Abstract
Psoriasis is a chronic, immune-mediated, inflammatory, and debilitating skin disease with significant impact on patients’ quality of life. Its pathogenesis is complex and not yet fully understood. However, the IL-23/IL-17 axis is currently considered the main pathogenic pathway in psoriasis. Guselkumab is a fully human immunoglobulin G1 λ (IgG1λ) monoclonal antibody (mAb) that binds to the p19 subunit of IL-23. It is the first of its class, already approved by the US Food and Drug Administration (FDA), as well as the European Medicines Agency (EMA) for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for either systemic therapy or phototherapy. Several clinical trials have demonstrated potential benefits of guselkumab over other already approved immunomodulators in terms of safety and efficacy. The results of the head-to-head trial ECLIPSE were recently released and are addressed in this review. They contribute to the increasing confidence in guselkumab, demonstrating great potential for long-term treatment of psoriasis. However, further long-term data and additional comparative studies will be essential for positioning guselkumab in the therapeutic armamentarium for psoriasis.
Keywords: antibodies, biological products, interleukin-23, monoclonal, psoriasis
Introduction
Psoriasis is a chronic, immune-mediated, inflammatory skin disease that affects over 125 million people worldwide,1–3 with significant impact on patients’ quality of life.4 Due to its systemic nature, psoriasis is associated with several medical comorbidities, including cardiovascular disease, inflammatory bowel disease, and psychiatric diseases.5–9
Psoriasis pathogenesis is complex and not yet fully understood. Even though, disease knowledge has significantly evolved in past years leading to the development of increasingly specific and efficacious targeted therapies.10–13
During the initiation phase of the psoriatic skin lesions, an increment of the production of TNF occurs and, as a result, activation of dermal dendritic cells.14,15 These cells are responsible for the increased production of IL-23 and the subsequent activation of distinct subgroups of IL-17 producing cells (T17) (helper T cells [Th17]; cytotoxic T cells [Tc17]; innate lymphoid cells [ILC3]; and γδ T cells).14,16–18 As IL-23 levels rise, secreted mainly from inflammatory dermal dendritic cells, T17 cells increase in number and produce large amounts of IL-17, specifically isoforms IL-17A and F, which drive the upregulation of many psoriasis-related genes.14,19 The clonal expansion of T17 cells and subsequent increased levels of IL-17 feedforward an inflammatory response and lead to keratinocyte hyperproliferation.14,15,20,21 Other types of cells, such as dermal macrophages and epidermal keratinocytes, were also associated with the increased production of IL-23 and may contribute to psoriatic lesions’ installation and maintenance.14,22 The discovery of the IL-23/IL-17 axis, widely considered the most critical pathogenic pathway on the development of psoriasis, and the development of drugs targeting this pathway shifted the paradigm of the management of this condition.15,20,23
The development of IL-17 inhibitors showed that the blockade of the pathway of this cytokine was associated with high levels of efficacy and rapid onset of action in moderate-to-severe psoriasis, but also better clinical responses than either TNF-α inhibitors or ustekinumab, a nonselective IL-23 inhibitor.24–31 However, several side effects such as neutropenia, candidiasis, and exacerbations of Crohn’s disease have been associated with these agents,24–31 reinforcing the need for new therapeutic solutions. More recently, selective IL-23 inhibitors such as guselkumab, tildrakizumab, and risankizumab have emerged, showing a very effective, durable, and safe profile32–40
This article intends to review the current literature on guselkumab in the management of psoriasis.
Guselkumab pharmacology
Guselkumab® (Janssen Biotech, Inc., Horsham, PA, USA) is a fully human immunoglobulin G1 λ (IgG1λ) monoclonal antibody (mAb) that binds to the p19 subunit of IL-23. It is first of its class to be approved by the US Food and Drug Administration (FDA)41 as well as the European Medicines Agency (EMA)42,43 for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for either systemic therapy or phototherapy. In light of its clinical efficacy in plaque psoriasis, guselkumab has also been or is currently being evaluated for the treatment of other diseases, namely generalized pustular psoriasis (GPP), erythrodermic psoriasis (EP), psoriatic arthritis, rheumatoid arthritis, and Crohn’s disease.
Pharmacodynamic properties
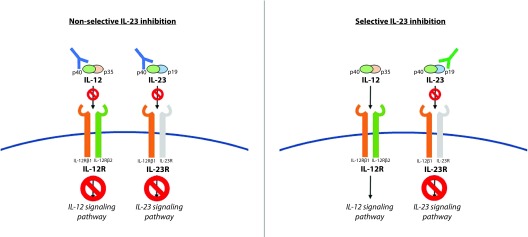
Guselkumab binds with both high affinity and high specificity to IL-23,43 preventing the interaction of the cytokine with its receptor on the surface of the cell. This action is responsible for blocking the initiation of the IL-23 pathway and the subsequent release of other proinflammatory cytokines (Figure 1).
Figure 1.
The IL-12 and IL-23 heterodimers are each composed of a common p40 subunit and a unique p35 and p19 subunit, respectively. The nonselective IL-23 inhibitors, such as ustekinumab, bind to the p40 subunit on IL-12 and IL-23, thereby inhibiting both signalling pathways. The selective IL-23 inhibitors, as guselkumab, bind to the p19 subunit on IL-23 and inhibit only the IL-23-mediated signalling pathway, allowing the IL-12 axis to remain intact.
