Abstract
Background
Wilms tumor (WT) is the most common type of pediatric renal malignancy, and is associated with poor prognosis. The aim of the present study was to identify microRNA (miRNA) signatures which might predict prognosis and categorize WTs into high- and low-risk subgroups.
Material/Methods
The miRNA expression profiles of WT patients and normal samples were obtained from the Therapeutically Applicable Research to Generate Effective Treatment database. Differentially expressed miRNAs between WT patients and normal samples were identified using the EdgeR package. Subsequently, correlations between differentially expressed miRNAs and the prognosis of overall survival were analyzed. Enrichment analyses for the targeted mRNAs were conducted via the Database for Annotation, Visualization, and Integration Discovery.
Results
A total of 154 miRNAs were identified as differentially expressed in WT. Of those, 18 miRNAs were associated with overall survival (P<0.05). A prognostic signature of 5 differentially expressed miRNAs (i.e., has-mir-149, has-mir-7112, has-mir-940, has-mir-1248, and has-mir-490) was constructed to classify the patients into high- and low-risk subgroups. The targeted mRNAs of these prognostic miRNAs were primarily enriched in Gene Ontology terms (i.e., protein autophosphorylation, protein dephosphorylation, and stress-activated MAPK cascade) and the Kyoto Encyclopedia of Genes and Genomes signaling pathways (i.e., MAPK, AMPK, and PI3K-Akt).
Conclusions
The 5-miRNA signature model might be useful in determining the prognosis of WT patients. As a promising prediction tool, this prognosis signature might serve as a potential biomarker for WT patients.
MeSH Keywords: MicroRNAs, Prognosis, Wilms Tumor
Background
Wilms tumor (WT) is a rare common type of pediatric renal malignancy, affecting approximately one in 10 000 children (aged <15 years) [1]. WT is associated with a poor prognosis. However, the rate of 5-year overall survival (OS) is constantly improving in response to the advancement of disease-associated therapies [2]. Prognostic tumor biomarkers play a vital role in the diagnosis and treatment of tumors. The identification of low-risk WT patients might lead to the administration of treatment with less morbidity. In addition, clinicians might apply more aggressive treatments in WT patients identified as at high risk of poor prognosis according to the results of the prognostic biomarker test. Therefore, novel biomarkers or prognostic models for the prediction of survival in WT are urgently warranted. This research regarding prognostic biomarkers might be an important step in improving the prognosis of patients with WT.
MicroRNAs (miRNAs) are single-stranded (18–25 nucleotide-long) noncoding RNAs which target mRNAs to control gene expression [3]. MiRNAs restrain protein synthesis through de-adenylation and repression of translation, and the subsequent degradation of mRNA targets [4]. The expression of >30% of human genes is regulated by miRNAs, indicating that miRNAs might play a pivotal role in numerous biological functions [5]. Aberrant expression of miRNAs is realized as an important step in the development of cancer [6]. Notably, the expression of miRNAs has been shown to be stable even in samples obtained from formalin-fixed paraffin-embedded tissues [4]. Such dysregulated miRNAs might be promising therapeutic targets. Previous studies reported that numerous key miRNAs (e.g., miR-483-5p [7] and miR-195 [8]) are closely related to the pathogenesis of WT. However, these studies were focused on a limited number of investigated miRNAs from a small number of patients or different miRNA-chip platforms. It is likely that multiple miRNAs (signature model) might provide more statistically predictive results. Thus, there is an urgent need to apply larger patient cohort data to the investigation of more specific prognostic classifiers in WT patients.
The Therapeutically Applicable Research to Generate Effective Treatment (TARGET) database has publicized large amounts of miRNA sequencing data, which might provide insight into the molecular mechanism involved in the progression and tumorigenesis of WT. The aim of the present study was to identify patterns of differentially expressed miRNAs (DEMs) between normal and WT samples via the use of advanced RNA sequencing analysis. Additionally, the prognostic value of these DEMs was evaluated. Furthermore, a 5-miRNA signature model was constructed to effectively predict the prognosis of patients with WT.
Material and Methods
Data collection and preprocessing
All miRNA expression data were downloaded from the publicly available TARGET database. The miRNA expression data and corresponding clinical information of 138 samples, including 6 normal samples and 132 WT samples, were analyzed in the present study. Considering that the data were obtained from the TARGET database, approval of the study by an ethics committee was not required. The EdgeR package in the R software (version 3.2.2) was used to analyze DEMs between normal and WT samples. The cutoff criteria were defined as |logFC(fold change)| >2 and a false discovery rate of < 0.05.
Risk stratification and receiver operating characteristic (ROC) curve
DEMs with a P<0.05 in the univariate Cox regression analysis were considered as candidate variables for inclusion in the multivariate Cox regression analysis. The risk scores of 127 patients with WT were calculated according to the predictive 5-miRNA signature model. The classification cutoff value between the low- and high-risk subgroups was defined as the median risk score. Differences in OS time between the low- and high-risk WT subgroups were compared via the log-rank test and Kaplan-Meier analysis. The receiver operating characteristic (ROC) curve and area under curve (AUC) values were used to evaluate the specificity and sensitivity of this 5-miRNA prognosis signature model. All the analyses were conducted using the R software (version: 3.3.2).
