Abstract
Dietary inclusions of baicalin and chlorogenic acid were beneficial for intestinal health in pigs. Nevertheless, it is unknown whether these plant-derived products had protection for intestine against bacterial challenge in chickens. This study was aimed at evaluating the potential mitigating effects of plant extracts (PE) from Flos lonicerae combined with Baikal skullcap (the active components are chlorogenic acid and baicalin) on intestinal disruption and dysbacteriosis induced by Salmonella pullorum in laying hens. A total of 216 41-week-old layers were randomly divided into 3 groups (6 replicates per group): negative control (NC), S. pullorum-infected positive control (PC), and the S. pullorum-infected group with supplementation of PE at 1000 mg/kg. All birds except those in NC were challenged with S. pullorum at the end of 4 weeks of the experiment. S. pullorum challenge impaired (P < 0.05) the production performance (egg production, feed intake, and feed efficiency) of laying hens, increased (P < 0.05) serum endotoxin content and frequency of Salmonella-positive organs, as well as up-regulated (P < 0.05) ileal expression of pro-inflammatory cytokines including IFNG, TNFA, IL8, and IL1B, whereas PE addition reversed (P < 0.05) these changes and increased (P < 0.05) ileal IL10 expression. Supplemental PE moderated ileal microbiota dysbiosis in challenged birds, characterized by a reduced abundance of Firmicutes along with increased abundances of Bacteroidetes (Bacteroides), Deferribacteres and several butyrate-producers such as Prevotellaceae, Faecalibacterium, Blautia, Butyricicoccus, Lachnoclostridium, and Olsenella, which may assist with energy harvesting and boost anti-inflammatory capacity of host. The decreased abundance of Firmicutes with the increased abundance of Bacteroidetes caused by PE addition had positive correlations with the decreased expression of ileal pro-inflammatory cytokines. The increased abundances of Bacteroidetes (Bacteroides) and Prevotellaceae following PE addition were also positively correlated with the improvement of performance (egg production and feed intake) of laying hens. Collectively, supplemental PE from Flos lonicerae in combination with Baikal skullcap alleviated S. pullorum-induced intestinal disruption and performance impairment in laying hens, which could be at least partially responsible by the modulation of gut microbial composition.
Keywords: gut microbiota, intestinal disruption, laying hen, plant extract, Salmonella pullorum
Introduction
Salmonella pullorum is a fowl-specific pathogen that is common in the poultry industry of China (Guo et al., 2018). S. pullorum challenge is responsible for considerable disorders such as intestinal inflammation and barrier dysfunction as well as compromised production performance of chickens (Wang et al., 2012; Wu Q.J. et al., 2018). Due to the increasing emergence of resistant bacteria and antibiotic residues in animal products, a stipulation had been drawn for the prohibition of antibiotics used in the feed of layers during the laying period, resulting in a necessity to seek for the available countermeasures to control or limit the adverse effects of S. pullorum challenge in laying hens. Recent studies have focused on the importance of plant extracts (PE) as potential approaches to alleviate bacteria-related intestinal dysfunction and performance compromise in broiler chickens (Bozkurt et al., 2016; Abudabos et al., 2017). However, little information was available regarding whether dietary addition of certain PE could alleviate S. pullorum-induced intestinal disruption and performance impairment in laying hens.
Flos lonicerae and Baikal skullcap are widely planted in China and used as the sources of traditional herbal medicines (Zhou et al., 2017). The extracts from Flos lonicerae and Baikal skullcap were associated with various biological properties such as heat-clearing, antioxidation and immune-regulation (Mo et al., 2012). The principal active components of Flos lonicerae and Baikal skullcap are respectively characterized as chlorogenic acid and baicalin (Kaplya et al., 2004), which were associated with antibacterial and anti-inflammation properties in vitro (Lou et al., 2011; Palocz et al., 2016; Fu et al., 2018; Wu S.C. et al., 2018). It was also reported that chlorogenic acid or baicalin addition modulated immune responses and improved intestinal homeostasis in pigs and mice (Chen et al., 2018b; Wu S.C. et al., 2018). A combination of the supplementation of chlorogenic acid and geniposide (a kind of glycoside that similar to baicalin in structure) was demonstrated to mitigate high-fat diet-induced intestinal inflammation and barrier dysfunction in mice (Peng et al., 2018). Accordingly, addition of the extracts from Flos lonicerae and Baikal skullcap may exert active roles in protecting the intestinal tract in chickens. The current study was aimed to verify the potential alleviation of them for S. pullorum-induced intestinal disruption in laying hens.
Salmonella challenge was suggested to result in disturbance of gut microbiota in chickens (Li et al., 2017), which plays a functional role in the course of bacterial infection and contributes to maintenance of multiple physiological processes of host (Ferreira et al., 2011; Susan et al., 2014). There was an evidence that the improvements in growth performance and intestinal health in chickens could be established by the modulation of gut microbiota (Crisol-Martínez et al., 2017), which helped to attenuate intestinal inflammation and recover intestinal mucosa from injury (Wang et al., 2016; Crisol-Martínez et al., 2017). Hence, it is necessary to decipher gut microbial composition in order to better understand the potential influence of its manipulation by dietary intervention on the production performance and intestinal health of host. Growing findings supported the important roles of gut microbiota in influencing the bioactivity and bioavailability of plant-derived products such as flavonoids and polyphenols (Ozdal et al., 2016). A modulation of gut microbiome in broilers was confirmed as a consequence of dietary addition with a certain kind of PE (Huang et al., 2018). However, it is largely unknown whether PE addition can moderate gut microbiota dysbiosis, thereby conducing to the protection of intestine against bacterial challenge in laying hens. In keeping with this, the present study was conducted to assess the remodeling of gut microbiota to explain the possible alleviation of S. pullorum-induced intestinal disruption of laying hens by the supplementation of PE from Flos lonicerae in combination with Baikal skullcap.
Materials and Methods
Animals and Experimental Design
The experimental animal protocol for this study was approved by the Animal Care and Use Committee of the Feed Research Institute of Chinese Academy of Agricultural Sciences. A total of 216 41-weeks-old Jinghong laying hens were randomly allocated into 3 groups, with 6 replicates of 12 birds each. Initial body weight and egg production were similar across all the replicates. All birds were acclimated to a basal diet and environment for 1 week. The treatment groups were as follows: negative control (NC) group, birds were fed a basal diet and free of challenge; positive control (PC) group, birds were fed a basal diet and challenged with S. pullorum; treatment (T) group, birds were fed a basal diet supplemented with 1000 mg/kg PE and challenged with S. pullorum. The PE was consisted by Flos lonicerae extract (containing 10% chlorogenic acid) and Baikal skullcap extract (containing 90% baicalin) at a ratio of 3:2 (the final concentrations of chlorogenic acid and baicalin were 60 and 360 mg/kg, respectively), enabling this product to function optimally on the basis of a preliminary unpublished work. The product was provided by Centre Technology Co., Ltd. (Beijing, China). All birds were housed in three-tier battery cages and exposed to 16 h of light/day. Room temperature was maintained between 18 and 22°C throughout the experiment. All birds were allowed free access to feed and water. The composition of basal diet based on National Research Council (National Research Council, 1994) is shown in Table 1. Egg weight and egg number were recorded daily from each replicate.
TABLE 1.
Composition of the basal diet (air-dry basis).
| Ingredients | Contents (g/kg of diet) |
| Corn | 654 |
| Soybean meal | 236 |
| Limestone | 89.3 |
| Sodium chloride | 3.0 |
| Dicalcium phosphate | 13.5 |
| Choline chloride (50%) | 1.0 |
| DL-Methionine (98%) | 1.0 |
| Multimineral1 | 2.0 |
| Multivitamin2 | 0.2 |
| Nutrient levels | |
| Metabolizable energy (MJ/kg) | 11.16 |
| Crude protein | 165.0 |
| Total phosphorus | 5.4 |
| Available phosphorus | 3.3 |
| Calcium | 33.1 |
| Lysine | 8.6 |
| Methionine | 3.7 |
1Supplied per kilogram of diet: Cu, 8 mg; Zn, 66 mg; Fe, 60 mg; Mn, 65 mg; Se, 0.3 mg; I, 1 mg. 2Supplied per kilogram of diet: vitamin A, 12,500 IU; vitamin D3, 4,125 IU; vitamin E, 15 IU; menadione, 2 mg; thiamin, 1 mg; riboflavin, 8.5 mg; pyridoxine, 8 mg; cobalamin, 5 mg; pantothenic acid, 50 mg; niacin, 32.5 mg; biotin, 2 mg; folic acid, 5 mg; choline, 500 mg.
Oral Challenge and Sampling
The S. pullorum strain (CVCC533, China Veterinary Culture Collection Center, Beijing, China) was cultured in lactose broth at 37°C for 16 h. To enumerate the bacteria, inoculum was diluted and plated on Salmonella-Shigella agar (Hopebio Biotechnology Co., Ltd., Qingdao, China) at 37°C for 24 h. At the end of week 4 of the experiment period, all birds except those in NC were orally gavaged with 1 mL of S. pullorum culture (1.0 × 109 CFU/mL) or the same amount of lactose broth. One bird per replicate was randomly selected for sample collection at 3 and 28 days post-infection (DPI). Individual blood were taken aseptically from the wing vein, serum samples were separated by centrifugation of blood at 3,000 rpm for 10 min at 4°C and stored at –20°C until analyzed. After blood collection, these birds were slaughtered rapidly, a little patch of heart, liver, and lung of each bird were harvested and preserved in –20°C for detecting the percentage of Salmonella-positive organs. Besides, the midpoint of ileal segment and ileal digesta of each bird were obtained and quick-freezed using liquid nitrogen, followed by the storage at −80°C until further analysis.
