Abstract
Background
Definitive surgical repair of persistent fistulas of the small intestine remains a surgical challenge with a high rate of re-fistulation and mortality. The aim of this study was to evaluate the type and incidence of complications after definitive surgical repair, and to identify factors predictive of severe postoperative complications or fistula recurrence.
Material/Methods
This was a retrospective study of 42 patients who underwent elective surgical repair of a persistent fistula of the small intestine. The analysis included preoperative and intraoperative parameters.
Results
The healing rate after definitive surgery was 71.4%. Postoperative complications developed in 88.1% of patients. The mortality rate was 7.2%. Fistula recurrence was recognized in 21.4% of cases. Overall, 93 complications occurred in 37 patients. The most common complications were septic (48.0%). Hemorrhagic and digestive tract-related complications accounted for 19.0% and 15.0% of all complications, respectively. Severe complications (Clavien-Dindo grade III–V) made up 28.0% of all complications. In univariate analysis, multiple fistulas (p=0.03), higher C-reactive protein level (p=0.01), and longer time interval from admission to definitive surgery (p=0.01) were associated with an increased risk of severe complications or fistula recurrence. In multivariate analysis, only multiple fistulas were an independent risk factor for severe complications or fistula recurrence (OR=8.2, p=0.04).
Conclusions
Fistula complexity determines the risk of severe postoperative complications or fistula recurrence after definitive surgical repair of the persistent small intestine fistulas. Inflammatory parameters should be normalized before definitive surgery.
MeSH Keywords: Digestive System Surgical Procedures, Intestinal Fistula, Postoperative Complications, Risk Factors
Background
Gastrointestinal (GI) fistulas are one of the most severe complications after abdominal surgery. Between 34.4% and 65.0% of these fistulas originate from the small intestine [1–8]. Patients with intestinal fistulas require a specialized and multidisciplinary approach that often results in prolonged hospitalization and high costs. The initial management for intestinal fistulas is conservative and includes treatment of sepsis, nutritional support, control of fistula output, and skin care. Medical treatment is successful in 15.6–69.9% of patients [5,9–12]. Patients with persistent intestinal fistulas require surgical reconstruction. Definitive surgical repair of the small intestine fistulas remains a real challenge, even for experienced surgeons. Re-fistulation after definitive surgery reaches 31.0% [2–7,9,12–19]. The rate of postoperative mortality is relatively high and varies between 3.5% and 14% [12,14,20]. However, there is a paucity of data on postoperative outcomes other than fistula recurrence or mortality following operative reconstruction of intestinal fistulas. In addition, most studies report mixed case series comprising fistulas affecting different parts of the gastrointestinal tract [1–8].
The aim of this study was to evaluate the type and incidence of complications after surgical reconstruction of the small intestine fistulas. We also attempted to identify factors useful for prediction of severe postoperative complications or fistula recurrence.
Material and Methods
Patients
Between January 2004 and December 2011, 84 consecutive patients with a small bowel fistula were treated at our institution. This study included patients with persistent fistulas of the small intestine who underwent a planned definitive surgical repair. The exclusion criteria were the following: fistula not affecting the small intestine, need of emergent laparotomy, and age under 18 years. All patients were initially managed conservatively. Medical treatment consisted of sepsis and fistula output control, nutritional support, and wound management. Conservative therapy proved successful in 36 patients (43.3%). Three patients died before definitive surgery could be performed. Another 3 patients underwent emergent surgery; 2 of them died and 1 had fistula recurrence which healed on conservative treatment. Patient selection for the study is shown in Figure 1. Overall, 42 patients who underwent definitive surgical repair for persistent fistulas of the small intestine were included in this study. The cohort of patients was divided into Group I (25 patients who had only mild or no complications), and Group II (17 patients who experienced postoperatively severe complications or fistula recurrence). The study was approved by the Institutional Ethics Review Board on 12 Nov 2013.
Figure 1.
