Introduction
Since its initial description as an occupational disease over a hundred years ago,6,43 the Hand-Arm Vibration Syndrome (HAVS) has been considered as one of the most disabling forms of persistent pain due to ergonomic injury.14,16,39 It is estimated that in the U.S. approximately 2.5 million workers are exposed daily to harmful vibration 45, whereas workplace prevalence of HAVS reaches up to 50% of the power tools users 45. HAVS is typically described as a persistent pain and tingling of the affected extremities. 13,39 Reduced tactile sensitivity or numbness are also observed. 13,39 Symptoms of autonomic dysfunction are often observed in HAVS 6,13,21,23,39,43,45,50, such as enhanced sympathetic activation and vasoconstriction of digital arteries 21, the so-called vibration-induced white finger or occupational (secondary) Raynaud’s phenomenon 6,13,21,23,43,45,50. Although all of these symptoms can be disabling, muscle pain is the most consistent complaint14,16 and the most important predictor for poor quality of life in HAVS patients.16 Unfortunately, pain management in HAVS is not based on controlled clinical trials and is devoid of mechanistic rationale.14,16,29,39
We have developed a preclinical model of HAVS wherein a rat’s hind limb is submitted to a bout of low-frequency mechanical vibration, comparable to that produced by power tools.7,10,36 This controlled ergonomic intervention induces long lasting mechanical hyperalgesia in the ipsilateral gastrocnemius muscle.2,7,9,10 Single fiber electrophysiology recordings from sensory afferents innervating the gastrocnemius muscle of rats exposed to this HAVS model display reduced threshold and, upon mechanical stimulation, long-lasting high-firing rates, consistent with nociceptor hyperexcitability.7 Using this model we have observed that knockdown of the transducer subunit glycoprotein 130 (gp130) of the interleukin 6 (IL-6) receptor, in nociceptors, prevented both hyperexcitability and concomitant muscle mechanical hyperalgesia, supporting a role for IL-6 in the induction of HAVS.7 While these findings are in line with the well-established role of IL-6 in preclinical models of persistent muscle pain,3,4,26,28 the underlying mechanism remains unknown.
Voltage-gated potassium channels (KV) play a prominent role in modulating the resting membrane potential, the shape of action potentials and repolarization. Thus, their expression is posited to play an important role in nociceptor excitability.44,51 Indeed, evidence shows that there is a decreased expression of KV channels in nociceptors after inflammation15,42,47 or nerve injury20,37,49, conditions where concomitant nociceptor hyperexcitability and pathological pain are observed.44,51 Interestingly, it has been shown that gp130-deficient nociceptors exhibit reduced excitability, increased KV currents and up-regulation of KV1.4 expression in dorsal root ganglion (DRG) neurons.24 Furthermore, mice bearing this conditional null mutation display high mechanical nociceptive threshold and reduced mechanical hypersensitivity in inflammatory, neuropathic and cancer pain models.5,35 These observations suggest that intracellular signaling through gp130 likely acts as an endogenous break for the expression of KV1.4,24 providing a plausible mechanism for IL-6 induced nociceptor hyperexcitability and mechanical hyperalgesia. Since HAVS exhibits some clinical and pathophysiological features consistent with an ongoing pro-inflammatory response,22 and our preclinical model is sensitive to knockdown of gp130 in muscle nociceptors,7 we tested the hypothesis that IL-6 contributes to vibration-induced muscle hyperalgesia signaling, through gp130, to decrease nociceptor expression of KV1.4.
Methods
Animals
Experiments were performed in adult male Sprague Dawley rats (250–450 g; Charles River Laboratories, Hollister, CA). Animals were housed in the animal care facility of the University of California, San Francisco, under environmentally controlled conditions (21°C–23°C; 12-hour alternating light–dark cycle; food and water ad libitum). Animal care and use adhered to the guidelines set by the National Institutes of Health and all studies were conducted in accordance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee at the University of California, San Francisco approved all experimental protocols. Concerted effort was made to minimize the number of animals used and their suffering.
Hand-arm vibration syndrome (HAVS) model
One hind limb of a rat was subjected to mechanical vibration with a laboratory vortex mixer, as previously described.2,7,9,10 Rats were anesthetized with 2.5% isoflurane in oxygen and their right hind limb affixed to the platform of a vortex mixer (Digital Vortex Genie II; Thermo Fisher Scientific, Waltham, MA) with Micropore surgical tape (3M Health Care, St. Paul, MN) so that the knee and ankle joint were both at 90°, without rotational torque on the leg. Each hind limb was vibrated at a frequency of 60 to 80 Hz with a 5 mm peak-to-peak displacement amplitude for 15 minutes. These vibration frequencies are within the range produced by hand-held power tools (35–150 Hz).36
Measurement of mechanical nociceptive threshold
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a Chatillon digital force transducer (model DFI2; Amtek Inc, Largo, FL), as previously described.4,9,10 Approximately 24 h after administration of the vibration protocol, rats were lightly restrained in a cylindrical acrylic restrainer that allows for easy access to the hind limb for mechanical nociceptive threshold testing. A 7-mm diameter probe was used to stimulate the belly of the gastrocnemius muscle with an increasing compression force. The nociceptive threshold was defined as the force (mN) at which the rat withdrew its hind leg. Rats were placed in restrainers where they were trained with the force transducer for 4 consecutive days before measuring baseline mechanical nociceptive threshold. Baseline nociceptive withdrawal threshold was defined as the mean of 3 readings taken at 5-min intervals. The magnitude of hyperalgesia was calculated as the percentage decrease from the baseline withdrawal threshold. Behavioral experiments were performed blind to treatment group. Upon completion of experiments rats were killed by carbon dioxide asphyxiation followed by bilateral thoracotomy.