Performance Measurement
Total feed intake was recorded for each replicate at the end of week 4 and 8 of the experiment. Egg production (calculated as an average hen-day production), egg weight, average daily feed intake (ADFI) and feed conversion ratio (FCR, defined as the ratio of feed intake to egg mass) were calculated out based on the periods of week 1–4, week 5–8, and week 1–8 of the experiment.
Egg Quality Determination
At the end of week 4 and 8 of the experiment, 4 eggs per replicate were randomly selected for the determination of egg quality. Eggshell-breaking strength was measured using an Egg Force Reader (Orka Technology Ltd., Ramat Hasharon, Israel). Eggshell thickness was expressed as the mean value of measurements obtained at 3 locations on the surface of the egg (i.e., the air cell, equator, and sharp end) using an Eggshell Thickness Gauge (ESTG1, Orka Technology Ltd., Ramat Hasharon, Israel). Albumen height, Haugh unit, and yolk color were determined with an Egg Analyzer (Orka Technology Ltd., Ramat Hasharon, Israel).
Serum Sample Analysis
Serum immunoglobulins (Ig) including IgY, IgA, and IgM were quantified separately with the commercial chicken-specific ELISA kits (Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). All the procedures were carried out according to the manufacturers’ instructions. The intra-assay precision and inter-assay precision (variable coefficient) were less than 8 and 10%, respectively. Serum endotoxin concentration was measured using a chromogenic substrate assay kit (Jiancheng Bioengineering Institute, Nanjing, China). Briefly, 0.1 mL serum was incubated with 0.1 ml Limulus amebocyte lysate at 37°C for 45 min. After several subsequent reactions, the samples were read spectrophotometrically at 545 nm. The endotoxin level was calculated based on a standard curve of endotoxin concentrations.
Bacteriological Analysis of Visceral Organs
The organ invasion after oral S. pullorum inoculation was measured as described previously (Shao et al., 2013). Briefly, samples of visceral organs including liver, heart and lung were weighed and homogenized separately with phosphate buffered solution (PBS). The homogenates of each organ were diluted 1:10 with a sterile solution of PBS and pre-enriched for 24 h at 37°C in lactose broth (Hopebio Biotechnology Co., Ltd., Qingdao, China). After this incubation, 100 μL of suspension was spread on Salmonella-Shigella agar (Hopebio Biotechnology Co., Ltd., Qingdao, China) for 24 h at 37°C, followed by the observation for the presence or absence of characteristic Salmonella colonies. Organ invasion rate was calculated by the ratio of Salmonella-positive samples to the total number of samples.
RNA Isolation and Real-Time Quantitative PCR
Total RNA was extracted from the ileum by using Trizol Reagent (TIANGEN Biotech. Co., Ltd., Beijing, China) following the manufacturer’s protocols. Extracted RNA was dissolved in RNase-free water and quantified using an UV/Visible spectrophotometer (Amersham Bioscience, Sweden) at an absorbance of 260 nm. The quality of RNA was estimated from the absorbance ratio at 260 to 280 nm and by determination of the 18S and 28S bands after electrophoresis in 1% agarose gels stained with ethidium bromide. The cDNA samples were obtained by reverse transcription of total RNA using TIANGEN QuantScript RT kit (TIANGEN Biotech. Co., Ltd., Beijing, China). Real-time PCR for measuring ileal gene expression was carried out using RealMasterMix-SYBR Green kit (TIANGEN Biotech. Co., Ltd., Beijing, China) in an iCycler iQ5 multicolor real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA, United States). The expression of ACTB (β-actin) was used as an internal control to normalize the amount of initial RNA for each sample. The primer sequences for the target genes (IL1B, interleukin 1beta; IL-8, interleukin 8; TNFA, tumor necrosis factor-alpha; IFNG, interferon-gamma; IL10, interleukin 10) and reference gene are shown in Table 2. The protocol for all the genes was as follows: 95°C for 5 min; 40 cycles of 95°C for 10 s, 60°C for 30 s. All measurements were carried out in duplicate. Amplification efficiency of each gene was validated by constructing a standard curve through serial dilutions of cDNA. Specificity of PCR products was evaluated by the analysis of melting curve. The results of relative mRNA expression of genes were calculated using the 2–ΔΔCt method (Livak and Schmittgen, 2001).
TABLE 2.
Sequences for real-time PCR primers.
| Genes1 | Primer sequence2(5′–3′) | Accession no. |
| ACTB | F: ATGATATTGCTGCGCTCGTT | L08165 |
| R: TCTTTCTGGCCCATACCAACC | ||
| IL1B | F: ACTGGGCATCAAGGGCTACA | Y15006.1 |
| R: GCTGTCCAGGCGGTAGAAGA | ||
| IL8 | F: GGCTTGCTAGGGGAAATGA | DQ393272.2 |
| R: AGCTGACTCTGACTAGGAAACTGT | ||
| TNFA | F: GCCCTTCCTGTAACCAGATG | GU230788.1 |
| R: ACACGACAGCCAAGTCAACG | ||
| IFNG | F: GAACTGGACAGGGAGAAATGAGA | NM_205149.1 |
| R: ACGCCATCAGGAAGGTTGTT | ||
| IL10 | F: CATGCTGCTGGGCCTGAA | AJ621614 |
| R: CGTCTCCTTGATCTGCTTGATG |
1ACTB, β-actin; IL1B, interleukin 1beta; IL8, interleukin 8; TNFA, tumor necrosis factor-alpha; IFNG, interferon-gamma; IL10, interleukin 10. 2F, forward; R, reverse.
Sequencing of Ileal Microbiota
Microbial DNA was extracted from the ileal content using NucleoSpin® DNA Stool kit (Macherey-Nagel company, Germany). The quality of extracted DNA were checked with gel electrophoresis. Bacterial 16S rDNA sequences spanning the variable regions V3–V4 were amplified using primer 338F (5′-ACT CCT ACG GGA GGC AGC A-3′) and 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′). Amplification by PCR consisted of the following procedures: denaturation at 95°C for 5 min, twenty-five cycles of 30 s at 95°C, 50°C for 30 s, and 72°C for 40 s, with a final extension at 72°C for 7 min. High-throughput sequencing of PCR products was carried out on the Illumina HiSeq2500 PE250 platform (Illumina, San Diego, United States) at Biomarker Technology Co., Ltd. (Beijing, China). The sequencing results has been submitted to the Sequence Read Archive of the NCBI (accession number: PRJNA535413). The raw paired-end reads from the original DNA fragments were merged using FLASH v1.2.7 (Magoc and Salzberg, 2011). All of the effective reads from each sample were clustered into operational taxonomic units (OTUs) based on a 97% sequence similarity identified by UCLUST in QIIME v1.8.0 (Edgar, 2010). Taxonomic classification at different taxonomic levels of OTU sequences were performed by comparing sequences to the GreenGene v13.8 database (DeSantis et al., 2006). Shannon and Simpson indices, Chao1 and ACE estimators were included in α-diversity analysis by using the MOTHUR v1.31.2 (Schloss et al., 2009). The principal coordinates analysis (PCoA) and partial least squares discriminant analysis (PLS-DA) plots based on unweighted Unifrac were used to estimate pairwise distances among samples and to establish β-diversity. Linear discriminant analysis (LDA) combined effect size measurements (LEfSe) and non-parametric t-test (with Metastats software) were employed to identify the biological differences among groups (White et al., 2009). Metagenome functional content from 16S rDNA was predicted using PICRUSt (Langille et al., 2013), based on KEGG and COG databases. Spearman correlation analysis was performed for the correlations between ileal cytokine expression and microbial composition as well as between performance parameters (feed efficiency, egg production and ADFI) and microbial composition.
Data Processing and Analysis
Data are presented as mean ± standard deviation and analyzed by one-way ANOVA using the general linear model procedure of SPSS 18.0 software. Differences among different groups were analyzed by Duncan’s multiple comparisons. Significance was set at P < 0.05 and 0.05 < P < 0.10 was considered as a trend toward significance. Data set on the percentages of Salmonella-positive organs were subjected to Chi-Square test.
Results
Production Performance and Egg Quality
Salmonella pullorum challenge resulted in reductions (P < 0.05) in egg production and ADFI coupled with an increase (P < 0.05) in FCR of laying hens from week 5 to 8 and week 1 to 8 of the experiment (Table 3). However, birds in PE group had greater (P < 0.05) egg production and ADFI from week 5 to 8 and week 1 to 8 of the experiment, which was concomitant with a lower (P < 0.05) FCR from week 5 to 8 than those in PC group. No alterations (P > 0.05) in egg quality at week 4 of the experiment were noted among groups. There was little change in egg quality except for a reduction (P < 0.05) of eggshell strength at week 8 of the experiment in PC group as compared with NC group. However, birds in T group showed an enhanced (P < 0.05) eggshell strength at week 8 of the experiment in comparison with those in PC group.
TABLE 3.