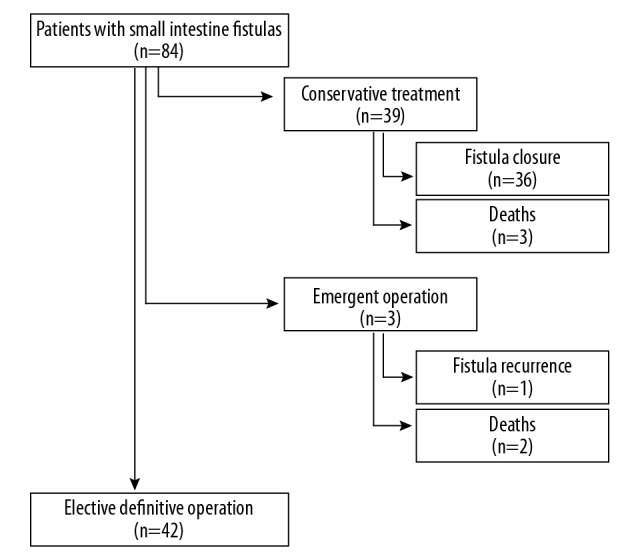
The flowchart shows selection of patients with small bowel fistulas for the study.
Classification of postoperative complications
This study included the complications that occurred within the first 30 postoperative days. The severity of complications was classified according to the Clavien-Dindo scale [21]. Clavien-Dindo grade I and II complications were defined as mild, and Clavien-Dindo grades III through V were classified as severe complications. Fistula recurrence was regarded as failure to cure, and as such was not reported as a complication. The spectrum and incidence of postoperative complications according to the Clavien-Dindo scale are summarized in Table 1. POSSUM and CR-POSSUM scores were calculated for each patient. In terms of POSSUM operative variables, all the operations were classified as major and elective. In addition, 1 point was assigned for simple suture repair of the fistula, 4 points for partial resection of the small intestine, and 8 points whenever a combined resection of the small and large intestines was performed.
Table 1.
The type and incidence of postoperative complications according to the Clavien-Dindo scale.
| Complication | Number |
|---|---|
| Grade I (34) | |
| SSI – bedside drainage | 27 |
| Wound hematoma – bedside evacuation | 4 |
| Eventration (dehiscence of all planes of the abdominal wall) | 1 |
| Urinary retention | 1 |
| Diarrhea | 1 |
| Grade II (33) | |
| Intestinal failure – TPN | 10 |
| Anemia – packed red cells transfusion | 8 |
| SSI treated with antibiotics | 3 |
| Arrhythmias treated pharmacologically | 2 |
| Diabetes | 1 |
| Hematuria | 1 |
| Hypothyroidism | 1 |
| End-jejunostomy syndrome | 1 |
| Intraabdominal collection treated with antibiotics | 1 |
| Depression | 1 |
| Pneumonia treated with antibiotics | 1 |
| Ileus – resolved spontaneously | 1 |
| Deterioration of mental status | 1 |
| Gastrointestinal bleeding | 1 |
| Grade III (15) | |
| Central venous catheter related infection (IIIa) | 8 |
| Subileus – Miller-Abbott tube insertion (IIIa) | 1 |
| Intraabdominal hemorrhage – reoperation (IIIb) | 3 |
| Intraabdominal abscess or peritonitis – reoperation (IIIb) | 2 |
| Billiary fistula (IIIb) | 1 |
| Grade IV (8) | |
| Respiratory failure (IVa) | 2 |
| Cardiac failure (IVa) | 1 |
| Septic shock – single organ disfunction (IVa) | 1 |
| Septic shock – multiple organ disfunction (IVb) | 2 |
| Multiple organ disfunction (IVb) | 2 |
| Grade V (3) | |
| Death | 3 |
SSI – surgical site infection; TPN – total parenteral nutrition.
Statistical analysis
The statistical analysis included preoperative and intraoperative parameters. Univariate analysis was performed using the Mann-Whitney U test. The factors which were at p<0.1 level in the univariate analysis were further included in the multivariate analysis. Multivariate analysis was performed using backward stepwise logistic regression. Hypothesis testing was performed using two-tailed α ≤0.05.
