Abstract
Background:
Hepatic ischemia-reperfusion injury (IRI) is a severe complication in liver transplantation, hepatectomy and hemorrhagic shock. As neuropeptides transmit the regulatory signal between nervous-immune systems communication, our previous study documented that pituitary adenylate cyclase-activating polypeptides (PACAP) depressed hepatic TLR4 immune response in liver IRI.
Methods:
Here, we focused on how PACAP suppressed hepatocellular damage and enhanced hepatocyte regeneration in a murine model of partial liver warm IRI.
Results:
Yes-associated protein (YAP), a cellular modulator of tissue regeneration, was readily induced in WT IR-livers. As its induction was failed in PACAP-deficient livers, PACAP supplement enhanced YAP expression in WT mouse, promoted its nuclear translocation and downstream anti-oxidative/regenerative genes expression both in vivo and in vitro. Further, verteporfin (VP), a YAP transcriptional inhibitor, abolished PACAP-mediated hepatoprotection significantly. Meanwhile, blockade of PKA-CREB signaling recreated liver damage in PACAP-protected liver, as well as impeded stimulation on YAP and its downstream gene expressions. Consistently, inhibition of PKA-CREB decreased PACAP promoted YAP expression in primary hepatocytes culture, and made them vulnerable to H2O2 stress in vitro. In addition, lysophosphatidic acid (LPA), another Hippo pathway inhibitor, failed to affect PACAP-mediated hepatoprotection or hepatocellular YAP induction. This implies that PACAP regulated YAP through PKA-CREB pathway at the transcriptional level rather than canonical hippo pathway.
Conclusion:
Our study discovered the neural modulation of PACAP-YAP axis in hepatic cytoprotection and homeostasis in liver IRI. These reveal a novel insight of neuropeptide PACAP in combating liver IRI in clinical patients.
Introduction
Hepatic ischemia-reperfusion injury remains the major problem in liver transplantation, hepatic resection and hemorrhagic shock.1–3 Indeed, IRI often leads to primary graft non function, which may predispose to late chronic rejection, and contributes to a shortage of organs available for transplantation.1–3 Our group was among the first to document that neuropeptide pituitary adenylate cyclase-activating polypeptides (PACAP) protected liver from IRI by modulating innate immune response.4
The nervous system communicates with immune system extensively to maintain homeostasis. When innate immunity recognizes the pathogen-associated molecular patterns (PAMPs),5,6 it produces inflammatory mediators, which transfer the peripheral signal to brainstem and anterior hypothalamus.7–10 Systemic neuroendocrine hypothalamus augments immunoreaction, while efferent vagus nerve optimizes the inflammation.7–10 PACAP is from vasoactive intestinal peptide (VIP)-glucagon-secretin family.11 Our previous study revealed that PACAP diminished TLR4 macrophage activation through cAMP-PKA pathway in liver IRI.3 Several studies revealed that PACAP may protect kidney cell and retinal pigment epithelial cells against oxidation in vitro.12–14 As three of PACAP receptors (VPAC1, VPAC2, and PAC1) are expressed on hepatocytes,15 we noticed that PACAP diminished hepatocellular necrosis and apoptosis in liver IRI,4 however, the underlying mechanism of PACAP on hepatocyte survival remained mystery.
The Yes-associated protein (YAP), one of the key components of the mammalian Hippo pathway, is critical in cell proliferation and survival.16,17 Translocating from cytoplasm to nucleus, YAP exerts its transcriptional function through TEA domain family (TEAD) transcriptional factors and followed repair/regeneration related genes, such as cyclin E, CTGF and FGF.16 Recent study found that acute activation of YAP de-differentiated adult hepatocytes into cells bearing progenitor characteristics in vivo.18 These hepatocyte-derived progenitor cells demonstrated self-renewal and engraftment capacity at the single cell level.18 Over-expression of hepatic YAP triggered massive hepatomegaly (4-fold increase in liver size) within 4 weeks.18
In this study, we elucidated that the emerging PACAP-YAP axis amplified hepatic regeneration/homeostasis in liver IRI. It warrants pivotal evaluation of neural immunomodulation as a potential treatment concept for liver IRI.
Materials and Methods
Murine partial liver IRI model.
C57BL/6 mice (WT, Charles River, San Diego, CA) and PACAP-deficient mice3 (C57BL/6 background) were used in an established murine model of partial warm liver IRI (70%).19, 20 In general, the arterial/portal vessels to the cephalad lobes were clamped for 90min. A single dose of PACAP38 (5nmol/mouse i.v., Sigma-Aldrich, St. Louis, MO) or YAP inhibitor verteporfin (VP; 20nmol/mouse i.v., Sigma-Aldrich) in PBS were given at 1h before ischemia. Some groups were infused with cAMP-PKA inhibitor H89 (20nmol/mouse i.v., Sigma-Aldrich) or CBP-CREB interaction inhibitor (20nmol/mouse i.v., MilliporeSigma, Billerica, MA) in dimethyl sulfoxide (DMSO). Mice were sacrificed at 6h of reperfusion; liver and serum samples were collected for analysis. All animal experiments were approved by UCLA Animal Research Committee.
Alanine aminotransferase (ALT).
Serum and culture medium ALT levels were measured for hepatocellular damage by ALT kit (Thermo Fisher Scientific, Chino, CA).
Histology.
Hematoxylin and eosin (H&E) stained liver specimens (4μm) were analyzed blindly in 10 high-power fields (HPFs).
Western blots.
Liver nuclear and cytoplasmic proteins were prepared by using Nuclear and Cytoplasmic Extraction kit (Thermo Fisher Scientific), and then separated by gel electrophoresis and transferred to nitrocellulose membrane. Anti-mouse YAP and GAPDH mAbs (Cell Signaling Technology) were used for probe.
Quantitative RT-PCR.
Platinum SYBR kit (Thermo Fisher Scientific) was used on QuantStudio3 (Thermo Fisher Scientific). The sequences of YAP, MnSOD, catalase, GSHPxs, ctgf and cyr61 primers were shown (Table S1). Gene expression was calculated by 2^(-Delta Ct). The ratio of target gene induction to housekeeping gene HPRT was calculated.
Caspase-3 activity assay.