Effects of plant extracts from Flos Lonicerae in combination with Baikal Skullcap on production performance1 and egg quality of laying hens.
| Time | NC2 | PC | T | P-value | |
| Egg production (%) | week 1–4 | 83.90 ± 5.16 | 83.94 ± 3.77 | 85.71 ± 4.15 | 0.724 |
| week 5–8 | 84.70 ± 0.74a | 62.37 ± 0.44c | 73.67 ± 1.24b | < 0.001 | |
| week 1–8 | 84.30 ± 2.83a | 73.15 ± 1.96c | 79.49 ± 2.34b | < 0.001 | |
| Egg weight (g) | week 1–4 | 64.87 ± 0.55 | 64.82 ± 1.78 | 64.94 ± 1.94 | 0.992 |
| week 5–8 | 66.04 ± 1.37 | 64.21 ± 1.81 | 64.41 ± 1.21 | 0.094 | |
| week 1–8 | 65.46 ± 0.95 | 64.52 ± 1.57 | 64.67 ± 1.54 | 0.469 | |
| ADFI (g) | week 1–4 | 117.65 ± 4.32 | 117.65 ± 4.32 | 117.68 ± 5.42 | 0.984 |
| week 5–8 | 117.88 ± 1.48a | 93.18 ± 3.56c | 105.26 ± 3.09b | < 0.001 | |
| week 1–8 | 117.77 ± 1.62a | 105.66 ± 3.04c | 111.47 ± 2.40b | < 0.001 | |
| FCR | week 1–4 | 2.25 ± 0.15 | 2.21 ± 0.07 | 2.23 ± 0.06 | 0.918 |
| week 5–8 | 2.21 ± 0.06c | 2.57 ± 0.20a | 2.51 ± 0.16b | 0.002 | |
| week 1–8 | 2.22 ± 0.09b | 2.39 ± 0.11a | 2.36 ± 0.11a | 0.025 | |
| Albumen height (mm) | week 4 | 6.37 ± 0.34 | 6.47 ± 0.43 | 6.91 ± 1.14 | 0.425 |
| week 8 | 7.11 ± 0.92 | 6.43 ± 0.75 | 6.89 ± 0.88 | 0.339 | |
| Haugh unit | week 4 | 77.26 ± 5.65 | 78.31 ± 6.16 | 79.28 ± 3.12 | 0.438 |
| week 8 | 81.99 ± 2.48 | 78.01 ± 2.80 | 79.63 ± 2.68 | 0.424 | |
| Yolk color | week 4 | 5.53 ± 0.37 | 6.00 ± 0.22 | 6.02 ± 1.01 | 0.295 |
| week 8 | 5.83 ± 0.54 | 6.10 ± 0.45 | 6.34 ± 0.62 | 0.272 | |
| Eggshell strength (N/m2) | week 4 | 33.50 ± 4.10 | 33.01 ± 1.89 | 36.41 ± 7.96 | 0.972 |
| week 8 | 35.13 ± 1.14b | 32.01 ± 0.97a | 34.80 ± 2.58b | 0.003 | |
| Eggshell thickness (mm) | week 4 | 0.423 ± 0.015 | 0.434 ± 0.014 | 0.437 ± 0.004 | 0.167 |
| week 8 | 0.432 ± 0.008 | 0.422 ± 0.008 | 0.430 ± 0.010 | 0.130 |
a,b,cValues with different superscripts within the same row differ significantly (P < 0.05). 1ADFI, average daily feed intake; FCR, feed conversion ratio. 2NC, negative control (birds were free of challenge); PC, positive control (birds were challenged with S. pullorum at the end of week 4 of the experiment); T, treatment group (PC + plant extracts addition at 1000 mg/kg).
Serum Biochemical Indices
Birds in T group had an increasing trend (P = 0.051) of serum IgY level with an elevated (P < 0.05) serum IgM level at 3 DPI than those in PC group (Table 4). In addition, serum IgY level at 28 DPI was increased in T group as compared to that in either NC or PC group. The S. pullorum-induced increase (P < 0.05) in serum endotoxin content at 3 DPI was reversed (P < 0.05) by PE treatment.
TABLE 4.
Effects of plant extracts from Flos Lonicerae in combination with Baikal Skullcap on serum indices1 of laying hens at 3 and 28 days post S. pullorum infection.
| Time | NC2 | PC | T | P-value | |
| IgY (mg/mL) | 3 days | 2.70 ± 0.86 | 1.95 ± 0.85 | 3.28 ± 0.86 | 0.051 |
| 28 days | 2.56 ± 0.69b | 1.83 ± 0.66b | 3.71 ± 0.95a | 0.003 | |
| IgA (mg/mL) | 3 days | 0.50 ± 0.04 | 0.52 ± 0.12 | 0.61 ± 0.13 | 0.168 |
| 28 days | 0.53 ± 0.10 | 0.47 ± 0.13 | 0.63 ± 0.88 | 0.052 | |
| IgM (mg/mL) | 3 days | 0.72 ± 0.24b | 0.79 ± 0.26b | 1.29 ± 0.23a | 0.002 |
| 28 days | 1.06 ± 0.37 | 0.72 ± 0.38 | 1.46 ± 0.76 | 0.087 | |
| Endotoxin (EU/mL) | 3 days | 0.50 ± 0.10b | 0.78 ± 0.13a | 0.52 ± 0.08b | 0.001 |
| 28 days | 0.48 ± 0.16 | 0.68 ± 0.12 | 0.52 ± 0.15 | 0.067 |
a,bValues with different superscripts within the same row differ significantly (P < 0.05). 1Ig, immunoglobulin. 2NC, negative control (birds were free of challenge); PC, positive control (birds were challenged with S. pullorum at the end of week 4 of the experiment); T, treatment group (PC + plant extracts addition at 1000 mg/kg).
Organ Invasion by Salmonella
The visceral organs including liver, heart and lung from NC group were all free of Salmonella after enrichment culture (Table 5), while 83.3% of the liver sample and 66.7% of the lung and heart samples taken in PC group at 3 DPI were positive for Salmonella. Birds in T group had lower (P < 0.05) percentage of Salmonella-positive liver sample at 3 DPI over that of PC group.
TABLE 5.
Effects of plant extracts from Flos Lonicerae in combination with Baikal Skullcap on the percentage (%) of Salmonella-positive organs of laying hens at 3 days post S. pullorum infection.
| NC1 | PC | T | P-value | |
| Liver | 0b | 83.3a | 16.7b | 0.025 |
| Lung | 0 | 66.7 | 50 | 0.095 |
| Heart | 0 | 66.7 | 50 | 0.095 |
a,bValues with different superscripts within the same row differ significantly (P < 0.05). 1NC, negative control (birds were free of challenge); PC, positive control (birds were challenged with S. pullorum at the end of week 4 of the experiment); T, treatment group (PC + plant extracts addition at 1000 mg/kg).
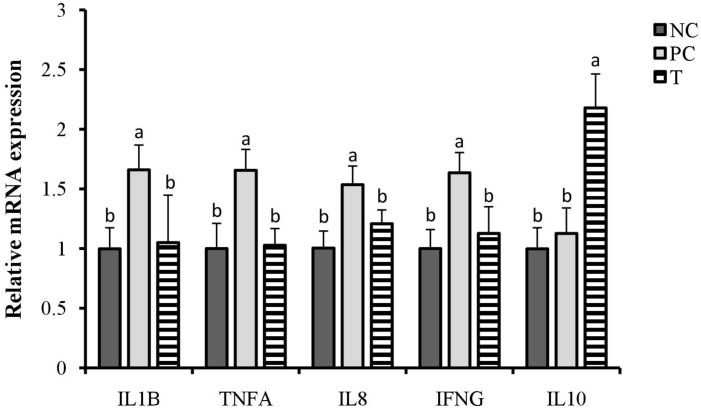
Relative mRNA Expression of Ileal Cytokines
There were increases (P < 0.05) in the relative expression of ileal IFNG, TNFA, IL8, and IL1B at 3 DPI of layers in response to S. pullorum challenge (Figure 1). However, they were all decreased (P < 0.05) in layers of T group when compared with those in PC group. Furthermore, an up-regulation (P < 0.05) in the relative expression of ileal IL10 at 3 DPI was observed in the birds from T group relative to PC group.
FIGURE 1.
Effects of plant extracts (PE) from Flos Lonicerae in combination with Baikal Skullcap on the relative expression of ileal cytokines of laying hens at 3 days post S. pullorum infection. Error bars represent the standard deviation. a,bTreatments with different letters were significantly different (P < 0.05). NC, negative control (birds were free of challenge); PC, positive control (birds were challenged with S. pullorum at the end of week 4 of the experiment); T, treatment group (PC + plant extracts addition at 1000 mg/kg).
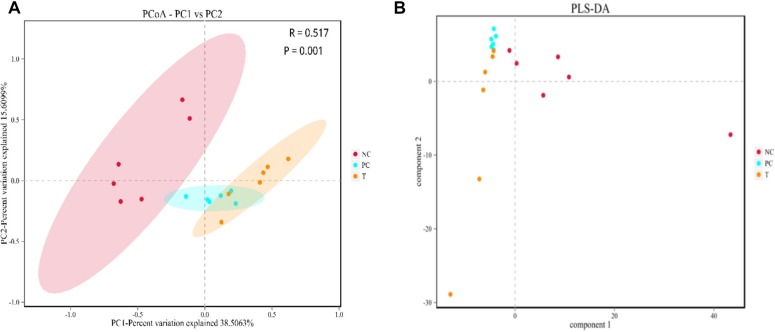
Diversity of Ileal Microbiota
The α-diversity including ACE and Chao1 estimators of ileal microbiota at 3 DPI were lower (P < 0.05) in the birds from PC group as compared with NC group (Supplementary Table S1). No change (P > 0.05) occurred in the α-diversity of ileal microbiota in response to PE treatment. To evaluate the similarity (β-diversity) of microbial community structure among groups, PCoA and PLS-DA were performed. PCoA plot showed a trend of separation of microbial communities at 3 DPI among groups (Figure 2A). PLS-DA plot also defined groups where the samples from different groups occupied distinct positions (Figure 2B).
FIGURE 2.
Beta-diversity analysis of ileal microbiota among groups at 3 days post S. pullorum infection. (A) principal co-ordinates analysis (PCoA). (B) Partial least squares discriminant analysis (PLS-DA). NC, negative control (birds were free of challenge); PC, positive control (birds were challenged with S. pullorum at the end of week 4 of the experiment); T, treatment group (PC + plant extracts addition at 1000 mg/kg).