Results
The median age of patients was 56 years (IQR: 46–67). Males constituted 57% of all patients. The fistulas developed after a primary surgical intervention for ileus (23.8%), complications of acute pancreatitis (23.8%), hernia (9.6%), gastrointestinal perforation (9.6%), and Crohn’s disease (4.8%). In 2 patients (4.8%) fistulas were internal and occurred spontaneously secondary to Crohn’s disease. Forty patients had enterocutaneous fistulas, of which 7 (18.9%) were high-output and 30 (81.1%) were low-output. Data on the amount of discharge were not available for 3 patients. Definitive surgery was performed after a median period of 38 days (IQR: 14–61) since admission to our institution. The median operative time was 187.5 min (IQR: 160–235). The surgical procedures used for repair of the fistulas included partial resection of the small intestine in 15 (35.7%) patients, partial resection of both the small and large intestine in 15 (35.7%) patients, and simple suture repair in 7 (16.6%) patients. In the remaining 5 (12%) cases, partial resection of the small and/or large intestine was combined with an additional simple suture repair. Eight of 42 patients needed colostomy (n=3) or ileostomy (n=5). Colostomies were created after inadvertent full-thickness injury of the large bowel during dissection, whenever primary repair was regarded as high-risk for leakage. Abdominal wall defects in 9 (21.4%) patients required mesh placement; a composite and polypropylene mesh were used in 1 and 8 patients, respectively. Intestinal fistulas healed in 30 (71.4%) patients after definitive surgery. Fistula recurrence was observed in 9 (21.4%) patients. The fistulas recurred after a median period of 9 days (IQR: 7–23.5). Subsequent conservative treatment was successful in 5 of these patients. In 1 case, the fistula healed after another reoperation. Two patients were discharged with residual low-output fistulas. One patient left the hospital with a high-out fistula against medical advice and was lost to follow-up. Three patients died (7.1%). The postoperative course was uneventful in only 5 (11.9%) patients. Postoperative complications occurred in 37 (88.1%) patients. Overall, 37 patients experienced 93 complications. A median of 2 complications per patient (IQR: 1–3) was recorded. Most complications (72%) were mild and corresponded to Clavien-Dindo grade I or II. Of these, 34 complications were grade I and 33 were grade II. Severe complications accounted for 28.0% of all complications, including 15 cases of grade III and 8 cases of grade IV. Postoperative death (i.e., Clavien-Dindo grade V complication) occurred in 3 cases. All the patients with fistula recurrence had at least 1 severe complication postoperatively.
Septic complications were the most common and accounted for 48.0% of all complications. Surgical site infection was recognized in 32 (76.2%) patients after a median of 5 postoperative days (IQR: 5–8). Thirty of these patients had superficial or deep surgical wound infections that were managed by wound irrigation and drainage; antibiotics were added in 3 of these patients. In 2 (4.8%) patients who had organ space surgical site infections, a relaparotomy was required; 1 patient manifested diffuse peritonitis on the third postoperative day and underwent peritoneal lavage for multiple intraperitoneal abscesses, and the other patient had surgical drainage of an intraabdominal abscess on the 28th postoperative day. Central venous catheter-related infections requiring its removal occurred in 8 cases after a median time of 21 days after operation (IQR: 7–27). Three patients developed septic shock postoperatively; 2 of these patients recovered on medical treatment, and the third patient underwent a relaparotomy due to diffuse peritonitis and there was an intestinal anastomotic leak intraoperatively. Hemorrhagic complications constituted 19.0% of all complications. Acute intraabdominal bleeding requiring emergent reoperation occurred in 3 patients; all these patients died after reoperation. Eight patients required transfusion of packed red blood cells due to postoperative anemia. In 4 patients, healing of the surgical incision was complicated by hematomas that needed bedside drainage. One patient experienced a transient hematuria that resolved spontaneously.
Digestive tract-related complications constituted 15.0% of all complications. Ten patients required prolonged parenteral nutrition postoperatively, 2 of these patients had short bowel syndrome and 1 patient manifested an end-jejunostomy syndrome defined as metabolic disturbances associated with a high-output end stoma. One patient suffered protracted diarrhea, and 2 patients experienced ileus that resolved on medical treatment and after Miller-Abbot tube insertion.
