Abstract
Adenomatous polyposis coli (APC) and KRAS proto-oncogene (KRAS) mutations frequently co-occur in non-small cell lung cancer. Inactivating APC mutations in colorectal carcinoma has been well characterized, leading to the approaches targeting on dysregulated APC pathway. However, it remains undetermined whether such approaches are also applicable to non-small cell lung cancer patients harboring similar mutations of APC. Dickkopf-related protein 2 (DKK2) is a Wnt antagonist. Our previous study has proved that anti-DKK2 antibody 5F8 suppressed the growth of colorectal carcinoma with APC mutations, illustrating a new target agent of APC-mutated tumors. This study aimed to investigate the potential of applying anti-DKK2 antibody to non-small cell lung cancer with APC mutations. We found significant upregulation of Dkk2 expression in APC-mutated lung cancers. Administration of DKK2 antibody inhibited cancer growth via modulating tumor immune microenvironment in lung cancer mouse models. Our study provided strong evidence supporting APC mutations-directed applications of anti-DKK2 targeted therapy in a wide range of cancer types, including lung cancer.
Subject terms: Tumour immunology, Lung cancer
Introduction
Lung cancer is one of the leading causes of cancer-related death, resulting in more than 1 million deaths worldwide annually1,2. Lung cancer can be histologically classified into four major categories: lung adenocarcinoma, squamous cell carcinoma (SCC), large cell carcinoma, comprising non-small cell lung cancer (NSCLC), and small cell3–9 carcinoma of the lung. Among these categories, NSCLC is more common (~80%). The top recurrent mutated genes in NSCLC include KRAS proto-oncogene (KRAS). It was reported that activating KRAS mutations were present in about 4% of SCC and 30% of adenocarcinoma10,11.
In human, a wide range of cellular processes are under the control of Wnt signaling pathways12–17. Briefly, the canonical Wnt/β-catenin pathway is initiated by two types of cell-surface receptors: low-density lipoprotein receptor-related proteins 5 and 6 (LRP5 and LRP6, respectively), and Frizzled proteins18. Dysregulation of Wnt signaling contributes to many human diseases, including cancer12–15. Adenomatous polyposis coli (APC) is one of the key components in canonical Wnt/β-catenin pathway19,20. APC mutations, primarily nonsense mutations and frameshift insertions and deletions encoding truncated proteins, were subsequently identified in a majority of sporadic colorectal adenomas and carcinomas21,22. Somatic APC mutations have furthermore been described in several other solid tumors, most prominently in gastric and pancreatic cancers22–24. In 2004, APC mutations have been reported in lung NSCLC and small-cell lung carcinoma25. Interestingly, APC mutations in lung cancer co-occurred with KRAS mutations in NSCLC, including adenocarcinoma and SCCs26.
Direct pharmacological strategies targeting KRAS-driven cancers are not clinically available27,28. However, the fact that APC and KRAS mutations frequently co-occur in NSCLC provides therapeutic opportunities to target altered APC pathway in such group of patients. Oncogenic APC mutations have been well characterized in colorectal carcinoma because they are dominant mutations that drive the development of colorectal cancer. Numerous therapeutically strategies targeting altered Wnt pathway in APC mutant colorectal cancers are under preclinical and clinical studies, including ours29. These approaches are potentially applicable to lung cancer patients harboring inactivating mutations of APC28.
Dickkopf-related protein 2 (DKK2)14,30, one of the Wnt antagonists, inhibits Wnt-β-catenin signaling by binding to LRP5 and LRP631. DKK2 has a less critical role in vertebrate development and adult life32–34. DKK2 deficiency leads to decreased blood glucose35 and a moderate reduction in bone mass32. In our recent study, we uncovered a function of DKK2 in promoting tumor progression by suppressing cytotoxic immune cell activation in colorectal carcinoma with APC mutations29. The loss of APC in intestinal tumor cells upregulated the expression of DKK2 through β-catenin pathway, which, together with its receptor LRP5, provided an unconventional mechanism for tumor immune evasion. The monoclonal antibody-mediated ablation of DKK2 impeded tumor progression and enhanced the effects of PD-1 blockade. This antibody presented a promising therapeutic effect on APC-mutated colorectal cancer29.
In the present study, we applied anti-DKK2 antibody to lung cancer mouse models driven by APC mutation. We found that anti-DKK2 antibody suppressed the tumor growth. We also found significant upregulation of Dkk2 expression in human lung cancer tissues with APC mutations. Taken together, we illustrated a new targeted strategy for NSCLC with APC mutations.
Results
Upregulation of DKK2 expression in human NSCLC with Apc mutation
Data on Dkk2 RNA expression of NSCLC were obtained from the TCGA data sets from OncoLnc. Two groups were separated according to the mutation status of APC: APCwt, and APCmutated groups. APC mutation includes 9 deep deletion, 17 truncating mutation, 34 missense mutation, and 1 inframe mutation. Upregulation of Dkk2 expression is found in APCmutated group compared with APCwt group (Fig. 1a). This suggests that DKK2 might have an important role in these NSCLCs with APC mutation. Our previous data indicated that gain-of-function mutations of PI3K also is linked to elevated Dkk2 expression29. Then, the APCwt group was further divided into APCwt with mutated PI3CA, and APCwt with wt PI3CA in lung SCC. The RNA level of Dkk2 is only associated with APC mutation status, excluding the PI3CA mutation status (Fig. 1b). Furthermore, analysis of the TCGA data sets showed that high Dkk2 expression correlates with poor survival rates (Fig. 1c). These results indicated loss of APC upregulate the expression of DKK2 in human NSCLC.
Fig. 1. High DKK2 expression is present in human non-small cell lung cancers with APC mutation and predicts poor survival.