Composition of Ileal Microbiota
The dominant phyla across all the groups were Firmicutes and Proteobacteria, which together contributed greater than 80% of the whole phyla (Figure 3A). S. pullorum challenge led to a reduced abundance of Proteobacteria with an increased abundance of Firmicutes at 3 DPI, when PE addition reduced the abundance of Firmicutes and increased Bacteroidetes abundance. Within Firmicutes, the majority belonged to the classes Bacilli, Clostridia and Gammaproteobacteria (Figure 3B). Family level analysis showed that the microbiota of layers was dominated by Lactobacillaceae, Peptostreptococcaceae, and Pasteurellaceae (Figure 3C). The increased abundance of Peptostreptococcaceae at 3 DPI in challenged birds was relieved by PE addition, which also increased the abundance of Bacteroidaceae. At genus level, the Lactobacillus and Romboutsia accounted for the largest proportion of the microbiota (Figure 3D). The abundance of Romboutsia at 3 DPI was increased in challenged birds but diminished by PE addition, which also induced an increase in Bacteroides abundance compared to PC. The averages of the relative abundance of bacterial groups at various taxonomic levels of layers at 3 DPI were exhibited in Supplementary Table S2.
FIGURE 3.
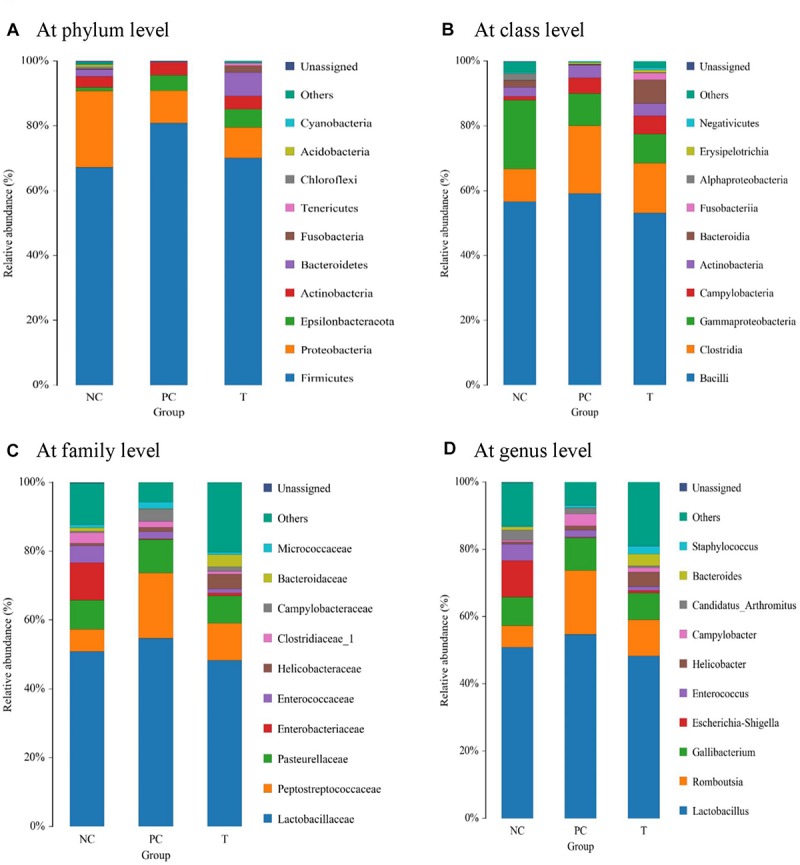
Analysis of ileal microbial composition at different taxonomic levels (A at phylum level, B at class level, C at family level, and D at genus level) of laying hens at 3 days post S. pullorum infection. NC, negative control (birds were free of challenge); PC, positive control (birds were challenged with S. pullorum at the end of week 4 of the experiment); T, treatment group (PC + plant extracts addition at 1000 mg/kg).
LEfSe analysis was applied to identify the relative richness (P < 0.05, LDA > 2.0) of bacterial members in groups (Figure 4). There were substantial members enriched in the ileum of NC birds at 3 DPI. Rothia was found to be enriched in birds of PC group, whereas the microbiota from T group was differentially enriched with Prevotellaceae, Desulfovibrionaceae (Desulfovibrio), Burkholderiaceae and Faecalibacterium. Non-parametric t-test was further employed to explore the differences in the microbial composition (Table 6). S. pullorum challenge reduced (P < 0.05) the abundances of Deferribacteres, Ruminococcus torques, Olsenella and Subdoligranulum at 3 DPI, and simultaneously tended to decrease (P < 0.10) the abundances of R. gauvreauii, Blautia, Butyricicoccus, Lachnoclostridium and Mesorhizobium. Bacillus and Rothia at 3 DPI appeared at higher (P < 0.05) abundances in PC group than those in NC group. There were elevations (P < 0.05) in the abundances of Blautia and Butyricicoccus with increasing trends (P < 0.10) of the abundances of Deferribacteres, R. gauvreauii, R. torques, Lachnoclostridium, Olsenella and Subdoligranulum at 3 DPI in response to PE addition, which also decreased (P < 0.05) the abundances of Bacillus and Rothia.
FIGURE 4.
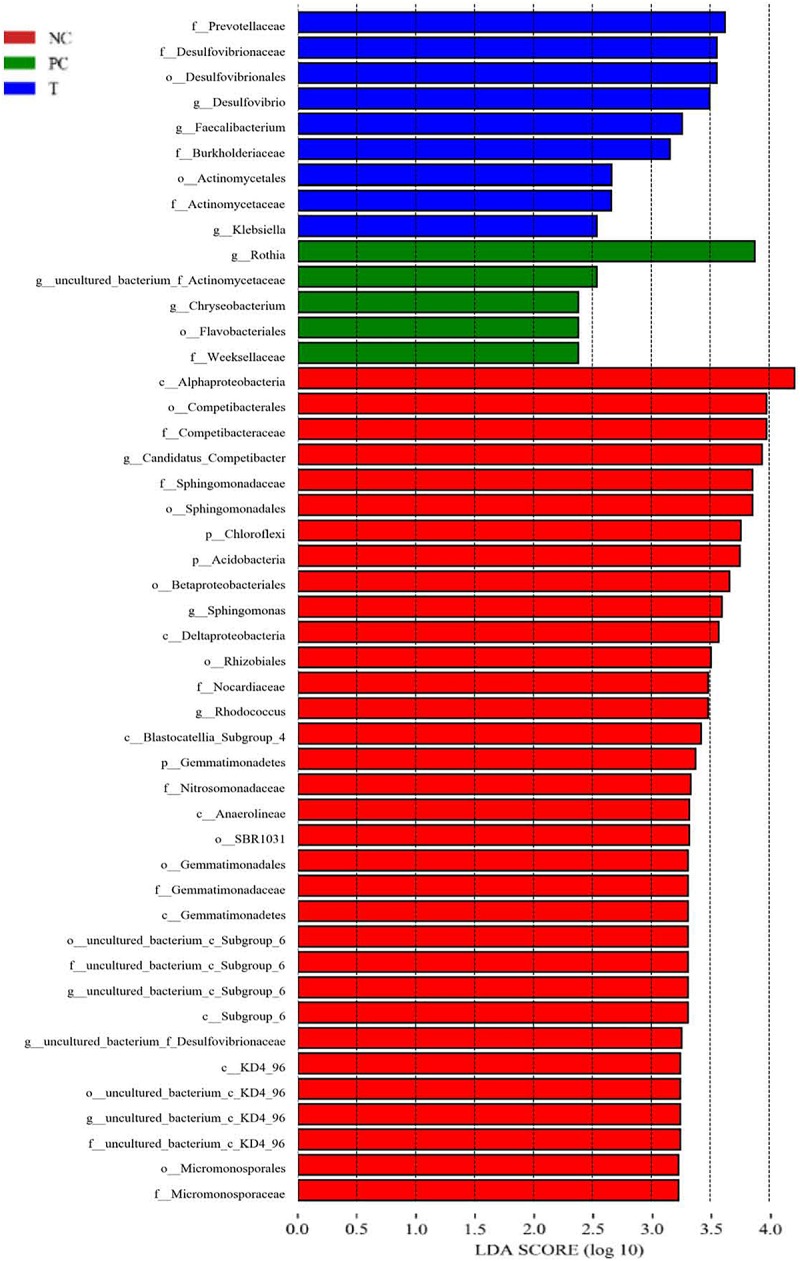
Linear discriminant analysis (LDA) combined effect size measurements (LEfSe) analysis of ileal microbiota in laying hens at 3 days post S. pullorum infection. Species with significant difference that have an LDA score greater than the estimated value (2.0). The length of the histogram represents the LDA score. NC, negative control (birds were free of challenge); PC, positive control (birds were challenged with S. pullorum at the end of week 4 of the experiment); T, treatment group (PC + plant extracts addition at 1000 mg/kg).
TABLE 6.
Differential species1 (%) identified from the ileal microbiota of laying hens from different groups.
| NC2 | PC | T |
P-values |
||
| NC vs. PC | PC vs. T | ||||
| Phylum | |||||
| Deferribacteres | 0.140 ± 0.134 | 0.001 ± 0.001 | 0.007 ± 0.003 | 0.035 | 0.067 |
| Genera | |||||
| R. gauvreauii_ | 0.0053 ± 0.0032 | 0.0009 ± 0.0006 | 0.0196 ± 0.0136 | 0.092 | 0.099 |
| R. torques | 0.0436 ± 0.0208 | 0.0039 ± 0.0015 | 0.0294 ± 0.0208 | 0.012 | 0.096 |
| Bacillus | 0.0057 ± 0.0024 | 0.0290 ± 0.0102 | 0.0043 ± 0.0022 | 0.005 | 0.007 |
| Blautia | 0.0195 ± 0.0137 | 0.0009 ± 0.0006 | 0.0089 ± 0.0039 | 0.098 | 0.017 |
| Butyricicoccus | 0.0097 ± 0.0060 | 0.0007 ± 0.0007 | 0.0321 ± 0.0170 | 0.062 | 0.027 |
| Lachnoclostridium | 0.045 ± 0.027 | 0.006 ± 0.001 | 0.032 ± 0.022 | 0.079 | 0.091 |
| Mesorhizobium | 0.0711 ± 0.0498 | 0.0021 ± 0.0011 | 0.0003 ± 0.0003 | 0.091 | 0.056 |
| Olsenella | 0.032 ± 0.014 | 0.002 ± 0.001 | 0.016 ± 0.011 | 0.007 | 0.077 |
| Rothia | 0.40 ± 0.24 | 1.70 ± 0.79 | 0.16 ± 0.07 | 0.043 | 0.021 |
| Subdoligranulum | 0.041 ± 0.021 | 0.002 ± 0.001 | 0.013 ± 0.009 | 0.012 | 0.096 |
1R. gauvreauii, Ruminococcus gauvreauii; R. torques, Ruminococcus torques. 2NC, negative control (birds were free of challenge); PC, positive control (birds were challenged with S. pullorum at the end of week 4 of the experiment); T, treatment group (PC + plant extracts addition at 1000 mg/kg).
