, J. Pediatr 137 (2000) 42–49. [DOI] [PubMed] [Google Scholar]
- [16].Butler MG, Lee J, Cox DM, et al. , Growth charts for Prader-Willi syndrome during growth hormone treatment, Clin. Pediatr 55 (2016) 957–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Accadbled F, Odent T, Moine A, et al. , Complications of scoliosis surgery in Prader-Willi syndrome, Spine 33 (2008) 394–401. [DOI] [PubMed] [Google Scholar]
- [18].Nakamura Y, Murakami N, Iida T, et al. , The characteristics of scoliosis in Prader-Willi syndrome (PWS): analysis of 58 scoliosis patients with PWS, J. Orthop. Sci 20 (2015) 17–22. [DOI] [PubMed] [Google Scholar]
- [19].Dos Santos C, Esioux L, Teinturier C, Tauber M, Goffin V, Bougneres P, A common polymorphism of the growth hormone receptor is associated with increased responsiveness to growth hormone, Nat. Genet 36 (2004) 720–724. [DOI] [PubMed] [Google Scholar]
- [20].Padidela R, Bryan SM, Abu-Amero S, et al. , The growth hormone receptor gene deleted for exon three (GHRd3) polymorphism is associated with birth and placental weight, Clin. Endocrinol 76 (2012) 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Butler MG, Roberts J, Hayes J, Tan X, Manzardo AM, Growth hormone receptor (GHR) gene polymorphism and Prader-Willi syndrome, Am. J. Med. Genet. A 161A (2013) 1647–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shim JS, Lee SH, Seo SW, Koo KH, Jin DK, The musculoskeletal manifestations of Prader-Willi syndrome, J. Pediatr. Orthop 30 (2010) 390–395. [DOI] [PubMed] [Google Scholar]
- [23].Coupaye M, Tauber M, Cuisset L, et al. , Effect of genotype and previous GH treatment on adiposity in adults with Prader-Willi syndrome, J. Clin. Endocrinol. Metab 101 (2016) 4895–4903. [DOI] [PubMed] [Google Scholar]