Abstract
Accurate knowledge of 13C isotopic signature (δ13C) of methane from each source is crucial for separating biogenic, fossil fuel and pyrogenic emissions in bottom-up and top-down methane budget. Livestock production is the largest anthropogenic source in the global methane budget, mostly from enteric fermentation of domestic ruminants. However, the global average, geographical distribution and temporal variations of the δ13C of enteric emissions are not well understood yet. Here, we provide a new estimation of C3-C4 diet composition of domestic ruminants (cattle, buffaloes, goats and sheep), a revised estimation of yearly enteric CH4 emissions, and a new estimation for the evolution of its δ13C during the period 1961–2012. Compared to previous estimates, our results suggest a larger contribution of ruminants’ enteric emissions to the increasing trend in global methane emissions between 2000 and 2012, and also a larger contribution to the observed decrease in the δ13C of atmospheric methane.
Subject terms: Stable isotope analysis, Carbon cycle, Atmospheric chemistry, Atmospheric chemistry
Global average, geographical distribution and temporal variations of the 13C isotopic signature of enteric fermentation emissions are not well understood. Here the authors established a global dataset and show a larger emission increase between the two periods (2002–2006 and 2008–2012) than previous studies.
Introduction
Methane has important anthropogenic emissions, and is the second largest driver of global radiative forcing (0.97 ± 0.16 W m−2) after CO21. Understanding the global methane budget and its sources is crucial for climate mitigation efforts. Both process-based (bottom-up) and atmospheric-based (top-down) methods are used to constrain the sources and sinks of methane. However, large uncertainties exist in both approaches, which limits the complete understanding of the global methane budget.
Measurements of atmospheric methane concentrations, including their trend and gradients between stations of the surface in situ network, together with a priori spatial and temporal patterns of source type information are used by atmospheric inversion systems to produce optimized estimates of broad source categories (the Global Carbon Project2) and of the global budget, including surface sources and atmospheric sinks. The measurements of the 13C stable isotope composition of atmospheric methane (i.e., δ13CCH4-atm) bring additional constraints for attributing methane emission sources3–6. The 13C/12C-ratio in atmospheric CH4 (δ13CCH4-atm; expressed in δ-notation relative to Vienna Pee Dee Belemnite (VPDB)-standard) is controlled by the relative contributions from different source types with distinctive isotope signatures, δ13CCH4-sources, and by the isotopic fractionation during reaction with atmospheric OH and chlorine radicals. Reference 6 and further ref. 7 revised the δ13C of methane sources and concluded that microbial sources, including wetlands, rice paddies, ruminant enteric fermentation and waste emissions, have a mean δ13CCH4 of ~ −61.7 ± 6.2‰, fossil-fuel sources have a mean δ13CCH4 of ~ −44.8 ± 10.7‰, and pyrogenic sources from biomass burning have a mean δ13CCH4 of ~−26.2 ± 4.8‰. The uncertainty and variability (in space and time) of these signatures directly affects the accuracy of source attribution by inversions. For example, the observed plateau of atmospheric methane concentration during 1999–2006, the renewed concentration-rise after 2006, and associated δ13CCH4-atm changes were used to quantify the role of different sources5. However, biases in the mean isotopic signatures of individual sources and how they change with time translate into potentially large uncertainties on the inferred trends of emission in this approach8.
Livestock production is the largest anthropogenic source in the global methane budget (103 [95–109] Tg CH4 yr−1 during 2000–20092). Enteric fermentation from ruminants dominates this source and accounts for emission of 87–97 Tg CH4 yr−1 during 2000–20099–12. Livestock manure management has a smaller contribution. Cattle, buffaloes, goats, and sheep are the main ruminant livestock types emitting CH4 and altogether represent 96% of the global enteric fermentation source9. Several methodologies are recommended by IPCC13 (Vol. 4, Chapter 10.3) to estimate national to global enteric methane emissions. The Tier 1 method that uses the livestock population data and default emission factors, the Tier 2 approach uses a more detailed country-specific data on gross energy intake and methane conversion factors for specific livestock categories, and the Tier 3 approach allows detailed parameterization of rumen fermentation. Given the large magnitude of ruminant emissions (FCH4-ruminant), its δ13C (δ13CCH4-ruminant) needs to be assessed as precisely as possible regionally for constraining the global mix of emissions using inversion models driven by atmospheric CH4 and isotope data.
Photosynthesis pathways differentiate C3 and C4 plants14. C4 plants contain more 13C than C3 plants relative to 12C. This difference in isotopic ratio causes methane emissions from ruminants with a higher C4 diet to be isotopically heavier (less negative δ13CCH4) than those with a C3 diet. Therefore, to assess the δ13CCH4-ruminant, it is critical to first differentiate the C3 vs. C4 feed composition in ruminant diet. To our knowledge, the proportions of C3 vs. C4 crops fed to ruminants (as concentrate feeds), as opposed to pig and poultry and their temporal changes, have not been investigated at national and global scale, since FAOSTAT only provides total feed crops for all livestock types grouped together. In addition, given the fact that C4 photosynthetic pathway predominates in warm season/low precipitation grass species, the strong increase in livestock number in tropical regions, such as South America, Africa, and South and Southeast Asia, should also increase the value of δ13CCH4-ruminant (i.e., heavier). Few studies have considered the impacts of shifting C4–C3 diet composition in 13C constraints on the global CH4 budget. Reference 6 estimated a global weighted mean δ13CCH4-ruminant value of −66.8 ± 2.8‰ using an observation-based C4–C3 diet fraction of the United States only and made assumptions for the rest of the world. The spatial distribution and temporal trends of livestock δ13CCH4-ruminant is thus a research gap.
In addition, atmospheric isotope signatures of CO2 (δ13CCO2-atm) decreased by −1.3‰ from 1960 to 2012 (see observations from the Scripps CO2 Program; http://scrippsco2.ucsd.edu/; data compiled in ref. 15) due to the increasing combustion of fossil carbon. This trend can cause the synchronized decrease of δ13C in plants16. Therefore, in addition to the shifting C4–C3 diet composition, the trend of δ13C in both C4 and C3 feeds will affect the temporal trends of livestock δ13CCH4-ruminant.
In this study, we establish a global, time-dependent dataset at national scale of the C3–C4 diet composition of domestic ruminants, the enteric methane emissions (FCH4-ruminant), and the flux weighted isotopic signature of the methane emissions (δ13CCH4-ruminant) over the period between 1961 and 2012. First, we separate the crop concentrate feeds consumed by ruminant vs. pigs and poultry using commodity and animal stocks statistics from FAOSTAT9. A simple feed model17 is used for this separation. Then we estimate the quantity of grass and occasional fodder and scavenged biomass based on ruminant energy requirement and grass-biomass use from previous studies, all with a distinction between C3 and C4. Then, using a relationship between δ13Cdiet and δ13CCH4-ruminant constructed in this study from δ13Cdiet and δ13CCH4-ruminant observations, we estimate the national- and time-dependent weighted isotopic signature of ruminant enteric methane emissions (δ13CCH4-ruminant) for the period of 1961–2012. Finally, we quantify the impact of the revised FCH4-ruminant and δ13CCH4-ruminant on δ13CCH4-source, and use a one-box model to quantify their effects on atmospheric CH4 concentration and δ13CCH4-atm. Table 1 provides a glossary of terms as used in this study.
Table 1.
Glossary of terms as used in this study
| Terms | Units | Explanations |
|---|---|---|
| δ13CCH4 | ‰ | The 13C isotopic signature of methane; i.e., the 13C/12C-ratio of CH4 expressed in δ-notation relative to Vienna Pee Dee Belemnite (VPDB)-standard |
| δ13CCH4-atm | ‰ | The 13C isotopic signature of atmospheric methane |
| δ13CCH4-sources | ‰ | The 13C isotopic signature of methane from different source types, such as microbial sources, fossil-fuel sources, and biomass burning |
| δ13CCH4-ruminant | ‰ | The 13C isotopic signature of ruminant enteric fermentation methane emissions |
| δ13Cdiet | ‰ | The 13C isotopic signature of ruminant diet |
| DM | kg | Dry matter |
| ECH4 | MJ (kg CH4)−1 | The energy content of methane |
| EGE | MJ (kg dry matter)−1 | The gross energy content of feeds |
| FCH4-ruminant | Tg CH4 yr−1 | The annual ruminant enteric fermentation methane emissions |
| FCR | kg dry matter (kg live-weight gain or kg eggs production)−1 | The feed conversion ratios |
| fdressing | % | The dressing percentage of livestock |
| fintensity | % | The farming intensity |
| fDE | % | The digestible fraction of gross energy contained in feeds (i.e., an indicator of digestibility) |
| GE | MJ | The gross energy intake/requirement by ruminants |
| ME | MJ | The metabolizable energy intake/requirement by ruminants |
| Q | kg dry matter | The total feed quantity used for different animal types |
| REM | % | The fraction of digestible energy available in diet used for maintenance |
| Weight | kg live-weight gain or kg eggs production | The total live weight of slaughtered animals or total weight of eggs production |
| Ym | % | The methane conversion factor |
Results
Feeds for domestic livestock
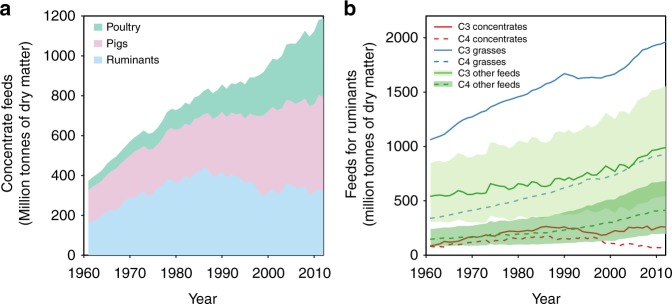
Annual concentrate feed commodities for livestock increased from 373 million-tons dry matter (Mt DM) in 1961 to 1186 Mt DM in 2012 (Fig. 1a). The interannual variation of the feed could be due to many factors, such as market price of feed (supply-side) and livestock products (demand-side), climate (mainly supply-side), and even epidemic disease (demand-side). We will only focus on decadal average and long-term trend in this study. The feed model estimated that poultry and pigs consumed about half (53%) of the concentrate feeds in 1960s, the rest being for ruminants. In 2000s, 68% of the concentrate feeds were used for poultry and pig production against 32% for ruminants. The increasing share of concentrate feeds for poultry and pigs could be due to the larger increase in the production of poultry and pigs (increased by 11.9, 5.1, and 4.5-folds for poultry meat, eggs and pig meat production, respectively, between 1961 and 2012) compared with that of ruminant (increased by 2.1-fold between 1961 and 2012), and the farming intensity change. Annual concentrate feeds consumed by ruminants represented 213 Mt DM yr−1 in the 1960s, peaked at 402 Mt DM yr−1 in the 1980s and then decreased to 332 Mt DM yr−1 in the 2000s. The C4 concentrate feeds comprise 35% (1980s) to 37% (1990s) of the total concentrate feed commodities for livestock.
Fig. 1.
The changes in concentrate feeds for poultry, pigs, and ruminants, and in the composition of feeds for ruminants over the period of 1961–2012. The concentrate feeds for poultry, pigs, and ruminants are presented as stacked area chart in (a). The feeds for ruminants are concentrates (including all crop concentrate feed commodities for ruminants, C3-based or C4-based), grass (including C3 and C4 grasses), and other feeds (i.e., stover and occasional including C3 and C4 part) following ref. 18. The light and dark green lines in (b) show the mean amount of C3 and C4 other feeds consumed by ruminants estimated in this study derived from Monte Carlo ensembles (n = 1000) from the range of uncertainty reported on feed digestibilities (i.e., fDE-s+o, fDE-concentrates, and fDE-grass; see Methods section) and on the fraction of digestible energy available in diet used for maintenance (REMs; REM parameter values themselves dependent on fDE; see Methods). The green shaded areas show the 95% confidence interval of the estimates. Source data are provided as a Source Data file
C3-based concentrate feeds constitute more than half of the ruminants’ concentrate feeds over the past five decades. The share of C4 in total ruminant’s concentrate feeds decreased from 43% in 1960s to 28% in 2000s. Concentrate feeds comprised only 8.0% [6.5–9.6%] of total dry matter consumption by ruminants in the 2000s, compared with 11.9% [9.9–13.9%] in the 1980s.