Functional Prediction of Ileal Microbiota
Presumptive functions of ileal microbiota of layers at 3 DPI were examined by using PICRUSt. According to the KEGG prediction (Supplementary Figure S1), the microbiota represented in the ileum was mainly associated with the following biological processes: amino acid metabolism, carbohydrate metabolism, energy metabolism and nucleotide metabolism. Metagenomic prediction based on COG categories revealed that the functional pathways enriched within the microbiota were those corresponding to general function prediction only, amino acid transport and metabolism, carbohydrate transport and metabolism, energy production and conversion as well as transcription. No differences (P > 0.10) were noted in the predicted pathways of the microbiota among groups.
Correlation Between Ileal Microbiota and Ileal Inflammation
A Spearman’s rank correlation analysis was employed to identify specific bacterial members associated with the differential expression of ileal cytokines among groups. The abundance of phylum Firmicutes showed highly positive correlations (P < 0.01) with the expression of IFNG, IL1B, TNFA and IL8 (Figure 5A). In contrast, the abundance of phylum Bacteroidetes was negatively correlated (P < 0.05) with TNFA and IL8 expression. The abundance of genus Bacteroides was positively correlated (P < 0.05) with IL1B, TNFA and IL8 expression (Figure 5B), but it also had a highly positive correlation (P < 0.01) with IL10 expression. Besides, Rothia abundance was negatively correlated (P < 0.05) with the expression of both TNFA and IL10.
FIGURE 5.
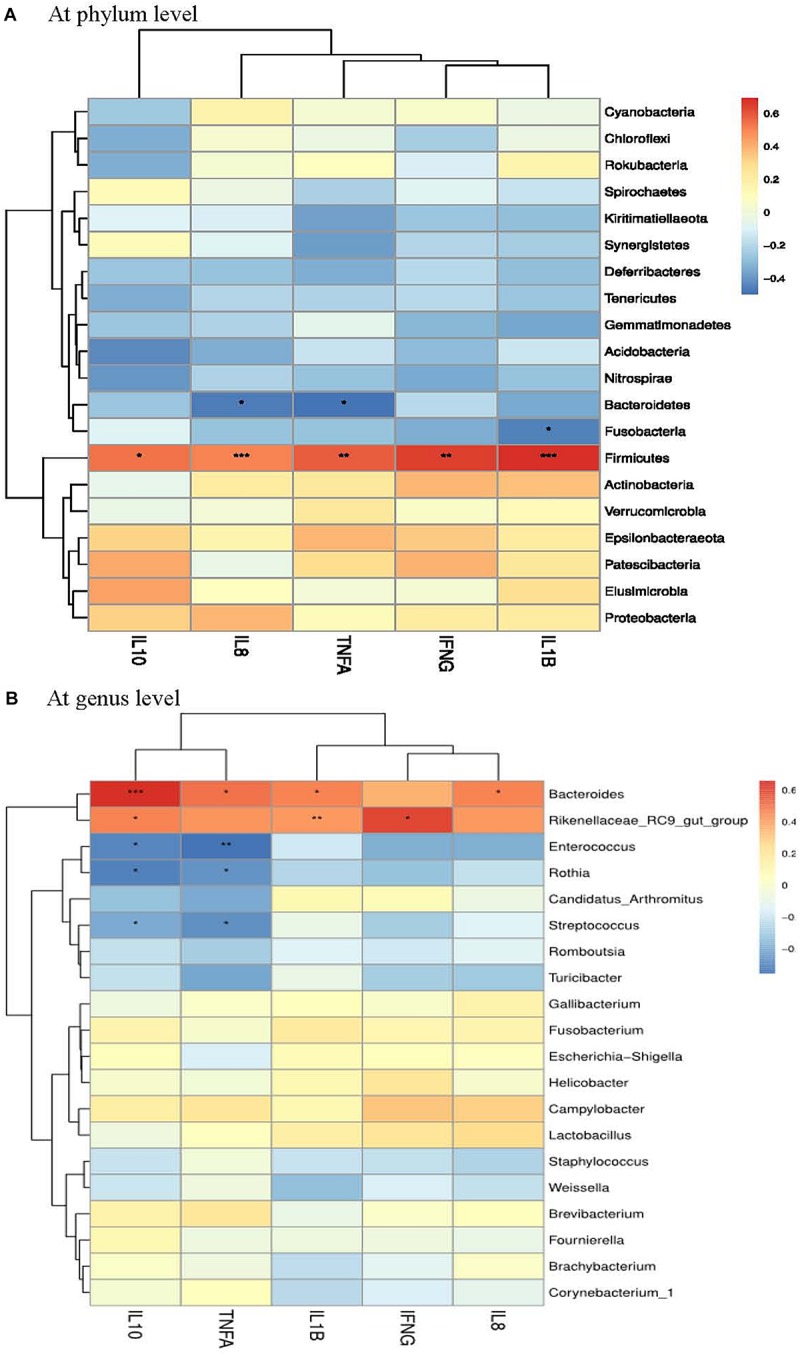
Correlation analysis between ileal cytokine expression and ileal microbial composition (A at phyum level and B at genus level) in laying hens at 3 days post S. pullorum infection. IL1B, interleukin 1beta; IL8, interleukin 8; TNFA, tumor necrosis factor-alpha; IFNG, interferon-gamma; IL10, interleukin 10. The red and blue panes, respectively, represent positive and negative correlations between ileal cytokine expression and microbiota (color intensity shows the Spearman’s r-value of correlation in each panel). The asterisks indicate significant correlations at P < 0.05 level (*P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001).
Correlation Between Ileal Microbiota and Production Performance
A Spearman’s rank correlation analysis was performed to evaluate the potential association between alterations in ileal microbiota and performance parameters of laying hens. There was a positive correlation between the phylum Deferribacteres and ADFI (Figure 6A), while the phylum Bacteroidetes was positively correlated with egg production and ADFI. The families Desulfovibrionaceae, Lachnospiraceae, Bacteroidaceae, Prevotellaceae and Ruminococcaceae had positive correlations with both egg production and ADFI (Figure 6B). Moreover, positive correlations were detected between Bacteroides and performance parameters (feed efficiency, egg production and ADFI) (Figure 6C).
FIGURE 6.
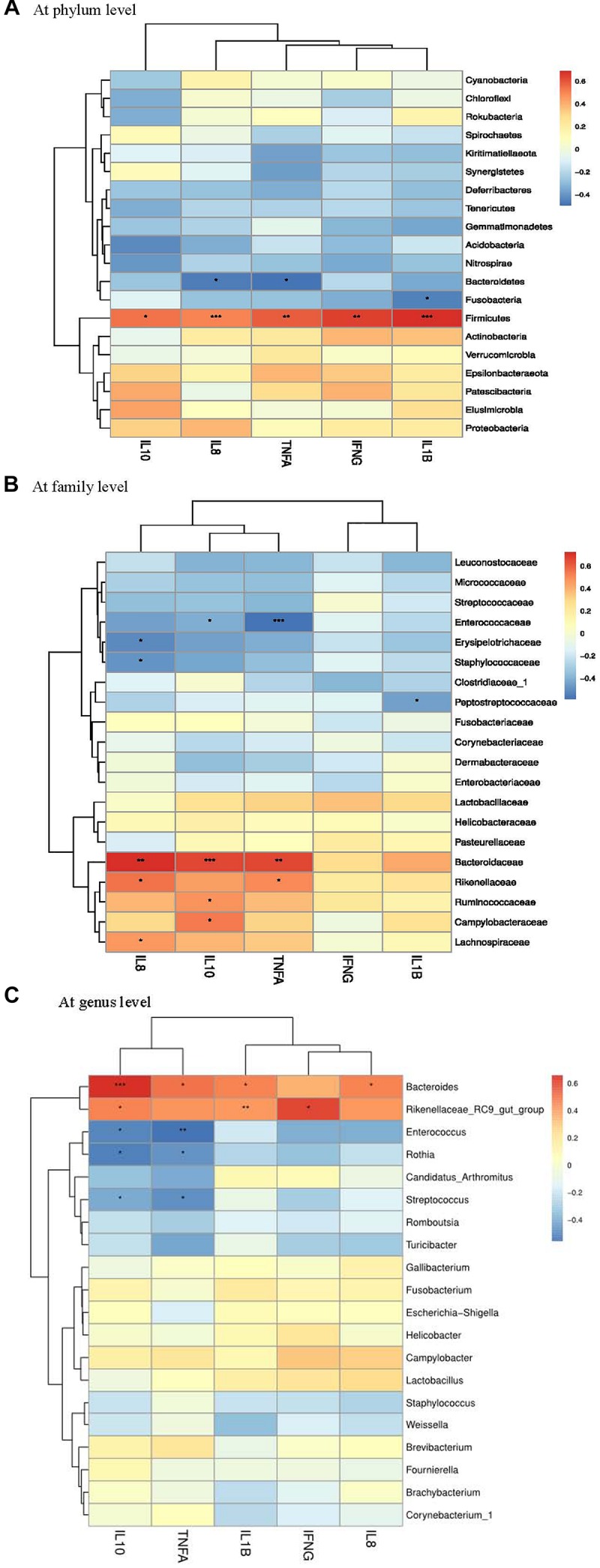
Correlation analysis between performance parameters and ileal microbial composition (A at phylum level, B at family level, and C at genus level) in laying hens at 3 days post S. pullorum infection. FE, feed efficiency; LR, laying rate (egg production); ADFI, average daily feed intake. The red and blue panes, respectively, represent positive and negative correlations between performance parameters and microbiota (color intensity shows the (Spearman’s r-value of correlation in each panel). The asterisks indicate significant correlations at P < 0.05 level (*P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001).)