Liver tissue samples were assessed by Caspase-3 cellular activity kit (MilliporeSigma).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL).
DNA fragmentation detection kit (MilliporeSigma) was used for TUNEL staining. TUNEL-positive cells were counted in 10 HPF/section under light microscopy.
Cell cultures.
After in situ collagenase digestion, mouse primary hepatocytes were separated by Percoll density centrifugation, and cultured for 24h. After pretreatment with PACAP (10nM), VP (20nM), 1-oleoyl lysophosphatidic acid (LPA, 100nM, Tocris Bioscience, Minneapolis, MN), H89 (10μM), or CREB inhibitor (10μM) for 1h, (PBS or DMSO as vehicle control), hepatocyte necrosis was triggered by hydrogen peroxide (H2O2, 0.1mM, Sigma-Aldrich).
Immunofluorescence.
Liver specimens and cultured hepatocytes were stained with YAP mAb (D8H1X, Cell Signaling Technology, Danvers, MA) followed with Alexa Fluor 594 donkey anti-rabbit (Thermo Fisher Scientific), Alexa Fluor 488 phospho-CREB mAb (Ser133, Cell Signaling Technology), and DAPI (Cell Signaling Technology). Liver superoxide were detected with ROS-sensing dye dihydroethidium (DHE; AnaSpec, Fremont, CA).
Statistical analysis.
All the data were presented as mean ± standard deviation, analyzed by Student’s-t test and ANOVA test. The Chi-square test was used to measure the correlation in categorical data. P<0.05 was considered significant.
Results
PACAP promoted YAP expression in liver IRI
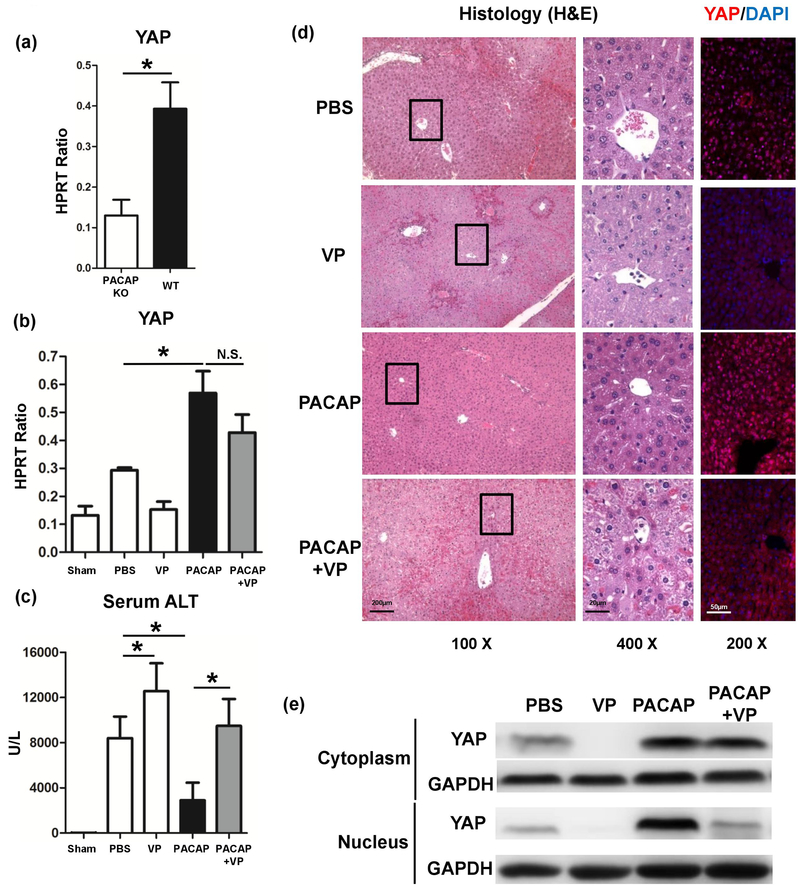
As exacerbated liver IRI was observed in PACAP KO littermates,3 we measured the YAP expression in these IR livers. Intriguingly, IR insult failed to induce YAP in PACAP KO livers, compared with WT group, (Fig. 1a: p<0.001), whereas PACAP therapy raised YAP mRNA level and nuclear translocation strongly in WT IR-livers (Fig. 1b, 1d, 1e: p<0.001). It indicated that PACAP is critical for YAP induction, while exogenous PACAP treatment could enhance YAP expression in liver IRI.
Fig. 1.
Liver samples were retrieved from B6 mice that were either sham operated or subjected to 90 minutes of partial warm ischemia, followed by 6h of reperfusion. (a) YAP expression in PACAP KO or WT IR livers. In some groups, WT mice were pretreated with PACAP, verteporfin (VP), PACAP+VP or PBS. Liver samples were analyzed for (b) YAP expression, (c) serum ALT level and (d) liver histology (representative H&E staining and immunofluorescence staining (YAP (red) and nuclear (DAPI, blue)); magnification: x100: scale bar represents 200μm; x400: scale bar represents 20μm; and x200: scale bar represents 50μm) (*p<0.001; n=10–12/group). (e) Western blots measured cytoplasmic and nuclear YAP and GAPDH in liver samples.
PACAP-induced YAP is critical for hepatocellular protection in liver IRI
To examine whether PACAP-mediated YAP is indispensable for abolishing liver IR-damage, we infused mice with verteporfin (VP), which blocking YAP-TEAD binding and suspending YAP transcription. VP recreated hepatocellular damage in PACAP-conditioned mice: increased sALT level (Fig. 1c: 9491±2379 vs. 2896±1567 U/L, p<0.001) and damaged liver architecture (widespread congestion, edema and necrosis), compared with PACAP-treated group (Fig. 1d). Of note, as VP mainly disrupted the YAP-TEAD binding, it did suppress YAP nuclear translocation (Fig. 1d, 1e), but not mRNA level (Fig. 1b, p=0.052). These data strengthened that PACAP-induced YAP is critical for hepatic protection in liver IRI.