Grass-biomass is the largest share of ruminants’ dry matter consumption comprising about 63% [52–74%] of the total dry matter intake. It is noteworthy that the share of C4 grasses in grass feed increased significantly from 24.3% in 1960s to 31.3% in 2000s mainly due to the rapid C4 grass feed increase in Latin America and Caribbean (Supplementary Fig. 1).
Other feeds represent the second largest share of total dry matter consumption by ruminants, ranging from 25.8% [14.0–38.4%] in 1980s to 29.1% [15.3–42.3%] in 2000s (Fig. 1b). The share of C4 other feeds in total other feeds follows that of the grass feed, given the assumptions made in Methods (the C3:C4 ratio of other feeds the same as the ratio of grasses for each country).
In total, the C4 diet of ruminant weighted by the fraction of each type of feed increased from 25.2% [24.8–25.8%] in the 1960s to 30.3% [30.2–30.4%] in the 2000s.
Relationship between δ13CCH4-ruminant and δ13Cdiet
Figure 2 shows the empirical relationship between δ13Cdiet and δ13CCH4-ruminant extracted from literature data (see Methods; Supplementary Tables 1 and 2). Here, we apply a linear regression, which results into the following equation:
| 1 |
with the standard errors of the fitted slope and intercept being 0.12 and 2.86‰, respectively (Fig. 2).
Fig. 2.
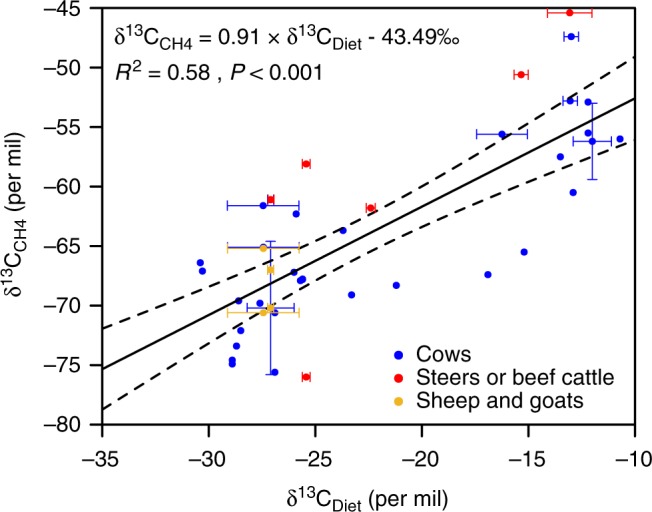
Linear regression between δ13C of diet (δ13Cdiet) and δ13C of CH4 from enteric fermentation of ruminants (δ13CCH4-ruminant). The dots denote 43 observations from six published documents54–59. δ13Cdiet are either obtained directly from literature or calculated based on the feed composition and the δ13C uncertainties of different feed categories (Supplementary Table 1 and 2; see Methods for detail). In the latter case, to account for the effect of decreasing δ13CCO2-atm on δ13C of feeds, we adjust the calculated δ13Cdiet to the year when the δ13CCH4-ruminant was measured assuming that the biomass used for feed grows in the same year as δ13CCH4-ruminant measurement and follows the annual δ13C of atmospheric CO2. The solid line represents the best-fit correlation from the observations considering uncertainties of δ13Cdiet and δ13CCH4-ruminant, and the dashed lines represent 95% confidence interval. The standard errors of the fitted slope and intercept are 0.12 and 2.86‰, respectively
Changes in FCH4-ruminant and δ13CCH4-ruminant
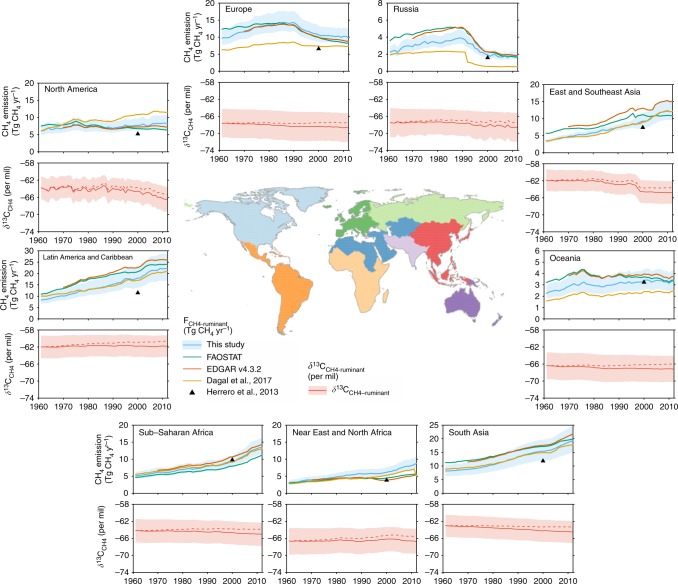
We estimated that global FCH4-ruminant has doubled from 48.5 ± 5.6 Tg CH4 yr−1 (mean ± 1-sigma standard deviation) in 1961 to 99.0 ± 11.7 Tg CH4 yr−1 in 2012 (Fig. 3a). The emissions’ increase mainly took place in the Latin American and Caribbean countries (+13.8 ± 2.0 Tg CH4 yr−1), East and Southeast Asia (+8.8 ± 1.3 Tg CH4 yr−1), Sub-Saharan Africa (+8.4 ± 0.7 Tg CH4 yr−1), Near East and North Africa (+5.6 ± 0.6 Tg CH4 yr−1), and South Asia (+11.1 ± 1.4 Tg CH4 yr−1) (Fig. 4). By contrast, FCH4-ruminant decreased in Europe and Russia during the period of 1990–2012 by 31% and 54%, respectively, making the emissions of 2012 lower than those of 1961 in these two regions.
Fig. 3.
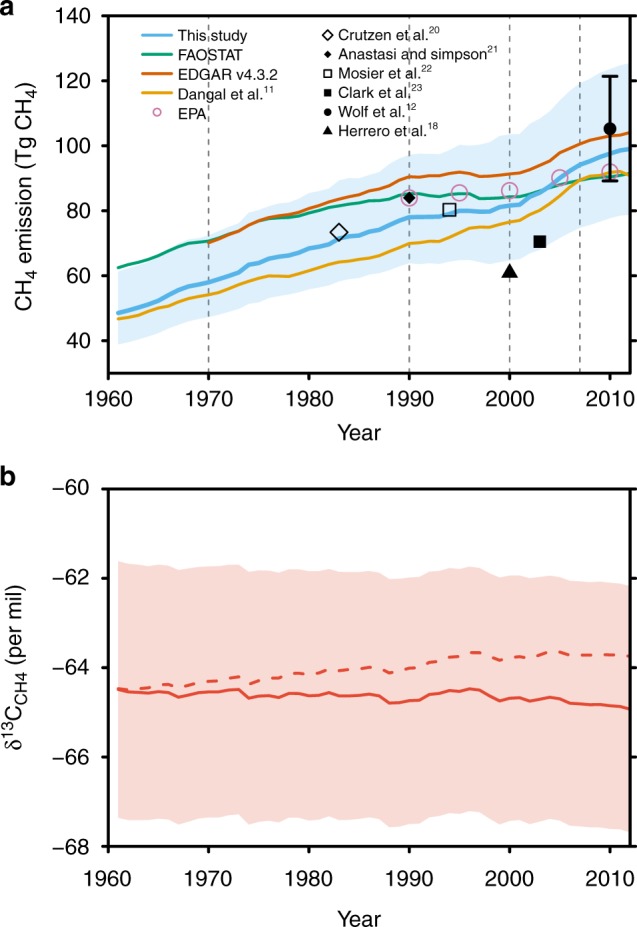
The changes in the global methane emissions from enteric fermentation of ruminants (FCH4-ruminant), and their globally weighted average isotopic signatures δ13CCH4-ruminant over the period of 1961–2012. The blue (a) and red (b) shaded areas show the 95% confidence interval of the estimates of FCH4-ruminant and δ13CCH4-ruminant, respectively. The red dashed line in (b) shows the δ13CCH4-ruminant without accounting for the effect of δ13CCO2-atm trend (see Methods). The dashed gray lines in (a) separate the periods that we consider the trend of FCH4-ruminant in main text and Table 2. Source data are provided as a Source Data file
Fig. 4.
The changes in the regional methane emissions from enteric fermentation of ruminants (FCH4-ruminant), and their weighted average isotopic signatures δ13CCH4-ruminant over the period of 1961–2012. The blue and red solid lines show the mean value of livestock emissions and their δ13CCH4 from this study, respectively. The blue and red shaded areas give the 95% confidence interval of those estimates. The red dashed lines show the δ13CCH4-ruminant without accounting for the decreasing δ13CCO2-atm trend (see Methods). Regions are classified following the definition of the FAO Global Livestock Environmental Assessment Model66. Western and eastern Europe are combined as Europe. The vertical-scale of the regional methane emissions has been adjusted so that the changes can be easily seen
The global mean δ13Cdiet decreased a little from −23.05‰ [−25.45 to −20.66‰] in 1961 to −23.53‰ [−25.74 to −21.31‰] in 2012 (data not shown). This global diet change together with the decreasing δ13C of feeds due to decreasing δ13CCO2-atm caused marginal change in the global mean δ13CCH4-ruminant (ranging from −64.49‰ [−67.36 to −61.62‰] in 1961 to −64.93‰ [−67.68 to −62.17‰] in 2012; Fig. 3b). However, δ13CCH4-ruminant has noticeable changes in several regions. There are δ13CCH4-ruminant increases in Near East and North Africa, Latin America, and Caribbean. Decreases in δ13CCH4-ruminant are found in North America since 1990, and in East and Southeast Asia during 1992–1996 caused by an increased reliance of C3 vs. C4 concentrates feed (in relative share; Supplementary Fig. 1).
Figure 5a shows the global distribution of national average δ13CCH4-ruminant in 2000s. Countries in tropical regions tend to have isotopically heavier ruminant methane emissions (less negative δ13CCH4-ruminant) due to a higher C4 plants proportion in the diet of animals. Country-level δ13CCH4-ruminant due to diet shift only shows large changes in opposite sign (heavier or lighter; Fig. 5b). Within the major livestock producing countries, δ13CCH4-ruminant decreased by −0.3‰ and −1.9‰ in the United States and China, respectively. In these two countries, the increase in poultry and pig numbers consumed most crop feeds, including maize, so that only few C4 crops feed became allocated to ruminants. The lower fraction of C4 diet explained the decrease of δ13CCH4-ruminant in these two countries. In Indonesia and Malaysia, the average δ13CCH4-ruminant showed a strong decrease of −1.8‰ and −1.2‰, respectively. More C4 crop feed was used there to feed the increasing poultry numbers (i.e., less C4 crops left for ruminant). By contrast, δ13CCH4-ruminant significantly increased from the 1960s to the 2000s in Brazil (+0.3‰), Argentina (+0.5‰) and Australia (+0.5‰), which are major livestock producing countries. This increase in the source signature is explained by the combined effect of increased C4 crop feed and C4 pasture grazing.
Fig. 5.