In patients with moderate-to-severe plaque psoriasis, the biopsy of skin samples from body regions affected with the disease, compared to nonlesional regions, before and after a single dose of guselkumab, showed that the drug is responsible for a significant (p<0.05) reduction in inflammatory dendritic cell and T-cell counts, and epidermal thickness at week 12 compared to baseline.32 Significant reductions in IL-17A serum levels since week 1 were observed when compared to baseline values (versus no change in placebo recipients) in patients considered as responders to guselkumab (with ≥50% improvement in the Psoriasis Area and Severity Index score [PASI 50] measured at week 12), with a response that was dose-dependent.32 Guselkumab has also shown a substantial impact in both the reduction of mRNA expression of IL-17F and IL-22, as well as in increased levels of INF-γ, produced by T-helper 1 (Th1) cells, thus proposing that the drug’s clinical performance relies primarily in the inhibition of the IL-23/Th17 pathway, allowing the IL-12/Th1 axis to remain intact32 (Figure 1).
Pharmacokinetic properties
Zhuang and colleagues37 conducted a first-in-human, phase I, randomized trial to assess the pharmacokinetics, immunogenicity, safety, and tolerability of guselkumab following a single intravenous (IV; 0.03–10 mg/kg) or subcutaneous (SC; 10–300 mg) administration in healthy subjects and patients with moderate-to-severe psoriasis. The pharmacokinetic profile of guselkumab was generally comparable between healthy subjects and patients with psoriasis. Investigators also noted that there was a dose-dependent increase in the mean maximum serum concentration and area under the zero-to-infinity serum concentration–time curve.37
Yao and colleagues44 established a population pharmacokinetics model using the information from three clinical trials involving guselkumab: the phase II X-PLORE (NCT01483599) and two phase III trials, namely VOYAGE 1 (NCT02207231) and VOYAGE 2 (NCT02207244). The final model showed an apparent clearance and apparent volume of distribution of 0.516 L/day, and 13.5 L, respectively. Mean half-life time values were consistent between both healthy subjects (12–19 days) and patients with psoriasis (15–17 days). The model-derived elimination half-life of the drug allowed for the conclusion that steady-state serum guselkumab concentrations occurred between weeks 12 and 14.44 However, Smolen and colleagues45 reported that the median serum levels of guselkumab would only reach the steady state by week 20, which is the information provided by EMA about the product.43
Therapeutic efficacy of guselkumab in psoriasis
Large, randomized, multinational, phase III trials (VOYAGE 1, VOYAGE 2, NAVIGATE [NCT02203032], and ECLIPSE [NCT03090100]) assessed the clinical efficacy of subcutaneous guselkumab in the treatment of moderate-to-severe plaque psoriasis in adults. Results from other trials support this data and will not be discussed further.33,46
Patients enrolled in the previously reported phase III clinical trials were individuals with ≥18 years of agewith moderate-to-severe plaque psoriasis for at least 6 months and had to be eligible for systemic therapy or phototherapy. Patients in VOYAGE trials and NAVIGATE had an initial Investigator Global Assessment (IGA) score of 3 or more, a minimum affected body surface area (BSA) of ≥10%, and a PASI score of 12 or more. These studies excluded patients with history or symptoms of active tuberculosis, other types of psoriasis (guttate, erythrodermic, or pustular), or who had been exposed to guselkumab or the active comparator (adalimumab in VOYAGE trials; ustekinumab in NAVIGATE; and secukinumab in ECLIPSE).34–36,38
VOYAGE trials
VOYAGE 1 was a phase III randomized, double-blind trial, which evaluated the effectiveness of guselkumab compared to placebo and adalimumab.34 For study design, see Table 1. The proportion of patients with an IGA score of 0/1 (cleared/minimal disease) and the proportion of patients with an at least PASI 90 response, both at week 16, were used to evaluate the clinical efficacy of guselkumab compared to placebo (as coprimary endpoints). To assess the efficacy of guselkumab compared to that of adalimumab, investigators used as major secondary endpoints the proportion of patients achieving an IGA score of 0/1, PASI 75, and PASI 90 responses at weeks 16, 24, and 48, respectively. A fixed sequence method of analyses was used to control the overall type 1 error rate.
Table 1.
Study design for each phase III trials.
| Clinical trial | Number of patients | Study design |
|---|---|---|
| VOYAGE 1 | 837 | Patients were randomized in a 1:2:2 ratio for one of three scenarios:
|
| VOYAGE 2 | 992 | Patients were randomized in a 2:1:1 ratio to:
|
| NAVIGATE | 871 | Patients received open-label ustekinumab (45 mg for patients ≤100 kg, 90 mg for patients >100 kg) at weeks 0 and 4. At week 16, 268 patients with an inadequate response to ustekinumab, considered as an IGA score of 2 or more, were randomized (double-blind) to:
|
| ECLIPSE | 1048 | Participants received one injection of active guselkumab and one injection of placebo when guselkumab was scheduled to be administered (weeks 0, 4, 12, and every 8 weeks thereafter through week 44) or two injections of placebo when no guselkumab was scheduled to be administered (weeks 1, 2, 3, 8, 16, 24, 32, and 40). Placebo injections were administered to maintain the blind. On the other hand, participants received two injections of secukinumab subcutaneously at weeks 0, 1, 2, 3, 4, and every 4 weeks thereafter through week 44. |
At week 16, comparing to the placebo group, a significantly higher proportion of patients receiving guselkumab had achieved an IGA score of 0/1 (85.1 versus 6.9%) and PASI 90 (73.3 versus 2.9%) response. In addition, a significantly higher proportion of patients achieving IGA 0, PASI 75, and PASI 100 was observed in guselkumab group versus the placebo group (Table 2).34 When compared to adalimumab at week 16, guselkumab was statistically superior, as observed by the percentage of patients achieving an IGA score of 0/1 (85.1 versus 65.9%), PASI 75 (91.2 versus 73.1%), and PASI 90 (73.3 versus 49.7%) responses. These responses maintained significance throughout week 24 (IGA 0/1 [84.2 versus 61.7%], PASI 75 [91.2 versus 72.2%], and PASI 90 [80.2 versus 53.0%]) and week 48 (IGA 0/1 [80.5 versus 55.4%], PASI 75 [87.8 versus 62.6%], and PASI 90 [76.3 versus 47.9%]). Similarly, the proportion of patients who achieved a PASI 100 response in the guselkumab group was significantly higher than in the adalimumab group at every checkpoint (weeks 16 [37.4 versus 17.1%], 24 [44.4 versus 24.9%], and 48 [47.4 versus 23.4%]). With the initiation of guselkumab at week 16, patients in the placebo crossover group achieved similar responses to those receiving guselkumab since week 0.34 Guselkumab was proven superior to both the placebo and/or adalimumab in the attainment of the coprimary endpoints and all major secondary endpoints (all p<0.001) in this clinical trial.34 For a more detailed analysis of the results, see Table 2.