PACAP-mediated YAP prevented hepatocyte death in IR injury
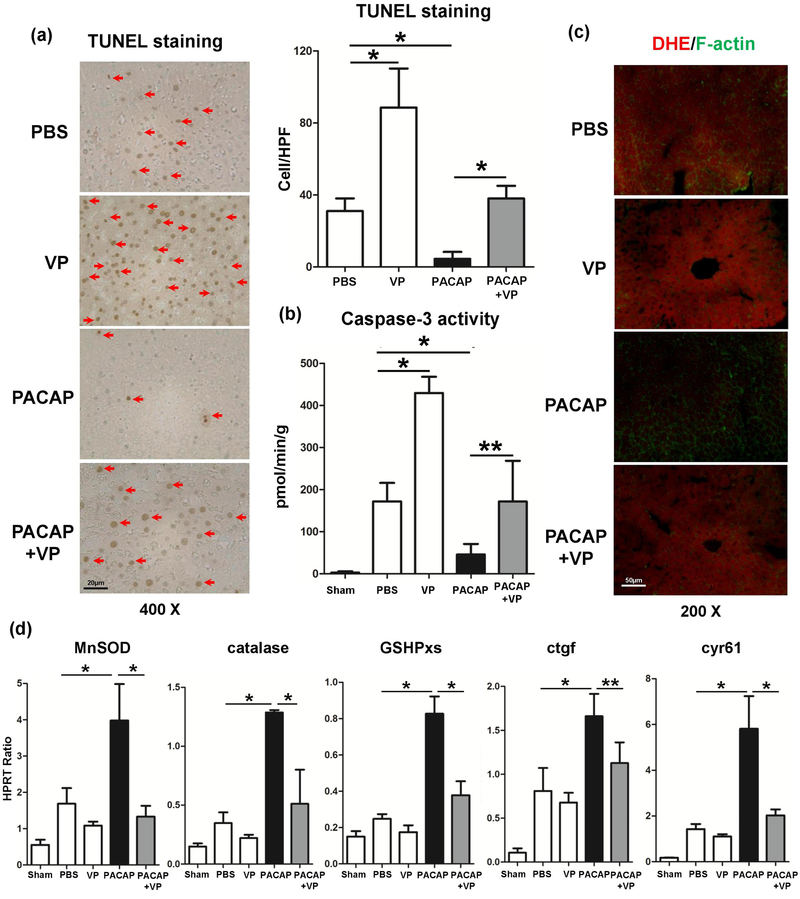
VP supplement abrogated PACAP-suppressed hepatocellular necrosis/apoptosis as evidenced by accumulation of TUNEL+ cells (Fig. 2a) and caspase-3 activity (Fig. 2b). Next, we analyzed the superoxide levels in hepatic tissue by using ROS-sensing dye dihydroethidium (DHE). Unlike IR-stressed control livers characterized by abundant ROS levels, these treated with PACAP showed weak DHE staining, barely oxidized by superoxide (Fig. 2c: massive red fluorescence vs. light red fluorescence, respectively). However, PACAP+VP treated liver tissues remained strong red fluorescence, indicating ROS accumulation (Fig. 2c). We then measured YAP-regulated downstream gene expression profile (anti-oxidative gene: MnSOD, catalase and GSHPxs; regenerative gene: ctgf and cyr61). These genes were uniformly elevated following PACAP treatment, and suppressed by VP supplement in liver IRI (Fig. 2d). This indicated that YAP was essential in PACAP mediated hepatocyte survival during IRI.
Fig. 2.
Necrosis/apoptosis and oxidative stress in IR livers (6 hours of reperfusion after 90 minutes of ischemia). (a) TUNEL-assisted detection of hepatic necrosis/apoptosis (red arrows) in ischemic liver lobes (magnification: x400, scale bar represents 20μm); (b) Caspase-3 activity; (c) ROS-sensing dye dihydroethidium (DHE) staining (strong red fluorescence indicating massive ROS production in PBS, VP and PACAP+VP groups, magnification: x200, scale bar represents 50μm); (d) qRT-PCR assisted detection of MnSOD, catalase, GSHPxs, ctgf and cyr61 (*p<0.001; **p<0.01; n=6–12/group).
PACAP facilitated YAP induction and downstream genes in hepatocyte
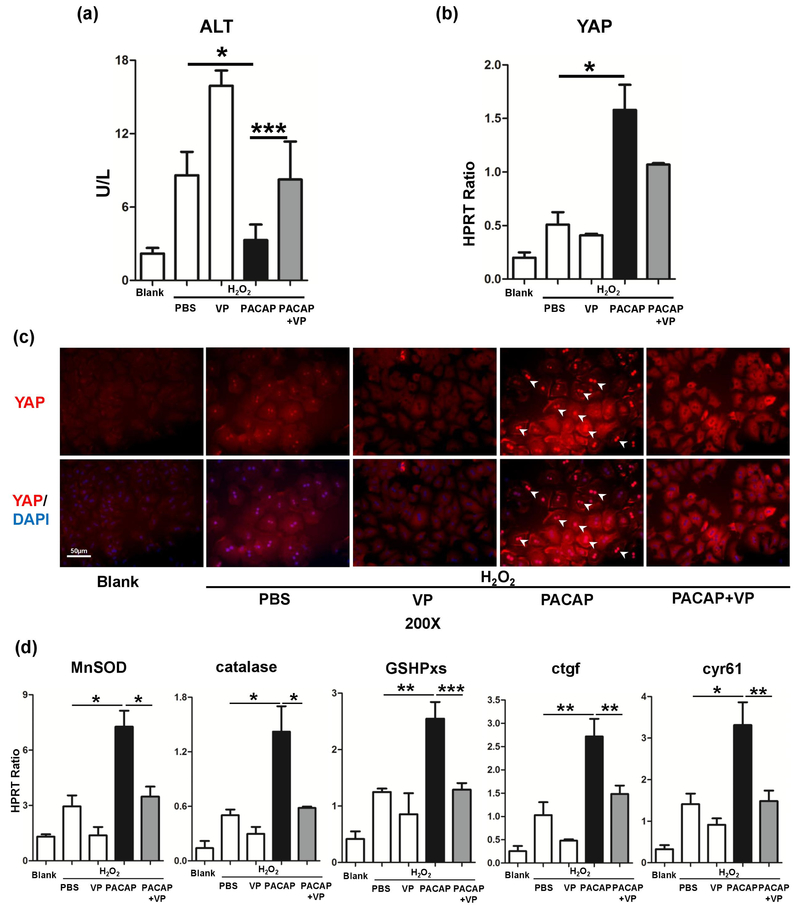
Primary hepatocytes were extracted from WT mice, and stimulated with H2O2. PACAP therapy diminished H2O2 stress induced hepatocyte damage (Fig. 3a: ALT 3.31±1.26 vs. 8.60±1.91 U/L, p<0.001). Consistent with in vivo results, PACAP dramatically promoted the YAP protein level in hepatocytes (Fig. 3b) and drove its nucleus accumulation, as shown by immunofluorescence image (Fig. 3c: bright red fluorescence in nucleus, indicated by white arrow). As PACAP significantly up-regulated YAP expression in H2O2 stressed hepatocytes, this nuclear YAP accumulation was mainly derived from newly transcripted YAP mRNA. In analogy with our in vivo finding, VP significantly neutralized PACAP-mediated cytoprotection in vitro (Fig. 3a: ALT 8.75±1.81 vs. 3.31±1.26 U/L, p<0.001). Interestingly, PACAP treatment not only enhanced YAP accumulation and nuclear translocation, but also promoted its function by elevating downstream gene expressions in H2O2-stressed hepatocytes. Here, PACAP significantly enhanced the induction of anti-oxidative genes (MnSOD, catalase and GSHPxs) and regenerative genes (ctgf and cyr61) (Fig. 3d). By specifically disrupting YAP-TEAD binding, VP significantly impaired these PACAP-elevated gene expressions (Fig. 3d).
