Abstract
Prader–Willi syndrome (PWS) is a neurodevelopmental genetic disorder characterized by intellectual disabilities and insatiable appetite with compulsive eating leading to severe obesity with detrimental health consequences. Transcranial direct current stimulation (tDCS) has been shown to modulate decision-making and cue-induced food craving in healthy adults. We conducted a pilot double blind, sham-controlled, multicenter study of tDCS modulation of food drive and craving in 10 adult PWS participants, 11 adult obese (OB) and 11 adult healthy-weight control (HWC) subjects. PWS and OB subjects received five consecutive daily sessions of active or sham tDCS over the right dorsolateral prefrontal cortex (DLPFC), while HWC received a single sham and active tDCS in a crossover design. Standardized psychometric instruments assessed food craving, drive and hyperphagia by self-report and caregiver assessment over 30 days. Robust baseline differences were observed in severity scores for the Three-Factor Eating Questionnaire (TFEQ) and Dykens Hyperphagia Questionnaire (DHQ) for PWS compared to HWC while obese participants were more similar to HWC. Active tDCS stimulation in PWS was associated with a significant change from baseline in TFEQ Disinhibition (Factor II) (Ƶ = 1.9, P < 0.05, 30 days) and Total Scores (Ƶ = 2.3, P < 0.02, 30 days), and participant ratings of the DHQ Severity (Ƶ = 1.8, P < 0.06, 5 days) and Total Scores (Ƶ = 1.9, P < 0.05, 15 days). These findings support sustained neuromodulatory effects and efficacy of tDCS to reduce food drive and behaviors impacting hyperphagia in PWS. Transcranial direct current stimulation may represent a straight-forward, low risk and low cost method to improve care, management and quality of life in PWS.
Keywords: Prader–Willi syndrome, transcranial direct current stimulation, hyperphagia, obesity
INTRODUCTION
Prader–Willi syndrome (PWS) is a neurodevelopmental disorder affecting between 350,000 and 400,000 people worldwide [Butler and Thompson, 2000]. It is the most common cause of syndromic obesity, with the majority of cases (about 70%) due to a paternal deletion of the chromosome 15q11–q13 region, maternal uniparental disomy 15 (UPD) in 25% of cases and imprinting defects in the remaining subjects [Bittel and Butler, 2005]. The clinical presentation varies with age and with neonatal hypotonia, a poor suck and feeding difficulties with failure to thrive in infancy, developmental delay, growth and other hormone deficiencies leading to short stature, small hands and feet and hypogonadism/hypogenitalism with an insatiable appetite leading to rapid weight gain and obesity in early childhood [Butler et al., 2006; Butler, 2011; McAllister et al., 2011; Angulo et al., 2015]. Hyperphagia is one of the most prominent and debilitating features of this disorder, and leads to significant morbidity among PWS subjects. Strict dietary restrictions and close monitoring of caloric intake are used to control weight gain in PWS [Butler et al., 2006]. Psychological food security in the presence of controlled food access has been shown to decrease food drive and craving in PWS, but additional treatments are needed to reduce burden and costs.
Although the mechanisms underlying hyperphagic behavior remain to be fully understood, increasing evidence suggests that the control of eating is related to neural networks associated with decision-making [Pignatti et al., 2012]. Among them, the dorsolateral prefrontal cortex (DLPFC), a key structure for the regulation and processing of food motivation and satiety signaling appears to play a crucial role by integrating incoming sensory and affective information that results in an emotional reaction to food stimuli [Rolls, 2000, 2005]. PWS is associated with reduced cortical and hippocampal grey and white matter volume compared to healthy weight controls which selectively impacts deletion the 15q11–q13 over the Prader–Willi syndrome UPD genetic subtype [Ogura et al., 2011; Honea et al., 2012]. Abnormal signaling and functional connectivity involving the hypothalamus, ventromedial prefrontal cortex, insula and extended amygdala have also been reported [Holsen et al., 2012; Honea et al., 2012; Zhang et al., 2015]. Recent studies comparing body mass index (BMI)-matched individuals with PWS participants showed hypoactivity of regions involved in inhibitory control including the DLPFC and orbitofrontal cortex, when exposed to food-related visual stimuli [Holsen et al., 2012]. Cortical inhibitory networks originating in the medial prefrontal cortex impacting food drive were hypoactive in PWS in both resting and post-meal states with hyperactive subcortical reward circuitry during the post-meal period [Honea et al., 2012; Zhang et al., 2015]. Reductions in activity within the inhibitory loop, combined with an enhanced activity of the subcortical reward circuitry, provide a plausible mechanistic explanation for food craving in PWS [Holsen et al., 2012].
Emerging techniques in brain stimulation such as transcranial direct current stimulation (tDCS) provide a safe, painless, inexpensive non-restrictive and non-invasive method to modify neuronal functioning and influence cognitive processes. The tDCS session modulates cortical activity through the application of a weak electric current to the scalp, which travels through the brain parenchyma from the positive (anode) to the negative (cathode) electrode. Active stimulation can promote long-lasting effects by either increasing cortical excitability under the anode or by decreasing it under the cathode [Nitsche and Paulus, 2000]. A number of studies highlighting the role of the DLPFC in decision-making have shown that anodal tDCS is capable of reducing craving for a number of substances including tobacco [Fregni et al., 2008a; Boggio et al., 2009] and food [Fregni et al., 2008b; Goldman et al., 2011]. A single session of anodal tDCS over the right DLPFC has shown a decrease in food craving in healthy participants [Fregni et al., 2008b; Goldman et al., 2011], while in obese subjects, anodal tDCS over the left DLPFC combined with aerobic exercise decreased the desire to eat more than either intervention alone [Montenegro et al., 2012]. In light of these findings, and given the fact that participants with PWS show hypoactivity of the DLPFC when presented with food-related cues [Holsen et al., 2012], we conjectured that modulating the activity of this cortical region could enhance its function in the decision-making process and consequently help reduce food craving in PWS.