Survival analysis
Prognostic DEM signatures were identified via the log-rank test and Kaplan-Meier analysis. The survival curves were constructed using the “survival” package in the R software (version: 3.3.2). The OS was defined as the survival endpoint in the present survival analysis. A P<0.05 denoted statistical significance.
Prediction of target genes and functional enrichment analysis
The online tools MiRTarBase [9] (http://mirtarbase.mbc.nctu.edu.tw), MiRDB [10] (http://www.mirdb.org/miRDB/), and TargetScan [11] (http://www.targetscan.org/) were used to predict target mRNAs for all prognostic miRNAs. To minimize false positives and screen most putative targets, target mRNAs, which were predicted by all 3 online programs, were selected as input for the functional enrichment analyses. Gene Ontology (GO) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of DEMs were performed via the Database for Annotation, Visualization, and Integrated Discovery in an attempt to elucidate the mechanism of tumorigenesis in WT.
Results
DEMs in WT
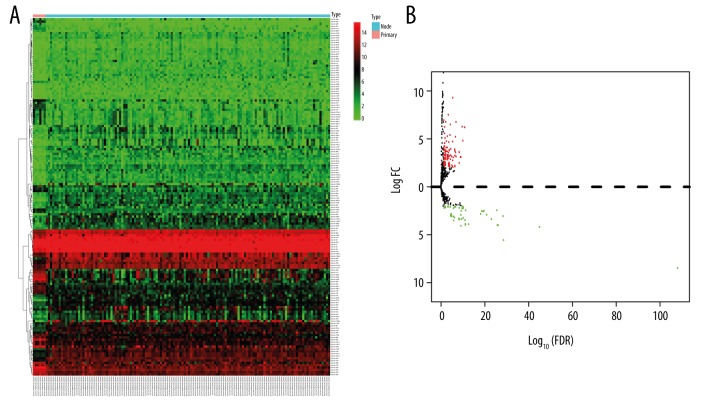
The miRNA expression profiles between normal and WT samples acquired from the TARGET database were analyzed. According to the cutoff threshold of |logFC(fold change)| >2 and a false discovery rate of <0.05, 105 upregulated and 49 downregulated DEMs were identified in WT. The heatmap plot and volcano plot of the related DEMs between normal and WT samples are presented in Figure 1. The top 10 upregulated and downregulated DEMs are shown in Table 1.
Figure 1.
Heatmap analysis (A) and volcano plot (B) of differentially expressed micoRNAs.
Table 1.
The top 10 upregulated and downregulated differentially expressed microRNAs.
| Genesymbol | LogFC | FDR | Change |
|---|---|---|---|
| hsa-mir-934 | −8.458976144 | 8.18E-109 | Down |
| hsa-mir-203a | −5.559616525 | 2.43E-29 | Down |
| hsa-mir-29c | −4.184218278 | 8.62E-46 | Down |
| hsa-mir-506 | −4.068436968 | 7.11E-10 | Down |
| hsa-mir-29a | −4.000404843 | 1.54E-26 | Down |
| hsa-mir-30a | −3.996893892 | 7.42E-27 | Down |
| hsa-mir-514a-1 | −3.966692055 | 6.42E-12 | Down |
| hsa-mir-514a-2 | −3.896476522 | 2.33E-13 | Down |
| hsa-mir-514a-3 | −3.863700282 | 5.84E-12 | Down |
| hsa-mir-203b | −3.503204575 | 1.32E-06 | Down |
| hsa-mir-383 | 9.298659023 | 4.06E-06 | Up |
| hsa-mir-1269a | 7.525185748 | 0.000415743 | Up |
| hsa-mir-1269b | 6.968194063 | 0.046671257 | Up |
| hsa-mir-767 | 6.775915413 | 0.006257893 | Up |
| hsa-mir-548f-1 | 6.766938092 | 1.24E-07 | Up |
| hsa-mir-2115 | 6.512683834 | 4.88E-05 | Up |
| hsa-mir-301b | 6.276573984 | 1.89E-10 | Up |
| hsa-mir-105-1 | 6.260382994 | 0.019280135 | Up |
| hsa-mir-483 | 6.210785374 | 1.38E-11 | Up |
| hsa-mir-105-2 | 5.578850103 | 0.025007756 | Up |
FC – fold change; FDR – false discovery rate.
Establishment of a five-miRNA signature model was associated with OS of WT patients
Univariate and multivariate Cox regression analyses were used to evaluate associations between the expression levels of DEMs and the OS of WT patients. Five DEMs (i.e., has-mir-149, has-mir-7112, has-mir-940, has-mir-1248, and has-mir-490) were selected from the stepwise multivariate Cox regression analysis to establish a predictive signature model. In the present study, this model was defined as follows: survival risk score=(0.263391572×expression value of has-mir-149) + (−0.229979121288481×expression value of has-mir-7112) + (0.427344897140062×expression value of has-mir-940) + (0.227391121990691×expression value of has-mir-1248) + (−0.147764549439855 x expression value of has-mir-490).