Drugs and reagents
Unless otherwise stated, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO). A function blocking IL-6 antibody (AF506, lots BC0617012 and BCZ0617012), produced in goats immunized with purified Escherichia coli-derived rrIL-6, was obtained from R&D Systems Inc (Minneapolis, MN). The lyophilized IL-6 antibody was diluted to 400 ng/µl in 0.1% bovine serum albumin (BSA) in Dulbecco’s phosphate-buffered saline without calcium and magnesium salts (DPBS, UCSF Cell Culture Facility), and injected into the belly of the gastrocnemius muscle 6 h after exposure to vibration in a dose of 50 µg (50 µl volume).
Intrathecal injection of antisense oligodeoxynucleotides
To evaluate the role of nociceptor expression of different targets in vibration-induced mechanical hyperalgesia, we attenuated their expression using intrathecal (i.t.) injections of antisense oligodeoxynucleotides (AS ODN) directed against gp130 or KV1.4 mRNAs. These ODN sequences have been previously reported to produce specific protein knockdown and behavioral changes consistent with such a decrease in protein expression in the rat.30,41
The 20-mer AS ODN sequence 5′-GCC ACC TCC ATG GTG GTA GT-3′ (GenBank accession number NM_012971.2) was used to produce specific knockdown of KV1.4 protein (Meiri et al., 1998). The control sense (SE) ODN sequence was 5′-ACT ACC ACC ATG GAG GTG GC-3′.30
The 19-mer AS ODN sequence 5’-TCC TTC CCA CCT TCT TCT G-3’ (ODN position within the complementary DNA sequence 1834–1852, GenBank accession number M92340), was directed against a unique sequence of rat gp130 mRNA.41 The MM ODN sequence, 5’-TAC TAC TCA CAT TCA TCA G-3’, corresponds to the gp130 subunit AS sequence with six mismatched bases denoted by boldface.
The ODNs were synthesized by Invitrogen (San Francisco, CA), dissolved in sterile 0.9% NaCl (B. Braun Medical Inc., Irvine, CA) to a concentration of 10 μg/μl and stored in 40 μl aliquots at −20°C until use. Immediately before injection aliquots were further diluted in sterile 0.9% NaCl to 4 μg/μl and 20 μl injected i.t. daily for three consecutive days under brief anesthesia with 2.5% isoflurane as previously reported.3,4,7,10 Tail flick was systematically checked to ensure proper i.t. injections.31
Tissue harvesting and Western blot analysis
Rats were euthanized by exsanguination, while under deep isoflurane anesthesia, 24 h after the vibration protocol. Ipsilateral L4 and L5 DRGs were quickly dissected out and immediately placed on dry ice, and then stored at −80°C until use for further analyses. The ipsilateral gastrocnemius muscle was dissected out and placed in a 50 ml centrifuge tube containing a homogenization buffer solution supplemented with a 2× protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Muscle tissue was then homogeneized using a Tissue Tearor™ homogeneizer, centrifuged for 15 min at 4°C and 3750 rpm and supernatants stored at −80°C. To determine whether vibration induces changes in the expression of KV1.4 and gp130 in DRGs, and IL-6 in the gastrocnemius muscle, Western blot analyses were performed, as previously described 1,9. Ipsilateral L4-L5 DRGs were transferred into cold homogenization buffer (150 mM NaCl, 10 mM EDTA, 2% sodium dodecyl sulfate, 50 mM Tris-HCl, pH 7.4), supplemented with a 2× protease inhibitor cocktail and then homogenized manually. Proteins were solubilized by incubating the DRG extracts at 37°C and 1400 rpm for 2 h in an Eppendorf Thermomixer (Eppendorf AG, Hamburg, Germany). Proteins were then extracted by a 15 min centrifugation 1400 rpm. The protein concentration of muscle and DRG extracts was determined using the micro BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL) with BSA as the standard. Forty (to assess gp130 and KV1.4 expression in L4-L5 DRG homogenates) or 100 (to assess IL-6 expression in gastrocnemius muscle homogenates) μg of protein per sample were mixed with 4x sample buffer [62.5 mM Tris. HCl, pH 6.8, 3% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.025% bromophenol blue), denatured at 90°C for 10 min and electrophoresed on a 4% to 15% precast polyacrylamide gel (Biorad, Hercules, CA) in 25 mM Tris containing 192 mM glycine and 0.1% sodium dodecyl sulfate. Proteins were then transferred to a nitrocellulose membrane using the semidry method (transfer time 1 h at 10 V). The nitrocellulose membranes were saturated by shaking in antibody dilution buffer (5% BSA in Tris-buffered saline containing 0.1% Tween 20 and 0.02% NaN3 [TBST]) for 1 h at room temperature (RT), cut in half at around 50kDa and then incubated with the primary antibodies: rabbit anti-gp130 (1:500; M20; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-KV1.4 (1:500, APC167, Alomone Labs, Jerusalem, Israel), rabbit anti-IL-6 (1:500; ab83339, Abcam, Cambridge, MA), rabbit anti-β-actin (1:1000; ab8227; Abcam), or rabbit anti-Grk2 (1:500, sc562, Santa Cruz) primary antibodies in TBST, at 4°C overnight. After 3 washes with TBST (RT, 15 min each), the membranes were probed with a donkey anti-rabbit horseradish peroxidase-conjugated antibody (1:2500, NA934V; GE Healthcare Life Sciences, Piscataway, NJ) for 2 h at RT. Western blots were washed 3 times with TBST (RT, 15 min each) and immunoreactivities visualized using a chemiluminescence detection kit (SuperSignal™ West Femto Maximum Sensitivity Substrate, Thermo Fisher Scientific). Results were analyzed using computer-assisted densitometry and immunoreactivity levels for each protein target were normalized with respect to the β-actin for gp130 and KV1.4, or Grk2 for IL-6, control levels. Grk2 is moderately but constitutively expressed by skeletal muscle tissue. Moreover, the Grk2 expression level is not affected by vibration injury. Therefore, Grk2 is an excellent reference candidate for the quantification of proteins whose muscular expression level is low to moderate and whose expression level is affected by vibration injury.