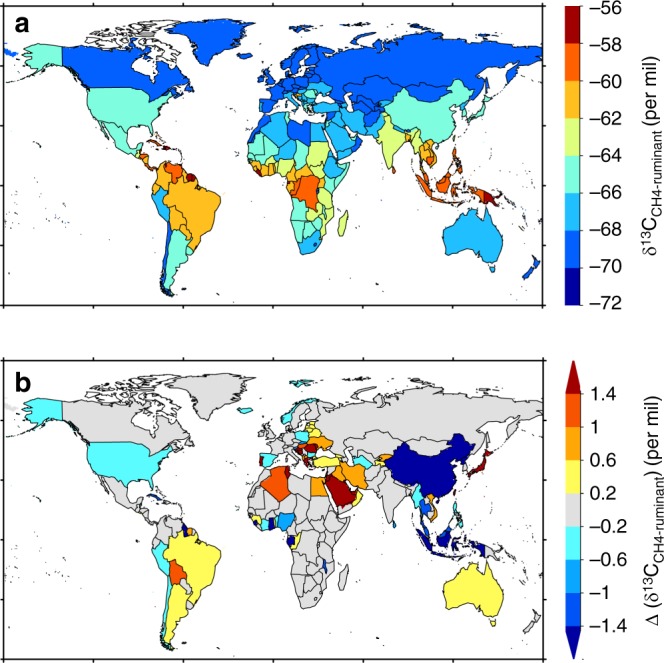
Country average δ13CCH4-ruminant in 2000s, and the changes in δ13CCH4-ruminant between 1960s and 2000s (∆ (δ13CCH4-ruminant)). The ∆ (δ13CCH4-ruminant) is here calculated from the diet shift only (i.e., changes in the relative C3–C4 fraction in feeds) without accounting for decreasing δ13CCO2-atm incorporated in the biomass of grazed plants (see Methods). A positive ∆ (δ13CCH4-ruminant) indicates an increase of δ13CCH4-ruminant from 1960s to 2000s from a higher fraction of C4 in the diet
Discussion
Global livestock feed data, including concentrates, grasses, stover, and occasional feeds, are available only for the year 200018. In this study, we reconstruct the consumption of concentrate crop feeds consumed by ruminant, the grazing of grass-biomass, and inferred the consumption of stover and occasional (other feeds) biomass as a residual to meet the metabolic energy requirement of the whole ruminant production sector over the period of 1961–2012 (Fig. 1). For 2000, we estimate that ruminants consumed 33% of the total concentrate feeds (317 Mt), 11% higher than the value given by ref. 18 (284 Mt). The difference could be due to the uncertainty in the feeds estimate for poultry and pigs in this study. The uncertainty could come from the feed conversion ratios (FCR) used here. For developed countries, we applied the FCR of poultry production derived from the industrial broiler system of the United States (intensive system with relatively low FCR). The FCRs of developing countries are assumed to be 20% higher than those of developed countries (derived from the FCR difference for pig production between the United States and China). On one hand, the FCR of poultry production other than broiler (like other types of chicken, duck, and turkey) is assumed to be the same as that of industrial broiler, which could bring uncertainty to the feed estimates. On the other hand, industrial and smallholder systems are assumed to have the same FCR for production in the simple feed model, due to the lack of the FCR information for smallholder system. Usually, smallholder system tends to have higher FCR than industrial system. This will cause the low feed requirement estimated by the simple feed model. In addition, the uncertainty could be due to the assumed proportion of backyard production that did not receive feed commodities collected in statistics (see Methods). In a simulation assuming that all poultry and pig productions receive feed commodities, the simple feed model estimates poultry and pigs consumed 78% of the total concentrate feeds (1013 Mt), with 22% eaten by ruminants (110 Mt).
Nevertheless, concentrate feeds comprised <10% of the total dry matter consumption by ruminants in the 2000s. The grass feed fraction in 2000 used in this study was directly derived from ref. 18. Stover and occasional feed used by ruminants is clearly the most uncertain term of the feed equation. They were estimated to be 1098 Mt in average for 2000, close to the estimate of ref. 18 (1131 Mt). But our estimates have large uncertainty ranging from 477 to 1976 Mt (95% confidence interval) due to the large uncertainty in digestibility of feeds. In summary, the simple feed model and assumptions on feed digestibility of ruminants’ feed can generally reproduce livestock feed consumptions at global scale well compared with ref. 18.
In this study, annual FCH4-ruminant was calculated based on IPCC Tier 2 methods. Compared with Tier 1 method that used the livestock population data and default emission factors, Tier 2 approach uses a more detailed country-specific data on gross energy intake and methane conversion factors for specific livestock categories, allowing the consideration of diet quantity and quality13. Table 2 shows the comparison of the global FCH4-ruminant estimated in this study and those from previous studies for the contemporary period (1980–2012; see also Fig. 3a). Our global estimates are generally within the range of previous estimates using IPCC Tier 1 and Tier 2 methods9–12,19–23. However, discrepancies exist between estimates using different methods and input data. IPCC Tier 2 method considers gross and net energy requirement and associated feed intake by different livestock categories (cattle, buffaloes, sheep, and goats) and sub-categories (dairy of non-dairy), and digestible energy availability of the diet (i.e., feed digestibility). For IPCC Tier 1 method, default emission factors for non-cattle livestock are from literature with indicated live-weight (see Tables 10.10 of ref. 13, Vol. 4, Chapter 10, pp. 10.28), and default region-specific emission factors for cattle used in a Tier 1 method (Tables 10.11 of ref. 13, Vol. 4, Chapter 10, pp. 10.29) are derived in fact from Tier 2 method and the data in Tables 10 A.1 and 10A.2 of ref. 13, Vol. 4, Chapter 10, pp. 10.72–10.75, which embodies livestock characteristics adjudged by expert opinions. Expert opinion necessarily limits confidence in those default emission factors to be representative of all cattle in large regions such as Africa, Middle East, and Asia. Compared with Tier 1 method, Tier 2 method (if related active data like body weight and feed digestibility are used) should allow a more accurate estimate of feed intake which is an important variable in estimating methane production from enteric fermentation (ref. 13, Vol. 4, Chapter 10, pp. 10.10). Reference 18, using Tier 3 approach with herd dynamics (i.e., age of first calving, replacement, and live-weight gain) and detailed parameterization of feed intake and rumen fermentation, estimated lower emissions in the year 2000 than all other studies (including our study; Fig. 3a). The highest emission comes from ref. 12 based on Tier 1 and accounting for recent changes in animal body mass, feed quality and quantity, milk productivity, and management of animals and manure.
Table 2.
Comparison of the global methane emissions from enteric fermentation of ruminants (FCH4-ruminant) and its trend
| Year | This study (Tg CH4 yr−1) | Estimates from previous studies (Tg CH4 yr−1) | Method/emission factors | Sources |
|---|---|---|---|---|
| Global FCH4-ruminant | ||||
| 1983 | 71.5 ± 7.8 | 73.4 | IPCC Tier 2 | Crutzen et al.20 |
| 1990 | 78.0 ± 8.4 | 84 | IPCC Tier 1 | Anastasi and Simpson21 |
| 1994 | 79.0 ± 8.6 | 80.3 | IPCC Tier 1 | Mosier et al.22 |
| 2000 | 81.6 ± 9.6 | 60.9 | IPCC Tier 3 | Herrero et al.18 |
| 86.3 | Mixed IPCC Tiersa | EPA, 201219 | ||
| 84.3 | IPCC Tier 1 | FAOSTAT9 | ||
| 91.3 | Hybrid IPCC Tier 1 | EDGAR v4.3.210 | ||
| 76.5 | IPCC Tier 2 | Dangal et al.11 | ||
| 2003 | 85.6 ± 10.0 | 70.5 | IPCC Tier 2 | Clark et al.23 |
| 2000s | 88.9 ± 10.4 | 96.0 ± 14.7 | Revised IPCC Tier 1 | Wolf et al.12 |
| 83.8 | IPCC Tier 2 | Dangal et al.11 | ||
| 2010 | 97.6 ± 11.4 | 92.0 | Mixed IPCC Tiersa | EPA, 201219 |
| 90.4 | IPCC Tier 1 | FAOSTAT9 | ||
| 103.1 | Hybrid IPCC Tier 1 | EDGAR v4.3.210 | ||
| 105.3 ± 16.1 | Revised IPCC Tier 1 | Wolf et al.12 | ||
| 91.8 | IPCC Tier 2 | Dangal et al.11 | ||
| Trend of global FCH4-ruminant | ||||
| Period | This study (Tg CH4 yr−2) | Estimates from previous studies (Tg CH4 yr−2) | Method/emission factors | Sources |
| 1961–2012 | 0.92 ± 0.12 | 0.50 | IPCC Tier 1 | FAOSTAT9 |
| 0.86 | IPCC Tier 2 | Dangal et al.11 | ||
| 1970–1989 | 0.95 ± 0.09 | 0.69 | IPCC Tier 1 | FAOSTAT9 |
| 0.94 | Hybrid IPCC Tier 1 | EDGAR v4.3.210 | ||
| 0.70 | IPCC Tier 2 | Dangal et al.11 | ||
| 1990–1999 | 0.33 ± 0.14 | −0.14 | IPCC Tier 1 | FAOSTAT9 |
| 0.05 | Hybrid IPCC Tier 1 | EDGAR v4.3.210 | ||
| 0.74 | IPCC Tier 2 | Dangal et al.11 | ||
| 2000–2012 | 1.61 ± 0.20 | 0.64 | IPCC Tier 1 | FAOSTAT9 |
| 1.19 | Hybrid IPCC Tier 1 | EDGAR v4.3.210 | ||
| 1.22 | Revised IPCC Tier 1 | Wolf et al.12 | ||
| 1.47 | IPCC Tier 2 | Dangal et al.11 | ||
Values from this study are shown as mean ± 1-sigma standard deviation
aU.S. EPA dataset is based on IPCC Tier 1 calculations supplemented with country-reported inventory data (ref. 19, pp.1), with most of the enteric CH4 emissions being from country-reported inventory data (Appendices of ref. 19, pp. G-8 to G-9). Given the fact that a majority of the reported data were derived from the UNFCCC flexible query system using higher IPCC Tiers, we called the method used by U.S. EPA data Mixed IPCC Tiers
The linear trend of global FCH4-ruminant found in this study (0.89 ± 0.11 Tg CH4 yr−2) is much higher than that from FAOSTAT9 (0.50 Tg CH4 yr−2), and is not different from that of ref. 11 (0.86 Tg CH4 yr−2) for the period of 1961–2012. For the period of 1970–1989, we estimate a trend of FCH4-ruminant (0.95 ± 0.09 Tg CH4 yr−2) similar to that from EDGAR v4.3.210 (0.94 Tg CH4 yr−2) and high than those from FAOSTAT9 and ref. 11 (Table 2). During 1990s, the emission trend from this study (0.33 ± 0.14 Tg CH4 yr−2) is larger than those from FAOSTAT9 (−0.14 Tg CH4 yr−2) and EDGAR v4.3.210 (0.05 Tg CH4 yr−2), but lower than that in ref. 11 (0.74 Tg CH4 yr−2). For the period of 2000–2012, we estimate a trend of (1.61 ± 0.20 Tg CH4 yr−2) which is higher than those from FAOSTAT9, EDGAR v4.3.210 and ref. 12, but similar to that from ref. 11 (1.47 Tg CH4 yr−2) using IPCC Tier 2 method. The differences of the trend estimates could come from several factors such as trends in milk yield and carcass weight, the statistics of ruminant numbers, and the assumptions of digestibility.
First, the trends from FAOSTAT9 are lower than all other estimates, mainly due to the fact that the estimate of FAOSTAT did not consider any trend of carcass weight and milk productivity. The increasing trend of carcass weight and milk productivity resulted into higher emissions per unit livestock as in all estimates other than those from FAOSTAT9 (see Methods). The EDGAR v4.3.2 inventory10 applies IPCC Tier 1 methods, but uses country-specific milk yield and carcass weight trend for cattle emissions (not for other animal types like sheep and goats; i.e., a hybrid Tier 1 method). This study and ref. 11 both account for country-specific milk yield and carcass weight trend for all ruminants.
Second, ruminant livestock numbers used in this study and EDGAR v4.3.210 are all from FAOSTAT9, while ref. 11 used additional data from subnational administrative regions in several countries, including the United States, Australia, Brazil, Canada, China, and Mongolia. This will affect the FCH4-ruminant and its trend.
Third, the method used to calculate FCH4-ruminant can affect the trend estimate. Time invariant default emission factors of IPCC Tier 1 method were used by FAOSTAT9 and EDGAR v4.3.210. This study and ref. 11 applied IPCC Tier 2 method considering gross and net energy requirement, and digestible energy availability of the diet (i.e., feed digestibility). But there are differences in the digestibility used. Here, we consider different digestibility according to time varying changes in the different feed types in each country (Eq. (10)) while ref. 11 used regional average feed digestibility derived from ref. 24 across the history.
Using IPCC Tier 2 method, our estimate and that from ref. 11 both give a large increase in emissions between 2002–2006 and 2008–2012, of 8.4 Tg CH4 yr−1 in ref. 12 (including manure management emissions; an increase of 6.9 Tg CH4 yr−1 for enteric emissions only), 8.6 Tg CH4 yr−1 in ref. 11 and 9.4 ± 2.0 Tg CH4 yr−1 in this study. These increases are larger than the estimate of in previous studies9,10,19 (an increase ranging from 3.6 to 6.5 Tg CH4 yr−1; Table S5 of ref. 8 for details). This suggests a larger contribution of enteric emissions to the recent increase in global methane emissions, and also a larger contribution to the recent decrease in the δ13C of atmospheric methane (lighter δ13CCH4-atm) given the lighter δ13CCH4-ruminant than δ13CCH4-atm (see Methods). It should be noted that there is larger trend of FCH4-ruminant in the period of 1999–2006 (1.7 ± 0.2 Tg CH4 yr−2) and that in the period of 2008–2012 (1.0 ± 0.1 Tg CH4 yr−2). The plateau of atmospheric methane concentration observed between late-1990s and mid-2000s therefore comes from other sources with larger emission decrease3 or/and from sink variability5.