The main goal of the present pilot study was to assess the efficacy of anodal tDCS, applied over the right DLPFC, in the activation of inhibitory control pathways modulating food craving and hyperphagia in PWS participants. This was evaluated through completion of the Three Factor Eating Questionaire (TFEQ) and the Dykens Hyperphagia Questionnaire by the adult participants and caregivers and self-report of food craving [Dykens et al., 2007]. Objective weight loss was also examined to validate potential changes in psychometric data concerning food motivation (e.g., craving and hyperphagia). In addition to PWS participants, two control groups consisting of healthy-weight subjects (HWC) and obese (OB) participants took part in this study. We hypothesized that PWS individuals undergoing active anodal tDCS of the right DLPFC would show a reduction in self-reported craving and hyperphagic behavior when compared to subjects undergoing sham stimulation with parallel effects in the obese group.
STUDY DESIGN
Participants
Participants were recruited at two sites, the Spaulding Rehabilitation Network Research Institute (SRN-RI), Harvard Medical School, and the University of Kansas Medical Center (KUMC), University of Kansas. All participants were between 18 and 64 years of age. The study included N=10 participants with PWS (5M:5F, mean age = 32±11 years) diagnosed clinically and confirmed with genetic testing (N = 7 with 15q11–q13 deletion; N 3 with maternal disomy 15 or imprinting defect (See Table I). Cognitive normal control participants were divided into two groups based on their BMI states. Healthy normal control subjects with a BMI less than 25 kg/m2 were assigned to the Healthy Weight Control (HWC) group (N = 11, mean age=28±11 years), while those with a exceeding 30 kg/m2 were assigned to the Obese (OB) group (N = 10, mean age = 47±11). Twenty participants were recruited at SRN-RI (2 PWS, 9 OB, 9 HWC), and 12 at KUMC (8 PWS, 2 OB, 2 HWC). Volunteers were excluded from study participation if they presented any of the following: history of neuropsychiatric disorders, epilepsy, unstable medical conditions, history of neurosurgery, significant visual or auditory impairment, contraindications to tDCS [Nitsche et al., 2008] or pregnancy. Both centers recruited participants from all three groups in order to balance the effects of study site on outcomes. A multicenter protocol was implemented and approved by the Institutional Review Boards at both sites and written informed consent was obtained from all participants or their legal representatives.
TABLE I.
Healthy, Obese, and Prader–Willi Syndrome Participant Baseline Characteristics
| Parameter (Baseline scores) | Healthy lean, N = 11 (mean ± SD) |
Obese, N = 11 (mean ± SD) |
Prader-Willi syndrome, N = 10 (mean ± SD) |
|---|---|---|---|
| Age, years | 27.8 ± 11 | 46.9 ± 11a | 32.5 ± 11a,b |
| Height, cm | 170 ± 11 | 173 ± 8 | 158 ± 14b |
| Weight, kg | 64.7 ± 13 | 110.4 ± 23a | 71.4 ± 18b |
| BMI | 22.1 ± 1.9 | 36.7 ± 7.2a | 28.6 ± 7.4a,b |
| Composite IQ (K-BIT2) | 113 ± 8.6 | 110 ± 34 | 62.3 ± 16a,b |
| Trail Making Test, Part A | |||
| Baseline pre- stimulation | 22.1 ± 8.2 | 30.4 ± 9.2a | 64.0 ± 31a,b |
| Trail Making Test, Part B | |||
| Baseline pre- stimulation | 39.8 ± 12 | 72.5 ± 25a | 188 ± 97a,b |
| Three-Factor Eating Questionnaire | |||
| Total Score | 19.8 ± 9.1 | 24.2 ± 8.4 | 31.8 ± 7.0ab |
| Factor I: Cognitive Restraint of Eating | 8.7 ± 4.6 | 9.8 ± 4.3 | 12.7 ± 3.3a |
| Factor II: Disinhibition | 4.9 ± 4.0 | 8.4 ± 3.6a | 9.4 ± 2.7a |
| Factor III: Hunger | 6.2 ± 3.1 | 5.9 ± 2.5 | 9.7 ± 2.8ab |
| Dykens Hyperphagia Questionnaire | |||
| Total Score | 21.1 ± 5.5 | 24.3 ± 8.3 | 30.2 ± 7.8a |
| Drive Score | 7.8 ± 2.9 | 7.6 ± 4.0 | 13.5 ± 4.1ab |
| Behavior Score | 10.7 ± 3.7 | 13.4 ± 5.4 | 13.1 ± 4.4 |
| Severity Score | 2.5 ± 0.8 | 3.3 ± 2.1 | 3.6 ± 1.8 |
| Food Craving Analogue Scale | |||
| Baseline pre- stimulation | 4.2 ± 3.1 | 3.9 ± 3.0 | 8.1 ± 2.8ab |
Significant difference 2-group comparison with lean using Mann–Whitney U, P < 0.05.
Significant difference PWS versus obese.
Randomization
We used a central randomization strategy across study sites and arms which resulted in a single-blinded placebo-controlled study design. Participants were assigned to the active or sham tDCS treatment groups according to the order of entrance in the study using computer-generated randomization list maintained by a study coordinator at each site not involved in other aspects of the trial. Randomization assignments for the PWS and obese subject groups used a ratio of 2:1 for active vs. sham tDCS given the lack of a priori information on the effects of the active intervention and based upon previous comparable studies [Peto, 1978].