Risk stratification and ROC curve analysis
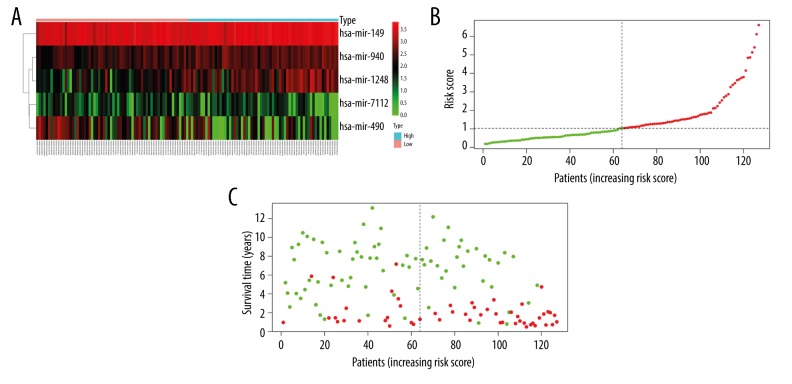
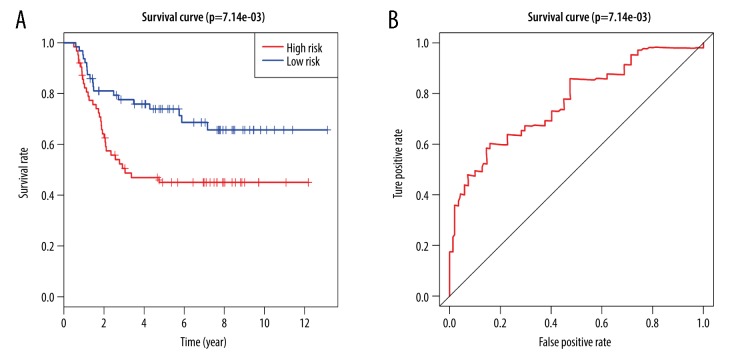
The 5-miRNA expression-based scores of 127 WT patients were calculated. According to the median risk score, these WT patients were assigned to a high-risk group (n=63) or a low-risk group (n=64) (Figure 2). In the current study, the AUC value of the ROC curve was applied to evaluate the prognostic power of the 5-miRNA signature model (Figure 3). Higher AUC values were indicative of greater predictive ability by the model. In addition, AUC values >0.70 denoted excellent model performance. In the signature model of the present study, the AUC value obtained from the ROC curve analysis was 0.767. This finding indicated good specificity and sensitivity of the 5-miRNA signature model in predicting the survival risk of WT patients.
Figure 2.
MicroRNA predictor-score analysis of Wilms tumor patients. (A) Heatmap of miRNA expression profiles of Wilms tumor patients. (B) MicroRNA predictor-score distribution. (C) Patients’ survival status.
Figure 3.
Kaplan-Meier and (receiver operating characteristic) ROC curves for the 5-miRNA signature model. (A) The Kaplan-Meier curves for entire Wilms tumor cohort divided by the optimum cutoff point. (B) The ROC curve for predicting overall survival in the Wilms tumor cohort.
Survival analysis
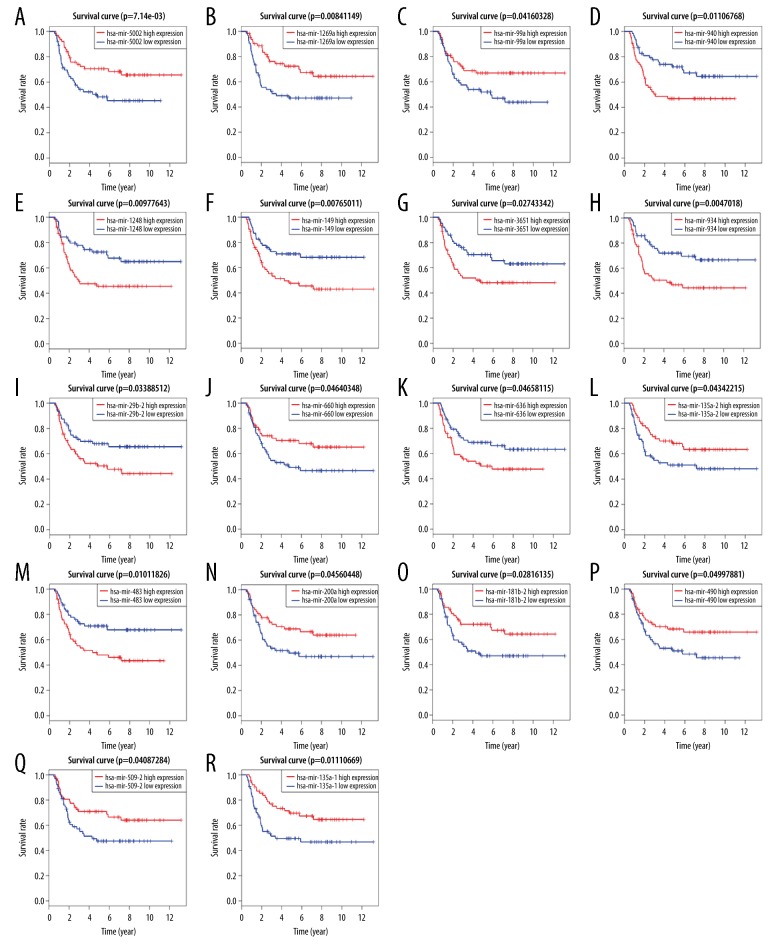