At regional scale, FCH4-ruminant estimated in this study is generally in agreement with those from FAOSTAT9 (using IPCC Tier 1 method), EDGAR v4.3.210 (using a hybrid IPCC Tier 1 method with partial consideration of the trends in livestock productivity), and ref. 11 (using IPCC Tier 2 method; Fig. 4). One major difference comes from Latin America and Caribbean, where we estimate a lower FCH4-ruminant than those from FAOSTAT9 and EDGAR v4.3.210. Our estimate is close to that from ref. 11 using IPCC Tier 2 method, and higher than that from ref. 18 using IPCC Tier 3 method. Differences can also be found among our estimates and those from ref. 11 and ref. 18 in North America, Europe, Russia, and Oceania. This could be due to the methods (IPCC Tier 1, 2 or 3 method), statistics of livestock numbers and live weight, and regional digestibility. For example, low estimates from ref. 11 in Europe, Russia, and Oceania could be due to the high feed digestibility used (derived from Table B13 of ref. 24). In Russia, the difference is due to the low ratio of non-dairy to dairy cattle in ref. 11 (data not shown), as non-dairy cattle emission intensity is higher than that of dairy cattle. Large differences can be found for emissions of the United States using various methods and statistics. For example, ref. 25 conducted an inventory of emissions in 1990s using subnational cattle numbers of different sub-groups, measured methane emission from each sub-group, and predominant type of diets. They reported that U.S. cattle emitted 6.6 Tg CH4 in 1998, which is similar to the estimates of this study (6.7 ± 0.8 Tg CH4 for all ruminants). Reference 12 recently reported U.S. enteric emission of 6.6 ± 1.0 Tg CH4 in 2012 using revised emission factors, which is similar to that of ref. 26 (6.2 Tg CH4) and our estimate (7.4 ± 0.9 Tg CH4). Using subnational livestock data and IPCC Tier 2 method, ref. 11 reported emissions larger than other estimates (9.4 Tg CH4 in 1998, and 9.9 Tg CH4 in 2012; also in Fig. 4).
Tropical and sub-tropical regions experienced much higher population growth than temperate regions in the past five decades27. People’s diet in tropical and sub-tropical regions was also shifted toward a more animal-based protein consumption9. Population growth and diet shift together resulted in a larger increase in ruminant numbers and feed requirements in regions like Latin America and Caribbean, Sub-Saharan Africa, Near East and North Africa, South Asia, and East and Southeast Asia (Supplementary Fig. 1). In addition to the local consumption, international trade also contributes to the feed requirement and FCH4-ruminant increase in regions like Latin America and Caribbean. Net export of ruminant meat increase from 0.65 million ton in 1961 to 2.1 million ton in 20129. Given the fact that concentrate feeds comprised only around 10% of the total ruminant feeds (see Results section), the C4 diet fraction in ruminant diet mainly depends on C3–C4 distribution of local feeds. As a result, larger increase in ruminants over tropical and sub-tropical regions (where C4 plant is relatively more dominant) compared with that of temperate regions, is the main cause of the global increase in the C4 diet fraction in the ruminant diet between 1960s and 2000s.
Latin America and Caribbean has the highest C4 diet fraction (Supplementary Fig. 1), and also has the largest increase in C4 diet fraction (from 43.9% [43.5–45.1%] in 1961 to 54.6% [54.2–54.9%] in 2012. This region makes the major contribution to the global increase in C4 diet fraction. The C4 diet also comprises a significant part of ruminant diet in the United States, Sub-Saharan Africa, South Asia, and East and Southeast Asia. Local C4 feeds are the dominant component of the C4 diet in these regions except the United States and China. C4 concentrates comprise 19% and 21% of the total ruminant feeds in the United States and China during the 1980s, respectively. However, due to the fast growth of poultry and pig numbers, more C4 concentrates in these two countries are used for poultry and pigs with less for ruminants simulated by our simple feed model. Thus, the C4 diet fractions in these two countries decreased in the past two decades.
The regional evolution of FCH4-ruminant follows the regional growth of ruminant numbers and feed consumptions, which is also a result of population growth and diet shift. In opposite to the vast FCH4-ruminant increase in most regions, large decreases in FCH4-ruminant are found in Europe and Russia between 1990 and 2012. For eastern Europe and Russia, the collapse of the Former USSR in early 1990s caused large decrease in livestock numbers and thus FCH4-ruminant. In western Europe, the decrease in livestock numbers and FCH4-ruminant comes from a series of policies: (1) the European Union has provided various incentives to farmers through the Common Agricultural Policy (CAP28) since 1962 to avoid the negative side-effects of some farming practices, and has shifted the incentives from price support to direct aid payments to farmers who withdraw land from production in 1992 (thus reduced livestock stocking levels); (2) in 1984, the European Community introduced milk production quotas that contributed to a reduction in the dairy cow population; (3) in 1991, the Nitrates Directive (91/677/EEC29) restricted the application of animal manure in nitrate vulnerable zones to a maximum of 170 kg N ha−1, which caps livestock density in pasture at some 1.7 livestock unit per ha (Annex 1 in ref. 30).
Large scatters exist in the data shown in Fig. 2. Due to lack of a more plausible relationship, the choice of linear relationship is a reasonable assumption and an interpretation of the data. Interestingly, the resulted equation is similar to the usual isotope effects modeled with first-order kinetics31, which is given by:
| 2 |
where α denotes the isotope effects, where α ≅ 1 and is denoted as (1+ε); Rdiet and RCH4 denote the 13C/12C molar ratio of diet (reactant) and ruminant enteric CH4 emissions (products of reaction), respectively. Using δ13Cdiet and δ13CCH4 (Rdiet/RVPDB-standard−1 and RCH4/RVPDB-standard−1, respectively) instead of Rdiet and RCH4, the Eq. (2) can be transformed to:
| 3 |
Eq. (3) can lead to:
| 4 |
which is usually linearized to:
| 5 |
Given the uncertainties in our regression coefficient for slope (0.91 ± 0.12), our linear regression (Eq. (1)) is compatible with Eq. (5) with a ε = 43.49 ± 2.86‰, where the resulted ε can be seen as the isotopic discrimination factor of the fermentation processes of livestock rumen. The resulted equation reflects the biochemical reactions for stable isotopes, which always produce depleted products (13C) while enriching the remaining substrates (12C) owing to the preference of enzyme systems to use lighter isotope substrates 12C.
Different δ13CCH4-ruminantdespite the same δ13Cdiet was found in the collected data (i.e., the large vertical scatter in Fig. 2). This isotopic variability could be due to the differences in first, exact feed composition (e.g., C3 feed: barley, wheat, soybean, alfalfa, straw, or C3 grass; C4 feed: maize grain, maize silage, or C4 grass), second, variation of feed δ13C in space and time, plant isotopic fractionation related to water use efficiency (WUE), and growing season δ13CCO2-atm when CO2 is fixed by plants and incorporated into biomass, third, energy content of feed, and fourth, the different ruminant species (cow, steer, goat, or sheep). For example, the feed composition given by the literature is sometimes coarse for some data points (i.e., points reporting a general C3 or C4 diet in Supplementary Table 2). In this study, we estimate δ13Cdiet using δ13C data collected for different feed categories, considering their uncertainty, and adjust them to the sampling year of δ13CCH4-ruminant using an adjustment factor derived from historical changes in δ13CCO2-atm from ref. 15 (see Methods). This adjustment partly accounts for the δ13Cdiet variability caused by different feed composition and different years of measurements. Spatial and temporal variability of δ13C in feed plants cannot be addressed given the sparse data. Energy content of feed might also affect the isotopic variability, through its relation to gut microbes conversion of intake into CH4. Given the fact that gut microbes preferably break down 12C components, high energy content of feed could potentially increase the conversion to CH4 by gut microbes, and cause heavier enteric CH4 emissions. Ruminant species and even the characteristics of individual animal could also be a source of the variations in δ13CCH4-ruminant. For example, as shown in Fig. 2, between animals fed by diet with similar δ13Cdiet, the δ13CCH4-ruminant can vary between different ruminant species (shown with different colors in Fig. 2) and within the same species (the dispersion of δ13CCH4-ruminant with similar δ13Cdiet shown with the same color in Fig. 2). The variation in δ13CCH4-ruminant caused by different ruminant species was also observed in a study using identical feed conditions for cows, sheep, and camels32, while the authors did not detect significant δ13CCH4-ruminant variation within each species.
We estimate that the annual mean δ13CCH4-ruminant slightly decreased from −64.49‰ [−67.36 to −61.62‰] in 1961 to −64.93‰ [−67.68 to −62.17‰] in 2012. This net small trend over 50 years is the result of two opposite mechanisms the increasing proportion of C4 grass and feeds (occasional and stover) consumed by ruminants (Fig. 1), and the decreasing δ13CCO2-atm that is incorporated in the biomass consumed by ruminants. The first mechanism tends to increase δ13Cdiet (less negative) of +0.74‰ over the last 50 years, while the second effect causes a decrease of δ13Cdiet of 1.18‰. Note that the magnitude of the decreasing trend of δ13C of biomass is not only parallel with the decreasing δ13CCO2-atm, but it is also controlled by trends of WUE over that period. There is evidence for an increase of WUE in temperate, boreal, and tropical forests33–36 over the last 50 years partly attributed to increasing CO2 in the atmosphere, but less so for C3 crops and grasses consumed by livestock37. Thus, in absence of direct observations at appropriate spatial and temporal scales to confirm an increased WUE for C3 plants consumed by livestock and given the fact that C4 plants are not expected to increase their WUE under elevated CO2, we assumed conservatively that WUE is constant, which may overestimate the negative trend of δ13CCH4-ruminant reflecting the trend of δ13CCO2-atm.
Our estimate is significantly lower than the data compilation from ref. 5 (−61‰), higher but within the uncertainty range of ref. 7 (−65.4 ± 6.7‰ with a median of −67.1‰), and at the upper bound of the 1-sigma standard deviation of value estimated by ref. 6 (−66.8 ± 2.8‰). The values given in ref. 7 from δ13CCH4-ruminant obtained in local studies were not weighted by the proportion of C3- versus C4-eating ruminants. The value estimated by ref. 6 is derived from data-driven δ13CCH4-ruminant estimates of −54.6 ± 3.1‰ for C4 plant-based diet, and of −69.4 ± 3.1‰ for C3 plant-based diet, and a global weighted average C4 diet fraction ranging from 1.5 to 19.6% (uniform distribution). The range is simply estimated by using the estimated C4 emission fraction of the United States (19.6%) as a global upper bound (i.e., the rest of the world have the same C4 emission fraction as the United States) and zero C4 emission fraction except the United States as a global lower bound (i.e., 1.5%). As comparison, our estimate is based on first, the refined national C3:C4 feed fraction and its associated δ13Cdiet considering the observation-based uncertainties in the δ13C of different feed categories and the impact of decreasing δ13CCO2-atm on the δ13C of feeds, and second, a data-driven relationship between δ13Cdiet and δ13CCH4-ruminant (see Methods). For the United States, we estimated a C4 feed fraction of 22.2 ± 0.3% for the period of 1980–2012, which is higher but within the uncertainty of the fraction assumed by ref. 6 (19.6 ± 5.9% during 1980–2012).
In this study, we assess the uncertainties of the composition and the δ13C of ruminant diet (δ13Cdiet), ruminant enteric methane emission (FCH4-ruminant), and its weighted δ13C (δ13CCH4-ruminant) through Monte Carlo ensembles (n = 1000) considering the uncertainties of the parameters used in calculation (see Methods and Supplementary Table 3). The parameters’ uncertainties considered here include the feed digestibility, the fraction of digestible energy available in diet used for maintenance (REMs), the methane conversion factor (Ym), the δ13C of feed categories, and the fitted linear regression between δ13Cdiet and δ13CCH4-ruminant constructed from observations.
We also acknowledge other uncertainties that are beyond our capacity of more precise estimation at current stage.