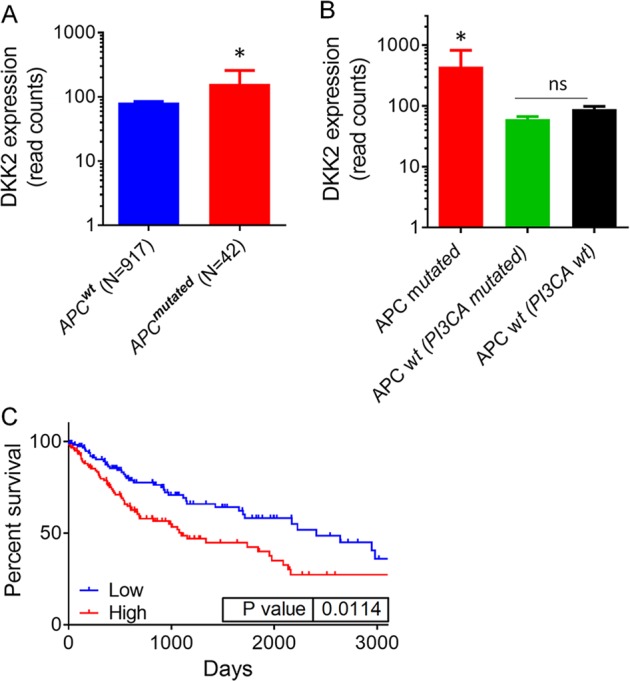
a Analysis of DKK2 expression in APCwt, and APCmutated non-small cell lung cancer groups. Data on DKK2 expression were obtained from the TCGA data sets from OncoLnc, then separate them into two groups according to the mutation status of APC. APC mutation includes 9 Deep Deletion, 17 Truncating Mutation, 34 Missense Mutation, and 1 Inframe Mutation. DKK2 read counts are RSEM RNA SeqV2 normalized. b Further divided APCwt group into two groups according to mutation status of PI3CA mutation in lung squamous cell carcinoma. c Analysis of the correlation of DKK2 expression and survival. Data on DKK2 expression, overall survival were obtained from the TCGA data sets from OncoLnc. The high and low DKK2 expressers were grouped on the basis an arbitrary cutoff percentile of 25%. Mantel–Cox log-rank tests were done using the GraphPad Prism 7 software. (*p < 0.05; **p < 0.01)
DKK2 is highly upregulated in KrasLSL-G12D; Apcfl/fl mouse lung tumor tissues
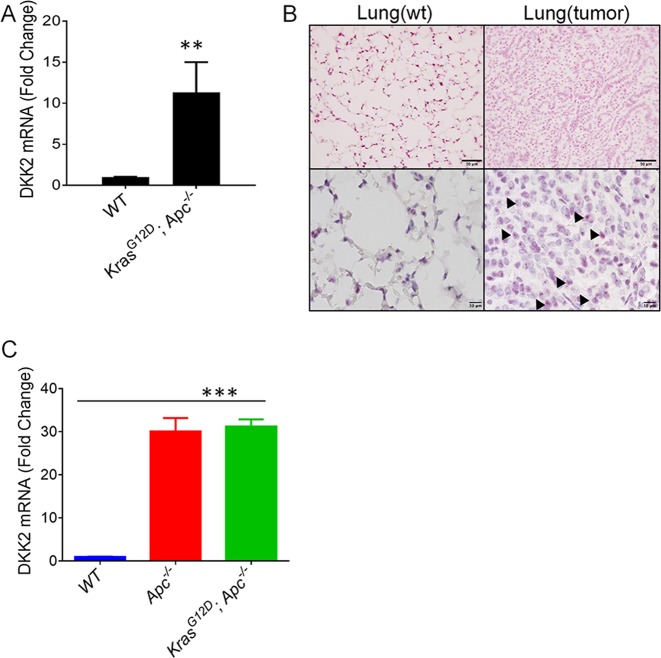
After KrasLSL-G12D; Apcfl/fl mice were generated, we administrate Cre adenovirus by nasal injection to induce mouse lung cancer progression. Lung tissues from WT and KrasLSL-G12D; Apcfl/fl were harvested for RNA extraction. Real-time PCR showed that Dkk2 expression was significantly upregulated in mouse lung cancer compared with normal lung tissue (Fig. 2a). Concordantly, in situ hybridization confirmed that Dkk2 expression is present in lung cancer tissues (Fig. 2b). We also isolated normal tissue or tumor organoids from WT, Apcfl/fl, and KrasLSL-G12D; Apcfl/fl mice, and performed real-time PCR. These results also indicated that loss of APC upregulates Dkk2 expression (Fig. 2c). Therefore, these results together supported the conclusion that APC loss drives DKK2 expression in both mouse and human NSCLC cells.
Fig. 2. Upregulation of DKK2 expression in KrasG12D; Apc−/− mouse lung tumor tissue.
a DKK2 RNA isolated from lung tumors from KrasG12D; Apc−/− and normal lung tissue from WT mice, respectively. DKK2 mRNA levels were determined by quantitative RT-PCR (data are presented as means ± sem; two-sided Student's t test, n = 3). b In situ hybridization to detect Dkk2 RNA expression is in lung cancer tissues and normal lung tissue. Arrow pointed DKK2 expression. Scale bars, 50 μm (top)/10 μm (bottom). c Dkk2 expression is upregulated by loss of APC in tumor organoids. Tumor organoids were isolated from WT, Apc−/−, and KrasG12D; Apc−/− mice (data are presented as means ± sem; two-sided Student's t test, n = 2). (*p < 0.05; **p < 0.01; ***p < 0.001)
Knockdown of DKK2 does not alter cell proliferation or apoptosis of LLC lung cancer cell in vitro
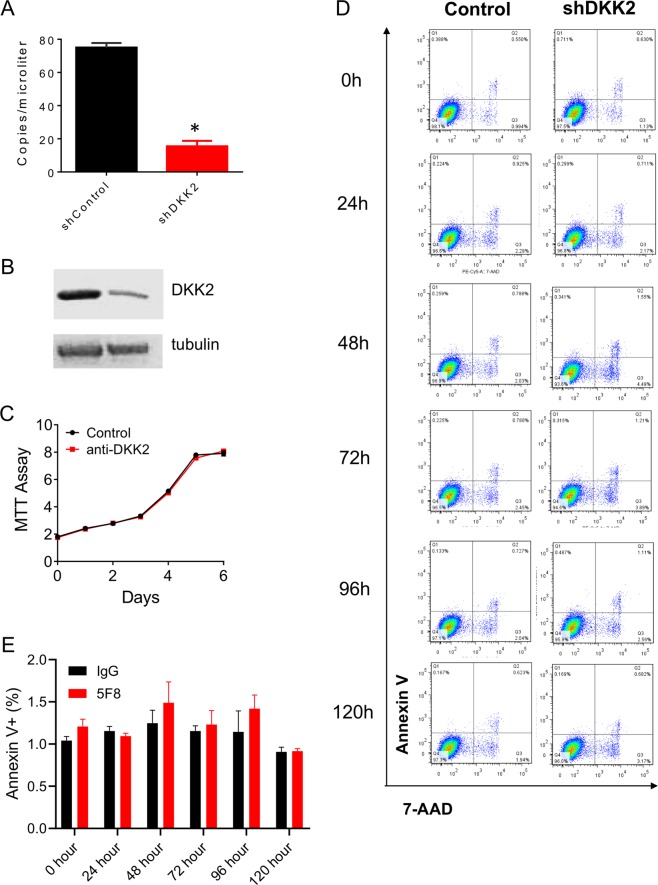
To investigate the role of DKK2 in lung cancer progression, we generated Dkk2 knockdown stable cell lines. QuantStudio™ 3D Digital PCR showed more than 80% knockdown efficiency in LLC stable cell lines (Fig. 3a), and western blot further confirmed that DKK2 protein level was downregulated (Fig. 3b). Next, we determined cell growth and apoptosis. MTT assay showed that there was no difference in cell growth after Dkk2 gene knockdown (Fig. 3c). Flow cytometry also indicated that knockdown of Dkk2 has no effect on cell apoptosis (Fig. 3d, e). These findings are consistent with our previous studies in colon cancer cell lines29, DKK2 does not affect tumor cell itself proliferation and apoptosis.