Table 2.
Summary of key results from clinical trials with guselkumab.
| Clinical trial | Proportion of patients achieving | ||||
|---|---|---|---|---|---|
| PASI 75 | PASI 90 | PASI 100 | IGA 0/1 | DLQI 0/1 | |
| VOYAGE 1 | At wk 16: Gus 91.2%; ADA 73.1%; PL 5.7% | At wk 16: GUS 73.3%; ADA 49.7%; PL 2.9% | At wk 16: GUS 37.4%; ADA 17.1%; PL 0.6% | At wk 16: GUS 85.1%; ADA 65.9%; PL 6.9% | At wk 16: GUS 56.3%; ADA 38.6%; PL 4.2% |
| At wk 24: GUS 91.2%; ADA 72.2% | At wk 24: GUS 80.2%; ADA 53.0% | At wk 24: GUS 44.4%; ADA 24.9% | At wk 24: GUS 84.2%; ADA 61.7% | At wk 24: GUS 60.9%; ADA 39.5% | |
| At wk 48: GUS 87.8%; ADA 62.6% | At wk 48: GUS 76.3%; ADA 47.9% | At wk 48: GUS 47.4%; ADA 23.4% | At wk 48: GUS 80.5%; ADA 55.4% | At wk 48: GUS 62.5%; ADA 38.9% | |
| (all p<0.001) | (all p<0.001) | (all p<0.001) | (all p<0.001) | (all p<0.001) | |
| VOYAGE 2 | At wk 16: GUS 86.3%; ADA 68.5%; PL 8.1% | At wk 16: GUS 70.0%; ADA 46.8%; PL 2.4% | At wk 16: GUS 34.1%; ADA 20.6%; PL 0.8% | At wk 16: GUS 84.1%; ADA 67.7%; PL 8.5% | At wk 16: GUS 51.7%; ADA 39.0%; PL 3.3% |
| At wk 24: GUS 89.1%; ADA 71.0% | At wk 24: GUS 75.2%; ADA 54.8% | At wk 24: GUS 44.2%; ADA 26.6% | At wk 24: GUS 83.5%; ADA 64.9% | At wk 24: GUS 57.6%; ADA 41.1% | |
| (all p<0.001) | (all p<0.001) | (all p<0.001) | (all p<0.001) | (all p<0.001) | |
| NAVIGATE | NR | At wk 28: GUS 48.1%; UST 22.6% | At wk 28: NR | At wk 28: GUS 31.1%; UST 14.3% | At wk 28: NR |
| (p<0.001) | (p=0.001) | ||||
| At wk 52: GUS 51.1%; UST 24.1% | At wk 52: GUS 20.0%; UST 7.5% | At wk 52: GUS 36.3%; UST 17.3% | At wk 52: GUS 38.8%; UST 19.0% | ||
| (p<0.001) | (p=0.003) | (p<0.001) | |||
| ECLIPSE | At wk 12: GUS: 89.3%; SEC 91.6% | At wk 12: GUS 69.1%; SEC 76.1% | At wk 12: NR | At wk 12: NR | NR |
| At wk 48: NR | At wk 48: GUS 84.5%; SEC 70.0% | At wk 48: GUS 58.2%; SEC 48.4% | At wk 48: GUS 85.0%; SEC 74.9% | ||
| (p<0.001) | |||||
Nonresponder imputation was used to assess binary endpoint missing data. All comparisons were made with guselkumab, and p-value represents the significance value of this comparison.
ADA, adalimuma; GUS, guselkumab; NR, not reported; PL, placebo; SEC, secukinumab; UST, ustekinumab; wk, week(s).