When estimating concentrate feeds intake by pigs and poultry, we account for the national livestock productivity, the different feed conversion ratio (converting production to feed requirement) and farming intensity (industrialized vs. backyard production) for developing and developed countries. Uncertainties in the feed conversion ratio have been discussed above. Besides, there could be uncertainties from other aspects. For example, the simple feed model is based on diet composition of Germany, which could cause inevitable uncertainties in the C3–C4 feed composition of pigs and poultry, and further affects uncertainties in C3–C4 concentrate feed for ruminants. There are also uncertainties in our assumptions on the logistic intensification and constant farming intensity of pig and poultry production in developing and developed countries, respectively, which will affect the time evolution of the concentrate feeds consumption by pigs and poultry. However, these uncertainties are currently not accessible due to lack of national-specific information on diet and farming intensity, and their historical changes.
In this study, we account for the impact of the global annual mean δ13CCO2-atm trend on the trend of δ13C of feed, δ13Cdiet and δ13CCH4-ruminant, while the potential effects of the latitudinal gradient and seasonality of δ13CCO2-atm (http://scrippsco2.ucsd.edu/graphics_gallery/isotopic_data/global_stations_isotopic_c13_trends) on the δ13C of feed (plants) are not considered. C4 photosynthesis is competitive under low atmospheric CO2 or high temperature/low water availability14. We constructed gridded C3–C4 grass (and other local feeds) distributions based on growing season temperature38. The impact of the rising CO2 (~76 ppm during 1961–2012) on the C3–C4 grass distribution is not considered due to lack of evaluated global estimation. Based on leaf photosynthetic rates, one may expect an increase in C3 grass species due to elevated CO2, thus a lower δ13Cdiet and a lower δ13CCH4-ruminant. But long-term CO2 enrichment experiments have not consistently shown a decrease of C4 species in mixed grasslands39 suggesting ecosystem-level mechanisms that maintain a fitness of C4 plants to elevated CO2. In addition, δ13C of C3 plants may have a dependence on mean annual precipitation40,41, which could potentially affect the spatial patter of the feed δ13C. However, a through meta-analysis on the effect on crops and grasses is needed before such relationship can be applied for assessing δ13C of feeds.
In the following section, we examine more in details the impact of our revised estimates FCH4-ruminant and δ13CCH4-ruminant on the trends of global atmospheric CH4 concentration and its isotopic composition using the time-dependent one-box model of the CH4 budget from refs. 42,43 (see Methods). A baseline simulation was run using the bottom-up reconstructions of the methane sources and tuning the historical atmospheric sink history for 12CH4 and 13CH4 to match atmospheric observations. Enteric methane emissions from EDGAR v4.3.2 were used in this baseline simulation as it is the most widely used prior inventory of atmospheric inversions2. Three perturbed runs were conducted to separate the effects of revised FCH4-ruminant, revised δ13CCH4-ruminant and revised δ13CCH4-ruminant changes between 1961 and 2012, respectively (see Methods for details in the model and the simulations). The purpose of the box-model simulations is to show the impact of our new estimates of livestock emissions on atmospheric trends, not to provide closure or re-analysis of recent changes in the budget for which adjustment of other sources (wetlands, fossil fuels) would be required.
With our revised FCH4-ruminant emissions and all other sources and sinks unchanged to their values from the baseline run, we simulate different trends in atmospheric CH4 compared with the baseline (Fig. 6a). During the period 1970–1989, our revised FCH4-ruminant produces a slightly smaller simulated trend of CH4 (15.8 ppb yr−1) than the baseline (16.2 ppb yr−1). For the period 1990–1999, our estimate of FCH4-ruminant (Table 2) produces similar trends of CH4 than the baseline. For the period after 2000, we obtain a larger trend of 4.1 ppb yr−1 compared with 3.0 ppb yr−1 in the baseline. This result suggests that our revised livestock emissions can explain a larger portion of the observed CH4 increase during 2000–2012, but that another source should be revised downwards by the same amount—or OH sink increased—to match with the observed CH4 trend.
Fig. 6.
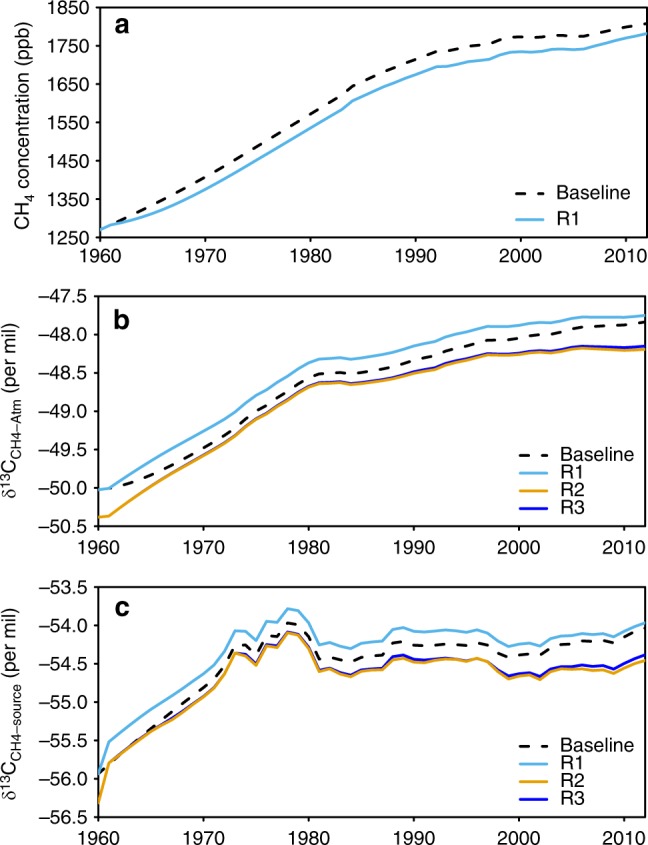
Global box model simulations of atmospheric CH4 concentration, the box model results on δ13CCH4-atm, and the isotopic source signature weighted by all sources (δ13CCH4-source). In the simulation of R1, only the revised FCH4-ruminant is used and δ13CCH4-ruminant set to default value of −62‰ previously used by ref. 5, 43; In R2, the revised FCH4-ruminant and δ13CCH4-ruminant are used (see Methods). R3 is the same as R2 but with constant δ13CCH4-ruminant at −64.49‰ for the period of 1961–2012. The differences between R1 and baseline are the effects of the revised FCH4-ruminant; the differences between R2 and R1 are the effects of the revised δ13CCH4-ruminant; the differences between R3 and R2 are the effects of the δ13CCH4-ruminant variation (i.e., the slightly decrease from −64.49‰ in 1961 to −64.93‰ in 2012). Source data are provided as a Source Data file
Our revised FCH4-ruminant (light blue line in Fig. 6c) alone, with δ13CCH4-ruminant set the default value from ref. 5,43 (−62‰), implies a higher global isotopic source signature (δ13CCH4-source weighted by all sources) due to our lower emissions than in EDGAR v4.3.210. The δ13CCH4-source differences with the baseline range from +0.11‰ in the 2000s to +0.18‰ in the 1970s. The smaller δ13CCH4-source difference in the 2000s comes from the fact that the revised FCH4-ruminant is closer to that of EDGAR v4.3.210 during that period. Using our new δ13CCH4-ruminant value shifts the global δ13CCH4-source by −0.38‰ during 1980–2012 (difference between brown and light blue lines in Fig. 6c), which counterbalances the effect of our lower FCH4-ruminant. This result suggests that both FCH4-ruminant and δ13CCH4-ruminant revisions have significant impacts on δ13CCH4-source. Further studies using isotopic mass balances should thus not only include the revised source estimates for ruminants, but also revised δ13CCH4-ruminant.
Both the revised FCH4-ruminant and δ13CCH4-ruminant affect the mean values and trends of δ13CCH4-atm in the box-model (Fig. 6b). From 1990 to 2012, compared with the baseline, the revised FCH4-ruminant alone (light blue line in Fig. 6c) produces a larger mean δ13CCH4-atm by +0.15‰. The revised δ13CCH4-ruminant alone produces a lower mean value of δ13CCH4-atm than the baseline by −0.37‰. The combined revised source and isotopic signature make the mean δ13CCH4-atm smaller than the baseline by −0.22‰. However, the decreasing δ13CCH4-ruminant has a very small effect on reconstructed δ13CCH4-atm (by −0.02‰ only). This finding implies that even with substantial shifts in diet and geographical distribution on ruminant CH4 emissions, the temporal changes in δ13CCH4-ruminant in the recent decades alone do not have significant effect on the reconstructed δ13CCH4-atm.
From 1990 to 2012, the box model prescribed with the revised FCH4-ruminant simulates a smaller increase in δ13CCH4-atm (change of +0.40‰ during 1990–2012; trend of +0.017‰ yr−1) compared with the baseline (change of +0.50‰; trend of +0.022‰ yr−1). Adding the new δ13CCH4-ruminant makes the increase in δ13CCH4-atm even smaller (change +0.31‰; trend of +0.013‰ yr−1). In other words, as compared with the baseline simulation using emissions from EDGAR v4.3.210 and default δ13CCH4-ruminant of −62‰, the updated FCH4-ruminant and δ13CCH4-ruminant from this study are responsible for a lower δ13CCH4-atm by −0.19‰ between 1990 and 2012, and by −0.08‰ after 2006 when the CH4 growth rate became positive again44,45 . This corresponds to more than half of the observed decrease of δ 13CCH4-atm (−0.15‰ between 2006 to 2012; derived from Table S4 of ref. 5). In conclusion, the box-model simulations have two main implications. Firstly, the revised CH4 emissions from ruminants have compensated δ13CCH4-atm trend that could have otherwise increased more largely driven by the increasing fossil-fuel-related emissions. Secondly, the revised δ13CCH4-ruminant to lower values gives a larger role of ruminant emissions in the recent δ13CCH4-atm trend than previously estimated, consistent with the results from ref. 5.
Methods
Data
FAOSTAT—Live Animals and Livestock Primary9 provides annual national statistics on live animal stocks, numbers of slaughtered animal and laying poultry, milking animals (producing milk) and slaughtered animals (producing meat), and correspondent milk yield for milking animals (dairy cows, sheep, goats, and buffaloes for milk), meat yield (carcass weight) for meat animals (i.e., poultry, pigs, beef cattle, sheep, goats, and buffaloes for meat), and egg yield for laying poultry. In this study, poultry species include chickens, ducks, geese, turkeys, and other birds from FAOSTAT. Data of the period 1961–2012 were used in this study.
Concentrate feeds for livestock over the period 1961–2012 were derived from FAOSTAT—Commodity Balances9. In total, 74 feed commodities were presented in FAOSTAT (Supplementary Table 4). These feed commodities were regrouped into seven groups as those in ref. 17 (maize, other cereals, oilseeds, cakes of oilseeds, brans, pulses, and others (mainly starch crops and sugars); Supplementary Table 4) and their dry matter content calculated using dry matter to biomass ratios (Supplementary Table 5).
Reconstructing the feedstuff for poultry and pigs
In this study, we adapted a simple feed model to determine the amount of concentrate feeds for poultry and pigs, and by difference to total feed commodities the amount that feeds ruminants. The details of the feed model and the methodology are described in Supplementary Note 1 (also see ref. 17). The input data for the feed model are concentrate feeds amounts, and animal stocks numbers and yields from FAOSTAT9. The 74 feed commodities (in dry matter) were grouped into seven groups of concentrate feeds categories: maize, other cereals, oilseeds, cakes of oilseeds, brans, pulses, and others (mainly starch crops and sugars; Supplementary Table 4). The supply of these concentrate feeds was distributed in priority to poultry and eggs producers, then to pigs according to their specific nutritional and energy demands46 established in the feed model (see Supplementary Note 1 for detail). Ruminant are assumed to receive all maize, other cereals, cakes of oilseeds, oilseeds, brans, and others feedstuff that are not consumed by poultry and pigs.
National- and time-dependent adjustments for farming intensity were applied to represent the situation that first, a share of the poultry and pig population are raised by smallholder farms as backyard production, and second, the fraction of backyard production in total production is decreasing in the recent decades following global intensification trend18. We assume that all smallholder farms backyard productions are based on local feed resources and did not use feed commodities included in FAOSTAT. In this study, the regional specific farming intensity in the year 2000 was derived from the fraction of smallholder production in literature (see Supporting information Sect. 4 of ref. 18). For developing countries, we assumed logistic increases in farming intensity during the period of 1960 and 2012, making the intensity value from literature reached by 2000 (Supplementary Fig. 2). The logistic increase is to mimic the intensification of pig and poultry production in developing countries characterized by a fast increase when intensification is low, and a slowing down at later stages when the level of intensification approaches the one of developed countries. An upper bound of 0.95 is set for farming intensity in developing countries, consistent with the maximum intensity indicated by Supporting information Sect. 4 of ref. 18. Farming intensities for developed countries were assumed to remain at the fraction of 2000 all the years through 1961 to 2012.