Fig. 3. DKK2 knockdown does not affect cell growth and apoptosis.
a DKK2 expression in LLC stable knockdown cells. DKK2 mRNA expression was determined by QuantStudio™ 3D Digital PCR System (data are presented as means ± sem; unpaired Student's t test, n = 3) using RNAs isolated from LLC cells with or without the shRNA. b Western analysis of DKK2 and tubulin levels were also shown. c MTT assays were performed to detect cell growth in LLC shscramble and shDKK2 cells. d Representative flow cytometry of 7-ADD and annexin V staining showed that there was no difference of cell apoptosis in shscramble and shDKK2 cells. e Quantification of annexin V+ cells. Data are presented as mean ± s.e.m. (*p < 0.05; **p < 0.01)
DKK2 knockdown inhibits tumor progression in vivo
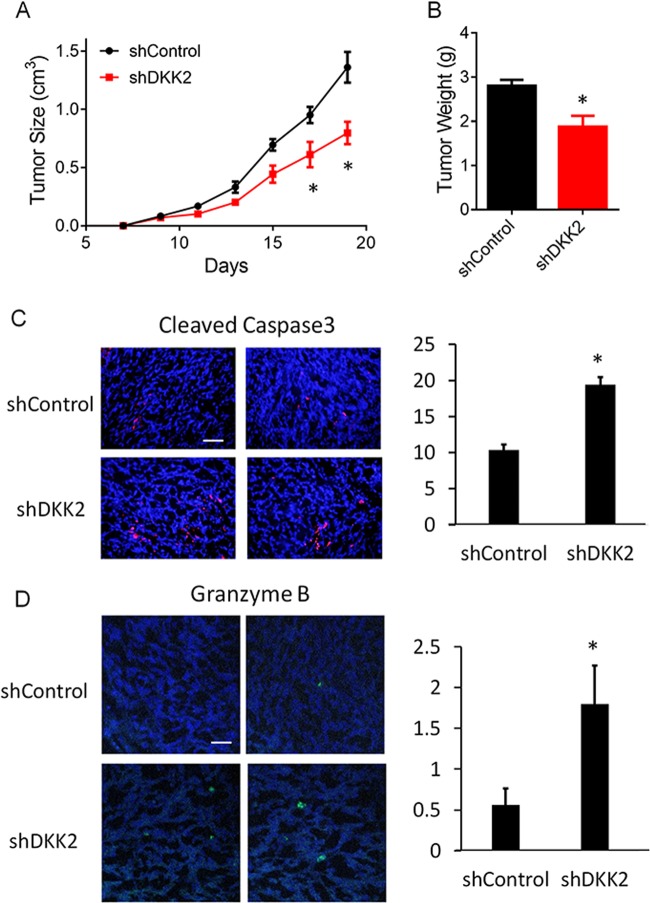
To study the effect of DKK2 on tumor progression in vivo, we performed tumor orthotopic experiment. Tumor cells with control shRNA or Dkk2 shRNA were implanted into flank of C57/BL mice. We monitored tumor size every other day, and found that the tumor burden was decreased dramatically in Dkk2 knockdown group (Fig. 4a). Tumor weight was also measured at the end-point of the day, which showed about 1/3 decrease in tumor mass (Fig. 4b). Because knockdown Dkk2 did not affect the growth of LCC cells in in vitro culture, the impaired tumor progression in the shDKK2 group might alter tumor microenvironment. Immunofluorescence staining of the LLC tumors revealed that DKK2 knockdown significantly increased the number of apoptotic cells (Fig. 4c). The cleaved caspase3 protein is a member of the cysteine-aspartic acid protease family36,37. Sequential activation of caspase3 plays a central role in the execution phase of cell apoptosis36,37. The cytotoxic granzyme B (GrB)/perforin pathway has been traditionally viewed as a primary mechanism that is used by cytotoxic lymphocytes to eliminate tumor cells36,37. Accordingly, we observed that Dkk2 knockdown increased the number of GZMB+ cells (Fig. 4d). These results implied that DKK2 may regulate the tumor immune microenvironment.
Fig. 4. LLC orthotopic tumor model on C57/BL mice with or without DKK2 knockdown.