Behavioral Measures
Food motivation was assessed in all participants using the Three-Factor Eating Questionnaire (TFEQ) by Stunkard and Messick [1985], a 51 item assessment tool developed to measure 3 dimensions (Factors) of human eating behavior [Cognitive Restraint of Eating (Factor I), Disinhibition (Factor II) and Hunger (Factor III) (Stunkard and Messick, 1985)]. Additionally, food motivation in PWS was assessed using the Dykens Hyperphagia Questionnaire, a 13-item informant measure of eating behaviors empirically tested and validated in participants with PWS [Dykens et al., 2007]. All participants also rated the intensity of their craving for food before and after each 30 min stimulation session on a visual numeric scale (VNS) that ranged from 0, which represented “not hungry at all,” to 10, which represented “very hungry.” Weight (kg) and height (cm) were also assessed using a commercially available electronic scale for weight and a standing stadiometer for height. In addition, IQ and cognitive functioning was assessed in PWS subjects using standard assessment tools including the Kaufman Brief Intellengence Test, 2nd edition (KBIT-2) [Kaufman and Kaufman, 2004] and Trail Making Test (TMT, Parts A and B) [Reitan, 1955]. The KBIT-2 is a brief, individually administered assessment of verbal and non verbal intelligence appropriate for age ranges 4–90 years that yields a standardized composite IQ score (mean = 100±15) with percentile ranks by age. The TMT is a neuropsy-chological assessment of visual attention and task switching that asks participants to connect a series of 25 dots as quickly and accurately as possible. TMT response time provides information on visual search and processing speed, scanning, mental flexibility and executive processing. The TMT was used to assess baseline cognitive functioning as well as the impact of tDCS processing speed and executive function. Mean response for Part A of 29 sec and Part B of 75 sec are considered normal. Mean response times >78 sec for Part A and >273 sec for Part B were regarded as deficient.
Transcranial Direct Current Stimulation (tDCS)
Both centers underwent joint training for all study techniques and procedures to ensure standardization across sites. A low-intensity DC Stimulator (Chattanooga Ionto™ iontophoresis system, Chattanooga Medical Supply Inc., Chattanooga, TN) was used to deliver 2.0 mA of direct current for 30 min through 2 saline-soaked electrodes (35 cm2). The anode was placed over the target area of stimulation, the right DLPFC, which corresponds to F4 in the International 10–20 System of Electrode Placement. The reference electrode, or cathode, was placed over the left supraorbital area using the same electrode montage used for active stimulation. A brief ramp-up and ramp-down phase of 15 sec was applied at the beginning and at the end of the sham stimulation sessions to facilitate blinding. Thus, in the administration of the sham tDCS condition, participants did receive mild electrical stimulation with somatosensation of an electrical stimulus in order to mimic the active arm and facilitate blinding in the placebo-tDCS condition. This technique has been shown to be a reliable method for blinding in sham-controlled studies involving tDCS of prefrontal targets in other studies [Palm et al., 2013].
Schedule of Events and Treatment
Obese and PWS subjects underwent mid-day stimulation sessions daily for five consecutive days with assessments at baseline, 5, 15, and 30 days following the last stimulation session. Healthy weight control subjects participated in a shorter crossover trial to reduce the physiological effects associated with cumulative sessions of tDCS since no therapeutic benefit was expected for this group. The crossover trial was a randomized counterbalanced design with two sessions of tDCS, one active and one sham, and a 15-day washout period between sessions.
Statistical Analysis
Descriptive data were presented as mean ± standard deviation by subject group for HWC, OB, or PWS participants, and study outcomes considered were change from baseline scores for the TFEQ, Dykens Hyperphagia Questionnaire, Food Craving Analogue Scale and weight assessments for active versus sham treated groups. Wilcoxon sign-ranked or Mann–Whitney U tests were utilized when appropriate. Statistical analyses including descriptive statistics were generated using the SAS statistical analysis software version 9.4 (SAS Inc., Cary, NC) and IBM SPSS Statistics (IBM Corporation, Chicago, IL).
RESULTS
Participant Characteristics, Response, and Tolerability
PWS participants had a standard IQ of 62.3±16 indicative of mild intellectual deficiency but significantly lower than seen in healthy weight controls or obese study participants with standard IQ scores in the normal range (Table I). Lean participants were significantly younger than obese and PWS participants. Weight and BMI measures were significantly higher in obese relative to lean and PWS participants, as expected based upon the study design. PWS participants were also significantly shorter in stature than obese participants. PWS participants scored significantly higher than lean participants on all four dimensions of the Three-Factor Eating Questionnaire, two dimensions of the Dykens Hyperphagia Questionnaire (Total and Drive Scores) and the Food Craving Analogue Scale. Baseline hyperphagia assessments for obese participants were more similar to lean than PWS participants showing a significant elevation for only one dimension of the TFEQ—Factor II (Disinhibition) at baseline.
The use of tDCS among lean, obese and PWS participants was generally well tolerated with no reports of significant adverse event. The most common side effects reported were tingling at the site of stimulation (32.1%), followed by skin redness (29.5%), sleepiness(12.5%), and headache (8.9%). There were no statistically significant differences in the number of side effects reported between the active and sham groups or between the three subject groups. No significant changes in weight were observed for the HWC controls who had limited exposure to tDCS, or in OB or PWS participants with several PWS subjects living in weight-controlled environments with strictly restricted access to food as a common management practice in adults with PWS.