The original feed model was constructed based on an average nutrient and energy requirement of animals for a whole year and the animal stocks at the time of enumeration9. In this study, we adapted the feed composition (Supplementary Note 1), and calculated feed requirements using animal productions (slaughtered for meat and eggs) and feed conversion ratios (FCR), as given by:
| 6 |
where Qn,i,j is the total feed quantity (in kg dry matter) used for animal type n (poultry for meat, poultry for eggs, pigs for meat) in country i and year j; Weightn,i,j is the total live weight of slaughtered animals (poultry and pigs for meat) and total weight of eggs in country i in year j; FCRn defines the feed requirement in kg dry matter per kg body weight gain or per kg egg production for animal type n in year i; and fintensity,i,j is the farming intensity in country i in year j.
During the recent decades, FCR of farm livestock keep decreasing due to the improvement in factors like nutrition and feeding practices (e.g., diet composition), health condition (reducing mortality), and killing weight (usually efficiency get worse after maturity). For developed countries, FCR = 1.95 kg dry matter (kg live-weight gain or kg eggs production)−1 in 2005 with a decreasing rate of −0.01 yr−1 for poultry live weight and eggs are used in this study. The FCR in 2005 is derived from the value for the United States Broiler Performance47, and the decreasing rate is assumed to generally fit the FCR evolution shown in the United States Broiler Performance. FCR = 3.28 kg dry matter (kg live weight gain)−1 in 1995 for pig live weight with a decreasing rate of −0.015 yr−1 are used in this study. The FCR in 1995 for pigs is derived from ref. 48 (compiled from statistics of the United States). The decreasing rate is roughly estimated using value of 1995 (3.28 kg dry matter (kg live-weight gain)−1) and of 2013 (just above 3 kg dry matter (kg live-weight gain)−1; see ref. 49), and assuming a linear change of the FCR. The FCRs of developing countries are assumed to be 20% higher than those of developed countries, given the example that China has 20% higher FCR for pigs than the United States49.
Weightn,i,j for poultry for meat (Weightpoultry,i,j), pigs (Weightpig,i,j), and eggs (Weightegg,i,j) in country i in year j are calculated as:
| 7 |
| 8 |
| 9 |
where Nk,i,j and Yk,i,j are the slaughtered numbers (in head) and the yield (in kg carcass weight per head) of poultry species k (chickens, ducks, geese, turkeys, and other birds) in country i in year j; Npig,i,j and Ypig,i,j are the slaughtered numbers (in head) and yield (in kg carcass weight per head) of pigs in country i in year j; Nlaying,k,i,j and Yegg,k,i,j are the laying numbers (in head) and yield (in kg eggs per head) of poultry species k (including hens and other birds) in country i in year j; fdressing,poultry and fdressing,pig are the dressing percentage of poultry and pig, respectively, representing the conversion factor between carcass weight and live weight. fdressing,poultry = 70% and fdressing,pig = 60%50 are used in this study.
Reconstructing the feeds for ruminants
After having allocated concentrate feeds to poultry and pigs, ruminants are assumed to receive the remaining quantities of feed commodities (Qconcentrates). In addition to feed commodities (concentrate feeds), ruminant livestock are mainly fed on grass but they also receive crop by-products (stover) and occasional feeds18. For grass-biomass consumed by ruminants (Qgrass), we used the global livestock production dataset of gridded grass-biomass use for the year 2000 from ref. 18 extrapolated by ref. 51 backward and forward in time during 1961–2012 using metabolisable energy (ME) requirement of ruminants in each country. The rest of the consumption of biomass by ruminant livestock is assumed to be met by local stover and occasional feeds (hereafter, as other feeds), following ref. 18.
The quantities of other feeds in country i in year j (Qs+o,i,j) are calculated as the solution of the following equation:
| 10 |
where MEruminant,i,j (MJ yr−1) is the total ME requirement of domestic ruminants in country i in year j derived from ref. 51; EGE-feed is the average gross energy (GE) density of the feed with a value of 18.45 MJ kg−1 of dry matter as suggested by 2006 IPCC Guidelines for National Greenhouse Gas Inventories (ref. 13, Vol. 4, Chapter 10); fDE-s+o, fDE-concentrates, and fDE-grass (in percent) are the digestible fractions of gross energy contained in other feeds, concentrate feeds, and grass-biomass, respectively. fDE-concentrates is set to 80% (mean value) corresponding to the feed digestibility of concentrate diet (ref. 13, Vol. 4, Chapter 10, pp. 14, with a range of 75–85% as 95% confidence interval). fDE-s+o and fDE-grass are set to 55% (mean value) corresponding to the feed digestibility of medium quality forage (ref. 13, Vol. 4, Chapter 10, pp. 14, range of 45–65% as 95% confidence interval). REMs+o, REMconcentrates, and REMgrass (in percent) are the fractions of digestible energy available in diet used for maintenance. REM parameter values themselves depend on fDE and are calculated following 2006 IPCC Guidelines for National Greenhouse Gas Inventories (ref. 13, Vol. 4, Chapter 10, Eqn. 10.14).
The isotopic signature of ruminant diet
The isotopic signature of ruminant diet in country i in year j (δ13Cdiet,i,j) is determined by the C3 and C4 fractions in ruminant diet, and can be calculated as:
| 11 |
where and are feed quantities (in kg dry matter) of C3-plant sources (including C3 concentrate feeds, C3 grasses, and C3 other feeds) and C4-plant sources (including C4 concentrate feeds, C4 grasses, and C4 other feeds) in country i in year j, respectively; and are the isotopic signature of different feeds for the year 2012; and is a factor to adjust the δ13Cdiet to year j given the fact that the δ13C of plant synchronized decreases along δ13CCO2-atm decrease16. is given by:
| 12 |
where and are isotopic signature of atmospheric CO2 for year j and the year 2012, respectively, which are derived from the observations of the Scripps CO2 Program (http://scrippsco2.ucsd.edu/) and compiled in ref. 15. Given the fact that the biomass used for feed are mainly the products of photosynthesis in the same year or growing season and subject to δ13CCO2-atm of the year, we assumed no time-lag between the changes in δ13Cdiet and that in δ13CCO2-atm. For and , we used the following values derived from literature and adjusted to for the year 2012: −25.10 ± 2.27‰ for C3 concentrate feeds; −28.25 ± 1.68‰ for C3 grasses and other feeds; −12.24 ± 0.34‰ for C4 concentrate feeds (mainly maize); and −13.3 ± 1.1‰ for C4 grasses and other feeds (Supplementary Table 1).
Concentrate feeds (including all feed commodities left for ruminants and their C3 or C4 type), grasses (including C3 and C4 grasses), stover and occasional (including C3 and C4 other feeds) are major components of ruminants’ diet to satisfy their ME requirement18. Maize (Qmaize,i,j), millet (Qmillet,i,j), sorghum (Qsorghum,i,j), and sugarcane (Qsugarcane,i,j) are the major C4 concentrate feeds for ruminant:
| 13 |
C3 concentrate feeds include concentrate feeds other than maize, millet, sorghum, and sugarcane:
| 14 |
where Qconcentrates,i,j is the remaining quantities of feed commodities after having allocated concentrate feeds to poultry and pigs. The quantities of millet (Qmillet,i,j) and sorghum (Qsorghum,i,j) are part of other cereals category, and the quantities of sugarcane (Qsugarcane,i,j) are part of others category (Supplementary Table 4). In each country each year, the fractions of millet and sorghum in the other cereals category and sugarcane in the others category are assumed to keep the same values than in the initial feed commodities from FAOSTAT (i.e., before applying the simple feed model) and in the feeds left for ruminants (i.e., after applying the simple feed model). Components of ruminant diet other than concentrate feeds (i.e., grass and other feeds) are assumed to be produced and consumed locally. Thus we assumed the C3:C4 ratio of other feeds (i.e., QC3s+o,i,j: QC4s+o,i,j) the same as the ratio of grasses for each country (i.e., QC3grass,i,j: QC4grass,i,j) following a similar geographical C3 vs. C4 distribution38 (see below).
For grass-biomass consumed by ruminants, we used the global livestock production dataset of gridded grass-biomass use for the year 2000 from ref. 18 extrapolated by ref. 51 at the resolution of 0.5° × 0.5° backward and forward in time during 1961–2012 using ME requirement of ruminants in each country. To distinguish between C3 and C4 grass-biomass consumed, we used the (0.5° × 0.5°) maps prepared for the MsTMIP model intercomparison52 of the relative fraction of C3 and C4 grasses. The MsTMIP gridded C3–C4 grass distribution is derived from the approach described in ref. 38 based on growing season temperature (see Sect. 3.6 of ref. 52 for more details). Decadal maps of the relative fraction of C3 and C4 grasses were obtained using gridded CRU–NCEP mean monthly precipitation and temperature data53 from 1960s to 2000s. The relative fraction of C3 and C4 in each grid cell of the grass-biomass use were averaged at country level to set the C3 (QC3grass,i,j) and C4 grass (QC4grass,i,j) consumed by ruminants.
Methane emissions from enteric fermentation of ruminants
Methane emission from enteric fermentation for country i in year j (FCH4-ruminant,i,j) is calculated using Eq. (15) adapted from IPCC Tier 2 algorithms (ref. 13, Vol. 4, Chapter 10, Eqn 10.21):
| 15 |
where GEruminant,i,j (in MJ) is the annual gross energy intake by ruminants in country i in year j; ECH4 is the energy content of methane with value of 55.65 MJ (kg CH4)−1; Ym,feed is the methane conversion factor expressed as the percent of gross energy in feed converted to methane; and 109 is to convert the unit of methane emission from kg to Tg (i.e., 1012 g). In this study, Ym = 6.5% ± 1.0% (the ± values represent the 95% confidence interval range) is used to represent wide-spread ruminant fed diets for cattle and mature sheep following IPCC guidelines13 (Vol. 4, Chapter 10, Tables 10.12 and 10.13), given the fact that globally, only little cattle in some developed countries is feedlot cattle with feed diets contain 90% or more concentrates. GEfeed,i,j is calculated as the gross energy content of different ruminant feeds (concentrates, grasses and other feeds):
| 16 |
δ13C of diet and δ13C of enteric methane emissions
We collected published documents with δ13CCH4-ruminant observations and, at the same time, with data on δ13Cdiet or information of diet composition (quantities of specific feed categories or proportion of C3 and C4 feeds). Forty-three data from six published documents were collected54–59, and used to derive the relationship between δ13Cdiet and δ13CCH4-ruminant. Within the 43 observations of δ13CCH4-ruminant, δ13Cdiet of 27 observations are obtained directly from the documents. For the rest 16 observations, feed compositions are provided (Supplementary Table 2). We calculated the δ13Cdiet as weighted average δ13C of feed compositions using the δ13C of different feed categories at the reference year 2012 (Supplementary Table 1), and adjusted the calculated δ13Cdiet to the year when the δ13CCH4-ruminant was measured (similar to Eqs. (11) and (12)). The δ13C of different feed categories were originally derived from literature with different years of sampling. To be comparable, they were first adjusted to the same year 2012 using the adjustment factor in Eq. (12). Mean value and uncertainty of the adjusted δ13C for each feed category was then calculated (Supplementary Table 1), and used to assess δ13Cdiet and its uncertainty through Monte Carlo ensembles (n = 10,000; Supplementary Table 2).
The resulted relationship between δ13Cdiet and δ13CCH4-ruminant and their uncertainty (Fig. 2) are used to estimate the δ13CCH4-ruminant of the global and national methane emissions from enteric fermentation (FCH4-ruminant) and its evolution since 1961.
Uncertainty estimates
In this study, we assessed the uncertainties of the quantity of other feeds for ruminant (Qs+o; i.e., feeds other than concentrates and grasses; Eq. (10)), the δ13C of ruminant diet (δ13Cdiet; Eq. (11)), ruminant enteric methane emission (FCH4-ruminant; Eq. (15)), and its weighted δ13C (δ13CCH4-ruminant, using the fitted equation of δ13Cdiet and δ13CCH4-ruminant obtained in this study; Fig. 2). All the above uncertainties were assessed through Monte Carlo ensembles (n = 1000) considering the uncertainties of the parameters used in calculation. The information on the uncertainty assessment in this study is listed in Supplementary Table 3, including the resulting estimates, corresponding equations, and the parameters, and their uncertainties considered in the uncertainty assessment.