A Kaplan-Meier univariate survival analysis was performed by combining information of gene expression profiles and the clinical information of WT samples. Among the 154 DEMs examined in this study, 18 miRNAs (i.e., hsa-mir-29b-2, hsa-mir-99a, hsa-mir-135a-1, hsa-mir-135a-2, hsa-mir-149, hsa-mir-181b-2, hsa-mir-200a, hsa-mir-483, hsa-mir-490, hsa-mir-509-2, hsa-mir-636, hsa-mir-660, hsa-mir-934, hsa-mir-940, hsa-mir-1248, hsa-mir-1269a, hsa-mir-3651, and hsa-mir-5002) were closely related to OS in WT patients (Figure 4). Low expression of hsa-mir-29b-2, hsa-mir-149, hsa-mir-483, hsa-mir-636, hsa-mir-934, hsa-mir-940, hsa-mir-1248, and hsa-mir-3651, was related to a high OS rate in WT patients. Notably, high expression of hsa-mir-99a, hsa-mir-135a-1, hsa-mir-135a-2, hsa-mir-181b-2, hsa-mir-200a, hsa-mir-490, hsa-mir-509-2, hsa-mir-660, hsa-mir-1269a, and hsa-mir-5002 was related to high survival rates in WT patients.
Figure 4.
(A–R) Prognostic significance of differentially expressed microRNAs.
Prediction of target mRNA and functional enrichment analysis
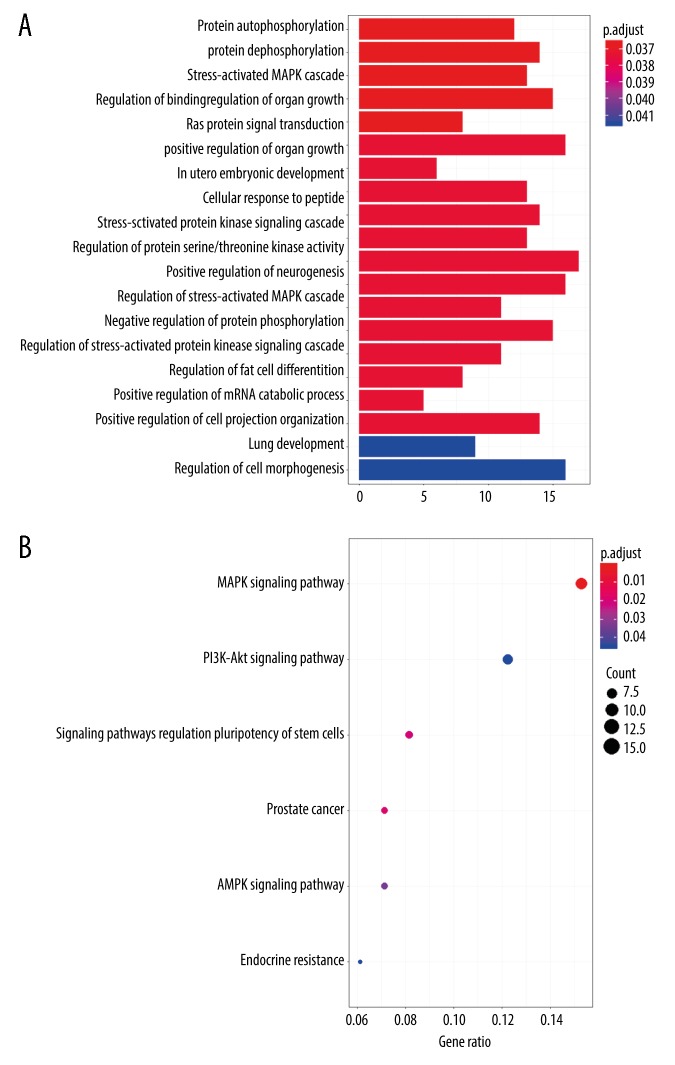
The online analysis tools TargetScan, miRDB, and miRTarBase were used to predict target mRNAs of prognostic DEMs. The overlapping target genes predicted by all 3 online programs were identified as target mRNAs. The results of the GO analysis showed that target mRNAs were mainly enriched in protein autophosphorylation, protein dephosphorylation, the stress-activated MAPK cascade, regulation of binding, and regulation of organ growth (Figure 5A, Table 2). According to the KEGG analysis, 6 statistically significant pathways were identified: MAPK signaling pathway, signaling pathways regulating the pluripotency of stem cells, AMPK signaling pathway, endocrine resistance, PI3K-Akt signaling pathway, and prostate cancer pathway (Figure 5B).
Figure 5.