Prader–Willi Syndrome Participants
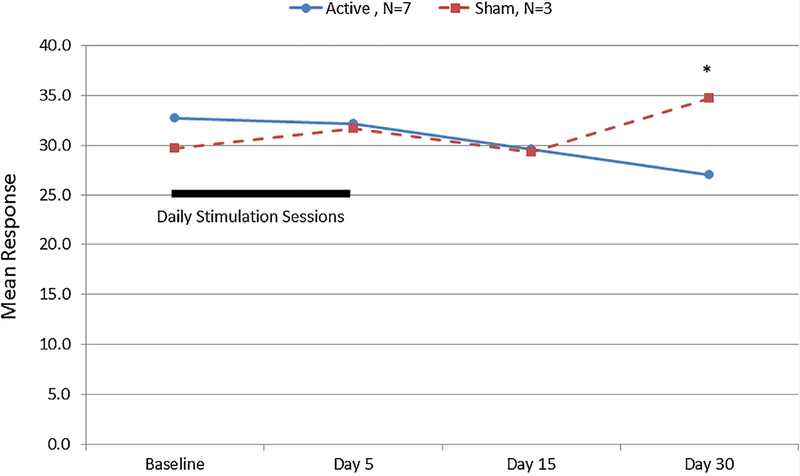
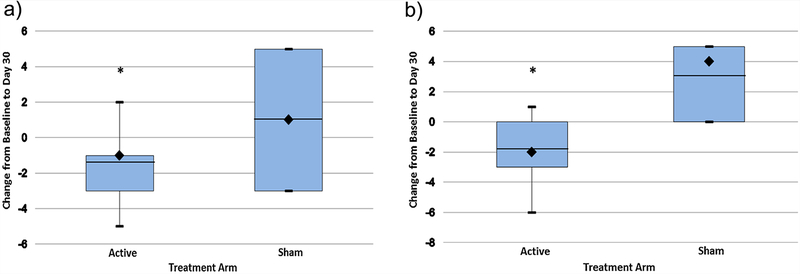
Assessment of the effects of tDCS on hyperphagia did identify significant changes associated with study participation in self-reported measures of disinhibition, severity and food craving among PWS subjects (Table II). The Three Factor Eating Questionnaire Total Score decreased over time for the active and sham PWS subject groups with a significant difference observed between active and sham groups at day 30 (Ƶ = 2.1, P < 0.05, See Fig. 1). A significant treatment group difference was found in the change from baseline Total and Disinhibition (Factor II) scores at 30 days (Ƶ = 2.3, P < 0.02, Fig. 2a; Ƶ = 1.9, P < 0.05, Fig. 2b). However, no consistent changes were noted for Factor I or Factor III TFEQ scores.
TABLE II.
Hyperphagia Response to Repeated Transcranial Direct Current Stimulation in Participants With Prader–Willi Syndrome
| Baseline (mean ± SD) |
Day 5 (mean ± SD) |
Day 15 (mean ± SD) |
Day 30 (mean ± SD) |
|
|---|---|---|---|---|
| Dykens Hyperphagia Questionnaire | ||||
| Total Score | ||||
| Active stimulation | 31.6 ± 8.7 | 28.3 ± 9.1 | 25.7 ± 6.3 | 25.3 ± 6.0 |
| Sham | 27.0 ± 5.0 | 32.7 ± 11.6 | 30.3 ± 0.6 | 28.3 ± 6.1 |
| Drive Score | ||||
| Active stimulation | 13.9 ± 4.1 | 11.1 ± 3.2 | 10.1 ± 2.2 | 8.6 ± 2.6 |
| Sham | 12.7 ± 4.9 | 13.7 ± 4.7 | 11.3 ± 1.2 | 10.3 ± 3.1 |
| Behavior Score | ||||
| Active stimulation | 13.9 ± 5.1 | 13.6 ± 5.9 | 12.0 ± 4.5 | 13.0 ± 3.8 |
| Sham | 11.3 ± 1.5 | 13.3 ± 6.7 | 12.7 ± 0.6 | 12.7 ± 2.9 |
| Severity Score | ||||
| Active stimulation | 3.9 ± 2.0 | 3.6 ± 1.4 | 3.6 ± 1.8 | 3.7 ± 2.3 |
| Sham | 3.0 ± 1.7 | 5.7 ± 0.6 | 6.3 ± 0.6 | 5.3 ± 0.6 |
| Three-Factor Eating Questionnaire | ||||
| Total Score | ||||
| Active stimulation | 32.7 ± 8.2 | 32.1 ± 6.1 | 29.6 ± 4.6 | 27.0 ± 4.7 |
| Sham | 29.7 ± 2.9 | 31.7 ± 6.5 | 29.3 ± 6.0 | 34.7 ± 2.5 |
| Factor I: Cognitive Restraint of Eating | ||||
| Active stimulation | 13.1 ± 3.7 | 13.4 ± 4.0 | 11.4 ± 2.7 | 10.9 ± 4.4 |
| Sham | 11.7 ± 2.5 | 13.0 ± 5.0 | 11.7 ± 4.2 | 12.7 ± 4.0 |
| Factor II: Disinhibition | ||||
| Active stimulation | 10.0 ± 3.1 | 9.0 ± 3.0 | 9.0 ± 2.1 | 8.1 ± 2.6 |
| Sham | 8.0 ± 1.0 | 8.7 ± 1.5 | 7.7 ± 1.2 | 11.0 ± 3.6 |
| Factor III: Hunger | ||||
| Active stimulation | 9.6 ± 3.1 | 9.7 ± 2.1 | 9.1 ± 3.8 | 8.0 ± 3.8 |
| Sham | 10.0 ± 2.6 | 10.0 ± 2.6 | 10.0 ± 2.6 | 11.0 ± 2.6 |
| Food Craving Analogue Scale | ||||
| Active stimulation (N=6)** | 8.8 ± 1.8 | 7.8 ± 2.4 | 4.1 ± 2.1 | 5.5 ± 4.0 |
| Sham | 9.0 ± 1.3 | 8.0 ± 3.0 | 8.3 ± 2.5 | 8.7 ± 1.9 |
| Weight (KG) | ||||
| Active stimulation | 71.9 ± 22.3 | 71.6 ± 22.4 | 72.6 ± 22.2 | 72.6 ± 22.6 |
| Sham | 69.2 ± 3.3 | 69.7 ± 3.3 | 69.3 ± 3.3 | 69.7 ± 3.9 |
| Trail Making Test Part A (Pre-stimulus measure) | ||||
| Active stimulation | 62.6 ± 27.9 | 56.8 ± 42.7 | 57.7 ± 29.8 | 51.6 ± 26.8 |
| Sham | 67.3 ± 44.3 | 65.0 ± 38.2 | 63.7 ± 37.1 | 57.3 ± 32.9 |
| Trail Making Test Part B (Pre-stimulus measure) | ||||
| Active stimulation | 199 ± 91 | 163±101 | 157 ± 92 | 155 ± 98 |
| Sham | 161±127 | 163± 127 | 146± 138 | 158± 132 |
The 2:1 randomization scheme yielded N = 7 active arm and N = 3 sham arm PWS participants.
One participant was excluded from the active arm of the Food Craving Scale for very low study responding. No significant differences were found between active and sham arms at baseline.