Fig. 3.
PACAP-induced YAP expression and its downstream genes in primary hepatocyte culture in vitro. Hepatocytes were pretreated with PBS, PACAP or VP 1 hour prior to H2O2 stress. (a) Supernatant ALT level; (b) YAP expression; (c) representative immunofluorescence staining of YAP (red) and nuclear (DAPI, blue): white arrow indicated YAP accumulation in nucleus (magnification: x200, scale bar represents 50μm); (d) qRT-PCR assisted detection of MnSOD, catalase, GSHPxs, ctgf and cyr61 gene expressions (*p<0.001; **p<0.01; n=6/group).
PACAP promoted YAP function via PKA
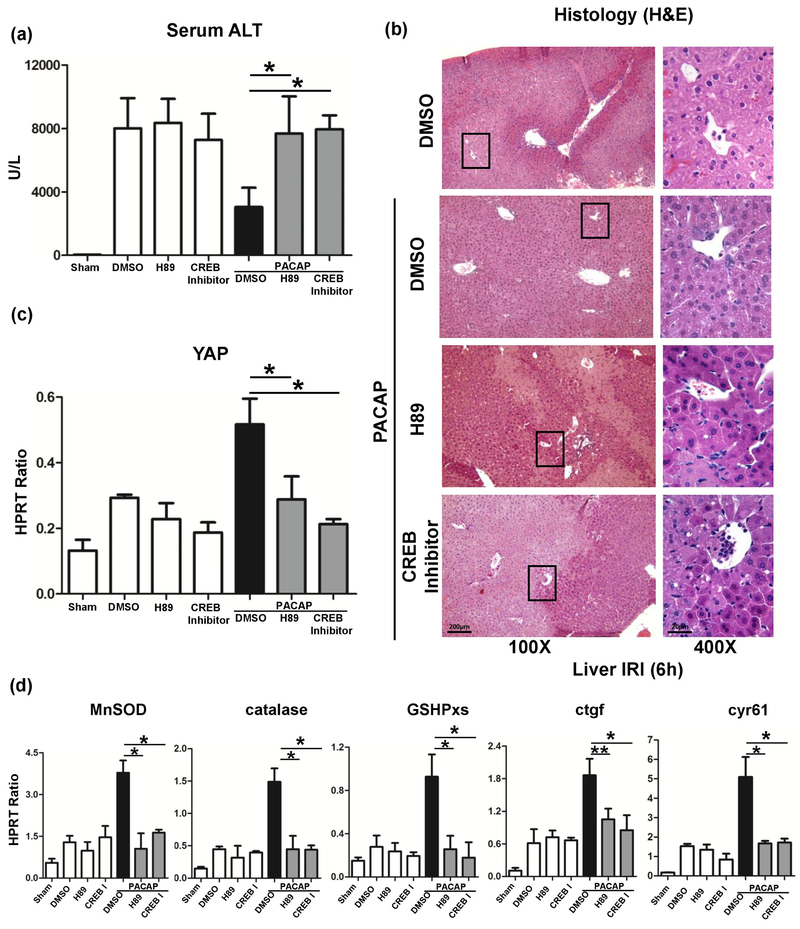
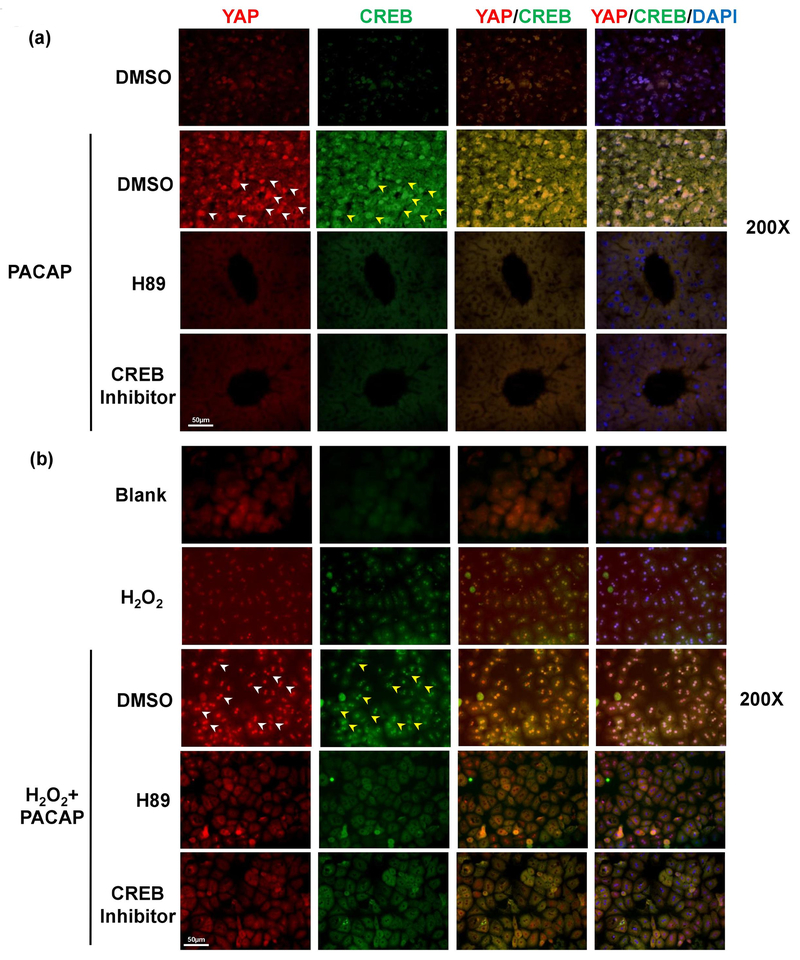
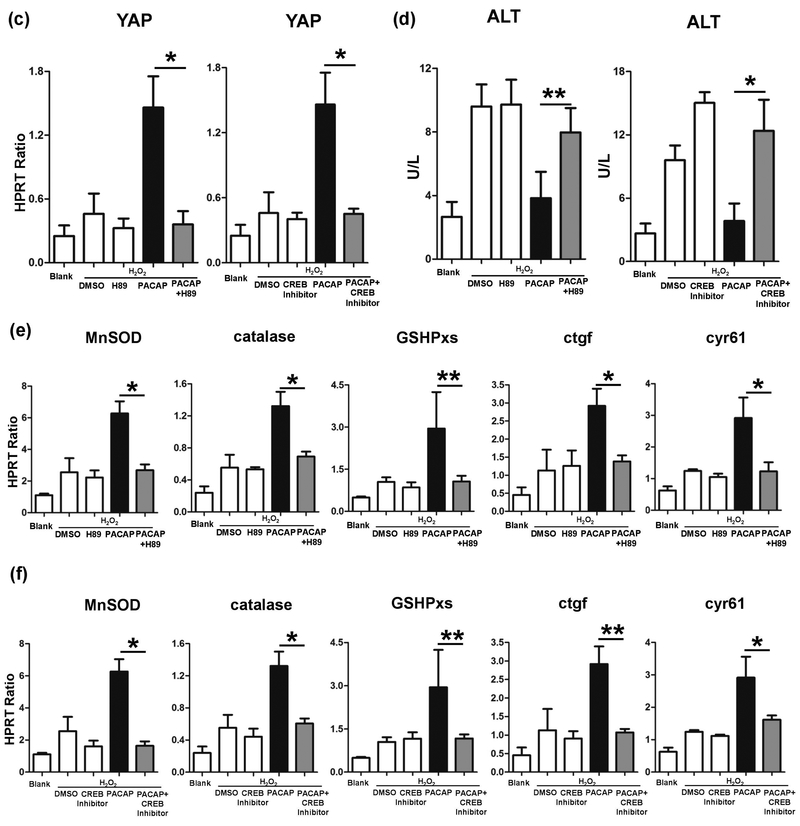
Previously, we have documented that PACAP-activated cAMP-PKA signaling protected liver against IR injury.3 Here, we employed H89, a PKA specific inhibitor, which restored liver IR-damage in PACAP-protected liver (Fig. 4a, 4b: sALT 7681±2347 [PACAP+H89] vs. 3044±1222 [PACAP], p<0.001). Strikingly, H89 suppressed PACAP-mediated YAP expression (Fig. 5a: light red fluorescence vs. strong red fluorescence in PACAP group, indicated by white arrow) and its downstream anti-oxidative/regenerative gene inductions in liver IRI (Fig. 4c, d). In H2O2-stressed primary hepatocyte cultures, H89 also abolished nucleus YAP accumulation/translocation (Fig. 5b: dense red fluorescence in PACAP