Functional Gene Ontology (A) and Kyoto Encyclopedia of Genes (B) enrichment analysis of targeted mRNAs.
Table 2.
The Enriched Gene Ontology Terms (top 10) and Kyoto Encyclopedia of Genes and Genomes of Target mRNAs.
| Category | Term | ID | FDR | Targeted mRNA |
|---|---|---|---|---|
| GO | Focal adhesion | GO: 0005925 | 0.015653541 | MYH9/ARHGAP31/ITGA3/CALR/GIT1/ARHGAP22/DLC1/GNA13/KLF11/EPHA2/STX16/ALCAM/YWHAE/ADD1/FGFR3 |
| GO | Cell-substrate adherens junction | GO: 0005924 | 0.015653541 | MYH9/ARHGAP31/ITGA3/CALR/GIT1/ARHGAP22/DLC1/GNA13/KLF11/EPHA2/STX16/ALCAM/YWHAE/ADD1/FGFR3 |
| GO | Cell-substrate junction | GO: 0030055 | 0.015653541 | MYH9/ARHGAP31/ITGA3/CALR/GIT1/ARHGAP22/DLC1/GNA13/KLF11/EPHA2/STX16/ALCAM/YWHAE/ADD1/FGFR3 |
| GO | Protein autophosphorylation | GO: 0046777 | 0.036590366 | AKT1/MINK1/FGFR1/MARK2/STK11/RAP2C/EPHA7/MAP3K9/TAOK1/EPHA4/FGFR3/MTOR |
| GO | Protein dephosphorylation | GO: 0006470 | 0.036590366 | CTDSP2/DUSP7/STK11/PTPRK/DLC1/UBASH3B/PTPRD/PPP1R15B/ARPP19/DUSP1/YWHAE/CTDSPL/MTMR3/MTOR |
| GO | Stress-activated MAPK cascade | GO: 0051403 | 0.036590366 | AKT1/MINK1/WNT7B/ZFP36L1/FOXM1/ZEB2/ZNF675/MAPKAPK2/MAP3K9/TAOK1/EPHA4/DUSP1/HMGB1 |
| GO | Regulation of binding | GO: 0051098 | 0.036590366 | AKT1/MARK2/CARM1/DISC1/UBASH3B/ZNF675/FOXC1/IGF1/EPHA4/SYAP1/PYGO2/RAPGEF2/HMGB1/STPG1/ADD1 |
| GO | Regulation of organ growth | GO: 0046620 | 0.036590366 | AKT1/FGFR1/CARM1/YAP1/FOXC1/IGF1/RBPJ/MTOR |
| GO | Ras protein signal transduction | GO: 0007265 | 0.037327922 | RAB11B/PLEKHG2/IQSEC3/ITGA3/NGFR/RAB7A/FOXM1/RAP2C/GRB2/OGT/DLC1/GNA13/RAB8B/RAB5B/IGF1/RAPGEF2 |
| GO | Positive regulation of organ growth | GO: 0046622 | 0.037327922 | AKT1/FGFR1/YAP1/IGF1/RBPJ/MTOR |
| KEGG | MAPK signaling pathway | hsa04010 | 0.000605175 | AKT1/MKNK2/FGFR1/NGFR/CACNB1/DUSP7/GRB2/EPHA2/MAPKAPK2/IGF1/TAOK1/FOS/DUSP1/RAPGEF2/FGFR3 |
| KEGG | Prostate cancer | hsa05215 | 0.018238418 | AKT1/FGFR1/GRB2/CCNE2/ZEB1/IGF1/MTOR |
| KEGG | Signaling pathways regulating pluripotency of stem cells | hsa04550 | 0.020225637 | AKT1/ZFHX3/FGFR1/WNT7B/GRB2/IGF1/SKIL/FGFR3 |
| KEGG | AMPK signaling pathway | hsa04152 | 0.033440098 | AKT1/RAB11B/STK11/SREBF1/SCD5/IGF1/MTOR |
| KEGG | Endocrine resistance | hsa01522 | 0.044793852 | AKT1/CARM1/GRB2/IGF1/FOS/MTOR |
| KEGG | PI3K-Akt signaling pathway | hsa04151 | 0.044793852 | AKT1/ITGA3/FGFR1/NGFR/STK11/GRB2/CCNE2/EPHA2/IGF1/YWHAE/FGFR3/MTOR |
GO – Gene Ontology, KEGG – Kyoto Encyclopedia of Genes; FDR – false discovery rate.
Discussion
WT is a type of pediatric renal malignancy. Poor prognosis remains the main cause of cancer-specific death in patients with WT [2]. The current treatment strategies against WT rely on attempts to investigate biomarkers reflecting prognosis and predict the risk of patients. MiRNAs are promising biomarkers for tumor diagnosis and prognosis. In recent years, miRNAs have been reported as prognostic biomarkers for the diagnosis and prognosis of WT patients. WT is a multifactorial and highly complex tumor disease, and numerous dysregulated miRNAs are involved in tumorigenesis and progression. Therefore, a model involving the combination of multiple miRNAs might provide more detailed and accurate information regarding the diagnosis and prognosis of WT versus a single miRNA.