For the quantity of other feeds for ruminant (Qs+o), we assessed the uncertainties due to feed digestibility (parameters fDE-s+o, fDE-concentrates, and fDE-grass) and associated REMs (REM parameter values themselves dependent on fDE). For the δ13C of ruminant diet (δ13Cdiet), we assessed the uncertainties due to the Qs+o and the δ13C of different feeds. For national and global methane emissions from enteric fermentation of ruminants (FCH4-ruminant), we assessed uncertainties due to the Qs+o and the methane conversion factor (Ym). For the weighted δ13CCH4 of ruminants (δ13CCH4-ruminant), we assessed the uncertainties due to the uncertainties of δ13Cdiet,i,j and the fitted linear regression between δ13Cdiet and δ13CCH4-ruminant constructed from observations.
Description of the one-box model
The one-box model used in study is described in detail by ref. 42,43, and was recently used for studying methane sources from observed δ13CCH4-atm5. We commence our model integration at an imposed steady state in 1700 as in refs. 42,43, considering the methane concentration being fairly steady at near ca. 1700. As in ref. 43, we used bottom-up constructions of the methane source inventory (as inputs; see below), and then used the inferred sink history to close the budget for individual isotopologues (i.e., 12CH4 and 13CH4).
Global methane mass balance can be shown as:
| 17 |
where S(t), λ(t), and C(t) are the global methane source (Tg yr−1), sink (yr−1), and tropospheric burden (Tg), respectively, at time t. The model is performed at 1-year time step, thus ignoring seasonality. Assuming the source and sink are held constant within each time step, the analytic integration of Eq. (17) can be given by:
| 18 |
where the tropospheric burden (C(t); i.e., Cend and Cbeg) and source history (S(t)) are both specified at annual time steps. C(t) is proportional to global mean concentration (in ppb; see below on the inputs) with a conversion factor of 2.767 Tg ppb−1 60,61. With bottom-up constructions of the methane source inventory (S(t) as inputs; Supplementary Fig. 3), the mass-balancing sink history (λ(t)) is deduced by solving Eq. (18) numerically in successive time steps of λ (Supplementary Fig. 3c). With the usual assumption of λ13 = αλ12, where α is the sink weighted fractionation factor given by ε = α − 1 = −6.9‰5, we could deduce λ13(t) and λ12(t) separately from λ(t) by imposing mass balance for total methane and for each methane isotopologue (with S(t) and its isotopologue components S13(t) and S12(t) as inputs; Sect. 4 of ref. 43). S13(t) and S12(t) are calculated from S(t) for each inventory component using a representative δ13C from Table 1 of ref. 43 (e.g., −62‰ for livestock enteric emissions; Supplementary Table 6).
To quantify the effects of the revised FCH4-ruminant and δ13CCH4-ruminant individually or together on atmospheric CH4 concentration and δ13CCH4-atm, the model was run in forward mode (see below for the details of the simulations). A baseline simulation started in 1700, using the bottom-up constructions of the methane source inventory (S13(t) and S12(t)) and the deduced mass-balancing sink history (λ13(t) and λ12(t)) for each methane isotopologue over the historical period (1700–2012). R1 simulation implemented a perturbation in the baseline that commences in 1960 with the revised FCH4-ruminant and kept the rest sources identical to the baseline simulation (e.g., δ13CCH4-ruminant set to default value of −62‰ previously used by ref. 5,43). In R2 simulation, the revised FCH4-ruminant and δ13CCH4-ruminant (including the slightly decrease from −64.49‰ in 1961 to −64.93‰ in 2012) were used. For the spin-up period of 1700–1960 in R2 simulation, the revised δ13CCH4-ruminant of 1961 (−64.49‰) was used. To run the R2 simulation, we first re-calculated S13(t) and S12(t) using revised δ13CCH4-ruminant, and re-deduced λ13(t) and λ12(t) as inputs. The R3 simulation is the same as R2 but with constant δ13CCH4-ruminant at −64.49‰ for the period of 1961–2012. Therefore, the resulted differences between R1 and baseline are the effects of the revised FCH4-ruminant; the differences between R2 and R1 are the effects of the revised δ13CCH4-ruminant; and the differences between R3 and R2 are the effects of the δ13CCH4-ruminant variation (i.e., the slightly decrease from −64.49‰ in 1961 to −64.93‰ in 2012). It should be noted that the purpose of the simulations is not to reproduce the observed δ13CCH4-atm, but to get a reasonable δ13CCH4-atm in 1960, and the purpose of the perturbed simulations R1, R2, and R3 is to test the effects of the FCH4-ruminant and δ13CCH4-ruminant revisions on the post-1960 trends from an unperturbed 1960.
In the original box model, the mass-balancing sink (λ(t)) during the historical period (1700–2012) was tuned to match exactly atmospheric observations (Supplementary Fig. 3c). Here, we want to illustrate the effect of changing ruminant emission and its 13C source signature, and the results from our sensitivity tests thus deviate from atmospheric observations. A re-tuning of the model to match observations of mean concentration (with our revised FCH4-ruminant for the period of 1961–2012) is beyond the scope of this study, but would only require a change in the global mass-balancing sink of 2.4% during 1961–2012 (from 4.9% in 1961 to <1% after 2010; see difference between red and black lines in Supplementary Figure 3c), which is well within the uncertainty of the atmospheric sink. An additional sensitivity test shows that the re-tuning of the global mass-balancing sink only has marginal effect on the results presented in the Discussion section.
Inputs for the box model include the global methane concentration (Supplementary Fig. 3), and the construction of the methane source inventory from 1700 (Supplementary Table 6). The global methane concentration since 1984 is derived from observations of a globally distributed network (NOAA-ESRL record44,62). Data for 1700–1983 are from ref. 63, converted to the NOAA04 scale64 and adjusted by 45% of the inter-polar gradient to adjust Antarctic to global values5.
For the construction of the methane source inventory, we combined the natural and anthropogenic emissions of 1700 derived from Table 1 of ref. 43, the historical anthropogenic emissions65 (1850–2000) used by the Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP), and the EDGAR v4.3.210 for the recent decades (Supplementary Table 6). Five natural emission sources are used in this study following Table 1 of ref. 43: wetlands, termites, oceans, wild animals, and geologic sources. We assumed that the natural emissions are kept constant across history. Nine anthropogenic sources are used including fossil fuel (industry), waste treatment and landfill, rice cultivation, livestock enteric fermentation, manure management, and four pyrogenic sources (agricultural waste burning, forest burning, C3 grass burning, and savanna (C4 grass) burning). The emissions of 1700 are derived from Table 1 of ref. 43 except pyrogenic sources (see below). Reference 65 provides gridded (0.5° × 0.5°) historical anthropogenic methane emissions from 1850 to 2000. This dataset includes fossil fuel, waste treatment and landfill, and different pyrogenic sources (agricultural waste burning, forest burning, and grass burning), but gathers rice cultivation, livestock enteric fermentation, manure management as a single agricultural sector source. To separate grass burning into C3 grass burning and savanna (C4 grass) burning, we applied the gridded C3–C4 grass distribution derived from the approach described in ref. 38 based on growing season temperature. The EDGAR v4.3.210 provides emissions from fossil fuel, waste treatment and landfill, rice cultivation, livestock enteric fermentation, manure management, and agricultural waste burning for the period of 1970–2012. To separate the agricultural sector emissions from ref. 65 into different components (for the period of 1850–1970), we applied the relative fractions of rice cultivation, livestock enteric fermentation, and manure management from EDGAR v4.3.210 for the year 1970. For pyrogenic emissions, we did not use fire emissions (wildfires, forest burning and savanna burning) of 1700 in the Table 1 of ref. 43. Instead, we used pyrogenic emissions of ref. 65, and assumed that they are constant between 1700 and 1850. For each of the anthropogenic source, we applied linear interpolation between 1700 (Table 1 of ref. 43) and 185065, and between 1850 and 1970 (for each decade) to obtain annual emissions. For the year since 1970, values from the EDGAR v4.3.210 were used.
Supplementary information
Acknowledgements
J.C. and P.C. acknowledge support from the ERC Synergy grant ERC-2013-SyG-610028 IMBALANCE-P. S.P. acknowledges support from the National Natural Science Foundation of China (grant number 41722101). H.T. and S.D. acknowledge support from NSF (grant numbers: 1903722, 1243232) and NKRDPC (grant number 2017YFA0604702).
Author contributions
P.C., J.C., and S.P. designed the study. J.C. and S.P. carried out the analysis. J.C., S.P., and P.C. wrote most of the paper. M.S., S.R.S.D, M.H., P.H., H.T., and P.B. contributed significantly to the final revisions of the paper.
Data availability
The data that support the findings of this study are available at the public Data Repository of the International Institute of Applied Systems Analysis (IIASA DARE; 10.22022/ESM/06-2019.45). The source data underlying Figs. 1, 3, and 6 are provided as a Source Data file. The source data underlying Fig. 2 are provided by Supplementary Table 2. The source data underlying Figs. 4 and 5 can be found in the IIASA DARE (10.22022/ESM/06-2019.45).
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks Hinrich Schaefer, Zhiliang Tan and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-11066-3.
References
- 1.Myhre, G. et al. in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker, T. F. et al.). (Cambridge University Press, Cambridge, UK and New York, NY, USA, 2013).
- 2.Saunois M, et al. The global methane budget 2000–2012. Earth Sys. Sci. Data. 2016;8:697–751. doi: 10.5194/essd-8-697-2016. [DOI] [Google Scholar]
- 3.Bousquet P, et al. Contribution of anthropogenic and natural sources to atmospheric methane variability. Nature. 2006;443:439. doi: 10.1038/nature05132. [DOI] [PubMed] [Google Scholar]
- 4.Mikaloff Fletcher Sara E., Tans Pieter P., Bruhwiler Lori M., Miller John B., Heimann Martin. CH4sources estimated from atmospheric observations of CH4and its13C/12C isotopic ratios: 1. Inverse modeling of source processes. Global Biogeochemical Cycles. 2004;18(4):n/a-n/a. [Google Scholar]
- 5.Schaefer H, et al. A 21st-century shift from fossil-fuel to biogenic methane emissions indicated by 13CH4. Science. 2016;352:80–84. doi: 10.1126/science.aad2705. [DOI] [PubMed] [Google Scholar]
- 6.Schwietzke S, et al. Upward revision of global fossil fuel methane emissions based on isotope database. Nature. 2016;538:88–91. doi: 10.1038/nature19797. [DOI] [PubMed] [Google Scholar]
- 7.Sherwood OA, Schwietzke S, Arling VA, Etiope G. Global inventory of gas geochemistry data from fossil fuel, microbial and burning sources, version 2017. Earth Syst. Sci. Data. 2017;9:639–656. doi: 10.5194/essd-9-639-2017. [DOI] [Google Scholar]
- 8.Saunois M, et al. Variability and quasi-decadal changes in the methane budget over the period 2000–2012. Atmos. Chem. Phys. 2017;17:11135–11161. doi: 10.5194/acp-17-11135-2017. [DOI] [Google Scholar]
- 9.FAOSTAT Online Statistical Service. (Food and Agriculture Organization (FAO), 2017); http://faostat3.fao.org.
- 10.EDGARv4.3.2. http://edgar.jrc.ec.europa.eu/ (2018).
- 11.Dangal SRS, et al. Methane emission from global livestock sector during 1890–2014: magnitude, trends and spatiotemporal patterns. Glob. Chang. Biol. 2017;23:4147–4161. doi: 10.1111/gcb.13709. [DOI] [PubMed] [Google Scholar]
- 12.Wolf J, Asrar GR, West TO. Revised methane emissions factors and spatially distributed annual carbon fluxes for global livestock. Carbon Balance Manag. 2017;12:16. doi: 10.1186/s13021-017-0084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories (eds Eggleston, H. S., Buendia, L., Miwa, K., Ngara, T. & Tanabe, K.) https://www.ipcc-nggip.iges.or.jp (Institute for Global Environmental Strategies Hayama, Japan, 2006).