Discussion
Previous studies evidenced that supplemental PE based on baicalin or chlorogenic acid was associated with an improvement in growth performance of animals (Chen et al., 2018a; Wu S.C. et al., 2018). Similarly, the current study revealed a mitigation of S. pullorum-induced impairment of production performance of laying hens in the presence of PE, indicated by the increased egg production and ADFI as well as feed efficiency. With regard to egg quality, the challenge resulted in a reduction of eggshell strength of layers. This might be derived from the Salmonella-induced compromised absorption of microelements such as manganese and zinc (Liu et al., 2012; Diaz-Ochoa et al., 2016), which are critical for eggshell formation (Gheisari et al., 2011). Thereby, the enhanced eggshell strength of layers might be the result of a restoration of S. pullorum-induced malabsorption of microelements following PE addition.
Immunoglobulins that produced by lymphocytes are critical for protecting against toxins and bacteria. Their levels are viewed as an important indicator of humoral immunity (Schroeder and Cavacini, 2010). In the current study, supplemental PE resulted in an increasing trend of serum IgY level with an elevated IgM level of birds at 3 DPI, indicating a reinforcement of humoral immunity that could be beneficial for the resistance of host to S. pullorum invasion. Endotoxin is part of the cell wall of gram-negative bacteria such as Salmonella and released into circulation when the intestinal barrier is destroyed. Serum endotoxin content can be served as an indicator for monitoring the extent of intestinal damage (Mani et al., 2012). Herein, an elevation in serum endotoxin content was observed in challenged birds, suggesting the idea of an impairment of gut barrier function as a result of S. pullorum challenge. In support of this view, translocations of Salmonella to the liver, lung and heart samples were observed following S. pullorum challenge. However, PE addition lowered serum endotoxin content and the frequency of Salmonella-positive liver sample in challenged birds, bringing an evidence for the attenuation of S. pullorum-induced intestinal disruption of laying hens following PE addition. Similar results were found in some previous studies (Chen et al., 2018b; Peng et al., 2018), in which supplemental baicalin or chlorogenic acid protected intestinal barrier against challenge in animals.
In concert with a previous study (Wei et al., 2018), the present study showed that S. pullorum challenge induced gut inflammation in laying hens, evidenced by the elevated expression of ileal pro-inflammatory cytokines inclusive of TNFA, IFNG, IL1B, and IL8. Although inflammatory responses play a crucial role in immune defense against infection (Withanage et al., 2005), the sharp increase of pro-inflammatory cytokines aggravated intestinal damage and induced high consumption of nutrients (Dinarello, 2000), which could subsequently transform into an impaired performance in animals (Pluske et al., 2018). Thereby, the induction of ileal inflammation mediated by pro-inflammatory cytokines conduced to the intestinal disruption and performance impairment in laying hens challenged with S. pullorum. However, there were reductions in the expression of ileal TNFA, IFNG, IL1B, and IL8 in challenged birds received a diet containing PE, providing a clue for the role of PE addition in remitting S. pullorum-induced intestinal inflammation, which might subsequently favor the attenuation of the impaired performance of laying hens. This is in accordance with the responses to baicalin and chlorogenic acid treatments in vitro and in animals, where gut inflammation was attenuated (Palocz et al., 2016; Chen et al., 2018b; Fu et al., 2018; Wu S.C. et al., 2018). It is of interest to notify an up-regulated expression of ileal IL10 in response to PE addition. IL10 is a key anti-inflammatory cytokine secreted by macrophages that serves to maintain immune balance by inhibiting the excessive production of pro-inflammatory cytokines (Corwin, 2000). As a result, an up-regulated IL10 expression could be associated with the alleviation of S. pullorum-induced ileal inflammation in laying hens fed with PE.
In attempts to better understand the origins of the mitigative effects of PE addition on the S. pullorum-induced intestinal disruption of layers, the focus of this study was on the analysis of gut microbiota, which could mediate the Salmonella-induced inflammatory environment of intestine in animals (Ferreira et al., 2011). Similar to a previous report (Price et al., 2010), the current study revealed that S. pullorum challenge induced a shift in microenvironment, favoring Firmicutes at the expense of Proteobacteria and Bacteroidetes, while PE addition increased the abundance of Bacteroidetes and reduced the abundance of Firmicutes. Increased Firmicutes and decreased Bacteroidetes were associated with gut inflammation and barrier dysfunction in animals (Rabot et al., 2016; Las-Heras et al., 2019). Besides, elevated Bacteroidetes conduced to the protection against Salmonella-induced gut inflammation (Ferreira et al., 2011). Analogously, this study showed that Firmicutes abundance had highly positive relationships with the expression of ileal pro-inflammatory cytokines (IL1B, IL8, TNFA and IFNG), while Bacteroidetes abundance was negatively correlated with the expression of IL8 and TNFA. In addition, although the genus Bacteroides was positively correlated with the expression of IL1B, IL8 and TNFA, it also had a highly positively correlation with IL10 expression, which may assist with anti-inflammatory response. Thereby, the increased abundances of Bacteroidetes (Bacteroides) along with the reduced abundance of Firmicutes in the ileum might contribute to the attenuation of S. pullorum-induced gut inflammation of layers following PE addition.
Several species were identified as biomarkers to distinguish ileal microbiota of layers among groups, such as Prevotellaceae, Desulfovibrionaceae, Burkholderiaceae and Faecalibacterium. Prevotellaceae is known for the production of succinate that facilitates energy metabolism of host through tricarboxylic acid cycle (De-Vadder et al., 2016). Prevotellaceae was also associated with short-chain fatty acids (SCFAs) generation in gut because of its capability to produce various polysaccharidases to degrade cellulose and insoluble starch, which was essential for animals to be able to digest plant-based diets (Wang et al., 2018), resulting in a positive association between gut Prevotellaceae and feed efficiency in animals (Quan et al., 2019). Faecalibacterium in chicken gut represented an important bacterial group closely related to the production of butyrate (Bjerrum et al., 2006), which serves as intestinal epithelial specific nutrient and energy component, favoring the suppression of inflammatory responses and recovery of intestinal injury via multiple ways (Singh N. et al., 2014). Desulfovibrionaceae was showed to be implicated in propionic acid production that aids in energy harvest of host (Liu et al., 2019). The functional roles of Burkholderiaceae in animal gut remain unclear. However, an increase in gut Burkholderia was accompanied by the improvements of intestinal structure and growth performance in chickens following a probiotic addition (Li et al., 2019), implying a benefit of Burkholderia for gut health of host. To sum up, the ileal enrichments of Prevotellaceae, Faecalibacterium and Burkholderiaceae could conduce to the improved performance along with the attenuated ileal inflammation in challenged birds following PE addition.
Further analysis revealed more differential species at various taxonomic levels among groups. For example, there was a restoration of PE addition in S. pullorum-induced reduction of ileal Deferribacteres that is viewed as a kind of iron-reducer (Miroshnichenko et al., 2003). Iron ion is a nutrient that often limits the growth of bacteria such as Salmonella, and animals typically try to reduce biologically available iron in order to avoid bacterial infection (Deriu et al., 2013). It was thus speculated that the increased Deferribacteres following PE addition contributed to the restriction of S. pullorum invasion, evidenced by a reduction of Salmonella-positive organ in layers. Blautia, Butyricicoccus, and Lachnoclostridium were proposed as butyrate-producers that implicated in the alleviation of gut inflammation (Zhang X. et al., 2015; Takahashi et al., 2016; Zhao et al., 2017; Pérez-Burillo et al., 2019). Ruminococcaceae contains substantial cellulolytic-degrading bacteria such as R. gauvreauii and R. torques, whose reductions were related to the occurrence of gut inflammation due to the lessened SCFAs production (Domingo et al., 2008; Takahashi et al., 2016; Halfvarson et al., 2017). Subdoligranulum and Olsenella also had abilities to ferment polysaccharides to SCFAs such as acetate and butyrate (Zhang J. et al., 2015; Ricaboni et al., 2017; Li et al., 2018). Besides, Subdoligranulum was capable of producing bacteriocins that can confine Salmonellla growth (Yang et al., 2013). An increased abundance of gut Subdoligranulum was confirmed to be accompanied by an improved production performance in laying hens (Guo et al., 2017). Rothia was viewed as a harmful bacterium that associated with gut inflammation occurrence (Bajer et al., 2017). In this study, PE addition triggered increases in Blautia, Butyricicoccus, Lachnoclostridium, R. gauvreauii, R. torques, Subdoligranulum, and Olsenella, concurrent with a reduction of Rothia, suggesting the ileal microbiota of birds fed with PE becomes more efficient in protecting intestine against S. pullorum-induced inflammation and assisting in energy harvest of host.