MiRNAs target mRNAs to control gene expression [3]. In the present study, a total of 105 upregulated and 49 downregulated DEMs were identified. Of the 154 DEMs detected, 18 DEMs were associated with OS in WT patients (P<0.05). Several DEMs detected via our analysis have been previously investigated in WT. For example, miR-483 was shown to cooperate with IGF2 and serve as an autonomous oncogene in WT [12]. The oncogenic mechanism of miR-483 was partially demonstrated by research findings indicating that miR-483 might modulate the proapoptotic protein (BBC3/PUMA) to protect cells from apoptosis [12]. Additionally, information regarding the regulatory role of hsa-mir-29b-2, hsa-mir-99a, hsa-mir-135a-1, hsa-mir-135a-2, hsa-mir-200a, hsa-mir-181b-2, hsa-mir-509-2, hsa-mir-636, hsa-mir-660, hsa-mir-934, hsa-mir-1248, hsa-mir-1269a, hsa-mir-3651, hsa-mir-149, hsa-mir-940, hsa-mir-490, and hsa-mir-5002 in WT is currently limited. A previous study showed that these DEMs might be related to oncogenesis. For example, miR-29b-2 was reported to improve prognosis in pancreatic ductal adenocarcinoma by targeting Cbl-b and promoting the expression of p53 [13]. In addition, miR-99a might downregulate 2 novel oncogenic proteins (i.e., E2F2 and EMR2), and repress stemness in lung cancer [14]. Moreover, miR-135a-1 might target EGFR to inhibit cell growth and migration in prostate cancer [15]. Furthermore, miR-200a was shown to target FOXA1 and act as a tumor suppressor on the survival, proliferation, and invasion of glioma cells [16]. Previous studies also reported that miR-200a is associated with the development of cancer (i.e., esophageal, breast, and endometrial), by targeting specific genes such as CRMP1, EPHA2, and PTEN [17–19]. Notably, miR-509-2 was differentially expressed in early-stage cervical cancer and might serve as a diagnostic biomarker in these patients [20]. Also, miR-636 acted as a tumor suppressor miRNA, which might affect the tumorigenesis of hepatocellular carcinoma through the downregulation of Ras [21]. Suppression of ANT2 by shRNA might restore the expression of miR-636 to play an anticancer role in hepatocellular carcinoma [21]. Of note, mir-660 might reduce the expression of MDM2 and stabilize the protein levels of p53 to act as a tumor suppressor miRNA in lung tumors [22]. Furthermore, miR-934 was significantly upregulated in estrogen receptor-negative breast cancer, and its expression was related to that of VGLL1 [23]. Suppression of miR-1248 might induce AGTR1-mediated cancer cell death to improve the outcome of chemotherapy against osteosarcoma [24]. MiR-1269a promotes tumor metastasis and forms a positive feedback loop with TGF-β signaling in colorectal cancer [25]. The role of these DEMs in the progression of WT is currently not well understood. Therefore, additional targeted molecular studies are required to evaluate their involvement in this process. In addition, further studies are warranted to illuminate the molecular and biological mechanisms of these DEMs.
GO and KEGG analyses of 18 prognostic DEMs (targeted mRNAs) were conducted to further investigate the related cellular mechanisms in WT. The results of the GO analysis showed that that target mRNAs were mainly enriched in protein autophosphorylation, protein dephosphorylation, the stress-activated MAPK cascade, regulation of binding, and regulation of organ growth. The results of the KEGG analysis indicated that target mRNAs were mainly enriched in the MAPK signaling pathway, signaling pathways regulating the pluripotency of stem cells, AMPK signaling pathway, endocrine resistance, PI3K-Akt signaling pathway, and prostate cancer pathway. In recent years, several biological processes and signaling pathways identified through our analysis have been investigated in WT. The stress-activated MAPK signaling pathway might serve as a tumor suppressor and common pro-oncogenic signal in the process of tumorigenesis [26,27]. Notably, a new candidate drug induced apoptosis of WT cells through the MAPK pathway [28]. Berberine was reported to activate AMPK signaling for the regulation of tumor progression and metastasis in WT [29]. Recent evidence indicated that the PI3K-AKT-p53 signaling pathway participated in the tumorigenesis of WT. This finding might represent a potential treatment strategy in the future [30]. The aforementioned evidence was partially consistent with the results obtained through the GO and KEGG analyses in the present study.
An increasing number of studies suggest that the expression of miRNAs in WT plays an important role in diagnosis and prognosis. Ludwig et al. identified circulating miRNAs (i.e., miR-130b-3p, miR-100-5p, and miR-143-3p) with a high diagnostic potential in WT [31]. Apart from circulating miRNAs, miRNAs in WT tissues have also been studied. For example, miR-483-5p was shown to be predictive of an adverse prognosis in WT [32]. Koller et al. investigated the expression levels of miR-204 and found that its expression was significantly decreased in 23 WT samples versus normal renal parenchyma [33]. An upregulated expression of miR-21 was reported to have a possible correlation with aggressive progression and poor prognosis in WT [34]. In the present study, we demonstrated that a miRNA signature model – instead of a single miRNA – constructed from the TARGET database might be a suitable prognostic biomarker. A 5-miRNA model was established, and this signature model was used to assign patients to different risk subgroups according to the characteristics of their tumors. Similar multi-miRNA prognostic models have been established in numerous types of tumors. For example, a 4-miRNA model (i.e., miR-4758, miR-876, miR-142, and miR-190b) was identified to serve as an effective independent prognostic factor for the prediction of OS in patients with endometrial cancer [35]. In addition, an 8-miRNA model was reported to effectively predict OS in WT and assist clinicians in screening WT patients at high risk of unfavorable OS.