- 14.Ehleringer JR, Cerling TE. C3 and C4 photosynthesis. Encycl. Glob. Environ. Change. 2002;2:186–190. [Google Scholar]
- 15.Graven H, et al. Compiled records of carbon isotopes in atmospheric CO2 for historical simulations in CMIP6. Geosci. Model Dev. 2017;10:4405–4417. doi: 10.5194/gmd-10-4405-2017. [DOI] [Google Scholar]
- 16.Francey RJ, Farquhar GD. An explanation of 13C/12C variations in tree rings. Nature. 1982;297:28–31. doi: 10.1038/297028a0. [DOI] [Google Scholar]
- 17.Ciais P., Bousquet P., Freibauer A., Naegler T. Horizontal displacement of carbon associated with agriculture and its impacts on atmospheric CO2. Global Biogeochemical Cycles. 2007;21(2):n/a-n/a. [Google Scholar]
- 18.Herrero M, et al. Biomass use, production, feed efficiencies, and greenhouse gas emissions from global livestock systems. Proc. Natl Acad. Sci. USA. 2013;110:20888–20893. doi: 10.1073/pnas.1308149110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United States Environment Protection Agency (U.S. EPA). Global Anthropogenic Non-CO2Greenhouse Gas Emissions: 1990–2030. Revised December 2012. https://www.epa.gov/global-mitigation-non-co2-greenhouse-gases/global-anthropogenic-non-co2-greenhouse-gas-emissions (United States Environment Protection Agency, Washington DC, 2012).
- 20.Crutzen PJ, Aselmann I, Seiler W. Methane production by domestic animals, wild ruminants, other herbivorous fauna, and humans. Tellus B. 1986;38:271–284. doi: 10.3402/tellusb.v38i3-4.15135. [DOI] [Google Scholar]
- 21.Anastasi C, Simpson VJ. Future methane emissions from animals. J. Geophys. Res.: Atmospheres. 1993;98:7181–7186. doi: 10.1029/92JD02816. [DOI] [Google Scholar]
- 22.Mosier AR, et al. Mitigating agricultural emissions of methane. Clim. Change. 1998;40:39–80. doi: 10.1023/A:1005338731269. [DOI] [Google Scholar]
- 23.Clark, H., Pinares-Patino, C. & De Klein, C. in Grassland. A Global Resource (ed. McGilloway) 279–293 (Wageningen Academic Publishers, Wageningen, The Netherlands, 2005).
- 24.Opio, C. et al. Greenhouse Gas Emissions from Ruminant Supply Chains—a Global Life Cycle Assessment 1–214 (Food and agriculture organization of the United Nations (FAO), Rome, 2013).
- 25.Westberg H, Lamb B, Johnson KA, Huyler M. Inventory of methane emissions from U.S. cattle. J. Geophys. Res. -Atmos. 2001;106:12633–12642. doi: 10.1029/2000JD900808. [DOI] [Google Scholar]
- 26.Hristov AN, et al. Discrepancies and uncertainties in bottom-up gridded inventories of livestock methane emissions for the contiguous United States. Environ. Sci. Technol. 2017;51:13668–13677. doi: 10.1021/acs.est.7b03332. [DOI] [PubMed] [Google Scholar]
- 27.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2015 Revision, World Population 2015 Wallchart. ST/ESA/SER.A/378 (2015).
- 28.European Common Agricultural Policy (CAP), https://ec.europa.eu/info/food-farming-fisheries/key-policies/common-agricultural-policy/cap-glance_en.
- 29.European Commission, Commission staff working document accompanying the document. Report from the Commission to the Council and the European Parliament on the Implementation of Council Directive 91/676/EEC Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources Based on Member State Reports for the Period 2008–2011 {COM(2013) 683 final} (European Commission, Brussels, 2013).
- 30.Webb, J. et al. Study on Variation of Manure N Efficiency Throughout Europe (AEA Technology Plc, Didcot, 2011).
- 31.Farquhar GD, J. R. Ehleringer A, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Phys. 1989;40:503–537. doi: 10.1146/annurev.pp.40.060189.002443. [DOI] [Google Scholar]
- 32.Schulze E, Lohmeyer S, Giese W. Determination of 13C/12C-ratios in rumen produced methane and CO2 of cows, sheep and camels. Isot. Environ. Heal. S. 1998;34:75–79. doi: 10.1080/10256019708036334. [DOI] [PubMed] [Google Scholar]
- 33.van der Sleen P, et al. No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 2014;8:24. doi: 10.1038/ngeo2313. [DOI] [Google Scholar]
- 34.Peñuelas J, Canadell JG, Ogaya R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 2011;20:597–608. doi: 10.1111/j.1466-8238.2010.00608.x. [DOI] [Google Scholar]
- 35.Giguère-Croteau C, et al. North America’s oldest boreal trees are more efficient water users due to increased CO2 but do not grow faster. Proc. Natl Acad. Sci. USA. 2019;116:2749–2754. doi: 10.1073/pnas.1816686116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank DC, et al. Water-use efficiency and transpiration across European forests during the Anthropocene. Nat. Clim. Change. 2015;5:579. doi: 10.1038/nclimate2614. [DOI] [Google Scholar]
- 37.Tang X, et al. How is water-use efficiency of terrestrial ecosystems distributed and changing on Earth? Sci. Rep.-UK. 2014;4:7483. doi: 10.1038/srep07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Still CJ, Berry JA, Collatz GJ, DeFries RS. Global distribution of C-3 and C-4 vegetation: carbon cycle implications. Glob. Biogeochem. Cycles. 2003;17:1006. doi: 10.1029/2001GB001807. [DOI] [Google Scholar]
- 39.Reich PB, Hobbie SE, Lee TD, Pastore MA. Unexpected reversal of C3 versus C4 grass response to elevated CO2 during a 20-year field experiment. Science. 2018;360:317–320. doi: 10.1126/science.aas9313. [DOI] [PubMed] [Google Scholar]
- 40.Kohn MJ. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc. Natl Acad. Sci. USA. 2010;107:19691–19695. doi: 10.1073/pnas.1004933107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diefendorf AF, Mueller KE, Wing SL, Koch PL, Freeman KH. Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc. Natl Acad. Sci. USA. 2010;107:5738–5743. doi: 10.1073/pnas.0910513107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lassey KR, Lowe DC, Manning MR. The trend in atmospheric methane δ13C and implications for isotopic constraints on the global methane budget. Glob. Biogeochem. Cycles. 2000;14:41–49. doi: 10.1029/1999GB900094. [DOI] [Google Scholar]
- 43.Lassey KR, Etheridge DM, Lowe DC, Smith AM, Ferretti DF. Centennial evolution of the atmospheric methane budget: what do the carbon isotopes tell us? Atmos. Chem. Phys. 2007;7:2119–2139. doi: 10.5194/acp-7-2119-2007. [DOI] [Google Scholar]
- 44.NOAA/ESRL. www.esrl.noaa.gov/gmd/ccgg/trends_ch4.
- 45.Nisbet EG, et al. Rising atmospheric methane: 2007–2014 growth and isotopic shift. Glob. Biogeochem. Cycles. 2016;30:1356–1370. doi: 10.1002/2016GB005406. [DOI] [Google Scholar]
- 46.KTBL (Kuratorium fur Technic und Bauwessen in der Landwitschaft). KTBL Pocket Book Agriculture 2000/01 (in German), 20th edn. (Kuratorium fur Technic und Bauwessen in der Landwitschaft, Munster, 2000).
- 47.National Chicken Council. https://www.nationalchickencouncil.org/about-the-industry/statistics/u-s-broiler-performance/ (2018).
- 48.Losinger WC. Feed-conversion ratio of finisher pigs in the USA. Prev. Vet. Med. 1998;36:287–305. doi: 10.1016/S0167-5877(98)00094-4. [DOI] [PubMed] [Google Scholar]
- 49.Rabobank Research. https://research.rabobank.com/far/en/sectors/farm-inputs/Pigs_Might_Fly.html (2015).
- 50.Verheijen, L. A. H. M., Wiersema, D., Hulshoff Pol, L. W. & De Wit, J. Management of Waste from Animal Product Processing, International Agriculture Centre, Wageningen, The Netherlands. http://www.fao.org/WAIRDOCS/LEAD/X6114E/x6114e04.htm (1996).
- 51.Chang J, et al. Combining livestock production information in a process-based vegetation model to reconstruct the history of grassland management. Biogeosciences. 2016;13:3757–3776. doi: 10.5194/bg-13-3757-2016. [DOI] [Google Scholar]
- 52.Wei Y, et al. The North American carbon program multi-scale synthesis and terrestrial model intercomparison project—Part 2: Environmental driver data. Geosci. Model Dev. 2014;7:2875–2893. doi: 10.5194/gmd-7-2875-2014. [DOI] [Google Scholar]
- 53.Viovy, N. CRU-NCEPv4, CRUNCEP dataset, http://dods.extra.cea.fr/data/p529viov/cruncep/readme.htm (2013).
- 54.Rust F. Ruminant methane delta (13C/12C) values: relation to atmospheric methane. Science. 1981;211:1044–1046. doi: 10.1126/science.7466376. [DOI] [PubMed] [Google Scholar]
- 55.Metges C, Kempe K, Schmidt H-L. Dependence of the carbon-isotope contents of breath carbon dioxide, milk, serum and rumen fermentation products on the δ13C value of food in dairy cows. Brit. J. Nutr. 1990;63:187–196. doi: 10.1079/BJN19900106. [DOI] [PubMed] [Google Scholar]
- 56.Levin I, Bergamaschi P, Dörr H, Trapp D. Stable isotopic signature of methane from major sources in Germany. Chemosphere. 1993;26:161–177. doi: 10.1016/0045-6535(93)90419-6. [DOI] [Google Scholar]
- 57.Klevenhusen F, Bernasconi SM, Kreuzer M, Soliva CR. Experimental validation of the Intergovernmental Panel on Climate Change default values for ruminant-derived methane and its carbon-isotope signature. Anim. Prod. Sci. 2010;50:159–167. doi: 10.1071/AN09112. [DOI] [Google Scholar]
- 58.Klevenhusen F, et al. Efficiency of monolaurin in mitigating ruminal methanogenesis and modifying C-isotope fractionation when incubating diets composed of either C3 or C4 plants in a rumen simulation technique (Rusitec) system. Brit. J. Nutr. 2009;102:1308–1317. doi: 10.1017/S0007114509990262. [DOI] [PubMed] [Google Scholar]
- 59.Bilek RS, Tyler SC, Kurihara M, Yagi K. Investigation of cattle methane production and emission over a 24-hour period using measurements of δ13C and δD of emitted CH4 and rumen water. J. Geophys. Res. -Atmos. 2001;106:15405–15413. doi: 10.1029/2001JD900177. [DOI] [Google Scholar]
- 60.Fung I, et al. Three-dimensional model synthesis of the global methane cycle. J. Geophys. Res. -Atmos. 1991;96:13033–13065. doi: 10.1029/91JD01247. [DOI] [Google Scholar]
- 61.Dlugokencky EJ, Masarie KA, Lang PM, Tans PP. Continuing decline in the growth rate of the atmospheric methane burden. Nature. 1998;393:447–450. doi: 10.1038/30934. [DOI] [Google Scholar]
- 62.Dlugokencky Edward J, Nisbet Euan G, Fisher R, Lowry D. Global atmospheric methane: budget, changes and dangers. Philos. T. R. Soc. A. 2011;369:2058–2072. doi: 10.1098/rsta.2010.0341. [DOI] [PubMed] [Google Scholar]
- 63.MacFarling Meure, C. et al. Law Dome CO2, CH4 and N2O ice core records extended to 2000 years BP. Geophys. Res. Lett. 33, 10.1029/2006gl026152 (2006).
- 64.Dlugokencky, E. J. et al. Conversion of NOAA atmospheric dry air CH4 mole fractions to a gravimetrically prepared standard scale. J. Geophys. Res.-Atmos. 110, 10.1029/2005jd006035 (2005).
- 65.Lamarque JF, et al. Historical (1850–2000) gridded anthropogenic and biomass burning emissions of reactive gases and aerosols: methodology and application. Atmos. Chem. Phys. 2010;10:7017–7039. doi: 10.5194/acp-10-7017-2010. [DOI] [Google Scholar]
- 66.GLEAM. FAO Global Livestock Environmental Assessment Model; http://www.fao.org/gleam/en/. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available at the public Data Repository of the International Institute of Applied Systems Analysis (IIASA DARE; 10.22022/ESM/06-2019.45). The source data underlying Figs. 1, 3, and 6 are provided as a Source Data file. The source data underlying Fig. 2 are provided by Supplementary Table 2. The source data underlying Figs. 4 and 5 can be found in the IIASA DARE (10.22022/ESM/06-2019.45).