Gut microbiota has profound impacts on the bioavailability of dietary components, playing prominent roles in host nutritional processes (Susan et al., 2014). It was thought that gut Firmicutes and Bacteroidetes were both closely related to chicken performance (Singh K.M. et al., 2014), due to their roles in polysaccharide decomposition and the subsequent generation of SCFAs that aid in host energy utilization (Postler and Ghosh, 2017). However, no influence was found of the change in the abundances of gut Firmicutes and Bacteroidetes on SCFAs production and energy harvest in mice (Murphy et al., 2010). Contradictorily, Singh K.M. et al. (2014) reported that broilers with high feed efficiency exhibited a higher abundance of Bacteroides in gut. Wang et al. (2019) observed an increased abundance of Bacteroidetes and a reduced abundance of Firmicutes accompanied by an improvement of growth performance in pigs. The above studies highlighted a complexity of the linkage between these two phyla and SCFAs-related energy harvest of host (Andoh et al., 2016). In this study, Bacteroidetes together with the families Bacteroidaceae, Desulfovibrionaceae, and Prevotellaceae elicited positive correlations with both egg production and ADFI. Bacteroides was also positively correlated with feed efficiency, egg production and ADFI. Thus, the alleviation of S. pullorum-induced compromised performance of laying hens following PE addition was likely associated with the improved gut microbiota, mainly characterized by the increased relative abundances of Bacteroidetes (Bacteroides), Desulfovibrionaceae and Prevotellaceae. Due to the limitation of the relative quantification of bacteria in analyzing gut microbiota-host interactions, the absolute quantification-based gut microbiota analysis might deserve to be further conducted, in order to validate the potential contribution of the modulated gut microbiota to the improved performance of laying hens following PE addition.
Conclusion
Supplemental PE alleviated S. pullorum-induced impairment in production performance and intestinal disruption by attenuating intestinal inflammation and barrier dysfunction in laying hens, which could be partially responsible by the capability to remodel gut microbial composition, particularly the enrichment of SCFAs-producing bacteria. This study can expand our fundamental knowledge concerning the roles of gut microbiota in mediating the various physiological functions of PE in animals.
Data Availability
The datasets generated for this study can be found in the Sequence Read Archive of the NCBI.
Ethics Statement
The animal study was reviewed and approved by the Animal Care and Use Committee of the Feed Research Institute of the Chinese Academy of Agricultural Sciences.
Author Contributions
W-wW implemented the experimental design and wrote the manuscript. H-jJ conducted the animal trial and performed the sample analyses. H-jZ and JW assisted with data analysis. S-gW and G-hQ contributed to the experimental design and preparation of the manuscript. All authors discussed the results and reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was financially supported by National Key R&D Program of China (2018YFD0500603), Beijing Innovation Consortium of Agriculture Research system (BAIC04-2018), the earmarked fund for Modern Agro-industry Technology Research System (CARS-40-K12), and the Agricultural Science and Technology Innovation Program (ASTIP).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01681/full#supplementary-material
References
- Abudabos A. M., Alyemni A. H., Dafalla Y. M., Khan R. U. (2017). The effect of phytogenics on growth traits, blood biochemical and intestinal histology in broiler chickens exposed to Clostridium perfringens challenge. J. Appl. Anim. Res. 46 691–695. 10.1080/09712119.2017.1383258 [DOI] [Google Scholar]
- Andoh A., Nishida A., Takahashi K., Inatomi O., Imaeda H., Bamba S., et al. (2016). Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J. Clin. Biochem. Nutr. 59 65–70. 10.3164/jcbn.15-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajer L., Kverka M., Kostovcik M., Macinga P., Dvorak J., Stehlikova Z., et al. (2017). Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J. Gastroenterol. 23 4548–4558. 10.3748/wjg.v23.i25.4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerrum L., Engberg R. M., Leser T. D., Jensen B. B., Finster K., Pedersen K. (2006). Microbial community composition of the ileum and cecum of broiler chickens as revealed by molecular and culture-based techniques. Poult. Sci. 85 1151–1164. 10.1093/ps/85.7.1151 [DOI] [PubMed] [Google Scholar]
- Bozkurt M., Ege G., Aysul N., Akşit H., Tüzün A. E., Küçükyılmaz K., et al. (2016). Effect of anticoccidial monensin with oregano essential oil on broilers experimentally challenged with mixed Eimeria spp. Poult. Sci. 95 1858–1868. 10.3382/ps/pew077 [DOI] [PubMed] [Google Scholar]
- Chen J. L., Li Y., Yu B., Chen D. W., Mao X. B., Zheng P., et al. (2018a). Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 96 1108–1118. 10.1093/jas/skx078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Yu B., Chen D. W., Huang Z. Q., Mao X. B., Zheng P., et al. (2018b). Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 59 84–92. 10.1016/j.jnutbio [DOI] [PubMed] [Google Scholar]
- Corwin E. J. (2000). Understanding cytokines part I: physiology and mechanisms of action. Biol. Res. Nurs. 2 30–40. 10.1177/109980040000200104 [DOI] [PubMed] [Google Scholar]
- Crisol-Martínez E., Stanley D., Geier M. S., Hughes R. J., Moore R. J. (2017). Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: linking gut microbiota and growth. Appl. Microbiol. Biotechnol. 101 4547–4559. 10.1007/s00253-017-8193-9 [DOI] [PubMed] [Google Scholar]
- Deriu E., Liu J. Z., Pezeshki M., Edwards R. A., Ochoa R. J., Contreras H., et al. (2013). Probiotic bacteria reduce Salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe 14 26–37. 10.1016/j.chom.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis T. Z., Hugenholtz P., Larsen N., Rojas M., Brodie E. L., Keller K., et al. (2006). Greengenes, a chimera-checked 16SrRNA gene database and work bench compatible with ARB. Appl. Environ. Microbiol. 72 5069–5072. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Vadder F., Kovatcheva-Datchary P., Zitoun C., Duchampt A., Backhed F., Mithieux G. (2016). Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 24 151–157. 10.1016/j.cmet.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Diaz-Ochoa V. E., Lam D., Lee C. S., Klaus S., Behnsen J., Liu J. Z., et al. (2016). Salmonella mitigates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration. Cell Host Microbe 19 814–825. 10.1016/j.chom.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. (2000). Proinflammatory cytokines. Chest 118 503–508. 10.1378/chest.118.2.503 [DOI] [PubMed] [Google Scholar]
- Domingo M. C., Huletsky A., Boissinot M., Bernard K. A., Picard F. J., Bergeron M. G. (2008). Ruminococcus gauvreauii sp. nov., a glycopeptide-resistant species isolated from a human faecal specimen. Int. J. Syst. Evol. Microbiol. 58 1393–1397. 10.1099/ijs.0.65259-0 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Ferreira R. B., Gill N., Willing B. P., Antunes L. C., Russell S. L., Croxen M. A., et al. (2011). The intestinal microbiota plays a role in Salmonella-induced colitis independent of pathogen colonization. PLoS One 6:e20338. 10.1371/journal.pone.0020338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S., Liu H., Xu L., Qiu Y., Liu Y., Wu Z., et al. (2018). Baicalin modulates NF-κB and NLRP3 inflammasome signaling in porcine aortic vascular endothelial cells infected by Haemophilus parasuis causing Glässer’s disease. Sci. Rep. 8:807. 10.1038/s41598-018-19293-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheisari A. A., Sanei A., Samie A., Gheisari M. M., Toghyani M. (2011). Effect of diets supplemented with different levels of manganese, zinc, and copper from their organic or inorganic sources on egg production and quality characteristics in laying hens. Biol. Trace Elem. Res. 142 557–571. 10.1007/s12011-010-8779-x [DOI] [PubMed] [Google Scholar]
- Guo J. R., Dong X. F., Liu S., Tong J. M. (2017). Effects of long-term Bacillus subtilis CGMCC 1.921 supplementation on performance, egg quality, and fecal and cecal microbiota of laying hens. Poult. Sci. 96 1280–1289. 10.3382/ps/pew389 [DOI] [PubMed] [Google Scholar]
- Guo X. D., Wang H. L., Cheng Y. L., Zhang W. T., Luo Q. P., Wen G. Y., et al. (2018). Quinolone resistance phenotype and genetic characterization of Salmonella enterica serovar Pullorum isolates in China, during 2011 to 2016. BMC Microbiol. 18:225. 10.1186/s12866-018-1368-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfvarson J., Brislawn C. J., Lamendella R., Vázquez-Baeza Y., Walters W. A., Bramer L. M., et al. (2017). Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2:17004. 10.1038/nmicrobiol.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Zhang Y., Xiao K. P., Jiang F., Wang H. C., Tang D. Z., et al. (2018). The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 6:211. 10.1186/s40168-018-0590-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplya O. A., Sherstoboev E. Y., Zueva E. P., Razina T. G., Amosova E. N., Krylova S. G. (2004). Effect of baikal skullcap extract administered alone or in combination with cyclophosphamide on natural cytotoxicity system in mice with lewis lung carcinoma. B. Exp. Biol. Med. 137 471–474. 10.1023/b:bebm.0000038156.50390.c2 [DOI] [PubMed] [Google Scholar]
- Langille M. G. I., Zaneveld J., Caporaso J. G., McDonald D., Knights D., Reyes J. A., et al. (2013). Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31 814–821. 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Las-Heras V., Clooney A. G., Ryan F. J., Cabrera-Rubio R., Casey P. G., Hueston C. M., et al. (2019). Short-term consumption of a high-fat diet increases host susceptibility to Listeria monocytogenes infection. Microbiome 7:7. 10.1186/s40168-019-0621-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. L., Wang J., Zhang H. J., Wu S. G., Hui Q. R., Yang C. B., et al. (2019). Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front. Physiol. 9:19. 10.3389/fphys.2018.01968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Hao H. H., Cheng G. Y., Liu C. B., Ahmed S., Shabbir M. A. B., et al. (2017). Microbial shifts in the intestinal microbiota of Salmonella infected chickens in response to enrofloxacin. Front. Microbiol. 8:1711. 10.3389/fmicb.2017.01711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. F., Zhang K., Yang H. Y. (2018). Pectin alleviates high fat (Lard) diet-induced nonalcoholic fatty liver disease in mice: possible role of shortchain fatty acids and gut microbiota regulated by pectin. J. Agric. Food Chem. 66 8015–8025. 10.1021/acs.jafc.8b02979 [DOI] [PubMed] [Google Scholar]