group, indicated by white arrow), decreased YAP gene expression (Fig. 5c), increased cell damage (Fig. 5d: ALT releasing: 7.97±1.93 [PACAP+H89] vs. 3.83±1.66 [PACAP] U/L, p<0.01), and decreased cytoprotection (Fig. 5e). It indicated that PACAP regulated hepatocellular YAP via PKA in liver IRI.
Fig. 4.
Effect of PKA-CREB inhibition on PACAP-mediated hepatic protection in liver IRI. PACAP, H89, CREB inhibitor or DMSO pretreated WT mice were subjected to 90 minutes ischemia. Liver samples were analyzed at 6 hours of reperfusion for (a) serum ALT level; (b) liver histology (representative H&E staining; magnification: x100, scale bar represents 200μm and x400, scale bar represents 20μm); (c) YAP expression; and (d) downstream gene expressions (*p<0.001; **p<0.01; n=6/group).
Fig. 5.
Effect of PKA-CREB inhibition on PACAP-mediated hepatocellular protection. Primary hepatocytes were pretreated with PACAP, H89, CREB inhibitor or DMSO 1 hour prior to H2O2 stress. Representative immunofluorescence staining of YAP (red), CREB (green) and nuclear (DAPI, blue): white arrow pointed at nuclear YAP accumulation, yellow arrow for nuclear CREB accumulation (magnification: x200, scale bar represents 50μm) in (a) liver IRI and (b) primary hepatocytes culture; (c) YAP expression; (d) supernatant ALT level; (e, f) downstream gene expressions (*p<0.001; **p<0.01; n=6/group).