Eight of 42 patients (19.0%) developed organ failure after surgical repair of the small intestine fistula. In 2 patients, prolonged mechanical ventilation was required due to respiratory failure. One patient had cardiac failure on the first postoperative day and required pharmacological support. In addition, 3 patients developed organ failure secondary to sepsis and 2 had an intraabdominal hemorrhage.
Univariate analysis, including the preoperative factors, is shown in Table 2. More than 1 fistula (p=0.029) and an elevated level of C-reactive protein (p=0.014) were significantly associated with risk of severe complications or fistula recurrence. Moreover, this risk was higher with a longer duration of hospitalization before definitive surgery (p=0.011). Other preoperative and intraoperative parameters in the univariate analysis did not influence the risk of severe complications or fistula recurrence (Tables 2, 3).
Table 2.
Risk factors of severe complications or fistula recurrence – univariate analysis: preoperative parameters.
| Parameter | Patients with mild or no complications (n=25) Group I |
Patients with severe complications or fistula recurrence (n=17) Group II |
p-Value |
|---|---|---|---|
| Fistula parameters | |||
| Fistula output on the day before surgery, ml/day; median (IQR) | 100 (300–50) | 150 (600–100) | 0.231 |
| Fistula output on the day before surgery, >500 ml/day; n (%) | 2 (8) | 5 (29) | 0.068 |
| Number of fistulas; median (IQR) | 1 (1–2) | 2 (4–1) | 0.029 |
| Number of fistulas >1, n(%) | 8 (32) | 11 (65) | 0.038 |
| Clinical parameters | |||
| Age, years; median (IQR) | 61.0 (67.0–47.0) | 55.0 (59.0–45.0) | 0.204 |
| Time since last operation, days, median (IQR) | 150.0 (332.0–73.0) | 85.5 (123.0–52.5) | 0.091 |
| Time since admission to definitive surgery, days median (IQR) | 23.0 (42.0–8.0) | 60.0 (89.0–34.0) | 0.011 |
| Systolic blood pressure, mmHg, median (IQR) | 120 (130–110) | 125 (130–110) | 0.725 |
| Mean blood pressure <70 mmHg, n (%) | 1 (4) | 1 (6) | 0.781 |
| Laboratory parameters | |||
| WBC >11×109/l, n (%) | 2 (8.0) | 4 (23.5) | 0.058 |
| RBC <3.8×1012/l, n (%) | 13 (52.0) | 10 (58.8) | 0.666 |
| HGB <10 g/dl, n (%) | 8 (32.0) | 7 (41.1) | 0.547 |
| HCT <30%, n (%) | 11 (44.0) | 7 (41.1) | 0.857 |
| PLT ×109/l, median (IQR) | 208 (351.0–208.0) | 348 (441.0–195.0) | 0.343 |
| Fibrinogen mg/dl, median (IQR) | 503 (564.0–395.5) | 558 (672.0–369.0) | 0.386 |
| Sodium <135 mmol/l, n (%) | 6 (24.0) | 7 (41.1) | 0.242 |
| Urea >48 mg/dl, n (%) | 5 (20.0) | 3 (17.6) | 0.850 |
| Creatinine >1.1 mg/dl, n (%) | 5 (20.0) | 1 (5.8) | 0.204 |
| Proteins <6.2 g/dl, n (%) | 7 (28.0) | 5 (29.4) | 0.852 |
| Albumins <3.0 g/dl, n (%) | 7 (28.0) | 9 (52.9) | 0.117 |
| Albumins g/dl, median (IQR) | 3.1 (3.4–2.7) | 2.8 (3.5–2.5) | 0.411 |
| Bilirubin >1.2 mg/dl, n (%) | 1 (4.0) | 1 (5.8) | 0.768 |
| AST > 40 U/l, n (%) | 6 (24.0) | 5 (29.4) | 0.696 |
| ALT >56 U/l, n (%) | 5 (20.0) | 2 (11.7) | 0.479 |
| CRP mg/l, median (IQR) | 7.6 (36.2–3.3) | 46.55 (89–11.4) | 0.014 |
| Scales | |||
| ASA III–IV, n (%) | 8 (32.0) | 9 (52.9) | 0.180 |
| APACHE II, median (IQR) | 8 (10–5) | 6 (7–4) | 0.053 |
Table 3.