Regional psoriasis was also evaluated and analysed in this trial. For this purpose, investigators used scores such as scalp-specific IGA (ss-IGA), Nail Psoriasis Severity Index (NAPSI), fingernail Physician’s Global Assessment (PGA) (f-PGA), and PGA of the hands and/or feet (hf-PGA). A significantly greater proportion of patients treated with guselkumab reached an ss-IGA score of 0/1 compared with placebo at week 16 (83.4 versus 14.5%; p<0.001). Using the same score, guselkumab also showed significantly higher efficacy than adalimumab at weeks 24 (84.5% in guselkumab group versus 69.2% in adalimumab group) and 48 (78.3% in guselkumab group versus 60.5% in adalimumab group) (both p<0.001).34 Compared to placebo at week 16, the guselkumab group had a higher mean per cent improvement in the NAPSI score and a greater proportion of patients achieving an f-PGA score of 0/1. Compared to adalimumab, the results in the f-PGA score were similar at week 24 but significant differences were observed at week 48 in patients treated with guselkumab (p=0.038).34 There were no differences between guselkumab and adalimumab groups concerning the NAPSI score, measured at weeks 24 and 48.34 A higher percentage of patients receiving guselkumab, comparing to those receiving placebo, achieved the hf-PGA score of 0/1 at week 16 (73.3 versus 14.0%; p<0.001). Regarding to the same score, guselkumab-treated patients also had better responses when compared to adalimumab-treated patients at week 24 (78.9 versus 56.8%; p<0.001) and week 48 (75.6 versus 62.1%; p<0.045).34
Health-related quality of life (HRQoL) was also evaluated with patient-reported outcomes using the Psoriasis Symptoms and Signs Diary (PSSD) and the Dermatology Life Quality Index (DLQI). Mean changes in the PSSD score from baseline for guselkumab-treated patients were higher than placebo at week 16 and higher than adalimumab at weeks 24 and 48 (p<0.001 for all three). It was possible to observe a significantly higher improvement from baseline DLQI in the group of patients who received guselkumab compared with placebo (−11.2±7.2 versus −0.6±6.4; p<0.001), with a concomitant higher proportion of patients achieving the DLQI score of 0/1 (56.3 versus 4.2%; p<0.001) at week 16. The investigators noted that guselkumab also outperformed adalimumab concerning both improvements from the baseline DLQI score (week 24 [−11.6±7.6 versus −9.5±7.9; p<0.001] and week 48 [−11.8±7.9 versus −9.2±8.3; p<0.001]) and achievement of the DLQI score of 0/1 (week 24 [60.9 versus 39.5%; p<0.001] and week 48 [62.5 versus 38.9%; p<0.001]).34
Still in VOYAGE 1, an open-label extension period began where patients who were having guselkumab as treatment agreed to continue to receive the drug every 8 weeks, whereas patients in the adalimumab group accepted to change to guselkumab at week 52 and every 8 weeks thereafter, after taking their last dose of adalimumab at week 47. At week 100, the proportion of patients who achieved an IGA score of 0/1, the IGA score of 0, PASI 75, PASI 90, and PASI 100 responses were 82.4, 53.8, 94.8, 82.1, and 49.0%, respectively. Placebo–guselkumab crossover and adalimumab–guselkumab crossover groups achieved similar results at week 100. It was concluded that efficacy was maintained through 2 years amongst patients who had continued treatment with guselkumab, and patients who changed from adalimumab to guselkumab after 1 year had improved efficacy at 2 years.47
In VOYAGE 2 trial, investigators assessed the efficacy and safety of guselkumab in moderate-to-severe psoriasis versus both placebo and adalimumab, including in the study one arm with discontinuation of guselkumab and another with switching adalimumab nonresponders to guselkumab35 (Table 1).
At week 16, comparing to the placebo group, a higher number of patients receiving guselkumab achieved an IGA score of 0/1 (84.1 versus 8.5%) and PASI 90 (70.0 versus 2.4%) response (coprimary endpoints) (both p<0.001). Guselkumab was also superior to adalimumab at weeks 16 and 24, as measured by the proportion of patients achieving an IGA score of 0/1 (week 16 [84.1 versus 67.7%] and week 24 [83.5 versus 64.9%]), PASI 75 (week 16 [86.3 versus 68.5%] and week 24 [89.1 versus 71.0%]), PASI 90 (week 16 [70.0 versus 46.8%] and week 24 [75.2 versus 54.8%]), and PASI 100 (week 16 [34.1 versus 20.6%] and week 24 [44.2 versus 26.6%]) responses (all p<0.001). From weeks 28 to 48, a higher persistence of response (PASI≥90) was observed in the guselkumab maintenance group versus the withdrawal group (88.6 versus 36.8%; p<0.001). For withdrawal patients, the median time to loss of PASI 90 response was 15.2 weeks.35 Regarding the group of patients considered as nonresponders to adalimumab who switched to guselkumab, 66.1% achieved PASI 90 at week 48, and 28.6% achieved PASI 100. Guselkumab improved patient-reported outcomes (DLQI and PSSD), similar to what was already demonstrated in VOYAGE 1.35 For a more detailed analysis of the results, consult Table 2. The efficacy of guselkumab versus both placebo and adalimumab was confirmed in VOYAGE 2 trial, because the results were similar to those observed in VOYAGE 1.35
Gordon and colleagues48 evaluated the consistency of guselkumab’s efficacy across several subpopulations of patients with psoriasis (defined by baseline demographics, disease characteristics, and previous exposure to treatments) using pooled information from VOYAGE 1 and VOYAGE 2 trials. Guselkumab provided significant benefit across almost all subpopulations, showing a more consistent response amongst lighter and heavier patients when comparing to adalimumab.48
NAVIGATE
NAVIGATE was a phase III, randomized, double-blind trial that assessed the clinical efficacy of guselkumab in patients with moderate-to-severe psoriasis who had a poor response to ustekinumab.36 For study design, consult Table 1. Amongst the initial 871 patients, 585 (67%) with an IGA score of 0/1 at week 16 continued to receive open-label ustekinumab.36 The investigators defined the number of visits in which randomized patients achieved an IGA score of 0/1 and at least a two-grade improvement in the IGA score (comparing to the IGA score at week 16) from week 28 through week 40 as the primary endpoint.36
The number of visits in which randomized patients achieved IGA of 0/1 was significantly higher in the group of patients receiving guselkumab versus the group receiving ustekinumab (1.5±1.6 versus 0.7±1.3; p≤0.001).36 Langley and colleagues36 also observed that a higher proportion of patients treated with guselkumab achieved an IGA score of 0/1 with an at least two-grade improvement at weeks 28 (31.1 versus 14.3%; p=0.001) and 52 (36.3 versus 17.3%; p<0.001). Investigators also noted that a greater proportion of patients treated with guselkumab, comparing to those receiving ustekinumab, achieved PASI 90 (51.1 versus 24.1%) and PASI 100 (20.0 versus 7.5%) responses (both p<0.005), as well as the DLQI score of 0/1 (38.8 versus 19.0%) at week 52.36 For a more detailed analysis of the results, see Table 2. This clinical trial gave us the opportunity to conclude that patients treated with ustekinumab who had a poor response at week 16 derived significant benefit from switching to guselkumab.36
ECLIPSE
A phase III randomized, double-blind, head-to-head trial called ECLIPSE was designed to assess guselkumab’s efficacy and safety compared to secukinumab in patients with moderate-to-severe plaque psoriasis. For study design, consult Table 1.