a, b C57/BL mice inoculated with LLC shControl and shDKK2 cells. Tumors were collected at day 19 and weighed (P < 0.05 for tumor growth at days 17 and 19, respectively; two-way ANOVA; two-tailed Student’s t test for tumor weight; n = 5). c Effects of DKK2 knockdown on tumor apoptosis by staining of the sections of cleaved caspase3. The sections were also counterstained with DAPI. Five independent sections per tumor and five tumors per group were examined (unpaired Student’s t test). Scale bars, 100 μm. Quantification of cleaved caspase3 staining was shown (right). Data are presented as mean ± s.e.m. d Granzyme B staining in tumor sections from shControl and shDKK2 groups. The sections were also counterstained with DAPI. Five independent sections per tumor and five tumors per group were examined (unpaired Student’s t test). Scale bars, 100 μm. Quantification of granzyme B staining was shown (right). Data are presented as mean ± s.e.m. (*p < 0.05; **p < 0.01)
DKK2 antibody administration inhibited lung cancer growth in vivo
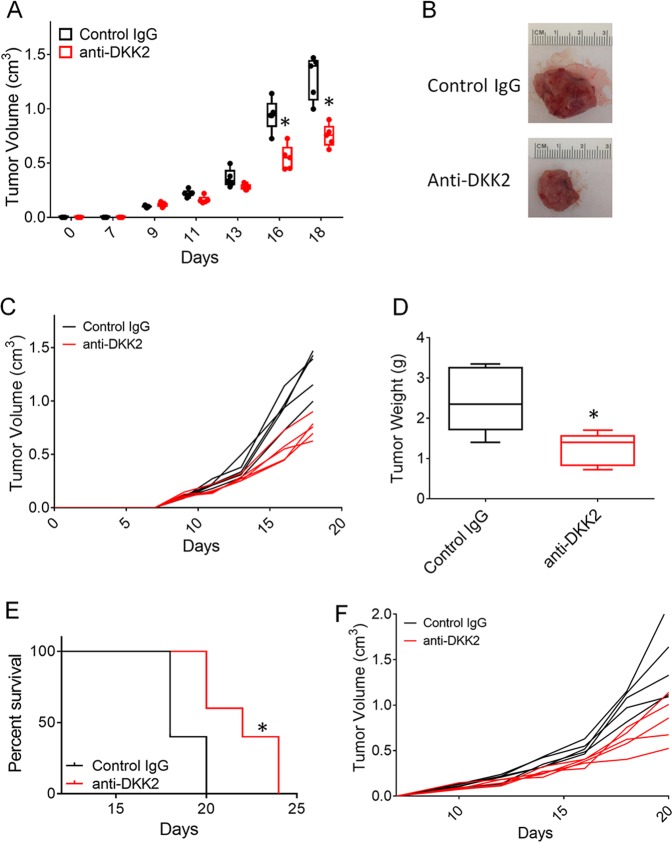
We have generated a specific monoclonal antibody (5F8) targeting DKK2 protein, which has been proved that efficiently impaired tumor progression in both colorectal cancer and melanomas29. In this study, we would like to test its antitumor effect in lung cancer mice models. The mice bearing LLC tumor cells were treated with control IgG or anti-DKK2 antibody every other day when tumor approached 200 mm3. Constant with previous study29, DKK2 blockade significantly reduced tumor burden (Fig. 5a–c). Tumor weight was cut down by 50% compared with control IgG treatment group (Fig. 5d). We also observed that the administration of anti-DKK2 antibody extended the survival of tumor-bearing mice in the LLC tumor model (Fig. 5e, f). These data supported that DKK2 could promote tumor progression.
Fig. 5. DKK2 antibody 5F8 treatment inhibited tumor growth on LLC orthotopic tumor model.
a–c C57/BL mice inoculated with LLC cells were treated with anti-DKK2 antibody (10 mg/kg i.p. every other day) starting at day 13 after inoculation. Tumors were collected at day 18 and weighed (P < 0.05 for tumor growth at days 16 and 18, respectively; two-way ANOVA; two-tailed Student’s t test for tumor weight; n = 5). a Tumor size were presented as groups of IgG control and anti-DKK2 antibody. Individual data points represent individual tumors. b Representative picture of tumor at end-point time. Individual tumor growth was shown as c. d Tumor weight at day 18. e Survival evaluation of C57/BL mice inoculated with LLC cells were treated with anti-DKK2 antibody (10 mg/kg i.p. every other day) starting at day 10 after inoculation. (P < 0.01 for anti-DKK2 antibody treated versus IgG-treated; two-sided log-rank Mantel–Cox test; n = 5). Individual tumor growth was shown as f. (*p < 0.05; **p < 0.01)
DKK2 blockade modulates tumor immune microenvironment
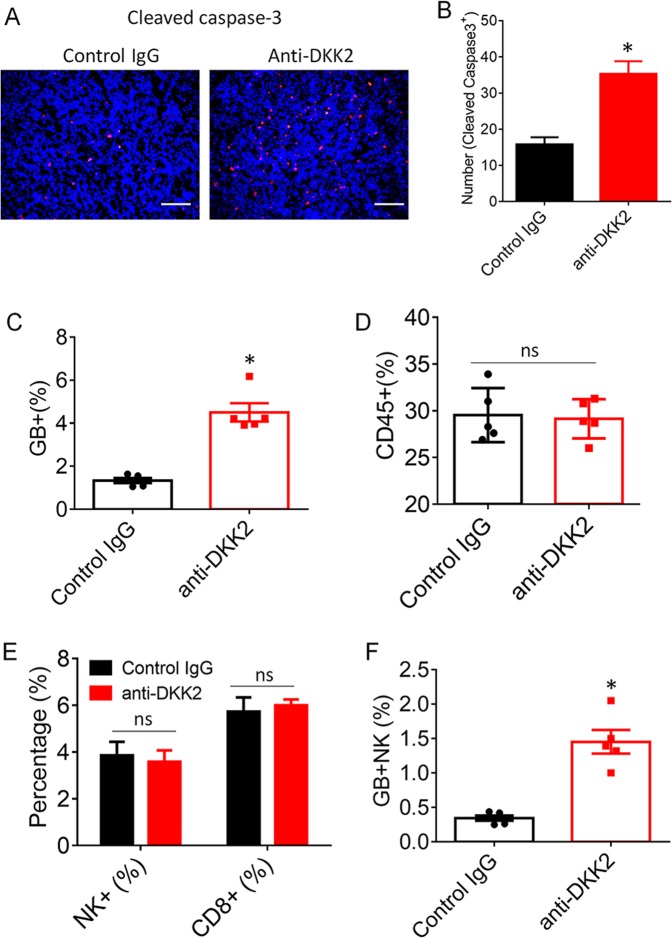
Furthermore, we performed the immunofluorescence staining of cleaved caspase3, which showed that the apoptotic cell numbers were increased to about three folds in anti-DKK2 antibody group, compared with those tumors treated with control IgG (Fig. 6a, b). Consistent with the immunostaining results, flow cytometry analysis of tumor-infiltrating lymphocytes (TILs) showed that anti-DKK2 antibody treatment increase the percentage of GZMB+ T cells (Fig. 6c). NK cells and CD8+ T lymphocytes are the cytotoxic immune cells that are capable of directly killing tumor cells through GrB/perforin pathway36,37. Although there were no differences between anti-DKK2-treated and isotype-treated samples in terms of the percentages of infiltrating TILs, CD8a+ T, and NK+ cells (Fig. 6d, e), we observed increased numbers of GZMB+NK+ cells in anti-DKK2-treated tumor samples compared with that in IgG-treated samples (Fig. 6f). These results indicate that the cytotoxic NK cells have significant roles in DKK2 blockade-mediated suppression of tumor formation. Clearly, DKK2 antibody could directly enhance activation of mouse NK cells.