Statistical analysis
Group data are expressed as mean ± SEM of n independent observations. Statistical comparisons were made using GraphPad Prism 6.0 statistical software (GraphPad Software Inc., La Jolla, CA). Comparisons between treatments were made by means of Student’s t test or two-way repeated measures analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. P < 0.05 was considered statistically significant.
Results
Vibration induces mechanical hyperalgesia and increased levels of IL-6 in muscle
Consistent with our previous reports,2,7,9,10 in the HAVS model rats exhibited a robust decrease in mechanical nociceptive threshold (i.e. hyperalgesia) in the gastrocnemius muscle 24 h after vibration compared to controls (−35.2 ± 1.7% vs −0.7 ± 0.8%, n=5/group, P < 0.001, Fig. 1A). In parallel experiments, an increase in local expression of IL-6 protein (33.7 ± 9.7% to Grk2 house keeping protein) was observed in extracts from the gastrocnemius muscle of HAVS rats compared to control (naïve) rats 24 h after exposure to vibration (n=6/group; P < 0.05, Fig. 1B,C). To assess the role of increased muscle levels of IL-6 in vibration-induced muscle hyperalgesia, we tested the effect of a local injection of a neutralizing anti-rat IL-6 antibody 6 h after vibration. This treatment (n=5), but not vehicle (n=5), significantly reversed the muscle hyperalgesia observed 6 h (−25.5 ± 2.3 vs −32.7 ± 1.9%, P < 0.05) and 24 h (−18.6 ± 2.2 vs −36.7 ± 0.9, P < 0.001) after vibration, confirming the involvement of local IL-6 mediating pain in the HAVS model (Fig. 1D).
Figure 1. Vibration induces muscle mechanical hyperalgesia and increased levels of IL-6 in the gastrocnemius muscle.
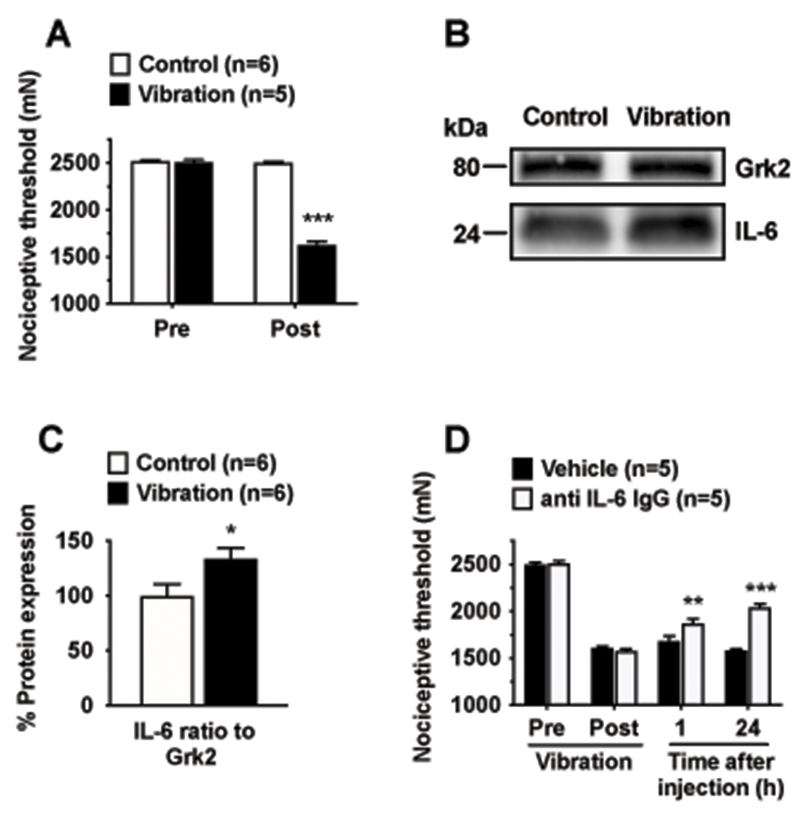
A. Hyperalgesia (i.e., reduction of mechanical nociceptive threshold) was observed in the gastrocnemius muscle in a group of rats submitted to the HAVS model, compared to control (naïve) rats. Two-way ANOVA showed significant effects for treatment (F1,5=383.8, P < 0.001), time (F1,5=222.1, P < 0.001) and treatment by time interaction (F1,5=464.3, P < 0.001). Post hoc analysis revealed significant differences between control and vibration exposed rats one day after vibration exposure; B. Representative Western blots showing IL-6 immunoreactivity in extracts from the ipsilateral gastrocnemius muscle of HAVS rats compared to control rats; C. Quantitative analysis (ratio of IL-6 to housekeeping protein Grk2) showed that, compared to controls, HAVS exhibited significantly increased IL-6 expression in the gastrocnemius muscle; D. Five hours after vibration (Post) nociceptive threshold was significantly reduced compared to baseline (Pre). A neutralizing antibody (anti-rat IL-6 goat IgG), but not vehicle (DPBS), injected into the gastrocnemius muscle 6 h after exposure to vibration significantly reduced the mechanical hyperalgesia. *P < 0.05; **P < 0.01; ***P < 0.001.