Risk factors of severe complications or fistula recurrence – univariate analysis: intraoperative parameters.
| Parameter | Patients with mild or no complications (n=25) Group I |
Patients with severe complications or fistula recurrence (n=17) Group II |
p-Value |
|---|---|---|---|
| Operative time, min; median (IQR) | 180 (225–155) | 210 (240–180) | 0.066 |
| Operative time >4 hours, n (%) | 2 (8) | 4 (23) | 0.163 |
| Length of the resected intestine, cm; median (IQR) | 30 (45–15) | 50 (85–3) | 0.232 |
| Duration of the systolic blood pressure within 80–100 mmHg, min; median (IQR) | 50 (70–20) | 70 (100–20) | 0.368 |
| Duration of the systolic blood pressure <80 mmHg, min; median (IQR) | 0 (15–0) | 10 (50–0) | 0.178 |
| Intestinal resection, n (%) | 22 (88.0) | 14 (82.3) | 0.612 |
| Stoma, n (%) | 4 (16.0) | 4 (23.5) | 0.546 |
| Scales | |||
| POSSUM, median (IQR) | 36 (42–33) | 40 (43–32) | 0.797 |
| Physiological points, median (IQR) | 20 (24–17) | 22 (25–16) | 0.887 |
| Operative points, median (IQR) | 16 (18–14) | 18 (18–14) | 0.527 |
| CR-POSSUM, median (IQR) | 16 (17–15) | 15 (17–14) | 0.103 |
| Physiological points, median (IQR) | 9 (10–8) | 8 (8–7) | 0.056 |
| Operative points, median (IQR) | 7 (8–7) | 7 (7–7) | 0.497 |
Multivariate analysis, including the preoperative parameters, showed that the presence of more than 1 fistula was an independent risk factor of severe complication or fistula recurrence. The odds of severe complications or fistula recurrence were approximately 8 times higher in patients with more than 1 fistula (p=0.044) (Table 4). The intraoperative parameters, including the POSSUM scores, were not associated with a higher risk of severe complications or fistula recurrence.
Table 4.
Risk factors of severe complications or fistula recurrence – multivariate analysis.
| Variable | Odds ratio | 95% confidence interval | p-value |
|---|---|---|---|
| Preoperative parameters | |||
| Number of fistulas >1 | 8.20 | 1.05–63.83 | 0.044 |
| Fistula output on the day before surgery >500 ml/day | 2.84 | 0.25–31.77 | 0.395 |
| WBC > 11×109/l | 7.34 | 0.18–286.29 | 0.286 |
| Time since admission to definitive surgery | 1.01 | 0.97–1.04 | 0.488 |
| APACHE II score | 0.79 | 0.57–1.09 | 0.161 |
| Time since last operation | 0.99 | 0.99–1.00 | 0.434 |
| Intraoperative parameters | |||
| Duration of the systolic blood pressure <80 mmHg | 1.03 | 0.99–1.08 | 0.084 |
| Operative time | 1.00 | 0.98–1.02 | 0.827 |
| Sum of physiological points on CR-POSSUM scale | 1.16 | 0.48–2.81 | 0.728 |
Discussion
This is one of the first studies reporting type, frequency, and severity of complications after definitive surgical repair of small bowel fistulas. In contrast to other studies, which reported a case-mix of fistula locations with 67–88% of cases originating from the small intestine [5–7,13], our series included only fistulas involving the small intestine. The rate of fistula recurrence after definitive operation was comparable to that reported in other studies [3,6,7,9,12–14,19,20]. Similarly, postoperative mortality of approximately 7.0% fell within the range reported by other groups [14,20]. A recent meta-analysis by Vries et al. [22] found that the weighted pooled recurrence rate after reconstructive surgery for enteric fistula was 19% (95% CI 15–24) with a mortality rate of 3% (95% CI 2–5). In our study, the majority of patients suffered at least 1 complication, whereas only 12% of patients had an uneventful postoperative course. In comparison, Owen et al. [7] observed complications in 87.6% of patients. Similarly, the morbidity rate reported by Ravindran et al. [18] was 86%. Such a high rate of postoperative complications after repair of the small intestine fistulas reflects both the difficult operative field and the complexity of surgical procedures in already debilitated and fragile patients.