Fig. 6. DKK2 antibody 5F8 treatment modulates tumor immune microenvironment.
a Effects of anti-DKK2 treatment on tumor apoptosis, and number of cleaved caspase3+ cells were evaluated by staining of the sections of tumor for cleaved caspase3. The sections were also counterstained with DAPI. Five independent sections per tumor and five tumors per group were examined (unpaired Student’s t test). Data are presented as mean ± s.e.m. All experiments were repeated twice. Scale bars, 100 μm. c–f LLC cells were injected subcutaneously into C57BL/6 mice. When tumors reached 600 mm3 on average, the mice were given one injection of antibody (10 mg/kg i.p.). Tumors were collected 24 h later for flow cytometry analysis (two-tailed Student’s t test; n = 5). Tumor-infiltrating lymphocytes are pregated as CD45+ cell from total tumor population. c Quantification of the percentage of granzyme B+ cells in tumors from IgG or 5F8-treated tumors. d Quantification of the percentage of CD45+ TILs in tumors from IgG or 5F8-treated tumors. e Quantification of the percentage of infiltrated CD8+ and NK1.1+ cells in LLC tumors from IgG or 5F8-treated tumors based on flow cytometry analysis. f Quantification of the percentage of infiltrated GB+; NK1.1+ cells. Data are presented as means ± sem (ns, not significant; n = 5; two-sided Student t test). (*p < 0.05; **p < 0.01)
Applicability of DKK2 blockade for treatment of KrasLSL-G12D; Apcfl/fl mouse lung cancer
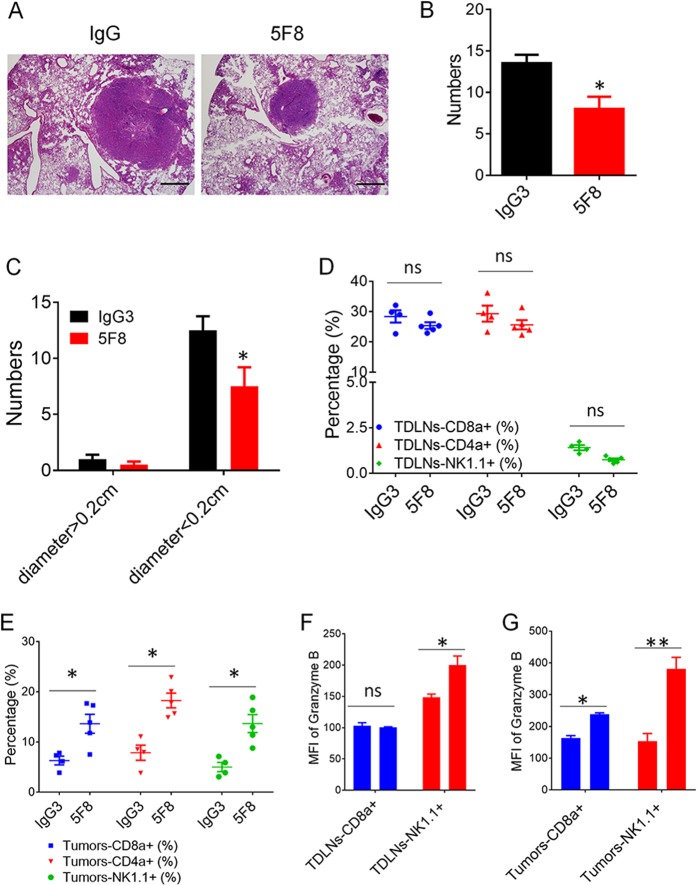
Then, we tested DKK2 blockade in a mouse lung cancer model of carrying an activating Kras mutation and loss of Apc. Treatment of these mice with anti-DKK2 antibody significantly reduced tumor number and tumor size (Fig. 7a–c), and enhanced activation of NK cells in both of tumor draining lymphoid node and tumor tissues (Fig. 7f, g). In addition, we observed that the percentage of infiltrating CD8+ T, NK+, CD4+ T cells also increased in anti-DKK2 antibody treated tumor (Fig. 7e), but no difference in tumor draining lymphoid node (Fig. 7d). The activation of infiltrating CD8+ T cells was also enhanced by anti-DKK2 antibody administration (Fig. 7g). These results, together with the presence of upregulated DKK2 expression in human NSCLCs with Apc mutation, and the correlation of high DKK2 expression with poor survival rates revealed by the TCGA suggest that DKK2 blockade may be applicable approaches for NSCLC cells harboring mutations of APC.
Fig. 7. Anti-DKK2 antibody (5F8) suppresses tumor progress and activate immune effector cells in lungs of KrasG12D; Apc−/− mice.
a–c The mice (8 weeks old) were instilled intranasally with 65 μl of MEM containing adenovirus expressing Cre (2 × 107 plaque-forming unites) and CaCl2. After 6 weeks, the mice were treated weekly with IgG or 5F8 (10 mg/Kg). After another 6 weeks, some of the lungs (n = 4 IgG and n = 4 5F8) are sectioned, and five histological sections from a lung of a representative IgG or 5F8-treated lung are also shown in a. Scale bars, 100 μm. Quantification of total tumor number were shown in b, and comparison of tumor number in IgG or 5F8 groups after separating tumor as big tumors and small tumors based on tumor diameter (0.2 cm) (c). (Data are presented as means ± sem; two-sided Student’s t test). d–f The rest of the lungs (n = 4 IgG and n = 5 5F8) were analyzed by flow cytometry (e, g). d, f Flow cytometry analysis of tumor draining lymph nodes. MFI, mean fluorescence intensity. Data are presented as means ± sem (**p < 0.01 versus IgG; n = 4–5; two-sided Student’s t test)
Discussion
In this study, we demonstrated that upregulation of DKK2 expression is present in both human NSCLC with APC mutation and mice lung tumors caused by Apc loss. We uncovered that increased DKK2 promoted lung cancer progression by modulating tumor immune microenvironment. By applying DKK2 monoclonal antibody 5F8, we observed that the 5F8 antibody had a strong tumor-suppressing effect in both orthotopic tumor model and genetically engineered mouse model of lung cancer. This study provides a novel therapeutic approach for NSCLC with APC mutation.