Vibration induces down-regulation of KV1.4 in nociceptors
To evaluate whether nociceptor hyperexcitability observed after vibration7 is associated with changes in the expression of KV1.4, Western blot analysis of L4-L5 DRG extracts was performed. A significant decrease in KV1.4 expression (−25.6 ± 10.1% to β-actin house keeping protein), was observed one day after exposure to vibration in HAVS rats compared to naïve controls (n=6/group, P < 0.05; Fig. 2A,B). To assess whether such a decreased expression is functionally meaningful (i.e., it is associated with mechanical hyperalgesia), we knocked down the expression KV1.4 in normal rats by means of an i.t. AS treatment and evaluated its effect on mechanical nociceptive threshold. One day after the last of 3 ODN injections, the nociceptive threshold was significantly reduced in the AS group compared to SE control group (−26 ± 1.6% vs 2.6 ± 1.2%, n=6/group; P < 0.0001; Fig. 2C). Compared to SE, the mechanical hyperalgesia induced by AS was significant for up to 7 days after the last ODN injection (−8.2 ± 0.7% vs 0.6 ± 1.1%, P < 0.0001; Fig. 2C), and fully recovered by day 10 (0.2 ± 0.7% vs 1.4 ± 1.2%, P < 0.0001; Fig. 2C), indicating that nociceptor down-regulation of KV1.4 is sufficient to produce muscle hyperalgesia.
Figure 2. Vibration down-regulates expression of KV1.4 in DRG innervating the gastrocnemius muscle.
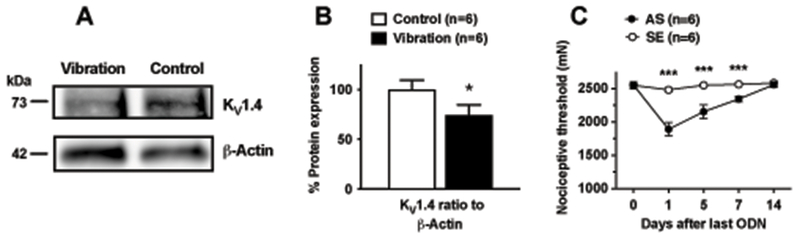
A. Representative Western blots showing KV1.4 immunoreactivity in extracts from the ipsilateral L4-L5 DRGs of HAVS rats compared to control (naïve) rats; B. Quantitative analysis (ratio to reference protein) showed that, compared to controls, HAVS rats exhibited significantly decreased KV1.4 expression in DRG innervating the gastrocnemius muscle (unpaired t test, t = 1.859 df = 10, P = 0.046). β-actin was used as the housekeeping reference protein; C. KV1.4 knockdown in DRG by i.t. AS, but not SE, ODN treatment produced significant mechanical hyperalgesia in the gastrocnemius muscle. Two-way ANOVA showed significant effects for treatment (F1,10=192, P < 0.001), time (F4,40=123.1, P < 0.001) and interaction (F4,40=77.8, P < 0.001). Post hoc analysis of each time point revealed significant differences between AS and SE treated rats from days 1 to 7 after last ODN injection. *P < 0.05; ***P < 0.001.
Vibration-induced down-regulation of KV1.4 is gp130 dependent
Evidence indicates that gp130-dependent IL-6 signaling regulates the expression of KV1.4 in nociceptors 24. We have shown that several proalgesic mediators, including glial cell-derived neurotrophic factor, IL-6 and tumor necrosis alpha, are likely involved in vibration-induced hyperalgesia.2,7,10 Therefore, we explored whether the down-regulation of KV1.4 observed in the HAVS model specifically depends on nociceptor gp130 expression. Preventive i.t. treatment with AS ODN directed against gp130, compared to MM control, attenuated vibration-induced mechanical hyperalgesia (−24.9 ± 2.9% vs −32.9 ± 2%, n=6/group, P < 0.01; Fig. 3A). Furthermore, Western blot analyses of DRG extracts from these rats showed that AS, compared to MM, attenuated both, the vibration-induced down-regulation of KV1.4 (increase of 37.3 ± 11.3%, n=6/group, P < 0.05), and gp130 expression (decrease of −59 ± 7.5%, n=6/group, P < 0.01), as shown by respective ratios to β-actin used as a house keeping protein (Fig. 3B–C).
Figure 3. Knockdown of gp130 attenuates vibration-induced muscle hyperalgesia and KV1.4 expression.