In our study, severe complications occurred in 40.5% of patients. The most common were septic complications, which accounted for almost half of all adverse postoperative events. Similarly, Owen et al. [7] reported septic episodes in 35.3% of patients managed surgically for gastrointestinal fistulas. In our series, infection usually involved the surgical wound (76.2%), whereas 4.8% of patients had an organ abscess or developed septic shock (7.1%). In contrast, Owen et al. [7] reported wound and organ infection after definitive surgical fistula repair in 16.4% and 14.4% of patients, respectively. The second most frequent septic complication in our study group was infection of the central venous catheter, which occurred in 19% of patients. Similarly, Owen et al. [7] found this complication in 26.1% of patients undergoing definitive surgery for enterocutaneous fistulas. Moreover, Visschers et al. [9] reported infection of the central venous catheter in 18.5% of patients with gastrointestinal fistulas; however, their series included patients treated both conservatively and surgically. The second most frequent postoperative complications in our study were hemorrhagic. Although a majority of patients required only transfusion of packed red blood cells, 3 patients underwent emergent relaparotomy for acute bleeding, and none of them survived. In comparison, Owen et al. [7] reported that 71.2% of patients after definitive surgery of gastrointestinal fistula in their study received blood products. The frequent need for blood transfusions in patients with enterocutaneous fistulas during the postoperative period might be attributed to extensive surgical dissection in already chronically ill people and anemic patients. In our study, approximately one-third of patients had a preoperative hemoglobin level below 10 g/dl. In such patients, even moderately complex operations will require blood transfusion, and acute bleeding might be life-threatening. Although digestive tract-related complications were less common and usually mild, some of them result in long-term sequelae. Two of our patients (4.8%) developed short bowel syndrome due to extensive intestinal resection. Both patients continue parenteral nutrition at home at 9 and 10 years following the definitive operation, respectively. Surprisingly, to the best of our knowledge, the rate of short bowel syndrome after definitive surgery for small bowel fistulas has not been reported in the literature.
One of the goals of this study was to determine which factors are predictive of severe complications or fistula recurrence after definitive surgery for small intestine fistula. Our statistical models included only pre- and intraoperative factors. We hypothesized that knowledge of these factors would allow adequate patient preparation for surgery, and would guide the surgeon’s decision making at the time of operation. In contrast to other authors, we decided not to include any postoperative factors in the analysis, because these should, in our opinion, be considered as complications. In this study, only the presence of multiple fistulas was independently associated with severe complications or fistula recurrence. Similarly, Mawdsley et al. [8] found out that fistula healing after definitive surgery was related only to the complexity of the fistula. In their study, the complex fistulas, which were defined as having several channels, affecting multiple intestinal loops, or passing through an abscess cavity, had a decreased likelihood of closure (OR=0.24; 95%CI 0.16–0.81; p=0.03). In comparison, Rahbour et al. [17] observed an increased risk of fistula recurrence in patients with comorbidities (OR=0.10; 95% CI: 0.02–0.49; p=0.02) and in patients who developed the fistula outside a specialist center (OR=0.14; 95% CI 0.03–0.67; p=0.01).
In the univariate analysis, severe complications or fistula recurrence were significantly associated with multiple fistulas (p=0.003), elevated CRP (p=0.01), and longer hospitalization preceding surgical treatment (p=0.01). Martinez et al. [23] found that a preoperative CRP level greater than 0.5 mg/dl was an independent risk factor of fistula recurrence after definitive surgery. Increased CRP level might indicate smoldering intraperitoneal infection, which warrants preoperative treatment; otherwise, it may be a source of infection postoperatively. In our study, 28.6% of patients had clinically silent interloop abscesses found intraoperatively. Similarly, Visschers et al. [9] showed that patients with sepsis developed recurrence of the fistula after definitive surgery more often than those who were not septic (16.0% vs. 1.9%; p=0.017).