Wnt signaling pathways control a wide range of cellular processes16. Dysregulation of Wnt-β-catenin signaling has been found contributing to many human cancers16,17. DKK2, a Wnt antagonist, inhibits Wnt-β-catenin signaling by binding to LRP6 or LRP5. Aberrant expression of DKK2 has been detected in many tumor tissues38. Several epigenetic alterations leading to a decline in the expression of DKK2 in tumors have been reported. For example, frequent epigenetic silencing of DKK2 has been found in ovarian carcinoma39, renal cancer40, and hepatocellular carcinoma41. However, increased DKK2 expression can promote proliferation and invasion via the Wnt signaling in prostate cancer42, Ewing sarcoma43, and colorectal cancer44. DKK2 not only regulates tumor cell proliferation, invasion, and apoptosis directly40,42,43, but also modify tumor microenvironment such as angiogenesis45. Our recent studies also showed that DKK2 promotes tumor progression by suppressing cytotoxic immune cell activation in colorectal carcinoma with APC mutations29. In the current study, we expanded our findings of DKK2 functions to human NSCLC with APC mutations. Our studies indicated that DKK2 plays similar roles in both colorectal and lung cancer when they carry APC mutants. Recently developed therapies targeting signature tumor driving events are largely applicable across tissues-of-origin. For example, Herceptin was originally designed to treat HER2-amplified breast cancer, but later was also approved for HER2-positive metastatic stomach cancer. Anti-PD1/PD-L1 showed durable effects on high mutation burden tumors across multiple cancer types. Recently, larotrectinib was granted approval for the treatment of solid tumors that have a neurotrophic receptor tyrosine kinase gene fusion, regardless of tissues-of-origin. As we showed previously and in this study, DKK2 antibody suppresses tumor growth in both APC mutant NSCLC and APC mutant colorectal cancer. It is still unknown whether DKK2 plays similar roles in other cancer types with reoccurring APC mutations, such as pancreatic cancer. Such studies will further extend applications of DKK2 blockade therapies.
Immunotherapy has become the fourth pillar for various cancer46,47. Although the currently approved checkpoint inhibitors including anti-PD1/PD-L1 and anti-CTLA4 have shown clinical efficacy for some tumors, few checkpoints can be counted on our finger46,47. In patients with NSCLC, only a small proportion of patients responded to monotherapy of anti-PD-1/PD-L1. Primary and acquired resistance to anti-PD-1/PD-L1 therapies that leads to failure of the therapy has blocked most of the patients from getting clinical benefit48. Large effects are being made to develop combinational strategies to overcome resistance to checkpoint inhibitors, majorly in cancer types that are considered high immunogenic, such as melanoma, NSCLC, and colorectal cancer. Recently, we have identified DKK2 as a novel tumor immune-suppressive protein in the microenvironment in colorectal cancer carrying APC mutation. We found that DKK2 provided resistance to PD-1 blockade in mouse models of colon cancer29,49. We then developed an anti-DKK2 antibody for the immunotherapeutic treatment of colorectal cancer with APC mutaions29,49. Combinational blockade of DKK2 and PD-1 outperformed on suppression of tumor growth than single antibody treatment in APC loss colon tumors29,49. APC and KRAS mutations frequently co-occur in NSCLC. In this study, we found in NSCLC, APC mutations drive high expression of Dkk2. DKK2 blockade introduced by anti-DKK2 antibody suppressed tumor growth and enhanced the effects of cytotoxic immune cells in APC mutant NSCLC, in consistent with the previous study in colorectal cancer29. While mechanisms for loss of APC and tumor suppressor PTEN regulate DKK2 have been described in colorectal cancer and melanoma29, there are more mechanisms in lung cancer yet to be discovered. These possibilities and the therapeutic potential of Dkk2 plus PD-1/PD-L1 blockade in PDX humanized mice model need warrant future investigations.
Methods
Mice
KrasLSL-G12D (B6.129S4-Krastm4Tyj/J), and Apcfl/fl (C57BL/6-Apctm1Tyj/J) mice were acquired from the Jackson Laboratory. Wild-type C57BL/6 mice were purchased from Envigo (Harlan). Mice were housed in specific-pathogen-free conditions and cared for in accordance with the US National Institutes of Health guidelines, and all procedures were approved by the Yale University Animal Care and Use Committee.
Gene-suppression studies using shRNA
The shRNAs were cloned into pLKO.1-puro lenti-viral vector (Addgene). Viral packaging and infection of cells were performed as previously described50,51. After viral infection, cells were selected with puromycin to generate stable cell lines. At least two batches of stable cell lines were generated for each experiment. Experiments were performed in triplicates and repeated at least twice using each batch of cells. The target sequences are:
5′-CAACAAGATGAAGAGCACCAAC-3′ (shScram),
5′-CTGCAAACCAGTGCTCCATCAG-3′ (shDKK2–1),
5′-GCGGGCCAAACTCAACTCCATC-3′ (shDKK2–2),
5′-ACTCCAAGATGCCTCATATAAA-3′ (shDKK2–3).
Quantitative RT-PCR
Total RNA was prepared and retrotranscribed as described50,51. The RT-PCR primers used are: mouse DKK2: 5′-TCAACTCCATCAAGTCCTCTC-3′ (forward) and 5′-TCACATTCCTTATCACTGCTG-3′ (reverse); mouse ACTIN: 5′-cctagaagcatttgcggtgg-3′ (forward) and 5’-gagctacgagctgcctgacg-3′ (reverse).
Histology
Murine organs were fixed in phosphate-buffered 4% formaldehyde and embedded in paraffin. Three to five micrometres thick sections were stained with hematoxylin and eosin.
Immunofluorescence staining on 5-μm paraffin sections using antibodies against cleaved caspase3 (Cell Signaling, #9664), Goat anti-Rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 568 (ThermoFisher, #A-11011), and FITC anti-human/mouse Granzyme B Antibody (BioLegend, #515403) was performed as described8,51,52.