FIG. 1.
Three Factor Eating Questionnaire Total Scores for participants with Prader–Willi syndrome after five days of transcranial direct current stimulation. Significant difference between active and sham treatment arms, Mann–Whitney U, P < 0.05. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ajmgb].
FIG. 2.
(a) Change in Three Factor Eating Questionnaire Total Score in participants with Prader–Willi syndrome after 30 days of transcranial direct current stimulation. Sample medians are indicated by black diamonds and means are indicated by the horizontal lines. Vertical lines show the maximum and minimum values. Active N = 7, Sham N = 3. Significant difference by Mann–Whitney U, P<0.05. (b) Change in Three Factor Eating Questionnaire Disinhibition (Factor II) Score in participants with Prader–Willi syndrome after 30 days of transcranial direct current stimulation. Sample medians are indicated by black diamonds and means are indicated by the horizontal lines. Vertical lines show the maximum and minimum values. Active N = 7, Sham N = 3. Significant difference by Mann–Whitney U, P<0.05. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ajmgb].
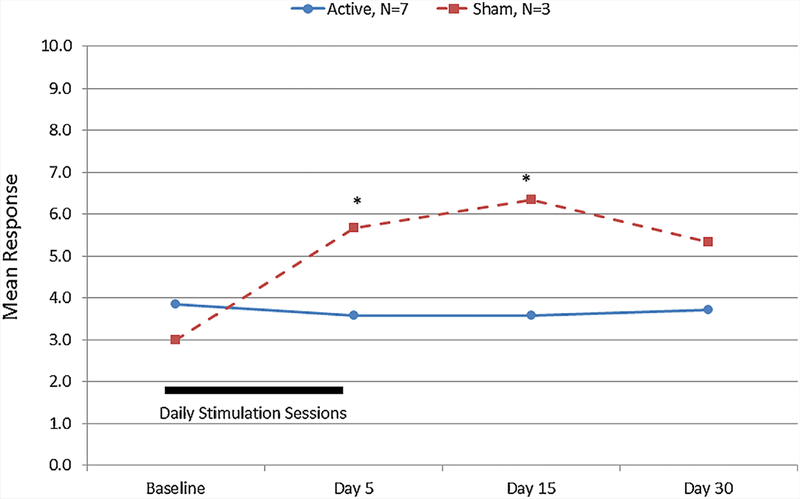
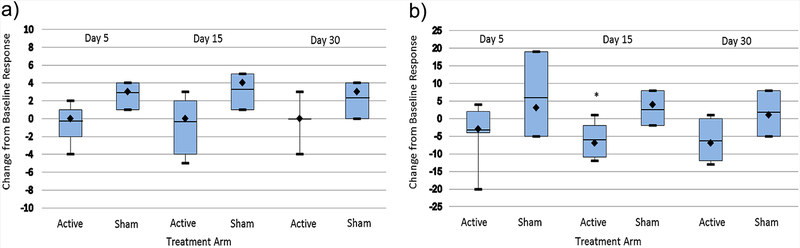
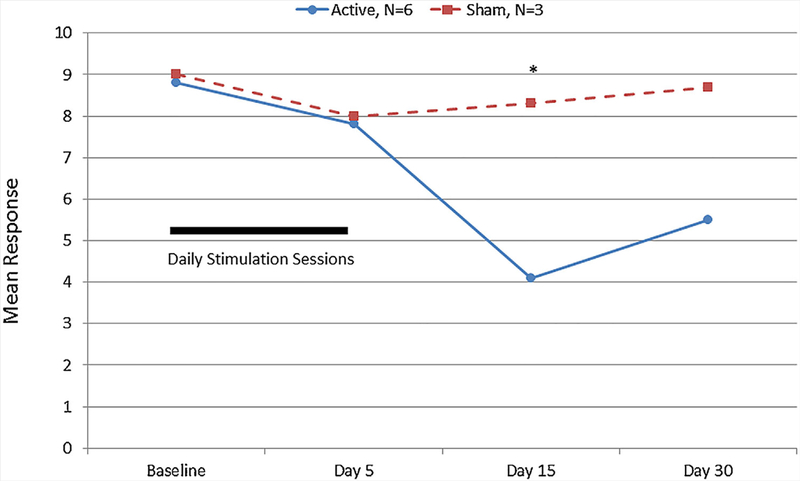
Participant reports from the Dykens Hyperphagia Questionnaire identified a significant difference between active and sham groups (Fig. 3) and a significant or near significant difference in the change from baseline responding for the active vs sham study group including Severity Score (Ƶ = 1.8, P < 0.06) after 5 days (See Fig. 4a) and the Total Score (Ƶ = 1.9, P < 0.05) after 15 days (See Fig. 4b). No significant differences were observed for the Behavior or Drive scores. Repeated exposure to food images was associated with a systematic increase in reported food cravings among all PWS participants (active and sham) pre- to post-stimulation session. Food craving differed significantly between the active vs the sham study arms at 5 and 15 days after initiation of tDCS treatments (See Fig. 5). PWS caretakers rated subjects in the active study arm significantly greater on the Dykens Hyperphagia Questionnaire at baseline than the sham arm with minimal changes noted throughout the study, but a near significant increase from baseline was observed for the Behavior score (Ƶ = 1.8, P < 0.06, 30 days) in the sham group which is similar to food craving and severity measures observed for PWS participants.
FIG. 3.
Dykens Hyperphagia Questionnaire Severity Scores for participants with Prader–Willi syndrome after 5 days of transcranial direct current stimulation. *Significant difference between active and sham treatment arms, Mann–Whitney U, P < 0.05. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ajmgb].
FIG. 4.