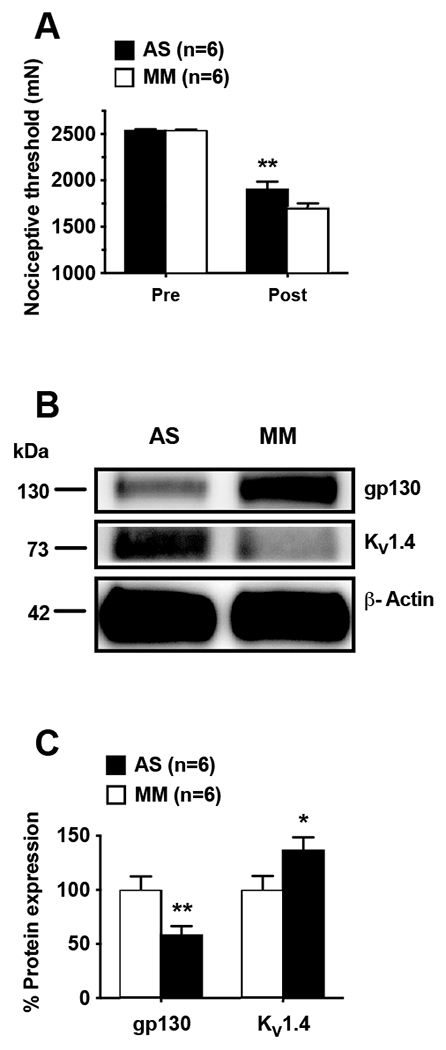
A. Compared to baseline mechanical nociceptive threshold (Pre), vibration produced significant muscle mechanical hyperalgesia (Post), which was attenuated by i.t. AS, but not MM, ODN directed against gp130; Two-way ANOVA showed significant effects for treatment (F1,10=6.275, P < 0.031), time (F1,10=253.1, P < 0.001) but not interaction (F1,10=4.869, P = 0.051). Post hoc analysis revealed significant differences between AS and MM treated rats one day after exposure to vibration. B. Representative Western blots showing reduced down-regulation of KV1.4 expression in rats treated with AS ODN against gp130. C. Quantitative analysis (ratio to reference protein) showed that, compared to MM, AS ODN significantly attenuated the down-regulation of KV1.4 and the expression of gp130 in L4-L5 DRG in HAVS model rats; unpaired Student t test (t = 2.187, df = 10) revealed significant differences between AS and MM treated rats in nociceptor expression of gp130 (P = 0.009) and KV1.4 (P = 0.026), one day after exposure to vibration. β-actin was used as housekeeping reference protein. *P < 0.05; **P < 0.01
Discussion
HAVS is an extremely painful and disabling occupational disease, which still lacks effective treatment. While sensory dysfunction and persistent pain, concomitant to nociceptor hyperexcitability,7 suggest a neuropathic component in this syndrome, paucity of knowledge of its pathophysiology has largely precluded the development of rationale, mechanism-based, therapies. The finding that vibration induces increased local expression of IL-6 in muscle, which in turn produces a decrease in the expression of KV1.4 α-subunit in muscle nociceptors, provides insight into the mechanism mediating the symptoms of HAVS and a possible novel treatment modality.
The rat model of HAVS
Our model of HAVS consists in a technically simple and reproducible intervention, which consistently produces a robust and persistent muscle pain reminiscent of that observed in HAVS patients 2,7,9,10. The stimulus used in this model, mechanical vibration, is relevant to the occupational disease HAVS, and its settings can be modified to explore the contribution of different parameters (vibration frequency, force, peak-to-peak amplitude, number of exposures, etc.) to the pain observed. Finally, this model is sensitive to putative antinociceptive interventions 2,7,10, allowing exploration of the mechanisms underlying pain in HAVS and the development of therapeutic strategies. This model, however, also has limitations that can preclude its utility as preclinical approach for the study of potential HAVS treatments. For instance, different exposures occur based on occupation (e.g., jackhammer operator versus dentist). In contrast to our preclinical model, the vibration injury more typically affects forelimbs of HAVS patients. Also in contrast to our model, active prehension of the vibrating tool is almost the rule in HAVS patients. Differences in pain phenotypes and inflammatory/immune responses have been observed between different rodent inbred strains 12,18. Thus, it cannot be ruled out that the IL-6 dependent mechanism observed in our model of HAVS could be, to some extent, strain-dependent. However, it is important to stress that all our data were obtained from outbred animals, minimizing the potential bias toward a strain-dependent phenomenon. While it is difficult to ascertain the importance of these variables for the “translatability” of our results, all these aspects should be considered as potential limitation for the findings derived from our model of HAVS.
Vibration increases IL-6 expression in the muscle
Consistent with our hypothesis, the exposure to a vibration protocol produced mechanical hyperalgesia in muscle concomitant to an increase in local levels of IL-6. It is well established that the expression of IL-6 in skeletal muscle increases in response to a number of stressors, including mechanical stimulation.46 While the mechanism underlying the changes in the expression of IL-6 in the gastrocnemius muscle induced by vibration was not explored, several phenomena may contribute. For instance, exposure to mechanical vibration produces vasoconstriction, vasospasm and ischemia, which contribute to HAVS,23,50 and are known to induce IL-6 expression in a number of tissues, including skeletal muscle.17,40 Alternatively, in vitro experiments have shown that mechanical vibration is able to induce enhanced expression of IL-6 in several cell types.27,33,34 This suggests that vibration directly activates mechanotransduction pathways, leading to intracellular signaling and IL-6 up-regulation. The potential contribution of these phenomena to HAVS warrants further studies.