Orthotopic tumor model
LLC Lewis lung carcinoma cells (0.5 × 106) were mixed with BD Matrigel (Matrix Growth Factor Reduced) (BD, #354230) in 100 μl and injected subcutaneously into the right flanks of the backs of female C57/BL mice (8–10 weeks old). Tumor growth was measured with calipers, and size was expressed as one-half of the product of perpendicular length and square width in cubic millimeters. For antibody treatment, control IgG3 antibody and anti DKK2 were diluted in PBS, and 100 μl (250 μg) was injected intraperitoneally (i.p.) at the intervals indicated in the figures. For survival tests, mice were euthanized when the tumor size exceeded 1000 mm3.
Flow cytometry
Cells in single-cell suspension were fixed with 2% PFA (Santa-Cruz, #sc-281692). After washing with a Flow Cytometry Staining Buffer (eBioscience, #00–4222–26), cells were stained with antibodies for cell-surface marker for 1 h on ice in the dark. For staining of intracellular proteins, the cells were washed and resuspended in the permeabilization buffer (BD, #554723) and stained by antibodies in the permeabilization buffer for 1 h on ice in the dark. The cells were then pelleted and resuspended in the Flow Cytometry Staining Buffer for flow cytometry analysis. Antibodies used for flow cytometry include: V450 Mouse Anti-Mouse CD45.2 (BD Horizon, #560697, Clone 104), APC anti-mouse NK1.1 Antibody (BioLegend, #108710, clone PK136), FITC anti-human/mouse Granzyme B Antibody (BioLegend, #515403, clone GB11), CD8a Monoclonal Antibody (53–6.7) PE-Cyanine7 (eBioscience, #25–0081–82, clone 53–6.7), CD4 Monoclonal Antibody (RM4–5), and PE (eBioscience, #12–0042–82, clone RM4–5).
In situ hybridization
In situ hybridization detection of DKK2 mRNA was carried out using the following reagents acquired from Advanced Cell Diagnostics, Inc., based on provided protocol: RNAscope® Target Retrieval Reagents (Cat #322000), RNAscope® Pretreat Reagents- H202 and ProteasePlus (Cat #322330), RNAscope® 2.5 HD Detection Reagent- RED (Cat #322360), RNAscope® Wash Buffer Reagents (Cat #310091), BioCare EcoMount (Cat #320409), ImmECatdgeTM Hydrophobic Barrier Pen (Cat #310018), and the mouse Dkk2 probe (#404841).
Mouse model of lung cancer
KrasLSL-G12D (B6.129S4-Krastm4Tyj/J), and Apcfl/fl (C57BL/6-Apctm1Tyj/J) mice were back crossed to C57/Bl background three generations, then KrasLSL-G12D mice were crossed with Apcfl/fl mice. For de novo lung cancer mice model, 8-week-old KrasLSL-G12D; Apcfl/fl mice were treated with 2 × 106 plaque-forming unites of adeno-Cre at 6–8 weeks of age as previously described8,51,52. Six weeks later, KrasLSL-G12D; Apcfl/fl mice were randomly divided into two groups (8–9 for each group) for intraperitoneal injection with either control IgG antibody or anti-DKK2 antibody every other day for another 6 weeks. Four pairs of mice were used for gross inspection and histopathological examination, and the rest of mice in each group were used for flow cytometry analysis.
Statistical analysis and study design
Minimal group sizes for tumor progression studies were determined via power calculations with the DSS Researcher’s Toolkit with an α of 0.05 and power of 0.8. Animals were grouped unblinded, but randomized, and investigators were blinded for the qualification experiments. No samples or animals were excluded from analysis. Assumptions concerning the data including normal distribution and similar variation between experimental groups were examined for appropriateness before statistical tests were conducted. Comparisons between two groups were performed by unpaired, two-tailed t-test. Comparisons between more than two groups were performed by one-way ANOVA, whereas comparisons with two or more independent variable factors were performed by two-way ANOVA followed by Bonferroni’s post hoc correction using Prism 6.0 software (GraphPad). Statistical tests were done with biological replicates. P < 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
This work was supported by the National Cancer Institute, Grant #1R13CA236284–01, and the National Natural Science Foundation of China, Grant #81770521.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by I. Amelio
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tianli Shen, Zhengxi Chen
Contributor Information
Xuejun Sun, Email: sunxy@mail.xjtu.edu.cn.
Qian Xiao, Email: qian.xiao@yale.edu.
Supplementary information
The online version of this article (10.1038/s41420-019-0204-4) contains supplementary material, which is available to authorized users.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis, C. E., Miller, K. D., Goding Sauer, A., Jemal, A. & Siegel, R. L. Cancer statistics for African Americans, 2019. CA: Cancer J. Clin.69, 211–233 (2019). [DOI] [PubMed]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao YJ, et al. LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc. Natl. Acad. Sci. USA. 2010;107:18892–18897. doi: 10.1073/pnas.1004952107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han, X. K. et al. Transdifferentiation of lung adenocarcinoma in mice with Lkb1 deficiency to squamous cell carcinoma. Nat. Commun.5, 10.1038/ncomms4261 (2014). [DOI] [PMC free article] [PubMed]
- 10.Suda K, Tomizawa K, Mitsudomi T. Biological and clinical significance of KRAS mutations in lung cancer: an oncogenic driver that contrasts with EGFR mutation. Cancer Metastasis Rev. 2010;29:49–60. doi: 10.1007/s10555-010-9209-4. [DOI] [PubMed] [Google Scholar]
- 11.Guin S, et al. Contributions of KRAS and RAL in non-small-cell lung cancer growth and progression. J. Thorac. Oncol.: Off. Publ. Int. Assoc. Study Lung Cancer. 2013;8:1492–1501. doi: 10.1097/JTO.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 13.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat. Rev. Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Q, Chen ZX, Jin XZ, Mao RY, Chen ZQ. The many postures of noncanonical Wnt signaling in development and diseases. Biomed. Pharmacother. 2017;93:359–369. doi: 10.1016/j.biopha.2017.06.061. [DOI] [PubMed] [Google Scholar]
- 17.Liu SH, et al. miR-221/222 activate the Wnt/beta-catenin signaling to promote triple-negative breast cancer. J. Mol. Cell Biol. 2018;10:302–315. doi: 10.1093/jmcb/mjy041. [DOI] [PubMed] [Google Scholar]