(a) Change from baseline Dykens Hyperphagia Questionnaire Severity Scores in participants with Prader–Willi syndrome after transcranial direct current stimulation. Sample medians are indicated by black diamonds and means are indicated by the horizontal lines. Vertical lines show the maximum and minimum values. Active N = 7, Sham N = 3. (b) Change from baseline Dykens Hyperphagia Questionnaire Total Score in participants with Prader–Willi syndrome after transcranial direct current stimulation. Sample medians are indicated by black diamonds and means are indicated by the horizontal lines. Vertical lines show the maximum and minimum values. Active N = 7, Sham N = 3. *Significant difference by Mann–Whitney U, P<0.05. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ajmgb].
FIG. 5.
Numerical Food Craving Rating for participants with Prader–Willi syndrome after 5 days of transcranial direct current stimmulation. One PWS participant with very low reported scores was excluded from the active study arm as an outlier. *Significant difference between active and sham treatment arms, Mann–Whitney U, P < 0.05. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ajmgb].
Healthy Weight Control and Obese Participant tDCS Response
A significant difference in the change from baseline was observed for the sham vs. active arm in the obese participant group for the TFEQ Total Score (Ƶ = 2.1, P < 0.04). No other changes in hyperphagia measures, weight or TMT scores were observed for active relative to sham participants from the HWC or OB participant groups (Table III).
TABLE III.
Response to Transcranial Direct Current Stimulation in Lean and Obese Individuals
| Lean Participants | ||||
|---|---|---|---|---|
| Three-Factor Eating Questionnaire | Factor I: Cognitive Restraint of Eating | Factor II: Disinhibition | Factor III: Hunger | Total Score |
| Active stimulation | 9.3 ± 4.8 | 5.3 ± 4.2 | 6.0 ± 3.7 | 20.5 ± 10.3 |
| Sham | 8.7 ± 4.6 | 4.9 ± 4.0 | 6.2 ± 3.2 | 19.8 ± 9.1 |
| Food Craving Analogue Scale | ||||
| Active stimulation | 4.4 ± 2.4 | Balanced design with a single tDCS exposure on visit 2 or 3, assessment pre-tDCS stimulation | ||
| Sham | 4.2 ± 3.1 | |||
| Obese participants | |||||
|---|---|---|---|---|---|
| Dykens Hyperphagia Questionnaire | Baseline (mean ± SD) | Day 5 (mean ± SD) | Day 15 (mean ± SD) | Day 30 (mean ± SD) | |
| #Total Score | |||||
| Active stimulation | 26.4 ± 7.8 | 21.0 ± 11.3 | 20.7 ± 5.0 | 24.3 ± 5.2 | |
| Sham | 20.5 ± 8.7 | 21.3 ± 9.1 | 11.5 ± 0.7 | 15.3 ± 5.1 | |
| Drive Score | |||||
| Active stimulation | 7.6 ± 3.8 | 6.0 ± 2.8 | 6.0 ± 0 | 6.7 ± 2.9 | |
| Sham | 7.8 ± 4.9 | 7.3 ± 4.6 | 4.5 ±0.7 | 6.0±3.5 | |
| Behavior Score | |||||
| Active stimulation | 15.6 ± 5.0 | 12.5 ± 9.2 | 12.3 ± 4.5 | 14.6 ± 3.4 | |
| Sham | 9.5 ± 3.9 | 11.3 ± 6.0 | 5.0±0 | 6.3 ± 1.2 | |
| Severity Score | |||||
| Active stimulation | 3.3 ± 2.6 | 2.5 ± 0.7 | 2.3 ± 0.6 | 3.0±1.2 | |
| Sham | 3.3 ± 1.0 | 2.8 ± 0.5 | 2.0 | 3.0±1.0 | |
| Three-Factor Eating Questionnaire | |||||
| Total score* | |||||
| Active stimulation | 24.7± 9.6 | 25.1 ± 9.4 | 23.6 ± 10.0 | 25.1 ± 10.7 | |
| Sham | 23.3 ± 6.9 | 19.5 ± 7.7 | 20.3 ± 7.7 | 16.3 ± 8.0 | |
| Factor I: Cognitive Restraint of Eating | |||||
| Active stimulation | 10.3 ± 4.6 | 11.6 ± 5.0 | 11.7 ± 4.1 | 12.6 ± 5.6 | |
| Sham | 9.0 ± 4.3 | 8.3 ± 2.5 | 9.7 ± 3.8 | 6.7 ± 3.2 | |
| Factor II: Disinhibition | |||||
| Active stimulation | 8.3 ± 4.4 | 7.4 ± 3.7 | 7.0±3.2 | 6.7 ± 3.8 | |
| Sham | 8.7 ± 2.4 | 7.0 ± 2.6 | 6.5 ± 2.6 | 5.7 ± 2.5 | |
| Factor III: Hunger | |||||
| Active stimulation | 6.1 ± 2.7 | 6.1 ± 3.2 | 4.9 ± 3.9 | 5.9 ± 3.9 | |
| Sham | 5.5 ± 2.5 | 4.3 ± 3.6 | 4.0 ± 2.4 | 4.0 ± 2.6 | |
| Food Craving Analogue Scale | |||||
| Active stimulation | 5.0 ± 3.2 | 5.4 ± 2.8 | 6.3 ± 2.0 | 5.2 ± 2.0 | |
| Sham | 2.1 ± 1.7 | 4.6 ± 4.5 | 5.0 ± 3.5 | 5.7 ± 3.3 | |
| Weight (KG) | |||||
| Active stimulation | 118.6 ± 24.5** | 118.3 ± 24.5 | 118.0 ± 25.4 | 117.0 ± 25.7 | |
| Sham | 95.7 ± 5.9 | 95.8 ± 6.3 | 95.0±7.2 | 95.8 ± 5.9 | |
Significant change from baseline responding at Day 30.
Significant difference Active vs. Sham at baseline
Mann–Whitney U test, P < 0.04; HWC N = 11; Obese active stimulation N = 7, Sham N = 3 or 4.