Vibration decreases expression of KV1.4, in an IL-6-dependent manner
We have previously shown that several proalgesic mediators, including glial cell-derived neurotrophic factor,2 IL-67 and tumor necrosis alpha,10 likely contribute to vibration-induced mechanical hyperalgesia. While our finding that neutralizing antibodies to IL-6 attenuated HAVS hyperalgesia confirmed a role for local IL-6, it did not clarify whether this cytokine does so by acting directly on muscle nociceptors, or indirectly, by acting on other cellular targets to decrease the nociceptive threshold. Furthermore, whether the down-regulation of KV1.4 observed here depends on IL-6 acting on nociceptor gp130 subunit was also unknown. We explored these issues by knocking down gp130 in nociceptors, which not only prevented vibration-induced mechanical hyperalgesia as previously observed,7 but also the vibration-induced down-regulation of KV1.4 in the nociceptor. Therefore, while several algogens may act on muscle nociceptors, contributing to hyperalgesia in HAVS, signaling trough gp130 is critical for decreased expression of KV1.4.
Although we did not study the mechanism underlying gp130-dependent decreased expression of KV1.4 α-subunit, previous studies point to transcriptional changes triggered by nociceptor injury. Indeed, small-size sensory neurons exhibit a marked decrease in KV1.4 mRNA after sciatic nerve transection49 or chronic constriction of the infraorbital nerve,25 neuropathic pain models where IL-6 plays a central role.19,52 Reciprocally, conditional deletion of gp130 in nociceptors increases voltage-gated A-type K+ currents and up-regulates Kcna4 mRNA expression, which encodes KV1.4, leading to decreased nociceptor excitability.24 More recently, it has been shown that nerve injury induces persistent upregulation of the microRNA cluster miR-17–92, which downregulates the expression of several KV α-subunits, including KV1.4 and KV4.3, and outward K+ currents, specifically A-type K+ currents.38 Of note, the HAVS model is also associated with nociceptor downregulation of the KV4.3 α subunit, which is involved in A-type K+ currents.9 Interestingly, in vitro studies in cholangiocarcinoma cell lines, which typically are associated to high levels of IL-6 in patients with this cancer, demonstrated that up-regulation of miR-17–92 cluster is induced by IL-6 in a gp130-dependent manner.53 Together, these observations suggest that intracellular signaling downstream to gp130 acts as a dynamic repressor for KV1.4 transcription, possibly involving miR-17–92, accounting for the IL-6 mediated induction of nociceptor plasticity observed in nerve injury models such as HAVS (see below).
Decreased expression of KV1.4 contributes to nociceptor hyperexcitability
A-type K+ currents are key determinants of neuronal excitability, contributing to the setting of resting membrane potential, spike latency and action potential waveform.44,51 Members of the KV1 and KV4 α subunit families contribute to A-type K+ currents, and decreased density of these currents has been observed in several preclinical models of persistent pain.8,48,49 While decreased expression of mRNA or protein for different KV α subunits, in the DRG, has been observed after nerve damage,20,32,37,49 we are unaware of any other study showing KV1.4 down-regulation after exposure to tissue injury comparable to mechanical vibration. However, consistent with our observations, models of chronic pain affecting deep tissue such as joint inflammation,42 bone cancer pain11 and visceral pain,15,54 which are associated with enhanced nociceptor excitability, display decreased KV1.4 expression and/or rapidly inactivating A-type K+ currents. Moreover, knockdown of KV1.4 in nociceptors produced muscle hyperalgesia in otherwise naïve rats, highlighting the importance of this KV α-subunit in the setting of normal mechanical nociceptive threshold. On the other hand, conditional deletion of gp130 in nociceptors produces increased conductance in A-type K+ currents and decreased neuronal excitability,24 and mice carrying this conditional deletion display enhanced mechanical nociceptive threshold and attenuated mechanical hyperalgesia in models of persistent pain.5,35 Together, these observations support the suggestion that attenuation of A-type K+ currents likely plays a central role in the induction of nociceptor hyperexcitability by IL-6, which likely explains the neuropathic-like responsiveness to mechanical stimulation observed in the HAVS model.7
Conclusions
In summary, mechanical vibration increases the expression of IL-6 in the exposed skeletal muscle. IL-6 downstream signaling, through gp130, decreases the expression of the α subunit KV1.4 in DRGs, leading to nociceptor hyperexcitability and persistent muscle mechanical hyperalgesia. Exploring these signaling pathways to identify key players underlying the transcriptional changes in KV1.4 expression is an important future direction. Finally, our data support the suggestion that analgesic therapies targeting IL-6 and or gp130 may provide an effective treatment for the chronic pain reported by patients with HAVS.
Acknowledgements:
This work was supported National Institutes of Health grant AR063312.
Footnotes
Conflict of interest statement: The authors report no conflict of interest.