The study demonstrated that guselkumab was superior to secukinumab for the primary endpoint assessed at week 48; that is, achievement of at least PASI 90 response (84.5% with guselkumab versus 70.0% with secukinumab; p<0.001).38 For a more detailed analysis of the results, see Table 2.
ECLIPSE incorporated six secondary endpoints evaluated at weeks 12 and 48. Investigators used a fixed statistical sequence procedure to control for multiple comparisons. At both weeks 12 and 48, 84.6% of patients receiving guselkumab achieved a PASI 75 response (versus 80.2% in the secukinumab group), demonstrating that guselkumab was not inferior to secukinumab in the first major secondary endpoint (p<0.001). However, it failed to demonstrate superiority and, with this in mind, p-values for all the subsequent major secondary endpoints were considered nominal.38
Another three major secondary endpoints were evaluated at week 48. Compared to secukinumab, a higher proportion of patients in guselkumab group achieved an IGA score of 0/1 (85.0 versus 74.9%), IGA of 0 (62.2 versus 50.4%), and PASI 100 (58.2 versus 48.4%) response.38
The remaining major secondary endpoints were assessed at week 12. Moreover, 89.3% of patients receiving guselkumab achieved a PASI 75 response, against 91.6% of patients receiving secukinumab. The proportion of patients achieving a PASI 90 response was 69.1% for guselkumab and 76.1% for secukinumab.38
Safety and tolerability of guselkumab
Guselkumab was generally well tolerated in adults with moderate-to-severe plaque psoriasis in all pivotal trials.34–36,38 Data from other phase I, II, and III trials support this information32,33,46 and will not be discussed further.
In VOYAGE 1, investigators reported comparable adverse events (AEs) amongst patients in the different treatment groups. Nasopharyngitis and upper respiratory tract infection were the most common adverse events reported.34 In addition to the fact that serious AEs and AEs leading to the discontinuation of the agent were uncommon, these events occurred in similar proportions for each treatment group throughout the study. Overall infections and infections requiring antibiotic treatment occurred at comparable rates across all treatment groups through week 48.34 By week 16, two major adverse cardiac events (MACEs) occurred, one in each of the guselkumab and adalimumab groups, and one patient receiving guselkumab developed a basal cell carcinoma. Between weeks 16 and 48, four cases of serious infections were reported: two in the group of patients receiving guselkumab (one thigh abscess and one cellulitis with postoperative wound infection) and two in the group of patients receiving adalimumab (one abdominal abscess and one fatal staphylococcal pneumonia). Two malignancies (breast and prostate cancers), both in the guselkumab group, and two basal cell carcinomas, one in each of the guselkumab and adalimumab groups, were observed up to week 48. No additional MACEs were reported after week 16. There were no differences between groups in the incidence of neutropenia and candidiasis, which was low in all treatment groups. There were no differences between groups regarding the incidence of laboratory abnormalities, which was also low. No Crohn’s disease-related events were reported.34
VOYAGE 2 corroborated the safety data observed in VOYAGE 1 regarding the comparable proportion of patients with one or more AE, AEs leading to discontinuation, and serious AEs in placebo and guselkumab groups through week 16.35 The most commonly reported events during placebo-controlled period (weeks 0–16) were nasopharyngitis, headache, and upper respiratory tract infection. That was the same pattern of AEs that were observed during the active-comparator period (weeks 0–28). No malignancies or nonmelanoma skin cancers (NMSCs) were reported through week 16. One MACE, specifically a myocardial infarction, occurred in the group of patients receiving the active-comparator adalimumab during placebo-controlled period. A higher proportion of patients treated with adalimumab had Injection site reactions (ISR) (6.9 versus 2.6%).35 Concerning to the active-comparator period, three serious infections were observed in each of the active treatment groups (guselkumab group: bronchitis, erysipelas, and soft tissue infection; adalimumab group: two cases of tuberculosis and one injection-site abscess). One malignancy (prostate cancer) and two NMSCs (one squamous cell carcinoma in the group of patients receiving guselkumab and one basal cell carcinoma in the placebo–guselkumab crossover group) were also reported. One patient in each of guselkumab- and adalimumab-treated groups developed a MACE through week 28.35 From weeks 28 to 48, one serious infection was reported in the maintenance group (appendicitis). During this randomized withdrawal and retreatment period (weeks 28–48), one case of MACE was reported in the placebo–guselkumab crossover group. One additional basal cell carcinoma and one squamous cell carcinoma were noted in the same placebo–guselkumab group through week 48. Similar to what was observed in VOYAGE 1 results, the incidence of laboratory abnormalities was low and comparable between groups.35
No new signs that would endanger the safety of the drug were identified during the long-term treatment (through 100 weeks) with open-label extension with guselkumab in VOYAGE 1 and VOYAGE 2.49
NAVIGATE brought no new safety data regarding patients who switched from ustekinumab to guselkumab without a washout period. Moreover, 64.0% of patients randomized to guselkumab had one or more AEs (versus 56.0% in ustekinumab group). Serious AEs were reported in 7% of patients treated with guselkumab (versus 5% with ustekinumab), and 2% of each group discontinued treatment due to an AE.36
In the ECLIPSE trial, the safety profiles observed for both drugs (guselkumab and secukinumab) were consistent with the information already presented in the respective registration trials. Similar percentage of patients reported at least one AE (77.9% with guselkumab versus 81.6% with secukinumab). Serious AEs were reported in 6.2% of patients receiving guselkumab (versus 7.2% of patients receiving secukinumab). Investigators documented six serious infections in guselkumab-treated patients (versus five in the secukinumab group).38
Immunogenicity
Zhuang and colleagues37 reported that 1 out of 20 (5.0%) patients in the second part of the phase I trial (which included patients with psoriasis) had positive antibodies to guselkumab. The incidence of antibodies to guselkumab reported in X-PLORE was 6.0%.33 However, they were nonneutralizing and had low titres.33 VOYAGE 1, VOYAGE 2, and NAVIGATE reported rates of antibody development of 5.3% through week 44, 6.6% through week 48, and 9.0% through week 60, respectively. No association between antibody incidence and reduced efficacy was noted.34–36
Other indications
A phase III, single‐arm, open‐label, multicentre trial conducted in Japan with 21 patients demonstrated the clinical benefit of using guselkumab in the treatment of other types of psoriasis, such as GPP and EP.50 Its efficacy was verified throughout the study period (52 weeks), and the safety profile of guselkumab was consistent with data already published in psoriasis. Sano and colleagues50 concluded that there would be a favourable risk–benefit profile for treating both GPP and EP patients with the selective IL-23 inhibitor. Nevertheless, further studies are required to confirm this effectiveness.