- 18.Niehrs C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 19.Polakis, P. Wnt signaling in cancer. Cold Spring Harb Persp. Biol. 4 (2012). [DOI] [PMC free article] [PubMed]
- 20.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fodde R. The APC gene in colorectal cancer. Eur. J. Cancer. 2002;38:867–871. doi: 10.1016/S0959-8049(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 22.Joyce JA, et al. Molecular genetic analysis of exons 1 to 6 of the APC gene in non-polyposis familial colorectal cancer. Clin. Genet. 1995;48:299–303. doi: 10.1111/j.1399-0004.1995.tb04113.x. [DOI] [PubMed] [Google Scholar]
- 23.Horii A, et al. Frequent somatic mutations of the APC gene in human pancreatic cancer. Cancer Res. 1992;52:6696–6698. [PubMed] [Google Scholar]
- 24.Fang D-C, et al. Mutation analysis of APC gene in gastric cancer with microsatellite instability. World J. Gastroenterol. 2002;8:787–791. doi: 10.3748/wjg.v8.i5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohgaki H, et al. APC mutations are infrequent but present in human lung cancer. Cancer Lett. 2004;207:197–203. doi: 10.1016/j.canlet.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Ding L, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ettinger DS, et al. Non-small cell lung cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 28.Benson AB, 3rd, et al. Colon cancer, version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017;15:370–398. doi: 10.6004/jnccn.2017.0036. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Q, et al. DKK2 imparts tumor immunity evasion through beta-catenin-independent suppression of cytotoxic immune-cell activation. Nat. Med. 2018;24:262–270. doi: 10.1038/nm.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niehrs C. Function and biological roles of the dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 31.Bao J, Zheng JJ, Wu D. The structural basis of DKK-mediated inhibition of Wnt/LRP signaling. Sci. Signal. 2012;5:pe22. doi: 10.1126/scisignal.2003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat. Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay M, et al. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development. 2006;133:2149–2154. doi: 10.1242/dev.02381. [DOI] [PubMed] [Google Scholar]
- 34.Gage PJ, Qian M, Wu D, Rosenberg KI. The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev. Biol. 2008;317:310–324. doi: 10.1016/j.ydbio.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, et al. Chemical and genetic evidence for the involvement of Wnt antagonist Dickkopf2 in regulation of glucose metabolism. Proc. Natl. Acad. Sci. USA. 2012;109:11402–11407. doi: 10.1073/pnas.1205015109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rousalova I, Krepela E. Granzyme B-induced apoptosis in cancer cells and its regulation (Review) Int. J. Oncol. 2010;37:1361–1378. doi: 10.3892/ijo_00000788. [DOI] [PubMed] [Google Scholar]
- 37.Wensink AC, Hack CE, Bovenschen N. Granzymes regulate proinflammatory cytokine responses. J. Immunol. 2015;194:491–497. doi: 10.4049/jimmunol.1401214. [DOI] [PubMed] [Google Scholar]
- 38.Shao YC, Wei Y, Liu JF, Xu XY. The role of dickkopf family in cancers: from bench to bedside. Am. J. Cancer Res. 2017;7:1754–1768. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Zhang S, Gu LY, Di W. Epigenetic silencing of DKK2 and Wnt signal pathway components in human ovarian carcinoma. Carcinogenesis. 2012;33:2334–2343. doi: 10.1093/carcin/bgs278. [DOI] [PubMed] [Google Scholar]
- 40.Hirata H, et al. Wnt antagonist gene DKK2 is epigenetically silenced and inhibits renal cancer progression through apoptotic and cell cycle pathways. Clin. Cancer Res. 2009;15:5678–5687. doi: 10.1158/1078-0432.CCR-09-0558. [DOI] [PubMed] [Google Scholar]
- 41.Fatima S, Luk JM, Poon RTP, Lee NP. Dysregulated expression of dickkopfs for potential detection of hepatocellular carcinoma. Expert Rev. Mol. Diagn. 2014;14:535–548. doi: 10.1586/14737159.2014.915747. [DOI] [PubMed] [Google Scholar]
- 42.Xu W, et al. Dickkopf 2 promotes proliferation and invasion via Wnt signaling in prostate cancer. Mol. Med. Rep. 2016;14:2283–2288. doi: 10.3892/mmr.2016.5502. [DOI] [PubMed] [Google Scholar]
- 43.Hauer K, et al. DKK2 mediates osteolysis, invasiveness, and metastatic spread in Ewing sarcoma. Cancer Res. 2013;73:967–977. doi: 10.1158/0008-5472.CAN-12-1492. [DOI] [PubMed] [Google Scholar]
- 44.Matsui A, et al. DICKKOPF-4 and -2 genes are upregulated in human colorectal cancer. Cancer Sci. 2009;100:1923–1930. doi: 10.1111/j.1349-7006.2009.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park H, et al. Distinct roles of DKK1 and DKK2 in tumor angiogenesis. Angiogenesis. 2014;17:221–234. doi: 10.1007/s10456-013-9390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azoury SC, Straughan DM, Shukla V. Immune checkpoint inhibitors for cancer therapy: clinical efficacy and safety. Curr. Cancer Drug Targets. 2015;15:452–462. doi: 10.2174/156800961506150805145120. [DOI] [PubMed] [Google Scholar]
- 47.Sanmamed MF, Chen LP. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sui Hongshu, Ma Ningxia, Wang Ying, Li Hui, Liu Xiaoming, Su Yanping, Yang Jiali. Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies. Journal of Immunology Research. 2018;2018:1–17. doi: 10.1155/2018/6984948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcais A, Walzer T. An immunosuppressive pathway for tumor progression. Nat. Med. 2018;24:260–261. doi: 10.1038/nm.4508. [DOI] [PubMed] [Google Scholar]
- 50.Jiang Y, et al. Snail and slug mediate tamoxifen resistance in breast cancer cells through activation of EGFR-ERK independent of epithelial-mesenchymal transition. J. Mol. Cell Biol. 2014;6:352–354. doi: 10.1093/jmcb/mju019. [DOI] [PubMed] [Google Scholar]
- 51.Xiao Qian, Jiang Yan, Liu Qingbo, Yue Jiao, Liu Chunying, Zhao Xiaotong, Qiao Yuemei, Ji Hongbin, Chen Jianfeng, Ge Gaoxiang. Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1. PLOS Genetics. 2015;11(5):e1005249. doi: 10.1371/journal.pgen.1005249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen ZX, Qiao J, Wang QR, Xiao Q. Data on tumor progression of c-mos deficiency in murine models of Kras(G12D) lung and Apc(Min) colorectal cancer. Data Brief. 2018;20:1124–1132. doi: 10.1016/j.dib.2018.08.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.