References
- [1].Alvarez P, Bogen O, Green PG, Levine JD. Nociceptor interleukin 10 receptor 1 is critical for muscle analgesia induced by repeated bouts of eccentric exercise in the rat. Pain 2017; 158: 1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alvarez P, Chen X, Bogen O, Green PG, Levine JD. IB4(+) nociceptors mediate persistent muscle pain induced by GDNF. J Neurophysiol 2012; 108: 2545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alvarez P, Green PG, Levine JD. Stress in the adult rat exacerbates muscle pain induced by early-life stress. Biol Psychiatry 2013; 74: 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. Eur J Neurosci 2010; 32: 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, Quarta S, Vogl C, Sailer CA, Uceyler N, Brockhaus J, Martini R, Sommer C, Zeilhofer HU, Müller W, Kuner R, Davis JB, Rose-John S, Kress M. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci 2009; 29: 13473–13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bovenzi M Hand-arm vibration syndrome and dose-response relation for vibration induced white finger among quarry drillers and stonecarvers. Italian Study Group on Physical Hazards in the Stone Industry. Occup Environ Med 1994; 51: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen X, Green PG, Levine JD. Neuropathic pain-like alterations in muscle nociceptor function associated with vibration-induced muscle pain. Pain 2010; 151: 460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chien LY, Cheng JK, Chu D, Cheng CF, Tsaur ML. Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci 2007; 27: 9855–9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Conner LB, Alvarez P, Bogen O, Levine JD. Role of Kv4.3 in Vibration-Induced Muscle Pain in the Rat. J Pain 2016; 17: 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibration-induced chronic musculoskeletal pain analyzed in the rat. J Pain 2010; 11: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Duan KZ, Xu Q, Zhang XM, Zhao ZQ, Mei YA, Zhang YQ. Targeting A-type K(+) channels in primary sensory neurons for bone cancer pain in a rat model. Pain 2012; 153: 562–574. [DOI] [PubMed] [Google Scholar]
- [12].Fecho K, Manning EL, Maixner W, Schmitt CP. Effects of carrageenan and morphine on acute inflammation and pain in Lewis and Fischer rats. Brain Behav Immun 2007; 21: 68–78. [DOI] [PubMed] [Google Scholar]
- [13].Forouharmajd F, Yadegari M, Ahmadvand M, Forouharmajd F, Pourabdian S. Hand-arm Vibration Effects on Performance, Tactile Acuity, and Temperature of Hand. J Med Signals Sens 2017; 7: 252–260. [PMC free article] [PubMed] [Google Scholar]
- [14].Handford M, Lepine K, Boccia K, Ruddick F, Alyeksyeyeva D, Thompson A, Holness DL, Switzer-McIntyre S. Hand-arm vibration syndrome: Workers’ experience with functional impairment and disability. J Hand Ther 2017; 30: 491–499. [DOI] [PubMed] [Google Scholar]
- [15].Hayashi Y, Takimoto K, Chancellor MB, Erickson KA, Erickson VL, Kirimoto T, Nakano K, de Groat WC, Yoshimura N. Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of Kv1.4 alpha-subunit in rats with cystitis. Am J Physiol Regul Integr Comp Physiol 2009; 296: R1661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].House R, Wills M, Liss G, Switzer-McIntyre S, Lander L, Jiang D. The effect of hand-arm vibration syndrome on quality of life. Occup Med (Lond) 2014; 64: 133–135. [DOI] [PubMed] [Google Scholar]
- [17].Huda R, Solanki DR, Mathru M. Inflammatory and redox responses to ischaemia/reperfusion in human skeletal muscle. Clin Sci (Lond) 2004; 107: 497–503. [DOI] [PubMed] [Google Scholar]
- [18].Isami K, Imai S, Sukeishi A, Nagayasu K, Shirakawa H, Nakagawa T, Kaneko S. The impact of mouse strain-specific spatial and temporal immune responses on the progression of neuropathic pain. Brain Behav Immun 2018; 74: 121–132. [DOI] [PubMed] [Google Scholar]
- [19].Khan J, Noboru N, Imamura Y, Eliav E. Effect of Pregabalin and Diclofenac on tactile allodynia, mechanical hyperalgesia and pro inflammatory cytokine levels (IL-6, IL-1β) induced by chronic constriction injury of the infraorbital nerve in rats. Cytokine 2018; 104: 124–129. [DOI] [PubMed] [Google Scholar]
- [20].Kim DS, Choi JO, Rim HD, Cho HJ. Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res Mol Brain Res 2002; 105: 146–152. [DOI] [PubMed] [Google Scholar]
- [21].Krajnak K, Dong RG, Flavahan S, Welcome D, Flavahan NA. Acute vibration increases alpha2C-adrenergic smooth muscle constriction and alters thermosensitivity of cutaneous arteries. J Appl Physiol (1985) 2006; 100: 1230–1237. [DOI] [PubMed] [Google Scholar]
- [22].Krajnak K, Waugh S. Systemic Effects of Segmental Vibration in an Animal Model of Hand-Arm Vibration Syndrome. J Occup Environ Med 2018; 60: 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krajnak K, Waugh S, Miller GR, Johnson C. Recovery of vascular function after exposure to a single bout of segmental vibration. J Toxicol Environ Health A 2014; 77: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Langeslag M, Malsch P, Welling A, Kress M. Reduced excitability of gp130-deficient nociceptors is associated with increased voltage-gated potassium currents and Kcna4 channel upregulation. Pflugers Arch 2014; 466: 2153–2165. [DOI] [PubMed] [Google Scholar]
- [25].Li N, Lu ZY, Yu LH, Burnstock G, Deng XM, Ma B. Inhibition of G protein-coupled P2Y2 receptor induced analgesia in a rat model of trigeminal neuropathic pain. Mol Pain 2014; 10: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain 2007; 8: 127–136. [DOI] [PubMed] [Google Scholar]