PACAP regulated YAP via PKA-CREB at the transcriptional level
CRE-binding protein (CREB) could be phosphorylated by activated PKA and then translocated into nucleus. We employed a specific CREB inhibitor to test how PACAP-PKA-CREB axis modulates YAP function. PACAP treatment promoted hepatocytes nucleus YAP accumulation/translocation (Fig. 5a, 5b: strong red fluorescence indicated by white arrow), and enhanced CREB activation (Fig. 5a, 5b: dense green fluorescence indicated by yellow arrow) in IR-insulted livers and H2O2-stressed primary hepatocytes cultures. However, CREB inhibitor supplement not only restrained nucleus CREB accumulation, but also arrested YAP expression in PACAP treated hepatocytes (Fig. 5a–c). In analogy with H89 effect, CREB inhibition recreated IR-mediated liver damage in PACAP treated animals (Fig. 4a, b: sALT 7958±875 [PACAP+CREB inhibitor] vs. 3044±1222 [PACAP], p<0.001). Furthermore, CREB inhibition diminished PACAP-induced YAP gene induction (Fig. 5a–c), increased cell damage (Fig. 5d: ALT releasing), and abolished its downstream protective gene expressions (Fig. 5e, f). Thus, PACAP regulated YAP expression via PKA-CREB signaling and mostly through direct transcriptional regulation by CREB.
PACAP elicited YAP by bypassing canonical Hippo pathway
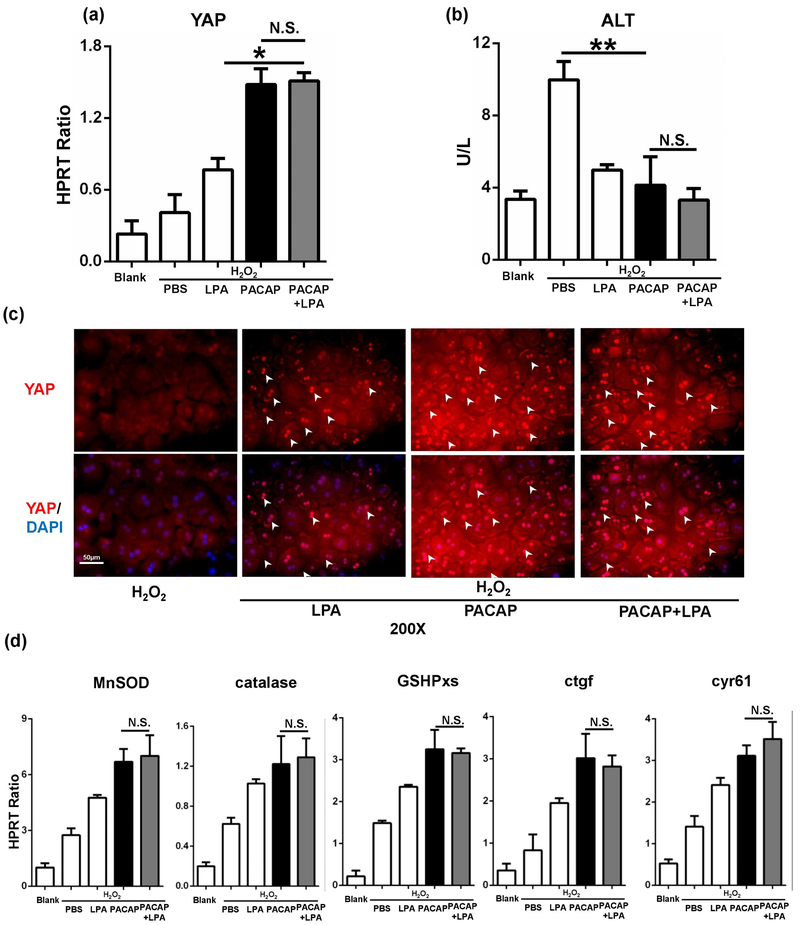
PKA may inhibit YAP function by activating the canonical Hippo pathway.16 To test the effect of PACAP on canonical Hippo pathway, we then employed lysophosphatidic acid (LPA) to inhibit Lats1, a key Hippo pathway component. Surprisingly, LPA treatment had no effect on PACAP-mediated YAP (Fig. 6a, p=0.96) or downstream gene expressions (Fig. 6d), and also failed to aggravate PACAP-mediated cytoprotection in H2O2-stressed hepatocytes (Fig. 6b: ALT releasing, p=0.45). Besides, inhibiting of canonical Hippo pathway had no effect on PACAP-mediated YAP accumulation/translocation in nucleus (Fig. 6c, white arrow). This indicated that PACAP induced YAP function may not through canonical Hippo pathway.
Fig. 6.
Effect of LPA on PACAP-mediated YAP regulation. Primary hepatocytes were pretreated with PACAP, LPA or PBS 1 hour prior to H2O2 stress. (a) YAP expression; (b) supernatant ALT level; (c) representative immunofluorescence staining of YAP (red) and nuclear (DAPI, blue): white arrow indicated YAP accumulation in nucleus (magnification: x200, scale bar represents 50μm); (d) downstream gene expressions (*p<0.001; **p<0.01; n.s.: nonsense; n=6/group).
Discussion
Previously, we have shown that PACAP inhibited innate immune response to protect liver from IR injury. We noticed that PACAP might improve hepatocyte survival in liver IRI, however, the underlying mechanism remains mystery. This study has discovered that: 1/ IR triggered YAP in WT IR-livers rather than PACAP KO; 2/ PACAP treatment amplified YAP expression/function and promoted anti-oxidative/regenerative gene inductions in hepatocytes, which is essential for PACAP-mediated cytoprotection in liver IR injury; and 3/ PACAP enhanced YAP function through PKA-CREB pathway at the transcriptional level, but not canonical Hippo pathway in liver IRI.
YAP is one of the key components of Hippo pathway, which is required for hepatocyte survival and bile duct development in mouse liver.21 In upstream Hippo pathway, Mst1/2 and Lats1/2 facilitated YAP phosphorylation, inhibited its translocation from cytoplasm to nucleus, and further negatively regulated YAP function.22 Transient activation of YAP is proved to promote tissue repair and regeneration in the context of injury, as evidenced in mouse models of myocardial infarction and colitis.23,24 YAP is also induced in liver after hepatectomy, which could facilitate liver regeneration under the control of hedgehog.25 At physiological condition, healthy adult hepatocyte displayed very low YAP level and the signal is diffused throughout the cell.26 We confirmed this low level of YAP in sham mouse livers. Interestingly, IR-mediated stress mildly promoted the YAP expression in our mouse model, and the effect lasting through 24h after reperfusion (self-repair phase). As YAP expression is minimal in PACAP KO mice, PACAP monotherapy significantly enhanced YAP expression in WT livers subjected to IR stress. This is consistent with PACAP-mediated liver cytoprotection against IR-induced tissue damage. Further, YAP inhibition, by a specific chemical inhibitor (VP), restored IR injury in PACAP-protected livers. As VP selectively blocked YAP binding TEAD, it did not affect PACAP-mediated YAP gene expression in liver IRI.