However, there were several limitations in the present study. Firstly, the miRNA expression data used for the signature model were obtained from a single database (TARGET). The performance of the 5-miRNAs signature model might be more accurately determined following additional validation with independent external miRNA expression data. The Gene Expression Omnibus and the Cancer Genome Atlas datasets were investigated for further validation. However, currently, there are no databases containing full survival information and miRNA expression data for further analysis. Secondly, this study applied bioinformatics data to construct the 5-miRNA signature model. Further experimental research validating the functions of the 5-miRNA signature model, and clinical studies investigating its predictive value are warranted.
Conclusions
We identified DEMs and constructed a 5-miRNA signature model, which might act as a potential predictor of prognosis in patients with WT. Significantly altered miRNAs might serve as prognostic biomarkers and therapeutic targets for WT. Further validation and functional studies are required to elucidate the molecular mechanisms of these miRNAs in WT.
Footnotes
Source of support: This study was supported by the Natural Science Foundation of Guangdong Province, China (Grant No. 2016A030310191); Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (Grant No. 20181062); the Fundamental Research Funds for the Central Universities (Sun Yat-sen University, Grant No. 17ykpy60); and the open project of the Key Laboratory of Tropical Disease Control (Sun Yat-sen University), the Ministry of Education (Grant No. 2018kfkt04)
Conflicts of interest
None.
References
- 1.Charlton J, Irtan S, Bergeron C, Pritchard-Jones K. Bilateral Wilms tumour: A review of clinical and molecular features. Exp Rev Mol Med. 2017;19:e8. doi: 10.1017/erm.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Routh JC, Grundy PE, Anderson JR, et al. B7-h1 as a biomarker for therapy failure in patients with favorable histology Wilms tumor. J Urol. 2013;189(4):1487–92. doi: 10.1016/j.juro.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Rupaimoole R, Slack FJ. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–22. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 4.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Ann Rev Biochem. 2010;79:351–79. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 5.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Osaki M, Okada F, Ochiya T. MiRNA therapy targeting cancer stem cells: A new paradigm for cancer treatment and prevention of tumor recurrence. Ther Deliv. 2015;6(3):323–37. doi: 10.4155/tde.14.122. [DOI] [PubMed] [Google Scholar]
- 7.Liu K, He B, Xu J, et al. MiR-483-5p Targets MKNK1 to suppress Wilms’ tumor cell proliferation and apoptosis in vitro and in vivo. Med Sci Monit. 2019;25:1459–68. doi: 10.12659/MSM.913005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu S, Fu W, Zhang L, et al. LINC00473 antagonizes the tumour suppressor miR-195 to mediate the pathogenesis of Wilms tumour via IKKalpha. Cell Prolif. 2018;51(1) doi: 10.1111/cpr.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou CH, Shrestha S, Yang CD, et al. MiRTarBase update 2018: A resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46(D1):D296–302. doi: 10.1093/nar/gkx1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong N, Wand X. MiRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015;43(Database issue):D146–52. doi: 10.1093/nar/gku1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker K, Kim KB. MiRTar Hunter: A prediction system for identifying human microRNA target sites. Mol Cells. 2013;35(3):195–201. doi: 10.1007/s10059-013-2165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veronese A, Lupini L, Consiglio J, et al. Oncogenic role of miR-483-3p at the IGF2/483 locus. Cancer Res. 2010;70(8):3140–49. doi: 10.1158/0008-5472.CAN-09-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Dong Q, Che X, et al. MicroRNA-29b-2-5p inhibits cell proliferation by directly targeting Cbl-b in pancreatic ductal adenocarcinoma. BMC Cancer. 2018;18(1):681. doi: 10.1186/s12885-018-4526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feliciano A, Garcia-Mayea Y, Jubierre L, et al. MiR-99a reveals two novel oncogenic proteins E2F2 and EMR2 and represses stemness in lung cancer. Cell Death Dis. 2017;8(10):e3141. doi: 10.1038/cddis.2017.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu B, Tao T, Wang Y, et al. hsa-miR-135a-1 inhibits prostate cancer cell growth and migration by targeting EGFR. Tumour Biol. 2016;37(10):14141–51. doi: 10.1007/s13277-016-5196-6. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Liu K, Yang P, et al. MicroRNA-200a functions as a tumor suppressor by targeting FOXA1 in glioma. Exp Ther Med. 2019;17(1):221–29. doi: 10.3892/etm.2018.