In a phase II randomized, double-blind, placebo-controlled trial, guselkumab demonstrated significant improvement in joints’ symptoms, physical function, enthesitis, dactylitis, and quality of life in patients with psoriatic arthritis.51 Two phase III trials are now underway to ascertain the efficacy and safety of guselkumab in patients who suffer from this pathological condition, either biologic-naive (NCT03158285) or previously treated with TNF-α inhibitors (NCT03162796).
Smolen and colleagues45 conducted a phase II trial that evaluated the safety and efficacy of guselkumab in patients suffering from active rheumatoid arthritis despite concomitant treatment with methotrexate. No statistically significant differences in the percentage of patients who achieved an American College of Rheumatology (ACR)-20 response between those who received guselkumab and the control group were found.45
To date, no published studies demonstrate the impact of guselkumab in the treatment of Crohn’s disease. However, ustekinumab, as a nonselective IL-23 inhibitor, has been shown to be effective treating this disease.52,53 Currently, a clinical trial is ongoing (GALAXI [NCT03466411]), and is recruiting patients and pretends to evaluate the efficacy and safety of guselkumab in participants with Crohn’s disease.
Discussion
As previously mentioned, the discovery of the IL-23/IL-17 axis has led to a substantial increase in our knowledge of the pathogenic immune events present in psoriasis and to shift the paradigm of the management of this condition. IL-17 inhibitors were approved previously than IL-23 selective inhibitors for the treatment of moderate-to-severe psoriasis. However, several complications have been associated with these agents, raising the need for different therapeutic solutions.
Although the inhibition of IL-23 alone rather the coinhibition of both IL-23 and IL-12 presents as a novel mechanism of action, the favourable safety profile of guselkumab has not come as a surprise, because long-term data were available for ustekinumab.53,54 However, allowing the IL-12/Th1 axis to remain intact, as is the case with guselkumab, may be a net-positive effect because the cytokines involved in this pathway contribute vitally to the hosts’ defence through their ability to stimulate both innate and adaptive immune effector cells against intracellular microbial infection and malignant cells.55–57 These cytokines also allow the initiation of a protective transcriptional program in keratinocytes that will limit skin inflammation mediated by T17 cells.15,58 Treatment with an IL-23p19 inhibitor led to long-term responses in some patients with just a single dose32 or after a withdrawal after 28 weeks of contact with the drug (in VOYAGE 2, 36.8% of patients rerandomized to placebo sustained PASI 90 at week 48).35 This clinical response can be explained in part by the impact of IL-12 in promoting transdifferentiation of Th17 cells into regulatory T cell or Th1 populations.15,59
Although IL-23 and IL-39 (another proinflammatory cytokine likely expressed in psoriatic skin) share the p19 subunit, whether guselkumab can bind to or inhibit human IL-39 is unknown, and further studies are required to explore the individual role of this cytokine.20
Several reviews have been published regarding the role of guselkumab in the treatment of psoriasis.60–63 However, recent divulgation of the results of ECLIPSE reinforces the role of the selective IL-23 inhibitor concerning other already approved immunomodulators, such as the active-comparator secukinumab used in trial.38 Even though secukinumab achieved a slightly faster onset of response, detailed analysis with the response-over-time curves showed that maximum response rates with guselkumab are achieved later – after 6 months – and are maintained over time through 1 year, translating into superiority for the primary endpoint of the study (PASI 90 response).38 The superiority of guselkumab in comparison to secukinumab is also apparent in the less frequent dosing regimen of the selective IL-23 inhibitor (guselkumab: initially at weeks 0 and 4, but then every 8 weeks versus secukinumab: administered weekly for 4 weeks followed by every 4 weeks as a maintenance dosing). From the patients’ point of view, characteristics such as less frequent drug administration or the option of self-administration contribute to better adherence and thus better clinical outcomes.
Conclusion
Guselkumab is a monoclonal antibody that selectively targets IL-23, and it is the first in its class to be approved to treat moderate-to-severe plaque psoriasis. Its efficacy and safety profiles were reinforced by recent studies such as ECLIPSE, demonstrating great potential for long-term treatment of psoriasis. Long-term data and additional comparative studies will be essential for positioning guselkumab in the therapeutic armamentarium for psoriasis.