In liver IRI, neutrophils first respond to parenchymal hypoxia, and generate ROS to promote hepatocellular necrosis.2,27 Inflammatory cytokine TNF-α, produced by macrophages could activate downstream procaspase 3, and leaded to hepatocyte apoptosis, featured as cellular shrinkage, chromatin condensation and apoptotic body formation.2,28 Indeed, PACAP diminished hepatocellular necrosis/apoptosis: decreased TUNEL+ cell number, suppressed caspase-3 activity and abolished ROS production (DHE staining) in IR-livers. In parallel, YAP-promoted anti-oxidant gene expressions (MnSOD, GSHPxs) were all significantly up-regulated in PACAP-treated group. Conversely, YAP inhibition diminished anti-oxidant gene expressions, exacerbated hepatodestruction, and promoted oxidative stress-mediated hepatocellular death in PACAP-treated IR livers. It is plausible that YAP function is essential for PACAP-mediated neural modulation.
We have reported that PACAP augmented macrophage cAMP/PKA to diminish destructive innate immune reactions.4 PKA phosphorylates CREB at Ser133 and facilitated its translocation to nucleus.29,30 As a transcriptional factor, phosphorylated CREB recruits CBP and p300 to mediate downstream gene expressions.31,32 Our results showed that PACAP activated CREB via PKA, as indicated by CREB accumulation in nucleus after PACAP therapy. In human liver cancer tissue and cell lines (HepG2), CREB promoted YAP transcriptional output by binding 608/439 region on YAP promoter.32 Our results indicated that either PKA or CREB inhibitor could interrupt PACAP-mediated YAP expression and accumulation in the nucleus. Inhibition of PKA-CREB totally reversed PACAP-mediated liver protection and blocked anti-oxidative and regenerative gene expressions. YAP transcriptional activity inhibitor VP abolished PACAP-hepatoprotection in liver IR injury, but failed to suppress YAP mRNA level significantly, which further documented that PACAP regulated YAP mainly at transcriptional level through PKA-CREB axis.
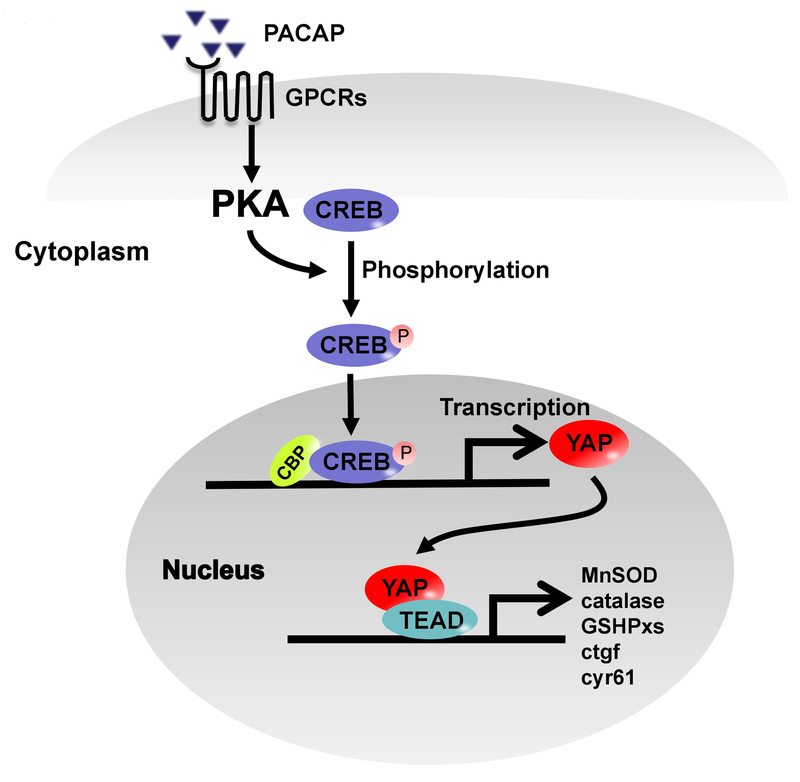
PKA may abolish YAP function by activating Lats1/2.16,33 We used LPA, a Lats1/2 inhibitor to screen how Hippo pathway affected PACAP-mediated YAP function in our model. Interestingly, LPA supplement had no effect on YAP expression, nucleus accumulation and downstream gene expressions in PACAP-pretreated mice. This revealed a composited regulation of PACAP on YAP at the level of transcription and post translation. Due to the relative low YAP protein level in primary hepatocytes, PACAP acts on YAP mainly through PKA-CREB signaling at the transcriptional level but not the canonical Hippo pathway (Fig. 7). Our pilot data indicated that PACAP ameliorated liver IRI and prolonged graft survival in a clinically-relevant mouse liver transplant model subjected to extended period of cold storage (4°C in University of Wisconsin solution for 20 h) (data not shown). As YAP activation in liver could de-differentiate adult hepatocytes and trigger massive hepatomegaly,18 PACAP-promoted YAP function may reveal a complete novel strategy to improve hepatocellular function against liver IRI in transplant recipients.
Fig. 7.
Scheme of molecular mechanisms of PACAP enhanced YAP expression/function through PKA-CREB signaling. PACAP binding to its receptors on hepatocytes triggers PKA-CREB pathway, which directly promotes YAP transcription, and downstream anti-oxidative/regenerative programs (MnSOD, catalase, GSHPxs, ctgf and cyr61).
In conclusion, current study documented the neural immunomodulation of PACAP-mediated YAP signaling in hepatic homeostasis and cytoprotection in liver IRI. PACAP monotherapy promoted hepatocellular YAP expression/function and downstream anti-oxidative/regenerative gene expressions, maintained hepatic homeostasis, and protected liver from IR injury. By activating PKA-CREB signaling pathway, PACAP enhanced YAP function at the transcriptional level but not via canonical Hippo pathway. Considering its metabolic property and action on both cytoprotection and immunosuppression, PACAP should be considered as a novel candidate agent in organ preservation and marginal graft recovery in future clinical trials.34
Supplementary Material
Funding:
The research has been supported by grants from : NIH R21 AI122155 and AI138165 (HJ), NIH PO1 AI120944, RO1 DK107533, DK102110, and DK062357 (JWKW), and The Dumont Research Foundation.