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zang Y, Tai Y, Wan B, Jia X. MiR-200a-3p promotes the proliferation of human esophageal cancer cells by post-transcriptionally regulating cytoplasmic collapsin response mediator protein-1. Int J Mol Med. 2016;38(5):1558–64. doi: 10.3892/ijmm.2016.2758. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q, Lu RL, Li JX, Rong LJ. MiR-200a and miR-200b target PTEN to regulate the endometrial cancer cell growth in vitro. Asian Pac J Trop Med. 2017;10(5):498–502. doi: 10.1016/j.apjtm.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Tsouko E, Wang J, Frigo DE, et al. MiR-200a inhibits migration of triple-negative breast cancer cells through direct repression of the EPHA2 oncogene. Carcinogenesis. 2015;36(9):1051–60. doi: 10.1093/carcin/bgv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q, Zeng X, Huang D, Qiu X. Identification of differentially expressed miRNAs in early-stage cervical cancer with lymph node metastasis across The Cancer Genome Atlas datasets. Cancer Manag Res. 2018;10:6489–504. doi: 10.2147/CMAR.S183488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang JY, Lee YS, Jeon YK, et al. ANT2 suppression by shRNA restores miR-636 expression, thereby downregulating Ras and inhibiting tumorigenesis of hepatocellular carcinoma. Exp Mol Med. 2013;45:e3. doi: 10.1038/emm.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortunato O, Boeri M, Moro M, et al. Mir-660 is downregulated in lung cancer patients and its replacement inhibits lung tumorigenesis by targeting MDM2-p53 interaction. Cell Death Dis. 2014;5:e1564. doi: 10.1038/cddis.2014.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castilla MA, Lopez-Garcia MA, Atienza MR, et al. VGLL1 expression is associated with a triple-negative basal-like phenotype in breast cancer. Endocr Relat Cancer. 2014;21(4):587–99. doi: 10.1530/ERC-13-0485. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Xu K, Liu P. Post-transcriptional control of angiotensin ii type 1 receptor regulates osteosarcoma cell death. Cell Physiol Biochem. 2018;45(4):1581–89. doi: 10.1159/000487719. [DOI] [PubMed] [Google Scholar]
- 25.Bu P, Wang L, Chen KY, et al. miR-1269 promotes metastasis and forms a positive feedback loop with TGF-beta. Nat Commun. 2015;6:6879. doi: 10.1038/ncomms7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: A new perspective. Cancer. 2014;120(22):3446–56. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa H, Hirata Y, Takeda K, et al. Apoptosis signal-regulating kinase 1 inhibits hepatocarcinogenesis by controlling the tumor-suppressing function of stress-activated mitogen-activated protein kinase. Hepatology (Baltimore, MD) 2011;54(1):185–95. doi: 10.1002/hep.24357. [DOI] [PubMed] [Google Scholar]
- 28.Li ZH, Tao YF, Xu LX, et al. A novel sphingosine kinase 1 inhibitor (SKI-5C) induces cell death of Wilms’ tumor cells in vitro and in vivo. Am J Transl Res. 2016;8(11):4548–63. [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Liu S. Berberine inhibits Wilms’ tumor cell progression through upregulation of Wilms’ tumor gene on the X chromosome. Mol Med Rep. 2013;8(5):1537–41. doi: 10.3892/mmr.2013.1665. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Xue E, Shao W. Andrographolide promotes vincristine-induced SK-NEP-1 tumor cell death via PI3K-AKT-p53 signaling pathway. Drug Des Devel Ther. 2016;10:3143–52. doi: 10.2147/DDDT.S113838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig N, Nourkami-Tutdibi N, Backes C, et al. Circulating serum miRNAs as potential biomarkers for nephroblastoma. Pediatr Blood Cancer. 2015;62(8):1360–67. doi: 10.1002/pbc.25481. [DOI] [PubMed] [Google Scholar]
- 32.Liu M, Roth A, Yu M, et al. The IGF2 intronic miR-483 selectively enhances transcription from IGF2 fetal promoters and enhances tumorigenesis. Genes Dev. 2013;27(23):2543–48. doi: 10.1101/gad.224170.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koller K, Pichler M, Koch K, et al. Nephroblastomas show low expression of microR-204 and high expression of its target, the oncogenic transcription factor MEIS1. Pediatr Dev Pathol. 2014;17(3):169–75. doi: 10.2350/13-01-1288-OA.1. [DOI] [PubMed] [Google Scholar]
- 34.Cui M, Liu W, Zhang L, et al. Clinicopathological parameters and prognostic relevance of miR-21 and PTEN expression in Wilms’ tumor. J Pediatr Surg. 2017;52(8):1348–54. doi: 10.1016/j.jpedsurg.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Wu YS, Lin H, Chen D, et al. A four-miRNA signature as a novel biomarker for predicting survival in endometrial cancer. Gene. 2019;697:86–93. doi: 10.1016/j.gene.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Shi Y, Yin Z, et al. An eight-miRNA signature as a potential biomarker for predicting survival in lung adenocarcinoma. J Transl Med. 2014;12:159. doi: 10.1186/1479-5876-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]