The optimal timing of definitive surgery for enterocutaneous fistulas remains unclear. Brenner et al. [13] showed that operations performed before the 6th week or between the 13th and 36th weeks after fistula formation were associated with the lowest recurrence rates (8% and 10%, respectively), whereas no fistula recurrence was observed if surgical intervention took place within the first 2 weeks. Furthermore, fistula recurrence rates increased up to 36% and 27% if operations were performed after 36 weeks or between the 7th and 12th weeks, respectively. In multivariate analysis, delay in surgical intervention of more than 36 weeks significantly increased the risk of fistula recurrence (OR=5.4; 95% CI: 1.8–16.4; p=0.003). Interestingly, patients with severe complications or fistula recurrence in our study were hospitalized longer prior to definitive surgery. Worse outcomes in this subgroup probably reflect the fact that more complex fistulas required longer periods of preoperative preparation.
In this study, the selected prognostic scales did not show any value for prediction of severe postoperative complications or fistula recurrence. Owen et al. [7] showed that ASA grade did not influence the risk of fistula recurrence, but patients with ASA grade 4 had an increased 1-year risk of death (OR=3.02; 95% CI 1.47–6.24; p=0.003). Similarly, Lynch et al. [14] found that ASA grade did not predict fistula recurrence.
This study has some limitations. First, it was retrospective and the patient population was limited. Second, fistula recurrence was regarded as failure to cure and as such we did not assign it any Clavien-Dindo grade. Failure to cure corresponds to the situation when the primary purpose of the operation is not achieved. Accordingly, recurrence of a fistula after definitive surgery should not be classified as a complication. Arguably, a postoperative fistula after other surgical procedures would be considered a complication.
Conclusions
Definitive surgery in patients with small bowel fistulas results in a high rate of postoperative morbidity. Presence of multiple fistulas determines the risk of severe postoperative complications or fistula recurrence after definitive surgical repair of persistent small intestine fistulas. Inflammatory parameters should preferably be normalized before definitive operation. ASA, APACHE II, POSSUM, and CR-POSSUM scales did not show any utility in prediction of severe postoperative complications.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Draus JM, Jr, Huss SA, Harty NJ, et al. Enterocutaneous fistula: Are treatments improving? Surgery. 2006;140(4):570–76. doi: 10.1016/j.surg.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Gyorki DE, Brooks CE, Gett R, et al. Enterocutaneous fistula: A single-centre experience. ANZ J Surg. 2010;80(3):178–81. doi: 10.1111/j.1445-2197.2009.05086.x. [DOI] [PubMed] [Google Scholar]
- 3.Hollington P, Mawdsley J, Lim W, et al. An 11-year experience of enterocutaneous fistula. Br J Surg. 2004;91(12):1646–51. doi: 10.1002/bjs.4788. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Ren J, Zhu W, et al. Management of enterocutaneous fistulas: 30-year clinical experience. Chin Med J (Engl) 2003;116(2):171–75. [PubMed] [Google Scholar]
- 5.Martinez JL, Luque-de-Leon E, Mier J, et al. Systematic management of postoperative enterocutaneous fistulas. Factors related to outcomes. World J Surg. 2008;32(3):436–43. doi: 10.1007/s00268-007-9304-z. [DOI] [PubMed] [Google Scholar]
- 6.Taggarshe Deepa BD, Jacobs M, McKendrick A, Mittal V. Management of enterocutaneous fistuale: A 10 years experience. World J Surg. 2010;2(7):242–46. doi: 10.4240/wjgs.v2.i7.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen RM, Love TP, Perez SD, et al. Definitive surgical treatment of enterocutaneous fistula: Outcomes of a 23-year experience. JAMA Surg. 2013;148(2):118–26. doi: 10.1001/2013.jamasurg.153. [DOI] [PubMed] [Google Scholar]