Acknowledgements
None.
Footnotes
Contributions: Both authors contributed equally to the preparation of this review. The named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: Miguel Nogueira has no conflicts of interest. Tiago Torres is a scientific consultant/speaker/clinical study investigator for AbbVie, Amgen, Boehringer Ingelheim, Biogen, Celgene, Janssen, LEO-Pharma, Eli-Lilly, MSD, Novartis, Pfizer, Samsung Bioepis, Sanofi. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at http://www.drugsincontext.com/wp-content/uploads/2019/06/dic.212594-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2019 Nogueira M, Torres T. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/guselkumab-for-the-treatment-of-psoriasis-evidence-to-date/
Provenance: invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 6 June 2019
References
- 1.Di Meglio P, Villanova F, Nestle FO. Psoriasis. Cold Spring Harb Perspect Med. 2014;4(8):1–30. doi: 10.1101/cshperspect.a015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths CEM, van der Walt JM, Ashcroft DM, et al. The global state of psoriasis disease epidemiology: a workshop report. Br J Dermatol. 2017;177(1):e4–e7. doi: 10.1111/bjd.15610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Federation of Psoriasis Associations. World Psoriasis Day. 2015. [Accessed 7 April 2019]. https://ifpa-pso.com/our-actions/world-psoriasis-day.
- 4.Rapp SR, Feldman SR, Exum ML, Fleischer ABJ, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 Pt 1):401–407. doi: 10.1016/S0190-9622(99)70112-X. [DOI] [PubMed] [Google Scholar]
- 5.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jindal S, Jindal N. Psoriasis and cardiovascular diseases: a literature review to determine the causal relationship. Cureus. 2018;10(2):e2195. doi: 10.7759/cureus.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y, Lee C-H, Chi C-C. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154(12):1417–1423. doi: 10.1001/jamadermatol.2018.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31(12):1999–2009. doi: 10.1111/jdv.14460. [DOI] [PubMed] [Google Scholar]
- 10.Sulzberger M, Witten V. The effect of topically applied compound F in selected dermatoses. J Invest Dermatol. 1952;19(2):101–102. doi: 10.1038/jid.1952.72. [DOI] [PubMed] [Google Scholar]
- 11.Mueller W, Hermann B. Cyclosporin A for psoriasis. N Engl J Med. 1979;301(10):555. doi: 10.1056/NEJM197909063011015. [DOI] [PubMed] [Google Scholar]
- 12.Winterfield LS, Menter A, Gordon K, Gottlieb A. Psoriasis treatment: current and emerging directed therapies. Ann Rheum Dis. 2005;64(Suppl 2):87–90. doi: 10.1136/ard.2004.032276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres T. Selective interleukin-23 p19 inhibition: another game changer in psoriasis? Focus on risankizumab. Drugs. 2017;77(14):1493–1503. doi: 10.1007/s40265-017-0794-1. [DOI] [PubMed] [Google Scholar]
- 14.Chan TC, Hawkes JE, Krueger JG. Interleukin 23 in the skin: role in psoriasis pathogenesis and selective interleukin 23 blockade as treatment. Ther Adv Chronic Dis. 2018;9(5):111–119. doi: 10.1177/2040622318759282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–653. doi: 10.1016/j.jaci.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatol Clin. 2015;33(1):13–23. doi: 10.1016/j.det.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27(1):485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 18.Res PCM, Piskin G, de Boer OJ, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One. 2010;5(11):e14108. doi: 10.1371/journal.pone.0014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaba LC, Krueger JG, Lowes MA. Resident and “inflammatory” dendritic cells in human skin. J Invest Dermatol. 2009;129(2):302–308. doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201(6):1605–1613. doi: 10.4049/jimmunol.1800013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 22.Yoon J, Leyva-Castillo JM, Wang G, et al. IL-23 induced in keratinocytes by endogenous TLR4 ligands polarizes dendritic cells to drive IL-22 responses to skin immunization. J Exp Med. 2016;213(10):2147–2166. doi: 10.1084/jem.20150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fragoulis GE, Siebert S, McInnes IB. Therapeutic targeting of IL-17 and IL-23 cytokines in immune-mediated diseases. Annu Rev Med. 2016;67(1):337–353. doi: 10.1146/annurev-med-051914-021944. [DOI] [PubMed] [Google Scholar]
- 24.Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of Ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 25.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 26.Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 27.Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286. doi: 10.1111/bjd.14493. [DOI] [PubMed] [Google Scholar]
- 28.Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177(4):1014–1023. doi: 10.1111/bjd.15666. [DOI] [PubMed] [Google Scholar]
- 29.Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. doi: 10.1016/j.jaad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths CEM, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551. doi: 10.1016/S0140-6736(15)60125-8. [DOI] [PubMed] [Google Scholar]
- 31.Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71(6):1183–1190.e3. doi: 10.1016/j.jaad.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 32.Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23–specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133(4):1032–1040. doi: 10.1016/j.jaci.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373(2):136–144. doi: 10.1056/NEJMoa1501646. [DOI] [PubMed] [Google Scholar]
- 34.Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 35.Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431. doi: 10.1016/j.jaad.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 36.Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178(1):114–123. doi: 10.1111/bjd.15750. [DOI] [PubMed] [Google Scholar]
- 37.Zhuang Y, Calderon C, Marciniak SJ, et al. First-in-human study to assess guselkumab (anti-IL-23 mAb) pharmacokinetics/safety in healthy subjects and patients with moderate-to-severe psoriasis. Eur J Clin Pharmacol. 2016;72(11):1303–1310. doi: 10.1007/s00228-016-2110-5. [DOI] [PubMed] [Google Scholar]
- 38.Langley R. Guselkumab demonstrates superior long-term responses to secukinumab at Week48 in the treatment of moderate to severe psoriasis: results from the ECLIPSE trial. 3rd Inflammatory Skin Disease Summit; Vienna, Austria. 2018. [Google Scholar]
- 39.Frampton JE. Tildrakizumab: a review in moderate-to-severe plaque psoriasis. Am J Clin Dermatol. 2019 Mar; doi: 10.1007/s40257-019-00435-9. [DOI] [PubMed] [Google Scholar]
- 40.Haugh IM, Preston AK, Kivelevitch DN, Menter AM. Risankizumab: an anti-IL-23 antibody for the treatment of psoriasis. Drug Des Devel Ther. 2018;12:3879–3883. doi: 10.2147/DDDT.S167149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markham A. Guselkumab: first global approval. Drugs. 2017;77(13):1487–1492. doi: 10.1007/s40265-017-0800-7. [DOI] [PubMed] [Google Scholar]
- 42.Ault A. Guselkumab (Tremfya) gets CHMP backing for plaque psoriasis. [Accessed 26 February 2019]. https://www.medscape.com/viewarticle/885745. Published 2017.