- [27].Ma X, Wehland M, Aleshcheva G, Hauslage J, Waßer K, Hemmersbach R, Infanger M, Bauer J, Grimm D. Interleukin-6 expression under gravitational stress due to vibration and hypergravity in follicular thyroid cancer cells. PLoS One 2013; 8: e68140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Manjavachi MN, Motta EM, Marotta DM, Leite DF, Calixto JB. Mechanisms involved in IL-6-induced muscular mechanical hyperalgesia in mice. Pain 2010; 151: 345–355. [DOI] [PubMed] [Google Scholar]
- [29].Matoba T, Kuwahara H. Treatments for hand-arm vibration disease in Japan. Kurume Med J 1990; 37 Suppl: S123–6. [DOI] [PubMed] [Google Scholar]
- [30].Meiri N, Sun MK, Segal Z, Alkon DL. Memory and long-term potentiation (LTP) dissociated: normal spatial memory despite CA1 LTP elimination with Kv1.4 antisense. Proc Natl Acad Sci U S A 1998; 95: 15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods 1994; 32: 197–200. [DOI] [PubMed] [Google Scholar]
- [32].Park SY, Choi JY, Kim RU, Lee YS, Cho HJ, Kim DS. Downregulation of voltage-gated potassium channel alpha gene expression by axotomy and neurotrophins in rat dorsal root ganglia. Mol Cells 2003; 16: 256–259. [PubMed] [Google Scholar]
- [33].Phusuntornsakul P, Jitpukdeebodintra S, Pavasant P, Leethanakul C. Vibration enhances PGE. J Periodontol 2018; 89: 1131–1141. [DOI] [PubMed] [Google Scholar]
- [34].Pravitharangul A, Suttapreyasri S, Leethanakul C. Iliac and mandible osteoblasts exhibit varied responses to LMHF vibration. Cell Biol Int 2018; 42: 1349–1357. [DOI] [PubMed] [Google Scholar]
- [35].Quarta S, Vogl C, Constantin CE, Üçeyler N, Sommer C, Kress M. Genetic evidence for an essential role of neuronally expressed IL-6 signal transducer gp130 in the induction and maintenance of experimentally induced mechanical hypersensitivity in vivo and in vitro. Mol Pain 2011; 7: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Radwin RG, Armstrong TJ, Vanbergeijk E. Vibration exposure for selected power hand tools used in automobile assembly. Am Ind Hyg Assoc J 1990; 51: 510–518. [DOI] [PubMed] [Google Scholar]
- [37].Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A 2001; 98: 13373–13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sakai A, Saitow F, Maruyama M, Miyake N, Miyake K, Shimada T, Okada T, Suzuki H. MicroRNA cluster miR-17–92 regulates multiple functionally related voltage-gated potassium channels in chronic neuropathic pain. Nat Commun 2017; 8: 16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shen SC, House RA. Hand-arm vibration syndrome: What family physicians should know. Can Fam Physician 2017; 63: 206–210. [PMC free article] [PubMed] [Google Scholar]
- [40].Shill DD, Polley KR, Willingham TB, Call JA, Murrow JR, McCully KK, Jenkins NT. Experimental intermittent ischemia augments exercise-induced inflammatory cytokine production. J Appl Physiol (1985) 2017; 123: 434–441. [DOI] [PubMed] [Google Scholar]
- [41].Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain 2008; 135: 98–107. [DOI] [PubMed] [Google Scholar]
- [42].Takeda M, Tanimoto T, Nasu M, Matsumoto S. Temporomandibular joint inflammation decreases the voltage-gated K+ channel subtype 1.4-immunoreactivity of trigeminal ganglion neurons in rats. Eur J Pain 2008; 12: 189–195. [DOI] [PubMed] [Google Scholar]
- [43].Taylor W, Wasserman D, Behrens V, Reynolds D, Samueloff S. Effect of the air hammer on the hands of stonecutters. The limestone quarries of Bedford, Indiana, revisited. Br J Ind Med 1984; 41: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tsantoulas C, McMahon SB. Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci 2014; 37: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].U.S. General Services Administration. Fact Sheet: Occupational Exposure to Hand-Arm Vibration (HAV). Available at: https://www.gsa.gov/cdnstatic/Hand-Arm_Vibration_Syndrome_01-06-2016.pdf. Accessed 11 March, 2019.
- [46].Welc SS, Clanton TL. The regulation of interleukin-6 implicates skeletal muscle as an integrative stress sensor and endocrine organ. Exp Physiol 2013; 98: 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wells JE, Rose ET, Rowland KC, Hatton JF. Kv1.4 subunit expression is decreased in neurons of painful human pulp. J Endod 2007; 33: 827–829. [DOI] [PubMed] [Google Scholar]
- [48].Xu GY, Winston JH, Shenoy M, Yin H, Pasricha PJ. Enhanced excitability and suppression of A-type K+ current of pancreas-specific afferent neurons in a rat model of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 2006; 291: G424–31. [DOI] [PubMed] [Google Scholar]
- [49].Yang EK, Takimoto K, Hayashi Y, de Groat WC, Yoshimura N. Altered expression of potassium channel subunit mRNA and alpha-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neuroscience 2004; 123: 867–874. [DOI] [PubMed] [Google Scholar]
- [50].Ye Y, Mauro M, Bovenzi M, Griffin MJ. Association between vasoconstriction during and following exposure to hand-transmitted vibration. Int Arch Occup Environ Health 2014; 87: 41–49. [DOI] [PubMed] [Google Scholar]
- [51].Zemel BM, Ritter DM, Covarrubias M, Muqeem T. A-Type K. Front Mol Neurosci 2018; 11: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, Ye DW, Tian YK. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation 2016; 13: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhu H, Han C, Lu D, Wu T. miR-17–92 cluster promotes cholangiocarcinoma growth: evidence for PTEN as downstream target and IL-6/Stat3 as upstream activator. Am J Pathol 2014; 184: 2828–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhu Y, Colak T, Shenoy M, Liu L, Mehta K, Pai R, Zou B, Xie XS, Pasricha PJ. Transforming growth factor beta induces sensory neuronal hyperexcitability, and contributes to pancreatic pain and hyperalgesia in rats with chronic pancreatitis. Mol Pain 2012; 8: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]