- Liu J. Z., Jellbauer S., Poe A. J., Ton V., Pesciaroli M., Kehl-Fie T. E., et al. (2012). Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11 227–239. 10.1016/j.chom.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. M., Li E. Y., Sun Z. Y., Fu D. J., Duan G. Q., Jiang M. M., et al. (2019). Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 9:287. 10.1038/s41598-018-36430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lou Z., Wang H., Zhu S., Ma C., Wang Z. (2011). Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 76 398–403. 10.1111/j.1750-3841.2011.02213.x [DOI] [PubMed] [Google Scholar]
- Magoc T., Salzberg S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27 2957–2963. 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani V., Weber T. E., Baumgard L. H., Gabler N. K. (2012). Growth and development symposium: endotoxin, inflammation, and intestinal function in livestock. J. Anim. Sci. 90 1452–1465. 10.2527/jas.2011-4627 [DOI] [PubMed] [Google Scholar]
- Miroshnichenko M. L., Slobodkin A. I., Kostrikina N. A., L’Haridon S., Nercessian O., Spring S., et al. (2003). Deferribacter abyssi sp. nov., an anaerobic thermophile from deep-sea hydrothermal vents of the Mid-Atlantic Ridge. Int. J. Syst. Evol. Microbiol. 5 1637–1641. 10.1099/ijs.0.02673-0 [DOI] [PubMed] [Google Scholar]
- Mo S. L., Liu W. F., Chen Y. L., Luo H. B., Sun L. B., Chen X. W., et al. (2012). Ligand- and protein-based modeling studies of the inhibitors of human cytochrome P450 2D6 and a virtual screening for potential inhibitors from the Chinese herbal medicine, Scutellaria baicalensis (Huangqin, Baikal Skullcap). Comb. Chem. High. T. Scr. 15 36–80. 10.2174/138620712798280826 [DOI] [PubMed] [Google Scholar]
- Murphy E. F., Cotter P. D., Healy S., Marques T. M., O’Sullivan O., Fouhy F., et al. (2010). Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 59 1635–1642. 10.1136/gut.2010.215665 [DOI] [PubMed] [Google Scholar]
- National Research Council (1994). Nutrient Requirements of Poultry: Ninth Revised Edition. Washington, DC: The National Academies Press; 10.17226/2114 [DOI] [Google Scholar]
- Ozdal T., Sela D. A., Xiao J. B., Boyacioglu D., Chen F., Capanoglu E. (2016). The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 8:78. 10.3390/nu8020078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palocz O., Paszti-Gere E., Galfi P., Farkas O. (2016). Chlorogenic acid combined with Lactobacillus plantarum 2142 reduced LPS-induced intestinal inflammation and oxidative stress in IPEC-J2 cells. PLoS One 11:e0166642. 10.1371/journal.pone.0166642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J. H., Leng J., Tian H. J., Yang T., Fang Y., Feng Q., et al. (2018). Geniposide and chlorogenic acid combination ameliorates non-alcoholic steatohepatitis involving the protection on the gut barrier function in mouse induced by high-fat diet. Front. Pharmacol. 9:1399. 10.3389/fphar.2018.01399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Burillo S., Pastoriza S., Fernández-Arteaga A., Luzón G., Jiménez-Hernández N., D’Auria G., et al. (2019). Spent coffee grounds extract rich in mannooligosaccharides promote a healthier gut microbial community in a dose dependent manner. J. Agric. Food Chem. 67 2500–2509. 10.1021/acs.jafc.8b06604 [DOI] [PubMed] [Google Scholar]
- Pluske J. R., Kim J. C., Black J. L. (2018). Manipulating the immune system for pigs to optimise performance. Anim. Prod. Sci. 58 666–680. 10.1071/AN17598 [DOI] [Google Scholar]
- Postler T. S., Ghosh S. (2017). Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 26 110–130. 10.1016/j.cmet.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. L., Totty H. R., Lee H. B., Utt M. D., Fitzner G. E., Yoon I., et al. (2010). Use of Saccharomyces cerevisiae fermentation product on growth performance and microbiota of weaned pigs during Salmonella infection. J. Anim. Sci. 88 3896–3908. 10.2527/jas.2009-2728 [DOI] [PubMed] [Google Scholar]
- Quan J. P., Cai G. Y., Yang M., Zeng Z. H., Ding R. R., Wang X. W., et al. (2019). Exploring the fecal microbial composition and metagenomic functional capacities associated with feed efficiency in commercial DLY pigs. Front. Microbiol. 10:52. 10.3389/fmicb.2019.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabot S., Membrez M., Blancher F., Berger B., Moine D., Krause L., et al. (2016). High fat diet drives obesity regardless the composition of gut microbiota in mice. Sci. Rep. 6:32484. 10.1038/srep32484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaboni D., Mailhe M., Vitton V., Bittar F., Raoult D., Fournier P. E. (2017). Olsenella provencensis sp. nov. Olsenella phocaeensis sp. nov. and Olsenella mediterranea sp. nov. increasing abundance isolated from the human colon. Hum. Microbiome J. 4 22–23. 10.1016/j.humic.2017.05.002 [DOI] [Google Scholar]
- Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75 7537–7541. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Cavacini L. (2010). Structure and function of immunoglobulins. J. Allergy Clin. Immun. 125 S41–S52. 10.1016/j.jaci.2009.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y. J., Guo Y. M., Wang Z. (2013). β-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult. Sci. 92 1764–1773. 10.3382/ps.2013-03029 [DOI] [PubMed] [Google Scholar]
- Singh K. M., Shah T. M., Beddy B., Deshpande S., Rank D. N., Joshi C. G. (2014). Taxonomic and gene-centric metagenomics of the fecal microbiome of low and high feed conversion ratio (FCR) broilers. J. Appl. Genet. 55 145–154. 10.1007/s13353-013-0179-4 [DOI] [PubMed] [Google Scholar]
- Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H., et al. (2014). Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40 128–139. 10.1016/j.immuni.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susan E. P., Paul W. O., Catherine S., Ross R. P., Fitzgerald G. F. (2014). Intestinal microbiota, diet and health. Br. J. Nutr. 111 387–402. 10.1017/S0007114513002560 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nishida A., Fujimoto T., Fujii M., Shioya M., Imaeda H., et al. (2016). Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion 93 59–65. 10.1159/000441768 [DOI] [PubMed] [Google Scholar]
- Wang J., Ji H., Wang S., Liu H., Zhang W., Zhang D., et al. (2018). Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 9:1953. 10.3389/fmicb.2018.01953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. C., Zhang T. T., Wen C., Jiang Z. Y., Wang T., Zhou Y. M. (2012). Protective effects of zinc-bearing clinoptilolite on broilers challenged with Salmonella pullorum. Poult. Sci. 91 1838–1845. 10.3382/ps.2012-02284 [DOI] [PubMed] [Google Scholar]
- Wang T. W., Teng K. L., Liu Y. Y., Shi W. X., Zhang J., Dong E. Q., et al. (2019). Lactobacillus plantarum PFM promotes intestinal development through modulation of gut microbiota in weaning piglets. Front. Microbiol. 10:90. 10.3389/fmicb.2019.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. W., Li Z., Han Q. Q., Guo Y. M., Zhang B., D’inca R. (2016). Dietary live yeast and mannan oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br. J. Nutr. 116 1878–1888. 10.1017/S0007114516004116 [DOI] [PubMed] [Google Scholar]
- Wei S., Wu K. L., Nie Y. J., Li X., Lian Z. X., Han H. B. (2018). Different innate immunity and clearance of Salmonella Pullorum in macrophages from White Leghorn and Tibetan chickens. Eur. J. Inflamm. 16 1–9. 10.1177/2058739218780039 [DOI] [Google Scholar]
- White J. R., Nagarajan N., Pop M. (2009). Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 5:e1000352. 10.1371/journal.pcbi.1000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withanage G., Wigley P., Kaiser P., Mastroeni P., Brooks H., Powers C., et al. (2005). Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect. Immun. 73 5173–5182. 10.1128/IAI.73.8.5173-5182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q. J., Zheng X. C., Wang T., Zhang T. Y. (2018). Effect of dietary oridonin supplementation on growth performance, gut health, and immune response of broilers infected with Salmonella pullorum. Irish Vet. J. 71:16. 10.1186/s13620-018-0128-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. C., Chu X. L., Su J. Q., Cui Z. Q., Zhang L. Y., Yu Z. J., et al. (2018). Baicalin protects mice against Salmonella typhimurium infection via the modulation of both bacterial virulence and host response. Phytomedicine 48 21–31. 10.1016/j.phymed.2018.04.063 [DOI] [PubMed] [Google Scholar]
- Yang J., Martinez I., Walter J., Keshavarzian A., Rose D. J. (2013). In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 23 74–81. 10.1016/j.anaerobe.2013.06.012 [DOI] [PubMed] [Google Scholar]
- Zhang J., Guo Z., Xue Z., Sun Z., Zhang M., Wang L., et al. (2015). A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. ISME J. 9 1979–1990. 10.1038/ismej.2015.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhao Y. F., Xu J., Xue Z., Zhang M., Pang X., et al. (2015). Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet induced obesity in rats. Sci. Rep. 5:14405. 10.1038/srep14405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Zhang Q., Ma W. N., Tian F., Shen H. Y., Zhou M. M. (2017). A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 8 4644–4656. 10.1039/c7fo01383c [DOI] [PubMed] [Google Scholar]
- Zhou W., Yin A., Shan J., Wang S., Cai B., Di L. (2017). Study on the rationality for antiviral activity of Flos Lonicerae Japonicae-Fructus Forsythiae herb couple preparations improved by chito-oligosaccharide via integral pharmacokinetics. Molecules 22:654. 10.3390/molecules22040654 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study can be found in the Sequence Read Archive of the NCBI.