Effects of tDCS on Cognitive Performance in PWS
Baseline cognitive performance as assessed by the TMT instrument Part A response times for PWS participants were longer than the average normal pace which indicated lower performance for all but one PWS subject (meanA = 64.0 sec, rangeA 19–109 sec). This speed decreased slightly over time (with practice) but did not differ significantly between active and sham groups (Table II). TMT Part B was more challenging than Part A and three PWS participants required the maximum time allotted for the test (300 sec). An overall response meanB = 188 sec (rangeB 51–300) was observed for Part B with these individuals but still within the normal range. There was no significant change in the TMT Part B times related to the tDCS exposure in PWS.
DISCUSSION
Prader–Willi syndrome (PWS) is a neurodevelopmental genetic disorder characterized by intellectual disabilities, an insatiable appetite with compulsive eating behaviors (hyperphagia) leading to severe obesity and detrimental health consequences. Hyperphagia is one of the most prominent and debilitating features of this disorder and leads to significant morbidity among participants and contributes negatively to their care and management. We investigated the effects of an emerging non-invasive brain stimulation technique, tDCS, on modulating food-motivated behaviors in participants with PWS and control subjects with healthy and abnormal weight status in a region of the brain thought to be related to decision-making and regulation of eating behavior, the DLPFC [Fregni et al., 2005, 2008a, b; Boggio et al., 2009; Goldman et al., 2011; Holsen et al., 2012; Honea et al., 2012; Zhang et al., 2015]. It was hypothesized that modulation of this area of the brain would be related to decreased food-craving, hyperphagia, and potential weight status in participants that received active tDCS modulation of this brain area in hopes of developing potential intervention techniques to modulate hyperphagia behavior in participants with PWS.
Scores for PWS participants were significantly elevated in all four dimensions of the Three-Factor Eating Questionnaire (TFEQ) at baseline consistent with the expected phenotype of chronic severe hyperphagia associated with this disorder. The measures were also significantly elevated on two dimensions of the Dykens Hyperphagia Questionnaire (Total and Drive Scores) as well as the Food Craving Analogue Scale supporting the hypothesis of primary disturbances in cognitive and emotional aspects and food preoccupation in PWS. Behavior and Severity measurements were higher than HWCs but did not meet criteria for statistical significance possibly due to the small sample size or increased variance in these measures. The controlled access environment associated with the care of PWS also may have reduced the number of items endorsed on the Behavioral and Severity dimensions of the Dykens Hyperphagia Questionnaire at baseline.
The tDCS appeared to be well-tolerated among all study subjects including those with PWS. This pilot investigation using tDCS to assess hyperphagia and food motivation in participants with PWS supported the use of active tDCS to augment food drive and behavioral inhibition in PWS, replicating and extending the results of prior investigations in the prefrontal cortex region [Goldman et al., 2011; Fregni et al., 2008b]. In addition to decreased food cravings and hunger ratings, results from the Dykens Hyperphagia Questionnaire demonstrated promising changes in hyperphagia behavior among PWS as reported by participants and caregivers. Statistically significant changes in the Dykens Hyperphagia Questionnaire (e.g., Severity subscale and Total Scores) were seen over 5 and 15-day periods—10 days after the last stimulation session which suggests persistent modulatory actions of food-related cognition and behavior in PWS. Parallel increases in Dykens Hyperphagia Severity Scores with Food Craving Ratings in the sham study arm suggest a possible state dependent relationship between the two measures possibly attributable to anticipatory factors since Severity Scores were normal at baseline. The repeated exposure to food images and frequent inquiries into hunger and drive for food during the course of the study might have led to anticipatory increases in symptomatology and possible frustration for study participants in the sham treatment arm. The data suggest that tDCS treatment attenuated these possible anticipatory effects. Similarly, PWS caretakers reported increased Behavior scores over time associated with the sham arm. The observed differences in baseline scores may reflect differences in the randomization or individual differences in the caretakers themselves which were largely stable over time.
Our tDCS treatments were designed to stimulate the area surrounding the anode in the right DLPFC but the cathode (reference electrode) placement over the left supraorbital area may inhibit activity in the surrounding area. The use of a reference electrode placed in another location is required in order to complete the electrical circuit. This placement is consistent with a range of published applications of tDCS technology. This application of tDCS did not appear to impact neuropsychological functioning or processing speed as assessed by the TMT supporting the specificity for food motivated behaviors without disruptive effects on central executive functions.
Obese participants in this study did not appear to respond to tDCS as observed in prior studies. The only significant change from baseline (TFEQ Total Score) was observed for sham rather than the active stimulation arm. The obese participants were older than the HWC subjects but age was not correlated with TFEQ ratings at baseline. Also TFEQ and Dykens Hyperphagia Questionnaire test scores for the obese subjects did not differ very much from the HWC at baseline. Never-the-less, these preliminary observations highlight the need for larger confirmatory testing.
No weight loss was observed among PWS participants during this relatively short period of treatment, but body mass and access to food are tightly controlled in PWS adults for safety, most often in a group home setting. Thus, changes in motivation and drive states may not immediately impact body mass, longer term standards are needed to monitor weight. It could be hypothesized that various aspects of food-intake decision making may be modulated by tDCS as has been noted previously in non-PWS subjects potentially leading to better weight management in PWS [Fregni et al., 2008b; Goldman et al., 2011]. Additionally, no significant weight loss was observed for obese or HWC subjects which may again reflect the short study duration and limited exposure to active stimulation (e.g., one session for the HWC experimental arm).
Extreme hyperphagia remains a severe and debilitating feature of PWS contributing to significant morbidity and mortality among those affected. Additionally, the management of hyperphagia behaviors in PWS requires rigorous monitoring, a costly medical care approach and is often associated with admission to specialized care facilities to control food access and provide dietary plans and exercise programs to assure psychological food security and manage expectations about food to minimize anxiety and disruptive behavior. Thus, there is a strong need for the development of new strategies to address this core feature of the disorder to improve manageability and assist families seeking assistance in treating this cardinal debilitating feature of PWS. If effective, tDCS could represent a significant advancement in the care and management of PWS possibly reducing the burden to individuals, families and society and improving quality of life.