- 43.European Medicines Agency. Tremfya 100 mg: product information and public assessment report. [Accessed 1 March 2019]. https://www.ema.europa.eu/en/medicines/human/EPAR/tremfya. Published 2017.
- 44.Yao Z, Hu C, Zhu Y, et al. Population Pharmacokinetic modeling of guselkumab, a human IgG1λ monoclonal antibody targeting IL-23, in patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58(5):613–627. doi: 10.1002/jcph.1063. [DOI] [PubMed] [Google Scholar]
- 45.Smolen JS, Agarwal SK, Ilivanova E, et al. A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann Rheum Dis. 2017;76(5):831–839. doi: 10.1136/annrheumdis-2016-209831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45(9):1053–1062. doi: 10.1111/1346-8138.14504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffiths CEM, Papp KA, Kimball AB, et al. Long-term efficacy of guselkumab for the treatment of moderate-to-severe psoriasis: results from the phase 3 VOYAGE 1 trial through two years. J Drugs Dermatol. 2018;17(8):826–832. [PubMed] [Google Scholar]
- 48.Gordon KB, Blauvelt A, Foley P, et al. Efficacy of guselkumab in subpopulations of patients with moderate-to-severe plaque psoriasis: a pooled analysis of the phase III VOYAGE 1 and VOYAGE 2 studies. Br J Dermatol. 2018;178(1):132–139. doi: 10.1111/bjd.16008. [DOI] [PubMed] [Google Scholar]
- 49.Reich K, Papp KA, Armstrong AW, et al. Safety of guselkumab in patients with moderate-to-severe psoriasis treated through 100 weeks: a pooled analysis from the randomized VOYAGE 1 and VOYAGE 2 studies. Br J Dermatol. 2019;180(5):1039–1049. doi: 10.1111/bjd.17454. [DOI] [PubMed] [Google Scholar]
- 50.Sano S, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45(5):529–539. doi: 10.1111/1346-8138.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391(10136):2213–2224. doi: 10.1016/S0140-6736(18)30952-8. [DOI] [PubMed] [Google Scholar]
- 52.Deepak P, Sandborn WJ. Ustekinumab and anti-interleukin-23 agents in Crohn’s disease. Gastroenterol Clin North Am. 2017;46(3):603–626. doi: 10.1016/j.gtc.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Verstockt B, Deleenheer B, Van Assche G, Vermeire S, Ferrante M. A safety assessment of biological therapies targeting the IL-23/IL-17 axis in inflammatory bowel diseases. Expert Opin Drug Saf. 2017;16(7):809–821. doi: 10.1080/14740338.2017.1338273. [DOI] [PubMed] [Google Scholar]
- 54.Papp KA, Griffiths CEM, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168(4):844–854. doi: 10.1111/bjd.12214. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Cao S, Kim S, et al. Interleukin-12: an update on its immunological activities, signaling and regulation of gene expression. Curr Immunol Rev. 2005;1(2):119–137. doi: 10.2174/1573395054065115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kleinschek MA, Muller U, Brodie SJ, et al. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol. 2006;176(2):1098–1106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- 57.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442(7101):461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 58.Kulig P, Musiol S, Freiberger SN, et al. IL-12 protects from psoriasiform skin inflammation. Nat Commun. 2016;7(1):13466. doi: 10.1038/ncomms13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gagliani N, Vesely MCA, Iseppon A, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523(7559):221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Salama ZT, Scott LJ. Guselkumab: a review in moderate to severe plaque psoriasis. Am J Clin Dermatol. 2018;19(6):907–918. doi: 10.1007/s40257-018-0406-1. [DOI] [PubMed] [Google Scholar]
- 61.Machado A, Torres T. Guselkumab for the treatment of psoriasis. BioDrugs. 2018;32(2):119–128. doi: 10.1007/s40259-018-0265-6. [DOI] [PubMed] [Google Scholar]
- 62.Yang EJ, Sanchez IM, Beck K, Sekhon S, Wu JJ, Bhutani T. Guselkumab for the treatment of moderate-to-severe plaque psoriasis. Expert Rev Clin Pharmacol. 2018;11(4):333–344. doi: 10.1080/17512433.2018.1445967. [DOI] [PubMed] [Google Scholar]
- 63.Galluzzo M, D’Adamio S, Campione E, Bianchi L, Talamonti M. A safety evaluation of guselkumab for the treatment of psoriasis. Expert Opin Drug Saf. 2018;17(7):741–751. doi: 10.1080/14740338.2018.1488963. [DOI] [PubMed] [Google Scholar]