Abbreviations:
- ctgf
connective tissue growth factor
- FGF
fibroblast growth factors
- GSHPxs
glutathione peroxidase
- LPA
lysophosphatidic acid
- MnSOD
manganese superoxide dismutase
- PACAP
Pituitary Adenylate Cyclase-Activating Polypeptides
- PKA
protein kinase A
- TEAD
TEA domian family
- YAP
Yes-associated protein
Footnotes
Disclosure: The authors declare that they have no conflict of interest.
References
- 1.Li CX, Man K, Lo CM. The Impact of Liver Graft Injury on Cancer Recurrence Posttransplantation. Transplantation. 2017;101(11):2665–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu L, Zhou H, Ni M, et al. Innate Immune Regulations and Liver Ischemia-Reperfusion Injury. Transplantation. 2016;100(12):2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kan C, Ungelenk L, Lupp A, et al. Ischemia-Reperfusion Injury in Aged Livers-The Energy Metabolism, Inflammatory Response, and Autophagy. Transplantation. 2018;102(3):368–377. [DOI] [PubMed] [Google Scholar]
- 4.Ji H, Zhang Y, Shen XD, et al. Neuropeptide PACAP in mouse liver ischemia and reperfusion injury: immunomodulation by the cAMP-PKA pathway. Hepatology. 2013;57(3):1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–787. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Yue S, Ke B, et al. Nuclear factor erythroid 2-related factor 2 regulates toll-like receptor 4 innate responses in mouse liver ischemia-reperfusion injury through Akt-forkhead box protein O1 signaling network. Transplantation. 2014;98(7):721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goehler LE, Gaykema RP, Hansen MK, et al. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85(1–3):49–59. [DOI] [PubMed] [Google Scholar]
- 8.Emch GS, Hermann GE, Rogers RC. TNF-alpha activates solitary nucleus neurons responsive to gastric distension. Am J Physiol Gastrointest Liver Physiol. 2000;279(3):G582–G586. [DOI] [PubMed] [Google Scholar]
- 9.Steinman L Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5(6):575–581. [DOI] [PubMed] [Google Scholar]
- 10.Brogden KA, Guthmiller JM, Salzet M, et al. The nervous system and innate immunity: the neuropeptide connection. Nat Immunol. 2005;6(6):558–564. [DOI] [PubMed] [Google Scholar]
- 11.Arimura A Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48(5):301–331. [DOI] [PubMed] [Google Scholar]
- 12.Ferencz A, Racz B, Tamas A, et al. Influence of PACAP on oxidative stress and tissue injury following small-bowel autotransplantation. J Mol Neurosci. 2009;37(2):168–176. [DOI] [PubMed] [Google Scholar]
- 13.Horvath G, Brubel R, Kovacs K, et al. Effects of PACAP on oxidative stress-induced cell death in rat kidney and human hepatocyte cells. J Mol Neurosci. 2011;43(1):67–75. [DOI] [PubMed] [Google Scholar]
- 14.Fabian E, Reglodi D, Mester L, et al. Effects of PACAP on intracellular signaling pathways in human retinal pigment epithelial cells exposed to oxidative stress. J Mol Neurosci. 2012;48(3):493–500. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen TD, Heintz GG, Wolfe MS. Structural characterization of PACAP receptors on rat liver plasma membranes. Am J Physiol. 1993;265(5 Pt 1):G811–G818. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Kim M, Lee S, et al. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32(11):1543–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao B, Li L, Guan KL. Hippo signaling at a glance. J Cell Sci. 2010;123(Pt 23):4001–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chestovich PJ, Uchida Y, Chang W, et al. Interleukin-22: implications for liver ischemia-reperfusion injury. Transplantation. 2012;93(5):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Xue Z, Zhang C, et al. Inhibition of Cyclin-dependent Kinase 2 Signaling Prevents Liver Ischemia and Reperfusion Injury. Transplantation. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang N, Bai H, David KK, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19(1):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13(1):63–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao D, Zhai P, Del Re DP, et al. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun. 2004;5:3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai J, Zhang N, Zheng Y, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24(21):2383–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swiderska-Syn M, Xie G, Michelotti GA, et al. Hedgehog regulates Yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64(1):232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yimlamai D, Christodoulou C, Galli GG, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157(6):1324–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palumbo T, Nakamura K, Lassman C, et al. Bruton Tyrosine Kinase Inhibition Attenuates Liver Damage in a Mouse Warm Ischemia and Reperfusion Model. Transplantation. 2017;101(2):322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi Y, Matsumura F, Liang J, et al. Neutrophil elastase and oxygen radicals enhance monocyte chemoattractant protein- expression after ischemia/reperfusion in rat liver. Transplantation. 1999;68(10):1459–1468. [DOI] [PubMed] [Google Scholar]
- 29.Delghandi MP, Johannessen M, Moens U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal. 2005;17(11):1343–1351. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa H, Jin MB, Ogata T, et al. Role of cyclic nucleotides in ischemia and reperfusion injury of canine livers. Transplantation. 2002;73(7):1041–1048. [DOI] [PubMed] [Google Scholar]
- 31.Naemi FM, Carter V, Kirby JA, et al. Anti-donor HLA class I antibodies: pathways to endothelial cell activation and cell-mediated allograft rejection. Transplantation. 2013;96(3):258–266. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Ma L, Weng W, et al. Mutual interaction between YAP and CREB promotes tumorigenesis in liver cancer. Hepatology. 2013;58(3):1011–1020. [DOI] [PubMed] [Google Scholar]
- 33.Yu FX, Zhang Y, Park HW, et al. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27(11):1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsung A, Nace GW, Geller DA. Pituitary adenylate cyclase-activating polypeptide: a neuromodulator of hepatic ischemia-reperfusion injury? Hepatology. 2013;57(3):878–880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.