- 8.Mawdsley JE, Hollington P, Bassett P, et al. An analysis of predictive factors for healing and mortality in patients with enterocutaneous fistulas. Aliment Pharmacol Ther. 2008;28(9):1111–21. doi: 10.1111/j.1365-2036.2008.03819.x. [DOI] [PubMed] [Google Scholar]
- 9.Visschers RG, Olde Damink SW, Winkens B, et al. Treatment strategies in 135 consecutive patients with enterocutaneous fistulas. World J Surg. 2008;32(3):445–53. doi: 10.1007/s00268-007-9371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haffejee AA. Surgical management of high output enterocutaneous fistulae: A 24-year experience. Curr Opin Clin Nutr Metab Care. 2004;7(3):309–16. doi: 10.1097/00075197-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Campos AC, Andrade DF, Campos GM, et al. A multivariate model to determine prognostic factors in gastrointestinal fistulas. J Am Coll Surg. 1999;188(5):483–90. doi: 10.1016/s1072-7515(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 12.Gupta M, Sonar P, Kakodkar R, et al. Small bowel enterocutaneous fistulae: The merits of early surgery. Indian J Surg. 2008;70(6):303–7. doi: 10.1007/s12262-008-0087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner M, Clayton JL, Tillou A, et al. Risk factors for recurrence after repair of enterocutaneous fistula. Arch Surg. 2009;144(6):500–5. doi: 10.1001/archsurg.2009.66. [DOI] [PubMed] [Google Scholar]
- 14.Lynch AC, Delaney CP, Senagore AJ, et al. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg. 2004;240(5):825–31. doi: 10.1097/01.sla.0000143895.17811.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez JL, Luque-de-Leon E, Ballinas-Oseguera G, et al. Factors predictive of recurrence and mortality after surgical repair of enterocutaneous fistula. J Gastrointest Surg. 2012;16(1):156–63. doi: 10.1007/s11605-011-1703-7. [DOI] [PubMed] [Google Scholar]
- 16.Mawdsley JE, Gibson P, Forbes A, Gabe SM. Technical report: Per-oral image-guided insertion of a jejunostomy feeding tube. Clin Radiol. 2004;59(10):951–53. doi: 10.1016/j.crad.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Rahbour G, Gabe SM, Ullah MR, et al. Seven-year experience of enterocutaneous fistula with univariate and multivariate analysis of factors associated with healing: Development of a validated scoring system. Colorectal Dis. 2013;15(9):1162–70. doi: 10.1111/codi.12363. [DOI] [PubMed] [Google Scholar]
- 18.Ravindran P, Ansari N, Young CJ, Solomon MJ. Definitive surgical closure of enterocutaneous fistula: Outcome and factors predictive of increased postoperative morbidity. Colorectal Dis. 2014;16(3):209–18. doi: 10.1111/codi.12473. [DOI] [PubMed] [Google Scholar]
- 19.Runstrom B, Hallbook O, Nystrom PO, et al. Outcome of 132 consecutive reconstructive operations for intestinal fistula-staged operation without primary anastomosis improved outcome in retrospective analysis. Scand J Surg. 2013;102(3):152–57. doi: 10.1177/1457496913490452. [DOI] [PubMed] [Google Scholar]
- 20.Rinsema W, Gouma DJ, von Meyenfeldt MF, et al. Primary conservative management of external small-bowel fistulas. Changing composition of fistula series? Acta Chir Scand. 1990;156(6–7):457–62. [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries FEE, Atema JJ, van Ruler O, et al. A systematic review and meta-analysis of timing and outcome of intestinal failure surgery in patients with enteric fistula. World J Surg. 2018;42(3):695–706. doi: 10.1007/s00268-017-4224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez JL, Luque-de-Leon E, Ferat-Osorio E, Estrada-Castellanos A. Predictive value of preoperative serum C-reactive protein for recurrence after definitive surgical repair of enterocutaneous fistula. Am J Surg. 2017;213(1):105–11. doi: 10.1016/j.amjsurg.2016.05.008. [DOI] [PubMed] [Google Scholar]