The tDCS is a promising emerging non-invasive technique that may have application in multiple areas of health behavior and change in PWS. This initial investigation using tDCS modulation of the DLPFC to assess hyperphagia and food motivation in participants with PWS demonstrated that active tDCS had a persistent modulatory impact on food drive and behaviors in these participants. However, this pilot investigation requires confirmatory follow-up studies with additional subjects to replicate and further clarify our findings and address the contributions of relevant confounds such as age and PWS genetic subtype which may impact food drive and responsivity to tDCS. The results support safety, tolerability and beneficial modulatory effects of this technique in participants with PWS as indexed by decreased hunger ratings and food drive assessed by validated measures of hyperphagia in participants with PWS. This is the first demonstration of modulated hyperphagia behavior in participants with PWS using this (or any other) technique and the findings are generally consistent with prior investigations using this methodology and technique in non-PWS subjects. Future studies examining the potential mechanism of altered hyperphagia behavior using tDCS would be greatly valued as would more general investigations of tDCS as a potential mechanism associated with weight loss in individuals with abnormal weight status.
ACKNOWLEDGEMENTS
We would like to thank Kelly Usrey for her role in the coordination of activities and participant recruitment and assessment at the University of Kansas Medical Center site. Funding support was provided by the Clara Schiller Perpetual Charitable Trust, Prader-Willi Syndrome Association (USA) and NICHD grant number HD02528. The authors have no conflicts of interest to declare.
Grant sponsor: Clara Schiller Perpetual Charitable Trust;Grant sponsor: Prader-Willi Syndrome Association (USA); Grant sponsor: NICHD; Grant number: HD02528.
REFERENCES
- Angulo MA, Butler MG, Cataletto ME. 2015. Prader-Willi syndrome: A review of clinical, genetic and endocrine findings. J Endocrinol Invest 38(12):1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Butler MG. 2005. Prader-Willi syndrome: Clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med 7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F. 2009. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett 463:82–86. [DOI] [PubMed] [Google Scholar]
- Butler MG, Thompson T. 2000. Prader-Willi syndrome: Clinical and genetic findings. The Endocrinologist 10:3S–16S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG. 2011. Prader-Willi syndrome: Obesity due to genomic imprinting. Curr Genomics 12:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Lee PDK, Whitman B. 2006. Management of Prader-Willi Syndrome. New York, NY: Springer. [Google Scholar]
- Dykens EM, Maxwell MA, Pantino E, Kossler R, Roof E. 2007. Assessment of hyperphagia in Prader-Willi syndrome. Obesity 15:1816–1826. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, Marcolin MA, Rigonatti SP, Silva MT, Paulus W, Pascual-Leone A. 2005. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res 166:23–30. [DOI] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS. 2008a. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: A randomized, sham-controlled study. J Clin Psychiatry 69:32–40. [DOI] [PubMed] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FA, Nitsche MA, Mecca T, Macedo EC, Pascual-Leone A, Boggio PS. 2008b. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite 51:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman RL, Borckardt JJ, Frohman HA, O’Neil PM, Madan A, Campbell LK, Budak A, George MS. 2011. Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving. Appetite 56:741–746. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Savage CR, Martin LE, Bruce AS, Lepping RJ, Ko E, Brooks WM, Butler MG, Zarcone JR, Goldstein JM. 2012. Importance of reward and prefrontal circuitry in hunger and satiety: Prader-Willi syndrome vs simple obesity. Int J Obes (Lond) 36:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Holsen LM, Lepping RJ, Perea R, Butler MG, Brooks WM, Savage CR. 2012. The neuroanatomy of genetic subtype differences in Prader-Willi syndrome. Am J Med Genet B Neuropsychiatr Genet 159B:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. 2004. Kaufman Brief Intelligence Test, Second Edition Bloomington, MN: Pearson, Inc. [Google Scholar]
- McAllister CJ, Whittington JE, Holland AJ. 2011. Development of the eating behaviour in Prader-Willi syndrome: advances in our understanding. Int J Obes (Lond) 35:188–197. [DOI] [PubMed] [Google Scholar]
- Montenegro RA, Okano AH, Cunha FA, Gurgel JL, Fontes EB, Farinatti PT. 2012. Prefrontal cortex transcranial direct current stimulation associated with aerobic exercise change aspects of appetite sensation in overweight adults. Appetite 58:333–338. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. 2000. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. 2008. Transcranial direct current stimulation: State of the art 2008. Brain Stimul 1:206–223. [DOI] [PubMed] [Google Scholar]
- Ogura K, Fujii T, Abe N, Hosokai Y, Shinohara M, Takahashi S, Mori E. 2011. Small gray matter volume in orbitofrontal cortex in Prader-Willi syndrome: A voxel-based MRI study. Hum Brain Mapp 32:1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm U, Reisinger E, Keeser D, Kuo MF, Pogarell O, Leicht G, Mulert C, Nitsche MA, Padberg F. 2013. Evaluation of sham transcranial direct current stimulation for randomized, placebo-controlled clinical trials. Brain Stimul 6:690–695. [DOI] [PubMed] [Google Scholar]
- Peto R 1978. Clinical trial methodology. Biomedicine 28:24–36. [PubMed] [Google Scholar]
- Pignatti R, Bertella L, Albani G, Mauro A, Molinari E, Semenza C. 2012. Decision-making in obesity: A study using the gambling task. Eat Weight Disord 11:126–132. [DOI] [PubMed] [Google Scholar]
- Reitan RM. 1955. The relation of the trail making test to organic brain damage. J Consult Psychol 19:393–394. [DOI] [PubMed] [Google Scholar]
- Rolls ET. 2000. The orbitofrontal cortex and reward. Cereb Cortex 10:284–294. [DOI] [PubMed] [Google Scholar]
- Rolls ET. 2005. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav 85:45–56. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. 1985. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 29:71–83. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang J, Zhang G, Zhu Q, Cai W, Tian J, Zhang YE, Miller JL, Wen X, Ding M, Gold MS, Liu Y. 2015. The neurobiological drive for overeating implicated in Prader-Willi syndrome. Brain Res 1620:72–80. [DOI] [PubMed] [Google Scholar]